Abstract
The antioxidant activity and nutritional composition of four dehydrated soups (vegetables, meat, chicken and fish) packaged in four formats — carton, plastic, and aluminium bags (the last with and without modified atmosphere) — were evaluated during 12 months’ storage. The results showed that all four soups had a good or very good antioxidant capacity as tested by the lipid peroxidation, deoxyribose, and Trolox equivalent antioxidant capacity (TEAC) tests. Of interest from a nutritional point of view was the finding that the lipid fraction of all the soups was below 1 %. The sodium content of the four soups and their ingredients was also analysed. By modifying some of the ingredients, a 25 % reduction in the sodium content of the soups was obtained, permitting them to be labelled as “sodium reduced”. The monosodium glutamate (MSG) content of the reformulated soups (lower sodium content) was below levels permitted by European legislation.
Keywords: Antioxidant, Nutritional, Dehydrated soup, Glutamate, Sodium content healthy
Introduction
Diet and nutrition are very important aspects for promoting and maintaining health throughout life. Soup, which stimulates saliva secretion and gastric peristalsis of the stomach, is recommended for inclusion in the human diet to help reduce energy intake and promote weight loss. The increase in satiety that it induces is due to the combined effect of delayed gastric emptying, which causes gastric distension and the rapid availability of the nutrients, which intensifies the glycaemic response (Clegg et al. 2013). Soup is used as a complementary food in diets. Soup has been evaluated for its effect in the regulation of plasmatic lipids (Lin et al. 2011), and even oxidative stress (Martínez-Tomás et al. 2012) due to the antioxidants it provides (Murcia et al. 2009).
However, processed foods, a term which includes soups, are the main contributors to salt (NaCl) ingestion by the general public and the relation between high sodium consumption, and pathologies like high blood pressure and cardiovascular diseases is increasingly evident (EFSA 2005). Yassibas et al. (2012) found that the frequent consumption of salty foods like pickled foods soups and sauces was associated with an increased risk of stomach cancer. For this reason, the general public is recommended to reduce their salt intake (RDI 5 g NaCl/day) and the food industry, especially, to reduce the salt content of processed foods (EFSA 2005). However, reducing the salt added to foods would affect the flavour intensity, which would probably entail a reduction in consumption (Mitchell et al. 2011). One alternative would be to substitute sodium chloride by other salts, such as potassium chloride or calcium chloride, although this would make food unsuitable for people with renal insufficiency. Another alternative would be to use flavour enhancers, such as MSG.
MSG (E621) is widely used in the food industry as a flavour enhancer, sometimes denominated “umami” or the fifth flavour. Sensory analyses have demonstrated that MSG increases the perception of salty flavours in food, and can particularly increase the palatability of soups formulated with a low sodium content (Insawang et al. 2012). Moreover, physiological and metabolic studies have shown that MSG speeds gastric emptying (Clegg et al. 2013).
The objective of this study was to evaluate the antioxidant activity and nutritional composition of four differently flavoured dehydrated soups (vegetables, meat, chicken and fish) and to ascertain whether these parameters were maintained during storage in different types of packaging (carton, plastic, and aluminium bags, the last with and without modified atmosphere). Our second goal was to see whether the sodium content of such soups could be decreased by 25 % to obtain healthier products.
Material and methods
Material
The chemicals used in the assays were of chromatographic quality and supplied by Sigma Chemical Co (Poole, Dorset, UK).
The samples under study, four dehydrated soups (vegetables, meat, chicken and fish) and their ingredients were provided by the company Paprimur SL, Murcia. Table 1 shows the list of ingredients of the soups, which were packaged in four forms: carton, plastic (polyethylene terephthalate, PET) and aluminium bags, the last in normal atmosphere and modified atmosphere conditions. In the modified atmosphere packaging (MAP) consisted of N2 (97 %) and CO2 (3 %).
Table 1.
Ingredient list of four dehydrated flavoured soups (vegetable, meat, chicken and fish)
| Soups | Ingredients |
|---|---|
| Vegetable | Salt, maltodextrin, sodium glutamate (E-621), disodium guanylate (E-627), disodium inosinate (E-631), modified corn starch, palm fat, onion, celery, garlic, parsley, hydrolyzed vegetable protein, yeast extract, vegetable flavouring, turmeric and caramel (E-150) |
| Meat | Salt, maltodextrin, sodium glutamate (E-621), disodium inosinate (E-631), disodium guanylate (E-627), modified corn starch, yeast extract, palm fat, meat flavouring, celery, onion, garlic, parsley, caramel (E-150) and turmeric |
| Chicken | Salt, maltodextrin, sodium glutamate (E-621), modified corn starch, hydrolyzed vegetable protein, palm fat, yeast extract, onion, garlic, celery, parsley, chicken flavouring, turmeric and caramel (E-150) |
| Fish | Salt, maltodextrin, sodium glutamate (E-621), disodium inosinate (E-631), disodium guanylate (E-627), modified corn starch, palm fat, onion, garlic, parsley, seafood flavouring, yeast extract fish, turmeric, caramel (E-150) and citric acid (E-330) |
The packaged samples were stored for a year at room temperature, imitating domestic storage, to assess their nutritional composition and oxidative behaviour. The four soups (in dehydrated form) and their ingredients were analyzed at the start and every 3 months, making a total of 5 time points.
Antioxidant evaluation
Peroxidation of phospholipid liposomes
The ability of samples to inhibit lipid peroxidation at pH 7.4 was tested using ox brain phospholipid liposomes, essentially as described in Murcia et al. (2009). The experiments were conducted in a physiological saline buffer (3.4 mM Na2HPO4-NaH2PO4 0.15 M NaCl), pH 7.4. In a final volume of 1 mL, the assay mixtures were made up with PBS, 0.5 mg/mL phospholipid liposomes, 100 μM FeCl3, 100 mg of samples, and 100 μM ascorbate (added last to start the reaction). The samples were incubated at 37 °C for 60 min, after which 1 mL each of 1 % (wt/v) TBA (thiobarbituric acid) and 2.8 % (wt/v) trichloroacetic acid were added to each mixture. The solutions were heated in a water bath at 80 °C for 20 min to develop the MDA(malondialdehyde)-TBA adduct [(TBA)2-MDA)]. The (TBA)2-MDA chromogen was extracted into 2 mL of butan-1-ol, and the extent of peroxidation was measured in the organic layer as absorbance at 532 nm.
Hydroxyl radical scavenging
In a final volume of 1.2 mL, the reaction mixtures contained the following reagents: 10 mM KH2PO4-KOH buffer (pH 7.4), 2.8 mM H2O2, 2.8 mM deoxyribose, 50 μM FeCl3 premixed with 100 μM EDTA (ethylenediaminetetraacetic acid) before addition to the reaction mixture, and 100 mg of the tested samples. Ascorbate (100 μM), where used, was added to start the reaction. The tubes were incubated at 37 °C for 1 h. The products of the hydroxyl radical (OH·) attack upon deoxyribose were measured as detailed in Jiménez-Monreal et al. (2008).
