Abstract
Many plants store fructan as reserve carbohydrate. Fructans naturally present in almost all plant foods, are also used as functional ingredients by the food industry to modify the texture and taste due to their properties as gelling agents, fat substitutes, soluble dietary fibers and low calorie sweeteners. Seven banana cultivars were analysed for fructans and Nendran banana was selected for the next set of experiments as it had the highest fructan content (1433.3 mg/100 g) among the cultivars studied. Low temperature ripening (16 °C) of Nendran banana resulted in higher fructan accumulation of these carbohydrates in cold conditions. Pectinase pre-treatment significantly increased yield of total fructans from 1.4/100 g to 6.5 g/100 g i.e., 370 %. Fructan composition was affected by processing, namely steaming and puree preparation in Nendran. The fructan composition data documented in this study will enable including banana, naturally high in fructans in the diet and will facilitate storage and processing for nutritional formulation for higher fructan consumption.
Keywords: Fructan, Nendran banana, Ripening, Processing, Composition
Introduction
Fructans are oligo and polysaccharides consisting of short chains of fructan units with the single D-glucosyl unit at the reducing end. In humans, there is no enzyme for digesting fructan. Fructans have many health benefits: stimulate the growth of beneficial microorganisms in the gut that limit the pathogens and reduce the risk of colon cancer (Kleessen et al. 2007), significantly increase stool frequency and prevent constipation (Hond et al. 2000), enhance calcium absorption (Roberfroid 2007) and bone mineralization in young adolescents (Abrams et al. 2005), control blood sugar level and insulin requirement (Ayman et al. 2004), reduce plasma levels of cholesterol and triacylglycerol (Beylot 2005) and stimulate the gastrointestinal immune system (Briet et al. 1995).
Fructans exhibit structural diversity in plants. In higher plants, fructans show more differences in fructosyl linkages and are classified into inulin, levan, inulin neoseries, levan neoseries and mixed levan (Vijn and Smeekens 1999). Inulin consists of linear (2–1)-β-D-frutosyl linkages and is present in chicory and Jerusalem artichoke. 1-Kestose or Isokestose is the shortest inulin molecule. Levan consists of linear (2–6)- linked β-D fructosyl units. 6-Kestose is the shortest levan molecule. Mixed type levan consists of both (2–1) and (2–6) β-D-fructosyl units. Inulin neoseries are linear (2–1) β-D- fructosyl units linked to both C1 and C6 of glucose of the sucrose molecule. The shortest molecule of inulin neoseries and mixed type levans is Neokestose. Levan neoseries are polymers of predominantly β (2–6)-linked fructosyl residues on either end of the glucose moiety of the sucrose molecule. Bifurcose is the shortest levan neoseries molecule. Although there are many types of fructosyl residues, in this paper, fructan molecule that consists of β (2–1) residue (1-kestose, nystose and inulin) from different cultivars of banana consumed in South India have been characterized.
India produces 28,455 million tonnes of bananas from an area of 796.5 ha. It is the largest producer of banana not only in Asia but also in the world and contributes 37.2 % to global production followed, by China (6.60 %) and Philippines (6.14 %). TamilNadu contributed 6736.4million tonnes from 130.4 ha with total productivity of 5.17 million tonnes per hectare in the year 2011–12 followed by Maharashtra, Gujarat and Andhra Pradesh (Indian Horticulture Database 2012). There are different cultivars of banana grown in different parts of the world and India grows the largest variety of bananas (20) which are available throughout the year. Banana is a moderate source of fructan (Van Loo et al. 1995). In banana, fructan is synthesized by the action of two different enzymes, 1-SST and 1-FFT. 1-SST transfers fructosyl residue from one sucrose molecule to another sucrose molecule leading to 1-kestose. 1-FFT transfers fructosyl residue from sucrose to 1-kestose thereby elongating the chain leading to 1-nystose. The basic molecule required for synthesizing fructan molecule is sucrose. The demand for fructans, in food industry is increasing steadily because of their functional properties. The objective of the present study was to investigate the distribution of fructans in different banana cultivars and to evaluate the effect of enzymatic treatment and ripening on fructan content from Nendran banana. Nendran banana was selected as it is the variety used specifically as a weaning food and also commonly processed and consumed. Most other varieties are consumed fresh.