Measurement of total antioxidant activity by TEAC assay
The ABTS·- (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical solution was generated from the following reagents: 2.5 mM ABAP (2,2′-azobis-(2-amidinopropane) dihydrochloride) and 20 mM ABTS2− stock solution in phosphate buffer solution (containing 100 mM phosphate and 150 mM NaCl, pH 7.4). These were incubated at 60 °C for 12 min, protected from light. The absorbance at 734 nm was measured to check ABTS·- formation (the results must be between 0.35 and 0.45). The antioxidant activity of the samples analyzed (40 mg mixed with 1960 μL of the radical solution) was measured at 734 nm for 6 min. The decrease in absorption at 734 nm (observed 6 min after the addition of each compound) was used to calculate the TEAC. A calibration curve was prepared with different concentrations of Trolox (hydrosoluble vitamin E analogue), the standard solution used to evaluate equivalent antioxidant capacity, according to the experimental conditions described in Jiménez-Monreal et al. (2008).
Rancimat test for oxidative stability
The Rancimat method (Metrohm model 743, Herisan, Switzerland) determines the IP (Induction Period) by measuring the increase in the volatile acidic byproducts released from the oxidizing oil at 120 °C. The concentration of the degradation products, which are transferred into distilled water, is assessed by measuring the conductivity. Longer IPs suggest a stronger activity of the added antioxidants. The relative activity of the antioxidants is expressed by the PF (Protection Factor), which is calculated by dividing the IP of sunflower oil with the added of samples by the IP of the control (sunflower oil alone) (Jiménez-Monreal et al. 2008).
Nutritional composition analysis
Moisture content
Moisture was determined according to the AOAC method Official Methods of Analysis reference 945.15 (AOAC 2000).
Ash content
Ash was determined according to the AOAC method Official Methods of Analysis reference 923.03 (AOAC 2000).
Protein content
For the determination of proteins, we followed the AOAC method using a Carlo Erba model AE 1108 elemental analyser with a Porapack QS (25 cm) gas chromatography (GC) column and a Thermic Conductivity Detector. Standard sample materials (EDTA, nicotinic acid, tryptophan, lysine and HCl) were placed in tared standard tin capsules for calibration and performance testing. Helium at 1.94 × 10−3 l s−1 was used as carrier gas; the reactor temperature was 1020 °C. The chromatographic oven temperature was 65 °C and the filament temperature was 190 °C. Samples weighing 1 mg were processed in these conditions, using V2O5, WO3 and MgO as additives. The range was from 0.01 to 100 %, with a standard deviation of 0.001 %. The results of the nitrogen content were multiplied by 6.25 to obtain the protein percentage (Murcia et al. 2003).
Fat content
Fat was determined by the Soxhlet method according to AOAC method Official Methods of Analysis reference 945.16 (AOAC 2000).
Carbohydrate content
The carbohydrate data were obtained by difference of rest of nutrients (lipids, proteins, moisture and ash).
Sodium and MSG content
Sodium analysis
The sodium content was determined by flame photometer (Make-Elico-Model CL220), which it is a simple and relatively inexpensive method. This technique uses a flame that evaporates the solvent and also sublimates and atomizes the metal and excites a valence electron to an upper energy state. The sample is introduced to the flame at a constant rate. A standard curve with a sodium concentration between 5 and 30 ppm was established daily. The sodium content in appropriately diluted food sample was determined against the standard curve (Chen et al. 2005).
To calculate the theoretical salt content, the sodium content mentioned in the technical specifications for each ingredient and the contribution of each of the ingredients to the final composition of the soups were taken into account.
MSG analysis
The MSG was analysed by high-performance liquid chromatography (HPLC). For the HPLC analysis, borate buffer (0.4 M) was added, followed by 20 μL of 2-mercaptoethanol and 20 μL of MSG made up to 10 mL. The derivatised MSG solution (20 μL) was injected into the HPLC column 1 min after preparation. The HPLC analysis was carried out using a Jasco PV-1580 instrument with a ThermoHypersil-Keystone Hypersil ODS column (250 × 4.6 mm, 5 μm) at room temperature. The excitation and emission wavelengths were set at 330 nm and 455 nm, respectively, to detect the derivatised MSG. An UV-visible detector KNK-029-757 Shimadzu was used. The analysis was carried out using a mobile phase of methanol phosphate buffer (35:65, v/v) at pH 5.8 (Muslin et al. 2012).
Statistical analysis
All experiments were carried out in triplicate. The results were analysed using the Statistical Package for Social Sciences Windows (SPSS) 22.0 and the analysis of variance (ANOVA) procedure. Fisher’s least significant difference (LSD) multiple range test was used to discriminate between means.
Results and discussion
Table 2 shows the antioxidant activity of the 4 dehydrated soups (vegetables, meat, chicken and fish) packaged in four different formats — carton, plastic, and aluminium bags (the last with and without modified atmosphere). The antioxidant assays used were the lipid peroxidation, deoxyribose, TEAC and Rancimat tests. The samples were stored for 12 months at room temperature, although Table 2 only shows the antioxidant activity evaluated at the initial time. As regards lipoperoxyl radical scavenging, the meat flavoured soup stood out from the other three (p < 0.05), with an inhibition percentages of around 70 %. The results also showed the capacity of the dehydrated soups to scavenge the hydroxyl radical, with values close to 90 % inhibition, values that were very similar in the vegetable, chicken and fish flavoured soups but lower, with significant differences, in the meat flavoured soup (p < 0.05). Good antioxidant activity was also observed from the TEAC values, which ranged between 6 and 8, with some significant differences (p < 0.05). Finally, as regard oxidative stability or oxidative protection, the Rancimat test showed that the four dehydrated soups had a protection factor close to 1, indicating low oxidative protection, although the fish flavoured soup had the highest value (p < 0.05).
Table 2.
Determination of antioxidant activity in four dehydrated flavoured soups (vegetable, meat, chicken and fish) packed in carton, plastic and aluminum bags (normal atmosphere) and aluminum bags (modified atmosphere), evaluated by different antioxidant assays (lipid peroxidation, deoxyribose, TEAC and Rancimat test)
| Antioxidant activity | |||||
|---|---|---|---|---|---|
| Packed | Carton | Plastic | Aluminum (normal atmosphere) | Aluminum (modified atmosphere) | |
| Soup | |||||
| Lipid peroxidationa | Vegetable | 50.0 ± 0.8a | 49.0 ± 1.8b | 57.4 ± 3.8b* | 48.8 ± 5.1a |
| Meat | 74.2 ± 6.1b | 78.4 ± 1.2c | 78.8 ± 2.1c | 72.2 ± 5.5b | |
| Chicken | 47.5 ± 2.8a | 41.3 ± 2.8a | 47.8 ± 2.7a | 42.3 ± 4.7a | |
| Fish | 44.5 ± 0.6a | 43.9 ± 1.3a | 45.4 ± 3.7a | 43.0 ± 0.9a | |
| Deoxyribosea | Vegetable | 93.1 ± 0.5b | 91.8 ± 0.7b | 93.1 ± 0.9b | 93.6 ± 0.1b |
| Meat | 89.2 ± 1.1a | 88.5 ± 1.2a | 89.0 ± 0.5a | 89.6 ± 0.7a | |
| Chicken | 92.0 ± 0.2b | 92.9 ± 0.9b | 92.2 ± 0.6b | 92.5 ± 0.6b | |
| Fish | 92.3 ± 0.4b | 93.1 ± 0.2b | 92.6 ± 0.5b | 92.3 ± 0.2b | |
| ABTS radical scavenging expressed as TEAC valueb | Vegetable | 6.9 ± 0.3a | 7.0 ± 0.2b | 8.3 ± 0.2c* | 6.5 ± 0.5a |
| Meat | 7.3 ± 0.2a | 6.2 ± 0.4a* | 7.3 ± 0.3b | 7.4 ± 0.4b | |
| Chicken | 6.9 ± 0.3a | 6.7 ± 0.4ab | 5.9 ± 0.5a | 7.5 ± 0.4b* | |
| Fish | 8.0 ± 0.4b | 6.5 ± 0.4ab* | 8.0 ± 0.5bc | 7.8 ± 0.2b | |
| Rancimat Testc | Vegetable | 0.98 ± 0.02ab | 0.99 ± 0.02b | 0.97 ± 0.03ab | 0.98 ± 0.01b |
| Meat | 0.95 ± 0.01a | 0.97 ± 0.01a* | 0.95 ± 0.02a | 0.95 ± 0.01a | |
| Chicken | 0.97 ± 0.00ab | 0.99 ± 0.02b | 1.00 ± 0.01bc* | 0.96 ± 0.02ab | |
| Fish | 1.01 ± 0.04b | 1.02 ± 0.01c | 1.02 ± 0.01c | 0.98 ± 0.01b | |
All determinations were performed in triplicate and values shown are mean ± standard deviation. Statistical differences was analysed by ANOVA (p < 0.05). Different letters indicate significant differences among soups by Multiple Range Test
*Statistical differences was analyzed by ANOVA (p < 0.05). Effect of packaging on each flavoured soup
aValues expressed as percent inhibition
bTEAC is the micromolar concentration of Trolox solution showing the equivalent antioxidant capacity of the particular substance at 24 h
cValues expressed as protection factor PF = induction time sample sunflower oil / sunflower oil induction time
The antioxidant activity varied according to the assay used (Table 2), and it was not possible to establish any order for such activity, perhaps due to the difference in the chemical composition of the samples (different bioactive compounds) and the different means and radicals used in the assays. For example, polyphenol antioxidant activity will depend to a great extent on the chemical properties (disposition of the phenolic hydrogens as radical scavengers) and the nature of the radical used; hence, compounds such as flavonols will show a higher inhibition against lipid peroxide radicals than flavones. In the case of quercetin, this flavonol is seen to be more effective in the DPPH (2,2-diphenyl-1-picrylhydrazyl) and TEAC tests (Nuutila et al. 2003). The antioxidant activity will also depend on the lifetime and reactivity, peroxyl radicals being more stable and less reactive and hydroxyl radicals the most reactive. For this reason, many bioactive components of food show a higher capacity to scavenge hydroxyl than peroxyl radicals (Gülçin 2012); in fact, for the soups a higher inhibition percentage was obtained in the deoxyribose assay (scavenging hydroxyl radical) than in the lipidic peroxidation assay (capturing the peroxyl radical). These results support the usefulness of using different methods to capture the free radicals for the proper evaluation of the antioxidant activity in food. Indeed, Tiveron et al. (2012) observed a higher antioxidant activity in some vegetable foods using the ABTS method than with the FRAP (ferric reducing antioxidant power) assay.
Our results concerning the good antioxidant activity of the four dehydrated soups analysed agree with those obtained by Murcia et al. (2009) in dehydrated soups.
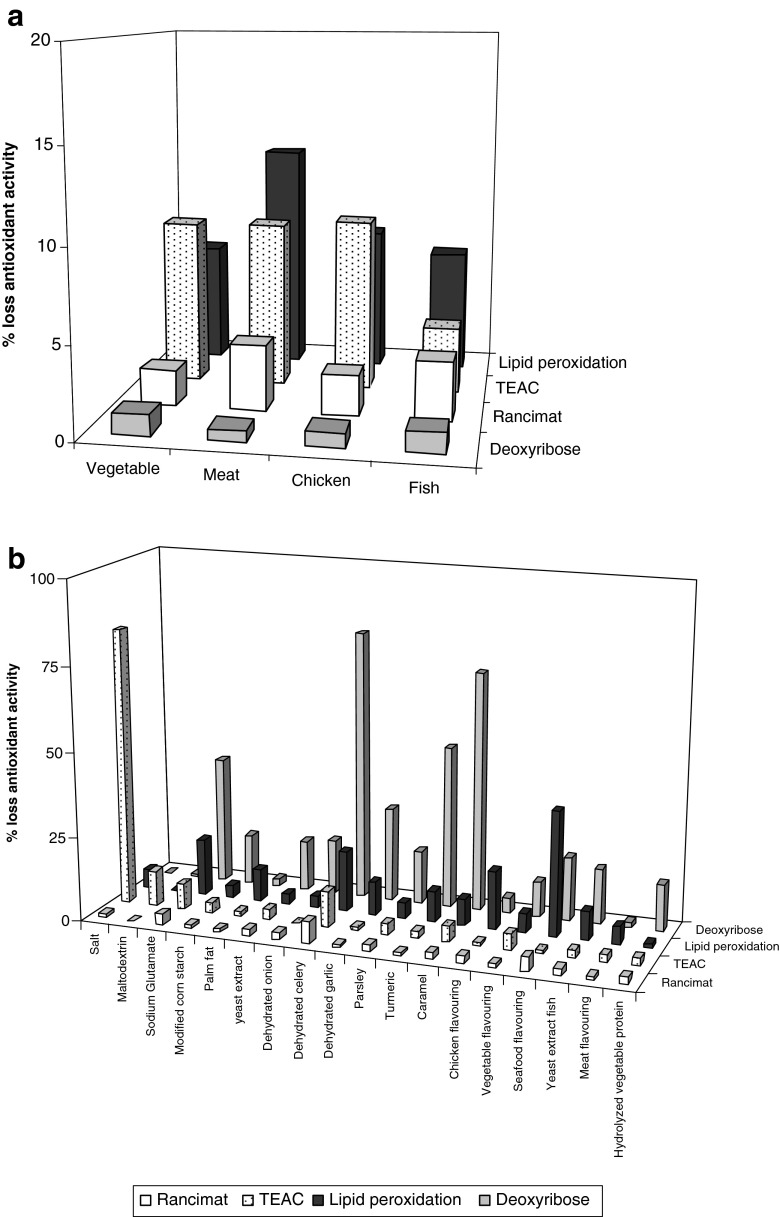
As regards changes in the antioxidant activity during 12 months’ storage (Fig. 1a), the antioxidant activity decreased, as measured by the capacity to scavenge the lipoperoxyl radical in the four soups, especially the meat flavoured dehydrated soup wich showed a loss of 12.9 % antioxidant activity. In the TEAC assay, the TEAC value diminished in all soups by around 9 %, except in the fish flavoured dehydrated soup, which showed the lowest losses (3.7 %). Finally, the losses of antioxidant activity were minimal in all four soups according to the Rancimat test (2.0–3.6 %) and deoxyribose assay (0.6–1.2 %). These results are agree with those of Jiménez-Monreal et al. (2008).