Material and methods
Selection of fruits
Seven banana cultivars i.e., Karpooravalli, Morris, Rasthali, Poovan, Hill banana, Red banana and Nendran used for this study were purchased from local markets and screened for fructans. Each variety was purchased from 10 different locations in and around Chennai city to ensure that variations in their fructan content were represented in the composite sample.
Sample preparation
Approximately 500 g of each cultivar from all the 10 outlets was cleaned, washed, peeled, cut and the edible portion taken. Equal edible portions (100 g) from each sample was pooled (10 × 100 g = 1 kg) and thoroughly mixed. From this 1 kg pooled sample, 500 g was blended in a food processor and the homogenized samples were stored frozen in air tight containers at −20 °C until further analysis. From the remaining 500 g, aliquots were used for moisture determination.
Screening and characterization
Moisture, titratable acidity and pH were carried out on the same day of sampling. All fruit samples were screened for the presence of total fructans using an enzyme kit. Characterization of fructans was done using HPLC for selected cultivars with high fructan content. All samples were also analysed for sugars (dextrose, fructose and sucrose) by HPLC (O’Donoghue et al. 2004) and inulin content by colorimetric assay (Ashwell 1957).
Ripening of Nendran banana
The effect of ripening on fructan content was studied in one fruit cultivar Nendran. The 1 day old harvested fruits were purchased in bulk from the wholesale fruit market in Chennai and ripened at two different temperatures - room temperature (28 ± 1 °C) and low temperature (16 ± 1 °C).
Two fruits were sampled from storage - daily from the room temperature lot and once in 5 days from the low tem-perature lot. Samples were blended in a food processor and aliquots of the pulp used for analysing moisture content on the same day. The remaining pulp was stored at −20 °C for further fructan characterization. The ripening study was repeated twice.
Enzymatic pre-treatment
In order to study the effect of enzyme treatment on fructan extraction, Nendran banana pulp substrate (2 g) was pretreated with four different enzymes independently and also in combination at their respective optimum temperatures - cellulase and hemicellulase at 40 °C; pectinase at 42.5 °C; and invertase at 55 °C for different time intervals of 0, 30, 60 and 90 min.
Steaming
One kilogram of Nendran cultivar got from one retail shop was divided into two equal portions (500 g each). One portion of the whole fruit was cleaned, peeled and homogenized in a blender and analyzed fresh for its moisture, pH, titratable acidity and fructan content. Another portion of the whole fruit was steam processed in a cooker for 15 min, cooled to room temperature (25 °C) and homogenized in a blender. The homogenized sample was analyzed for its moisture, pH, and titratable acidity on the same day. Both the fresh and steamed portions (each 500 g) were stored in air-tight containers at −20 °C for further analysis.
Puree preparation
Two kilograms of Nendran got from one retail shop were divided into two portions (500 g and 1.5 kg each). One portion of whole fresh fruit (500 g) was peeled, weighed, homogenized and then analyzed for its moisture, pH, titratable acidity and fructan content. Another portion (1.5 kg) was blanched for 5 min at 80 °C. After cooling, the skin was removed and the fruit was pulped in a homogenizer. Five hundred gram portion was taken and analyzed for its moisture, pH, titratable acidity and fructan content. The puree was prepared from the remaining pulp by heating to increase the brix value from 28° to 34° Brix and then cooled to room temperature. Both the fresh pulp and the prepared puree were stored in air tight containers at −20 °C for further analysis.
Analytical methods
All reagents used were of analytical grade. Standard sugars like glucose, fructose and sucrose were purchased from Merck, USA and kestose and nystose were purchased from Sigma Aldrich, USA. Enzyme kits for fructan estimation were obtained from Megazyme International Ireland Ltd., Wicklow, Ireland.