Fig. 1.
Loss of antioxidant activity during storage (12 months) in four dehydrated soups (a) and in their ingredients (b) expressed as percentage of losses
In our study, no variations were observed in the antioxidant activity between different containers (carton, plastic and aluminium bags with and without modified atmosphere), in the deoxyribose assay and lipid peroxidation (except the vegetable flavoured soup in normal atmosphere aluminium packaging, p < 0.05) in agreement with Pérez-Vicente et al. (2004) who observed no differences in foods stored in glass, brik cartons and brik aluminium, and Raitio et al. (2012) using containers with normal atmosphere and with MAP. However, the TEAC value of the meat flavoured and fish flavoured soups (p < 0.05) was lower in plastic bag than the rest of packed. Finally, the aluminium bag was the best (p < 0.05) in vegetable flavoured soup (normal atmosphere) and chicken flavoured soup (MAP). In the Rancimat assay, the best PF values with significant differences (p < 0.05) were found in the plastic bag for meat flavoured soup and in the normal atmosphere aluminium package for chicken flavoured soup.
Table 3 (which only shows the antioxidant activity evaluated at the initial time) represents the antioxidant activity determined in the ingredients for the 4 flavoured soups studied using the lipid peroxidation, deoxyribose, TEAC and Rancimat tests. In the lipidic peroxidation assay, dehydrated onion, palm fat and meat flavouring stood out, with inhibition percentages above 85 % (p < 0.05). In the deoxyribose assay, maltodextrin and palm fat showed very good OH radical scavenging capacity, with 93.2 and 91.8 %, respectively, differing significantly from the rest of the ingredients (p < 0.05). In the TEAC assay, all the ingredients except maltodextrin, glutamate and modified corn starch (p < 0.05) showed a good or very good antioxidant activity. However, in the Rancimat test, practically all of them showed a protection factor close to 1 (low oxidative protection capacity), with dehydrated garlic and celery, parsley, caramel and turmeric showing the best results in this respect (p < 0.05).
Table 3.
Determination of antioxidant activity in the ingredients of four dehydrated flavoured soups (vegetable, meat, chicken and fish), evaluated by different antioxidant assays (lipid peroxidation, deoxyribose, TEAC and Rancimat test)
| Antioxidant activity | ||||
|---|---|---|---|---|
| Ingredients | Lipid peroxidationa | Deoxyribosea | TEACb | Rancimatc |
| Salt (NaCl) | 59.3 ± 1.6f | -a | 6.8 ± 0.3c | 0.92 ± 0.08ab |
| Maltodextrin | -a | 93.2 ± 0.1 k | 3.8 ± 0.3b | 0.97 ± 0.04de |
| Sodium glutamate | 39.1 ± 1.9e | 47.4 ± 3.4 g | 1.3 ± 0.3a | 0.94 ± 0.02bcd |
| Modified corn starch | 27.5 ± 0.4d | 28.1 ± 3.6d | 3.4 ± 0.2b | 1.00 ± 0.06gh |
| Palm fat | 87.2 ± 1.0 h | 91.8 ± 1.4 k | 7.5 ± 0.4d | 1.00 ± 0.05fgh |
| Yeast extract | 41.5 ± 2.8e | 61.2 ± 2.2 h | 9.5 ± 0.3 fg | 1.01 ± 0.05 h |
| Dehydrated onion | 94.4 ± 0.6i | 34.0 ± 3.6e | 9.5 ± 0.1 fg | 0.92 ± 0.03abc |
| Dehydrated celery | 56.1 ± 3.2f | 16.0 ± 3.2b | 8.5 ± 0.4e | 1.07 ± 0.02j |
| Dehydrated garlic | 58.4 ± 2.1f | 37.9 ± 4.7f | 9.6 ± 0.2 g | 1.05 ± 0.14ij |
| Parsley | 58.6 ± 1.6f | -a | 9.6 ± 0.1 g | 1.05 ± 0.12ij |
| Turmeric | 69.5 ± 5.0 g | 28.4 ± 2.5d | 9.6 ± 0.1 g | 1.04 ± 0.14i |
| Caramel | 69.8 ± 1.8 g | 21.8 ± 3.8c | 8.2 ± 0.4e | 1.05 ± 0.04ij |
| Chicken flavouring | 12.7 ± 2.8b | 80.3 ± 1.0j | 9.6 ± 0.1 g | 0.97 ± 0.04efg |
| Vegetable flavouring | 42.2 ± 4.7e | 73.5 ± 1.0i | 8.5 ± 0.3e | 0.94 ± 0.07bc |
| Seafood flavouring | 21.1 ± 1.0c | 62.0 ± 1.8 h | 9.8 ± 0.1 g | 0.93 ± 0.06abc |
| Yeast extract fish | 55.9 ± 4.4f | 61.4 ± 2.4 h | 9.7 ± 0.2 g | 0.95 ± 0.04cde |
| Meat flavouring | 87.1 ± 3.7 h | 80.5 ± 0.6j | 9.8 ± 0.1 g | 0.97 ± 0.03ef |
| Hydrolyzed vegetable protein | 68.1 ± 1.9 g | 62.8 ± 3.4 h | 9.2 ± 0.3f | 0.91 ± 0.03a |
All determinations were performed in triplicate and values shown are mean ± standard deviation
Statistical differences was analysed by ANOVA (p < 0.05). Different letters indicate significant differences among soups by Multiple Range Test
aValues expressed as percent inhibition
bTEAC is the micromolar concentration of Trolox solution showing the equivalent antioxidant capacity of the particular substance at 24 h
cValues expressed as protection factor PF = induction time sample sunflower oil / sunflower oil induction time
- No antioxidant activity was detected
Foods with the highest concentration of antioxidants are of vegetable origin (Gülçin 2012) — fresh vegetables, dehydrated vegetables or even non-dehydrated soups (Murcia et al. 2009). When these ingredients (as maltodextrin, MSG (Kraboun et al. 2013), palm fat (Leow et al. 2013), dehydrated celery, turmeric and parsley, dehydrated onion and garlic (Murcia et al. 2009)) are used in our dehydrated soups, all of them valued with different antioxidant methods, could be responsible for the total antioxidant activity (Table 3).
The synergistic or antagonistic interactions between the different components present in the food may increase or decrease the final antioxidant activity of the product. Also thermal and mechanical treatments during the processing of foodstuffs can also affect the antioxidant activity, e.g. the heating step of canning can generate pro-oxidant components in celery (Murcia et al. 2009). The food matrices can be broken, facilitating the release solubilization of bioactive compounds, increasing their bioavailability (Maiani et al. 2009); also, the dehydration used may cause a loss of vitamin C (Gupta and Prakash 2008).
The evolution of the antioxidant activity of individual ingredients during the 12 months of storage is shown in Fig. 1b, expressed as a percentage of loss of antioxidant activity. In the case of lipoperoxyl radical scavenging, the highest losses were observed in seafood flavouring (37 %), dehydrated celery (18 %), chicken flavouring (17.3 %) and sodium glutamate (16.8 %). For the hydroxyl radical scavenging activity, losses were observed in dehydrated celery (80 %), caramel (71.1 %), turmeric (48.2 %) and sodium glutamate (37.8 %). Finally, in the TEAC and Rancimat assays, no important losses were observed, except in the salt, which showed a decrease of 82.3 % in antioxidant activity in the TEAC assay.
Compounds like melanoidins present in garlic can be generated during storage in a Maillard reaction (Martínez-Tomé et al. 2004), increasing the antioxidant activity, and the presence of metal ion chelators can act synergically with other antioxidant compounds, as seen in dehydrated onion and garlic (Gamboa-Santos et al. 2012), probably as a result of an increase in molecular mobility that allows the combination of radicals and their stabilization. However, in other studies of dehydrated food, an increase in the concentration of free radicals has been observed during storage, possibly because of an increase in lipidic oxidation (Murcia et al. 2009) or because of the loss of ascorbic (peroxyl radical scavenger) and beta carotenes with time (Singh et al. 2003a).
Table 4 shows the nutritional composition of the four dehydrated soups packed in a carton during 12 months storage. The main component was ash, with a mean value of 66 %, the meat and fish-flavoured soups having the highest values (p < 0.05), followed by the carbohydrate component, with a mean value of 22 %, the fish flavoured soup showing the highest value (p < 0.05). The results pointed to an average content of 9 % protein, except the fish flavoured soup where it was lower (p < 0.05). The least abundant components were lipids, with a mean value close to 1, with significant differences between them (p < 0.05).
Table 4.
Determination of the nutritional composition in four dehydrated flavoured soups (vegetable, meat, chicken and fish) packed and stored in carton for 12 months
| Percentage of Nutritional composition | ||||
|---|---|---|---|---|
| Soups | First day | 12 months | p | |
| Moisture | Vegetable | 2.1 ± 0.2a | 1.9 ± 0.3a | * |
| Meat | 1.5 ± 0.1a | 1.8 ± 0.2a | ||
| Chicken | 1.7 ± 0.6a | 2.1 ± 0.4a | ||
| Fish | 1.5 ± 0.2a | 2.4 ± 0.4a | ||
| Lipids | Vegetable | 0.8 ± 0.1a | 1.2 ± 0.3a | |
| Meat | 0.9 ± 0.1b | 1.0 ± 0.1a | ||
| Chicken | 1.1 ± 0.2c | 1.1 ± 0.1a | ||
| Fish | 0.9 ± 0.1b | 1.2 ± 0.2a | ||
| Proteins | Vegetable | 8.0 ± 0.8b | 8.8 ± 0.6bc | * |
| Meat | 9.7 ± 0.6c | 8.3 ± 0.8b | ||
| Chicken | 10.0 ± 0.4c | 9.8 ± 0.1c | ||
| Fish | 4.2 ± 0.5a | 5.0 ± 0.4a | ||
| Ash | Vegetable | 65.5 ± 0.5a | 66.3 ± 0.4a | |
| Meat | 67.1 ± 0.4b | 67.9 ± 0.3c | ||
| Chicken | 66.3 ± 0.4a | 66.5 ± 0.3ab | ||
| Fish | 67.2 ± 0.4b | 67.0 ± 0.5b | ||
| Carbohydrates | Vegetable | 23.7 ± 0.7b | 22.5 ± 0.4b | |
| Meat | 20.7 ± 1.1a | 20.7 ± 1.1a | ||
| Chicken | 21.1 ± 1.1a | 20.7 ± 0.6a | ||
| Fish | 25.7 ± 0.4c | 24.5 ± 0.6c | ||
All determinations were performed in triplicate and values shown are mean ± standard deviation
Statistical differences was analysed by ANOVA (p < 0.05). Different letters indicate significant differences among soups by Multiple Range Test
*Statistical differences were analyzed by ANOVA (p < 0.05). Storage effect: sample analyzed on first day with respect to the sample analyzed after 12 months
In general, the nutritional composition of soups will vary depending on the ingredients used, the moisture content (between 1.8 and 2.6 %), proteins (up to 14.7 %) (Singh et al. 2003b), crude fat (from 21.3 to 24.6 %), ash (from 44.7 to 38.6 %) and carbohydrates (from 17.2 to 21.6 %) as seen in chicken soup, mushroom, pork and fish (Chiang et al. 2007).
Our study of the nutritional composition of the four dehydrated soups studied identified no significant variations resulting from the storage time during 12 months of storage, except the moisture and protein contents in meat flavoured soup. Singh et al. (2003a), who evaluated proteins in dehydrated food, observed no significant differences during storage and Raitio et al. (2011) observed no significant changes in the water content.
Table 5 showed the sodium content of the four dehydrated soups packed in carton and their ingredients, as detected by flame photometry (% of real sodium) and the sodium values of the soups calculated by specific techniques (% theoretical sodium). Table 5 also shows the sodium content of the four reformulated soups and the reduction achieved in an attempt to make them healthier.
Table 5.