Moisture content was determined by oven drying and expressed as percentage. The pH of the diluted sample was determined using a pH meter (Susima A1-plus). Titratable acidity was determined by titrating known amount of the appropriately diluted and filtered sample against standardized sodium hydroxide solution (AOAC 2000) and expressed as anhydrous citric acid percentage. Brix measurement was obtained at a temperature of 28 °C using a portable hand refractometer with the measuring range of 0–32° Brix (Advance Research Instruments, Model: HR-032).
Total fructans
The method for extracting fructans fully described in the Megazyme Fructan HK Assay procedure was followed (AOAC 2003). Two samples (A and B) were treated as follows: Sample A was treated with purified fructanase, which hydrolyzed fructan to fructose and glucose, while sample B was treated with blank. The concentration of glucose plus fructose was measured with a hexokinase/phosphoglucose isomerase (PGI) / glucose 6-phosphate dehy-drogenase system. The fructan content was then measured by the difference between sample A and B and expressed on the basis of fresh fruit weight (mg/100 g edible portion) and dry weight (g/100 g edible portion).
Sugar analysis
Fruit sample (2 g) was extracted based on the method described by O’Donoghue et al. (2004) with modifications. Distilled water (4.5 mL) was added to the fruit sample and held for 10 min at 75 °C to extract the sugars. To the slurry, 7.5 mL methanol was added to give a final 62.5 %(v/v) MeOH solution and extracted for 15 min at 55 °C. The slurry was then passed through a 0.2 μm Millex-GV syringe driven filter. Extracts were stored at −20 °C until further use.
Inulin determination
The sample was extracted in 80 % eth-anol for 6 h to remove free sugars. The residue was filtered and extracted again in 80 % ethanol for 10 min. The pooled extracts were combined and volume was made-up to 50 mL and analyzed for fructose. The residue was dried and hot water extracted. Inulin content was estimated by using Resorcinol reagent (Ashwell 1957). Fructose was used to draw the standard graph and the amount of inulin was expressed in terms of g fructose / 100 g edible portion.
Statistical analysis
All analyses were carried out in triplicates. Statistical analysis was performed using Graph Pad Prism 5.0 (GraphPad Software Inc., San Diego, CA). The correlation coefficients for enzymatic treatment were analysed by t-test.
Results and discussion
The moisture content of edible portion of banana cultivars ranged from 51.7 to 72.8 % (Table 1). Nendran and Morris had lower moisture compared to Red banana and Poovan. All banana cultivars were acidic (pH between 4.5 and 5.6), Poovan being most acidic, while Karpooravalli was least acidic (Table 1).
Table 1.
Fructan content of banana cultivars
| Banana cultivar | Moisture (%) | pH | Titratable acidity (% anhydrous Citric acid) | Fructan (FW) mg/100 g | Fructan (DW) g/100 g |
|---|---|---|---|---|---|
| Hill banana | 68.6 ± 0.5 | 5.3 ± 0.01 | 0.25 ± 0.02 | 696.4 ± 2.7 | 2.22 ± 0.06 |
| Karpooravalli | 67.3 ± 1.1 | 5.6 ± 0.07 | 0.13 ± 0.03 | 665.7 ± 23.5 | 2.04 ± 0.07 |
| Morris | 51.7 ± 0.8 | 5.4 ± 0.04 | 0.33 ± 0.02 | ND | ND |
| Nendran | 55.1 ± 0.3 | 4.6 ± 0.05 | 0.42 ± 0.01 | 1433.3 ± 20 | 3.19 ± 0.04 |
| Poovan | 71.4 ± 0.3 | 4.5 ± 0.02 | 0.46 ± 0.03 | ND | ND |
| Rasthali | 68.4 ± 0.1 | 4.8 ± 0.06 | 0.39 ± 0.33 | 470.0 ± 37.4 | 1.49 ± 0.01 |
| Red banana | 72.8 ± 1.2 | 5.2 ± 0.03 | 0.33 ± 0.01 | ND | ND |
FW Fresh weight, DW Dry weightl, ND Not detected
* Moisture, titratable acidity and fructan content, Mean ± S.D (n = 3)
Total fructan was detected in fresh fruit of only four cultivars - Rasthali, Karpooravalli, Hill banana and Nendran (Table 1). Nendran contained the maximum fructan, about two to three times of the other cultivars. It is to be noted that all these four varieties are traditionally valued for their health promoting characteristics and Nendran and Hill banana are used as weaning food.