Determination of sodium content in four dehydrated flavoured soups (vegetable, meat, chicken and fish) packed in carton and in their ingredients by flame photometry (% actual sodium) and the values calculated from the technical (% theoretical sodium) and sodium content in four reformulated soups as well as the percentage reduction achieved
| % Sodium | ||||
|---|---|---|---|---|
| Soup | Actual | Theoretical | Reformulated | %reduction reformulated |
| Vegetable | 23.4 ± 1.8 fg | 26.5 | 17.4 | 25.6 |
| Meat | 25.9 ± 1.3i | 26.5 | 17.9 | 30.8 |
| Chicken | 23.8 ± 1.4gh | 26.5 | 17.8 | 25.2 |
| Fish | 25.1 ± 1.6hi | 26.1 | 17.3 | 31.1 |
| Ingredients | ||||
| Salt (NaCl) | 37.3 ± 0.8j | 39.3 | ||
| Maltodextrin | 0.005 ± 0.003a | - | ||
| Sodium glutamate | 11.6 ± 0.9c | 12.3 | ||
| Modified corn starch | 0.3 ± 0.2a | 0.01 | ||
| Palm fat | 0.6 ± 0.2a | 0.5 | ||
| Yeast extract | 20.5 ± 0.9e | 15.0 | ||
| Caramel | 0.07 ± 0.04a | - | ||
| Chicken flavouring | 22.1 ± 0.8f | - | ||
| Vegetable flavouring | 18.9 ± 0.4de | 18.9 | ||
| Seafood flavouring | 8.1 ± 1.2b | - | ||
| Yeast extract fish | 17.9 ± 0.5d | 17.9 | ||
| Meat flavouring | 22.8 ± 1.0 fg | - | ||
| Hydrolyzed vegetable protein | 19.3 ± 0.9de | 16.7 | ||
All determinations were performed in triplicate and values shown are mean ± standard deviation
Statistical differences was analysed by ANOVA (p < 0.05). Different letters indicate significant differences among soups by Multiple Range Test
- No information on sodium content in the technical specifications
The dehydrated soups studied had a sodium content (Table 5) of between 23.4 and 25.9 % as seen by flame photometry, with significant differences (p < 0.05) between them. The sodium content of the ingredients used in soup making varied between 0.005 and 37.3 %. The sodium present in soups was due to the main ingredient, salt NaCl, which was found in similar proportions in the four soups, although other ingredients also contributed with a moderate sodium content, e.g. the meat flavouring and chicken flavouring, hydrolysed vegetable protein (HVP), vegetable flavouring, yeast extract, and MSG mainly. The different proportions of ingredients present in the four studied soups and even the absence of some of them must also be taken into account; for example, HVP was not present on the meat and fish flavoured soup, and chicken, meat and vegetables flavourings are not used in the fish flavoured soup, or chicken flavouring and meat flavouring are not used in the vegetable flavoured soup. However, most ingredients are found in low quantities in all the soups, so their impact on the total sodium content would be low.
Table 6 shows the sodium content of the ingredients of the four dehydrated soups (vegetable, meat, chicken and fish) in the original formulas and in the reformulated ones with their reduced sodium content. The table also shows the increased and decreased percentages of some of the ingredients: for example, as the salt decreased (37.2 % reduction) so the values of MSG increased (50 % increase), as did the content of other minor ingredients (maltodextrin, 42.8 %; starch, 200 %; chicken flavouring 200 %; vegetable flavouring 42.4 %; seafood flavouring 50 %; meat flavouring 88.9 %; fish yeast extract 100 %). In this way a reduction of between 25.2 and 31.1 % sodium was attained in the 4 dehydrated soups.
Table 6.
Determination of sodium content in the ingredients of the four dehydrated flavoured soups (vegetable, meat, chicken and fish) in the original formula and reformulated soup with 30 % sodium reduction and the percentage modified ingredients
| Ingredients | % sodium | ||
|---|---|---|---|
| Original formula | Reformulated product | % modified | |
| Salt (NaCl) | 23.1 ± 0.6 | 14.5 | −37.2 |
| Maltodextrin | 0.07 × 10−2 ± 0.00 | 0.1 × 10−2 | +42.8 |
| Sodium glutamate | 1.4 ± 0.1 | 2.1 | +50 |
| Modified corn starch | 0.01 ± 0.00 | 0.03 | +200 |
| Palm fat | 0.01 ± 0.00 | 0.01 | - |
| Yeast extract | 0.4 ± 0.0 | 0.5 | - |
| Caramel | 1.7 × 10−4 ± 0.00 | 2 × 10−4 | +17.6 |
| Chicken flavouring | 0.1 ± 0.0 | 0.3 | +200 |
| Vegetable flavouring | 0.3 ± 0.1 | 0.5 | +42.4 |
| Seafood flavouring | 0.08 ± 0.01 | 0.1 | +50 |
| Yeast extract fish | 0.1 ± 0.0 | 0.3 | +100 |
| Meat flavouring | 0.2 ± 0.0 | 0.3 | +88.9 |
| Hydrolyzed vegetable protein | 0.3 ± 0.1 | 0.3 | - |
All determinations were performed in triplicate and values shown are mean ± standard deviation
- No changes in sodium content
Thus, when the four soups were reconstituted for consumption, the sodium content was 0.46 g/100 g, 0.52 g/100 g, 0.47 g/100 g and 0.50 g/100 g, in the vegetable, meat, chicken and fish soups, respectively. The sodium content after reformulation was 0.27 g/100 g, 0.29 g/100 g, 0.28 g/100 g and 0.28 g/100 g respectively. Mitchell et al. (2011) reformulated a vegetable soup whith an initial sodium content of 0.37 g/100 g, while the corresponding “low in sodium” soup had 0.18 g/100 g, which was lower than the sodium content of our soups because of the nature of the ingredients — flavouring and extracts in our soups and basically lyophilized vegetables (potato, carrot, onion) with their lower sodium content in the soups studied by Mitchell et al. (2011).
Table 7 showed the MSG content of the four dehydrated soups (vegetables, meat, chicken and fish) packed in carton, as determined by HPLC (% of real MSG) and the values calculated using technical specifications (% theoretical MSG), as well as the MSG content of the reformulated soups compared with the initial soups.
Table 7.
Determination of monosodium glutamate content in four dehydrated flavoured soups (vegetable, meat, chicken and fish) packed in carton by HPLC (% actual monosodium glutamate) and the values calculated from the technical (% theoretical monosodium glutamate) and GMS content in reformulated soups and percentage increase of the GMS in the reformulated soups
| Soups | % Monosodium glutamate | |||
|---|---|---|---|---|
| Packed in carton | Actual | Theoretical | Reformulated | % increase reformulated |
| Vegetable | 12.53 ± 0.10c | 12.70 | 19.5 | 53.5 |
| Meat | 10.87 ± 0.17a | 11.50 | 17.0 | 47.8 |
| Chicken | 13.20 ± 0.18d | 12.50 | 17.0 | 36.0 |
| Fish | 11.80 ± 0.20b | 13.11 | 18.5 | 41.1 |
All determinations were performed in triplicate and values shown are mean ± standard deviation
Statistical differences was analysed by ANOVA (p < 0.05). Different letters indicate significant differences among soups by Multiple Range Test
Taking into account that one of the ingredients used in the four dehydrated soups studied to reduce the salt content was MSG because it enhances the flavour, and that this ingredient increased by 36–53.5 % in the reformulated versions, analyses were carried out to ensure that the new values were within the legal limits. The results showed values of between 10.87 and 13.20 % with significant differences (p < 0.05). European law (Reglamento UE 2011) fixes a maximum limit of 10 g/kg of MSG and its salts in food products, except for non-processed foods and seasoning which have no specific maximum. The MSG content of the reformulated soups in our study was between 17 and 19.5 g MSG/100 g of dehydrated soup, which, when the soups are reconstituted for consumption, represented a content of 2 g of MSG/kg of product, which is well within the permitted value.
The optimum concentration of MSG, as a flavour enhancer of food, as established by Walker and Lupien (2000) is between 0.2 and 0.8 % which is in accordance with our results. Other authors mention values of between 0.15 and 0.2 %, in dehydrated meat soups (Fernández Pérez et al. 2004).
IN SUMMARY, dehydration treatments such as lyophilization produce food of good quality and dehydrated soups usually maintain the organoleptic and nutritional characteristics of the mixture used (Wang et al. 2010).
In Spain, 88.2 % of the population ingests 9.8 g/day of sodium, which is above the recommended amount of 5 g/ day (Ortega et al. 2011). A moderate decrease in salt intake (only 10 %) would contribute to reducing strokes and heart attacks, as well as the risk of cardiovascular pathologies (He and MacGregor 2011).
Several studies have found that the main sources of sodium ingestion in the population are in bread and soup (Huybrechts et al. 2012). To reduce the salt intake of the population, the food industry should take urgent steps to reduce the quantity of salt added to their processed foods (EFSA 2005).
As regards the sodium content, according to the FDA (U.S. Food and Drug Administration) (2014) the term “healthy” can be applied to those foods that contain less than 480 mg of sodium per portion; if such foods are labelled “low in sodium” they must have a maximum of 140 mg, while foods labelled as “sodium reduced or less sodium” must have at least 25 % less sodium than the referenced food. According to this and bearing in mind the obtained results, our reformulated soups (vegetables, meat, chicken and fish) had sodium levels that were reduced by 25.6, 30.8, 25.2 and 31.1 %, respectively, and could be sold as “sodium reduced or less sodium”, while maintaining their antioxidant properties and without suffering losses in nutritional value.
Acknowledgments
This study was financed by a project the Centre for the Development of Industrial Technology (CDTI, Ref. 11577) with the collaboration of Paprimur S.L. Spanish Ministry of Health and Consumption Affairs (Projects 05/1276, 08/1259, 11/01791, 14/00636, Red Predimed-RETIC RD06/0045/1004, and CIBEROBN CB12/03/30038), Grant of support to research groups no. 35/2011 (BI Govnt.) and EU FEDER funds.
Contributors
M.M-T. and M.A.M designed research; A.M.J-M., M.M., M.L.L. and V.G-M. conducted research; M.B. and A.M.J-M. analyzed data; M.A.M, M.M-T. and A.M.J-M. wrote the paper. M.M-T had primary responsibility for final content. All authors read and approved the final manuscript.
Conflict of interest
The authors declare no competing financial interest.
Abbreviations
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)
- HVP
Hydrolyzed vegetable protein
- MAP
Modified atmosphere packaged
- MSG
Monosodium glutamate
- TEAC
Trolox equivalent antioxidant capacity.
Footnotes
Research highlights
-soups had good antioxidant capacity in lipid peroxidation, deoxyribose and TEAC tests
-from a nutritional point of view, the lipid fraction of all the soups was below 1 %
-when reformulated, the four soups contained 25 % less sodium
-glutamate content of reformulated soups was below the levels permitted by legislation
References
- AOAC (2000) Official Methods of Analysis (17th ed.), AOAC International, Methods 945.15, 923.03 and 945.16. Washington, DC
- Chen MJ, Hsieh YT, Weng YM, Chiou R. Flame photometric determination of salinity in processed foods. Food Chem. 2005;91:765–770. doi: 10.1016/j.foodchem.2004.10.002. [DOI] [Google Scholar]
- Chiang P, Yen C, Mau J. Non volatile taste components of various broth cubes. Food Chem. 2007;101:932–937. doi: 10.1016/j.foodchem.2006.02.041. [DOI] [Google Scholar]
- Clegg ME, Ranawana V, Shafat A, Henry CJ. Soups increase satiety through delayed gastric emptying yet increased glycaemic response. Eur J Clin Nutr. 2013;67:8–11. doi: 10.1038/ejcn.2012.152. [DOI] [PubMed] [Google Scholar]
- EFSA Opinion of the scientific panel on dietetic products, nutrition and allergies on a request from the commission related to the tolerable upper intake level of sodium. EFSA J. 2005;209:1–26. [Google Scholar]
- FDA (2014) http://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm316876.htm. Accessed September 2014
- Fernández Pérez V, Trapiador J, Martín M, De Castro L. Optimization of the drying step for preparing a new commercial powdered soup. Innovative Food Sci Emerg. 2004;5:361–368. doi: 10.1016/j.ifset.2004.05.001. [DOI] [Google Scholar]
- Gamboa-Santos J, Soria AC, Corzo-Martínez M, Villamiel M, Motilla A. Effect of storage on quality of industrially dehydrated onion, garlic, potato and carrot. J Food Nutr Res. 2012;51:132–144. [Google Scholar]
- Gülçin I. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012;86:345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- Gupta S, Prakash J. Influence of antioxidant components on activity of dehydrated green leafy vegetables. Food Sci Technol Res. 2008;14:104–109. doi: 10.3136/fstr.14.104. [DOI] [Google Scholar]
- He FJ, Macgregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet. 2011;378:380. doi: 10.1016/S0140-6736(11)61174-4. [DOI] [PubMed] [Google Scholar]
- Huybrechts I, De Keyzer W, Lin Y, Vandevijvere S, Vereecken C, Van Oyen H, Tilleman K, Bellemans M, De Maeyer M, De Backer G, De Henauw S. Food sources and correlates of sodium and potassium intakes in Flemish pre-school children. Public Health Nutr. 2012;15:1039–1046. doi: 10.1017/S1368980011002497. [DOI] [PubMed] [Google Scholar]
- Insawang T, Selmi C, Cha’on U, Pethlert S, Yongvanit P, Arrejitranusorn P, Boonsiri P, Khampitak T, Tangrassameeprasert R, Pinitsoontorn C, Prasongwattana V, Gershwin ME, Hammock BD. Monosodium glutamate (MSG) intake is associated with the prevalence of metabolic syndrome in a rural Thai population. Nutr Metab. 2012;9:50–56. doi: 10.1186/1743-7075-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Monreal AM, Martínez-Tomé M, Egea I, Romojaro F, Murcia MA. Effect of industrial processing and storage on antioxidant activity of apricot (Prunus armeniaca v. bulida) Eur Food Res Technol. 2008;227:125–134. doi: 10.1007/s00217-007-0701-1. [DOI] [Google Scholar]