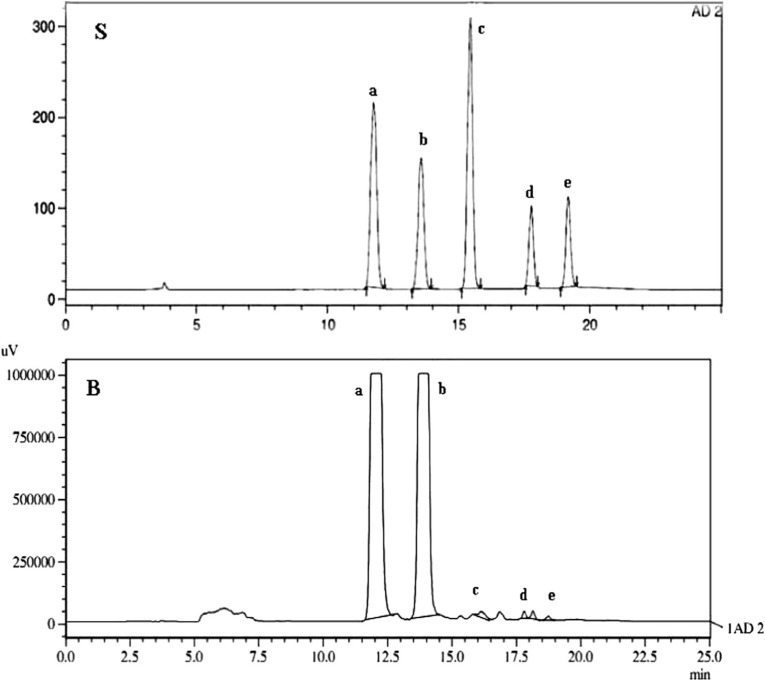
Glucose and fructose were the main sugars present in all samples (Fig. 1). Hill banana contained least monosaccharides than other fruits analyzed and is generally less sweet to taste (Table 2). Sucrose was detected in all fruits analyzed, except Rasthali. Nendran and Hill banana alone were found to contain kestose, while nystose was not detected. Inulin content of these two cultivars was relatively high, with wide variations which were also reflected in total fructan present (Table 3).
Fig. 1.
HPLC-ELSD chromatogram profile for standard (S) & Banana (B) illustrating the location of sugars and fructan fractions in using the Ashahipak NH2P-4E column; with water and acetonitrile as mobile phase. Peaks: a, Fructose; b, Dextrose; c, Sucrose; d, 1-kestose; e, Nystose
Table 2.
Sugars in banana cultivars quantified by HPLC
| Food | Fructose mg/100 g | Glucose mg/100 g | Sucrose mg/100 g | Kestose mg/100 g | Nystose mg/100 g |
|---|---|---|---|---|---|
| Hill banana | 54.8 ± 0.9 | 68.2 ± 1.8 | 43.0 ± 0.8 | 17.1 ± 0.7 | ND |
| Karpooravalli | 702.8 ± 2.3 | 5403.4 ± 0.7 | 856.9 ± 0.7 | ND | ND |
| Nendran | 8467.5 ± 1.5 | 9786.2 ± 1.3 | 756.2 ± 0.5 | 61.2 ± 0.4 | ND |
| Rasthali | 848.5 ± 1.6 | 1118.6 ± 1.4 | ND | ND | ND |
Sugars, Mean ± S.D (n = 3)
ND Not detected
Table 3.
Comparison of FOS content in banana cultivars
| Present study (FW) g/100 g | Agopian et al. 2008 (FW) g/100 g | ||||||
|---|---|---|---|---|---|---|---|
| Banana cultivars | GF2 | GF3 | Inulin | TF | Banana cultivar | GF2 | GF3 |
| Hill Banana | 0.17 | ND | 0.23 | 0.69 | Ouro | 0.29 | ND |
| Karpooravalli | ND | ND | 0.17 | 0.66 | Nanicao | 0.41 | ND |
| Morris | - | - | - | ND | Prata | 0.56 | 0.03 |
| Nendran | 0.07 | ND | 0.85 | 0.47 | Maca | 0.25 | ND |
| Poovan | - | - | - | ND | Mysore | 0.55 | ND |
| Rasthali | ND | ND | 0.19 | 1.43 | Pacovan | 0.44 | ND |
| Red Banana | - | - | - | ND | Terra | 0.77 | ND |
| Figo | 0.28 | ND | |||||
TF present in lower amount than detectable value in banana cultivars. GF3, GF4 and Inulin was not analysed in banana cultivars
FW Fresh Weight, GF 2 1-kestose, GF 3 Nystose, TF Total fructans, ND Not Detected, - Not analyzed
Wide differences in FOS content of banana cultivars have been reported by different groups as presented in Table 3. While Campbell et al. (1997) from Ohio reported 1.09 mg/100 g of dry mass, Hograrth et al. (2000) also from Ohio documented 430 and 600 mg/100 g fruit weight at different stages of maturity and Homme et al. (2001) from France reported 130 mg/100 g in banana puree. In contrast, Muir et al. (2007) did not detect fructan in Australian banana. As observed from Table 3, banana cultivars from Brazil had high and varied content of kestose, while nystose was detected only in one cultivar - Prata (Agopian et al. 2008). These differences in the fructan content can be attributed to several factors such as soil, cultivar, stage of ripening and storage conditions.
Effect of ripening in Nendran
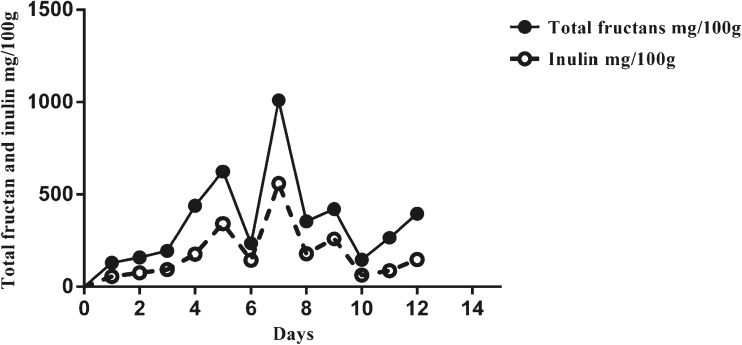
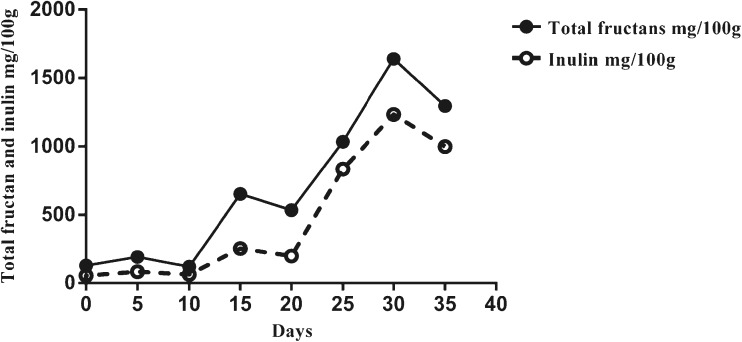
All analyzed results are the means of duplicate sets of Nendran banana stored at 16 ± 1 and 28 ± 1 °C. In the first batch of fruits ripened at 16 °C, fructan peaked on the 25th day, while in the second it peaked on the 30th day. In contrast at 28 °C, the peak fructan was observed on the 7th day in both batches. The pulp to peel ratio increased steadily in fruits stored at both room (28 ± 1 °C) and low (16 ± 1 °C) temperature whereas, the moisture content decreased and then increased during the final stages of ripening (Data not shown).