- Kraboun K, Tochampa W, Chatdamrong W, Kongbangkerd T. Effect of monosodium glutamate and peptone on antioxidant activity of monascal waxy corn. Int Food Res J. 2013;20:623–631. [Google Scholar]
- Leow SS, Sekaran SD, Sundram K, Tan Y, Sambanthamurthi R. Oil palm phenolics attenuate changes caused by an atherogenic diet in mice. Eur J Nutr. 2013;52:443–456. doi: 10.1007/s00394-012-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Tsai JS, Hung LB, Pan BS. Plasma lipid regulatory effect of compounded freshwater clam hydrolysate and Gracilaria insoluble dietary fibre. Food Chem. 2011;125:397–401. doi: 10.1016/j.foodchem.2010.09.016. [DOI] [Google Scholar]
- Maiani G, Caston MJP, Catasta G, Toti E, Cambrodon IG, Bysted A, Granado-Lorencio F, Olmedilla-Alonso B, Valoti M, Böhm V, Mayer-Miebach E, Behsnilian D, Schlemmer U. Carotenoids: actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol Nutr Food Res. 2009;53:194–218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- Martínez-Tomás R, Pérez-Llama F, Sánchez-Campillo M, González-Silvera D, Cascales AI, García-Fernández M, López-Jiménez JA, Zamora Navarro S, Burgos MI, López-Azorín F, Wellner A, Avilés Plaza F, Bialek L, Alminger M, Larqué E. Daily intake of fruit and vegetable soups processed in different ways increases human serum b-carotene and lycopene concentrations and reduces levels of several oxidative stress markers in healthy subjects. Food Chem. 2012;134:127–133. doi: 10.1016/j.foodchem.2012.02.078. [DOI] [Google Scholar]
- Martínez-Tomé M, Murcia MA, Frega N, Ruggieri S, Jiménez AM, Roses F, Parras P. Evaluation of antioxidant capacity of cereal brans. J Agric Food Chem. 2004;52:4690–4699. doi: 10.1021/jf049621s. [DOI] [PubMed] [Google Scholar]
- Mitchell M, Brunton NP, Wilkinson MG. Impact of salt reduction on the instrumental and sensory flavor profile of vegetable soup. Food Res Int. 2011;44:1036–1043. doi: 10.1016/j.foodres.2011.03.007. [DOI] [Google Scholar]
- Murcia MA, Martínez-Tomé M, Vera A, Morte A, Gutierrez A, Honrubia M, Jiménez AM. Effect of industrial processing on desert truffles Terfezia claveryi Chatin and Picoa juniperi Vittadini: proximate composition and fatty acids. J Sci Food Agric. 2003;83:535–541. doi: 10.1002/jsfa.1397. [DOI] [Google Scholar]
- Murcia MA, Jiménez-Monreal AM, García-Diz L, Carmona M, Maggi L, Martínez-Tomé M. Antioxidant activity of minimally processed (in modified atmospheres), dehydrated and ready-to-eat vegetables. Food Chem Toxicol. 2009;47:2103–2110. doi: 10.1016/j.fct.2009.05.039. [DOI] [PubMed] [Google Scholar]
- Muslin NZ, Ahmad M, Heng LY, Saad B. Optical biosensor test for the screening and direct determination of glutamate in food samples. Sensors Actuators B Chem. 2012;161:493–497. doi: 10.1016/j.snb.2011.10.066. [DOI] [Google Scholar]
- Nuutila AM, Puupponen-Pimiä R, Aarni M, Osksman-Caldentey KM. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003;81:485–493. doi: 10.1016/S0308-8146(02)00476-4. [DOI] [Google Scholar]
- Ortega RM, López-Sobater AM, Ballesteros JM, Pérez-Farinós N, Rodríguez-Rodríguez E, Aparício A, Perea JM, Andrés P. Estimation of salt intake by 24 h urinary sodium excretion in a representative sample of Spanish adults. Br J Nutr. 2011;105:787–794. doi: 10.1017/S000711451000423X. [DOI] [PubMed] [Google Scholar]
- Pérez-Vicente A, Serrano P, Abellán P, García-Viguera C. Influence of packaging material on pomegranate juice colour and bioactive compounds, during storage. J Sci Food Agric. 2004;84:639–644. doi: 10.1002/jsfa.1721. [DOI] [Google Scholar]
- Raitio R, Orlien V, Skibsted LH. Storage stability of cauliflower soup powder: the effect of lipid oxidation and protein degradation reactions. Food Chem. 2011;128:371–379. doi: 10.1016/j.foodchem.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Raitio R, Orlien V, Skibsted LH. Effects of palm oil quality and packaging on the storage stability of dry vegetable bouillon paste. Food Chem. 2012;132:1324–1332. doi: 10.1016/j.foodchem.2011.11.112. [DOI] [PubMed] [Google Scholar]
- Reglamento (UE) (2011) n° 1129/2011 de la Comisión de 11 de noviembre por el que se modifica el anexo II del Reglamento (CE) n° 1333/2008 del Parlamento Europeo y del Consejo para establecer una lista de aditivos alimentarios de la Unión
- Singh G, Kawatra A, Sehgal S, Pragati S. Effect of storage on nutritional composition of selected dehydrated green leafy vegetable, herb and carrot powders. Plant Food Hum Nutr. 2003;58:1–9. doi: 10.1023/B:QUAL.0000040362.70675.30. [DOI] [PubMed] [Google Scholar]
- Singh S, Ghosh S, Patil GR. Development of a mushroom-whey soup powder. Int J Food Sci Technol. 2003;38:217–224. doi: 10.1046/j.1365-2621.2003.00661.x. [DOI] [Google Scholar]
- Tiveron AP, Melo PS, Bergamaschi KB, Vieira TMFS, Regitano-d’Arce MAB, Alencar SM. Antioxidant activity of Brazilian vegetables and its relation with phenolic composition. Int J Mol Sci. 2012;13:8943–8957. doi: 10.3390/ijms13078943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R, Lupien JR. The safety evaluation of monosodium glutamate. J Nutr. 2000;130:1049–1052. doi: 10.1093/jn/130.4.1049S. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang M, Mujumdar AS. Effect of food ingredient on microwave freeze drying of instant vegetable soup. LWT-Food Sci Technol. 2010;43:1144–1150. doi: 10.1016/j.lwt.2010.03.007. [DOI] [Google Scholar]
- Yassibas E, Arslan P, Yalçin S. Evaluation of dietary and life-style habits of patients with gastric cancer: a case–control study in Turkey. Asian Pac J Cancer Prev. 2012;13:2291–2297. doi: 10.7314/APJCP.2012.13.5.2291. [DOI] [PubMed] [Google Scholar]