Total fructan content of Nendran banana attained its peak value at day-7 in case of room temperature storage (28 °C) and day-30 in case of low temperature (16 °C) (Figs. 2 and 3). In low temperature storage, all analyzed parameters were delayed by 15 days. Gradual increase in fructans was noted during ripening at both temperatures. Both fructan and inulin levels accumulated significantly (p < 0.05) by 58.5 and 29.4 % respectively, during ripen-ing at low temperature, compared to room temperature storage. All sugars accumulated to a larger extent at low temperature than at room temperature - fructose and glucose by 17.4 and 59.4 % respectively; 1-kestose by 33.4 % and sucrose by 25.6 % (Data not shown).
Fig. 2.
Total fructan and inulin content of fruits stored at room temperature (28 ± 1 °C)
Fig. 3.
Total fructan and inulin content of fruits stored at low temperature (16 ± 1 °C)
The results of the present study are similar to the results published by Agopian et al. (2009). However differences in cultivars, degree of ripening, and the presence of fructan outside the inulin neo series could explain the discrepancies reported. The results also suggest that the start of 1-kestose accumulation is highly dependent on the specific amount of sucrose accumulated since kestose was not present in the unripe banana.
Effect of enzymatic pretreatment in Nendran
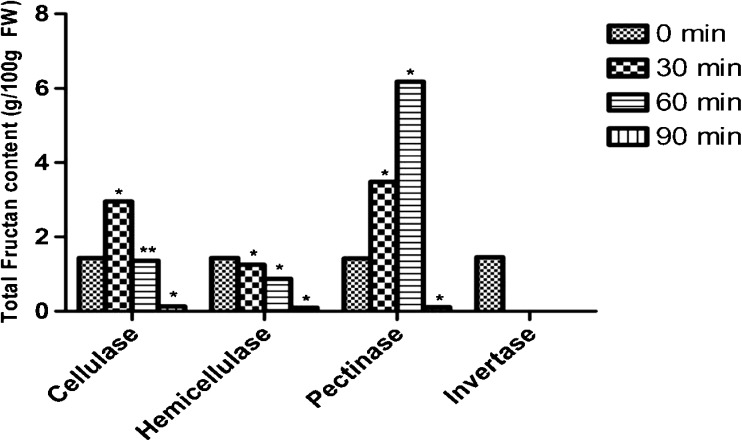
Pectinase pretreatment alone significantly increased total fructans from initial 1.4/100 to 6.5 g /100 g i.e., 370 % when compared to other enzymes (Fig. 4). The degradation of pectic substances, which are predominant structural constituents of primary cell wall, could explain this. They are the sole poly-saccharides in middle lamella, along with some cellulose micro fibrils, while they may be virtually absent in secondary walls. Cellulase, hemicellulase and pectinase combined pre-treatments increased total fructans from initial 1.4/100 to 6.2 g /100 g i.e., by 334 %. Evidently, single cell wall degrading enzyme, pectinase could enhance fructan extraction significantly than the combination.
Fig. 4.
Effect of enzyme pretreatments on fructan content. * Significantly different when P < 0.05 from control ** Not significantly different when P < 0.05 from control
Pectinase treatment caused reduction in fructose content, while sucrose showed a slight increase. Glucose did not change whereas; slight decrease in kestose was observed. Inulin content of the fruit increased from initial 850.2 to 2780.3 mg / 100 g corresponding to an increase of 227 %. Our experiments with enzyme-aided extraction of fructan from Nendran demonstrate the potential for recover-ing natural prebiotic fructan effectively from other fruits.
Effect of steam processing in Nendran
Moisture content and titratable acidity decreased for steamed fruits, where as pH increased. Steaming decreased acidity and fructan in Nendran (Table 4). The loss in fructan observed in the present study is similar to the result reported by Hograrth et al. (2000), who documented a decrease in fructan content in processed banana, grapes and tomato.
Table 4.
Analysis of fresh and steamed fruits (Nendran)
| Fresh fruits | Steamed fruits | % Change (+/−) | |
|---|---|---|---|
| Moisture content (%) | 59.3 ± 0.7 | 57.6 ± 0.4 | −2.9 |
| Titratable acidity (% anhydrous citric acid) | 0.43 ± 0.04 | 0.31 ± 0.02 | −27.9 |
| pH | 4.6 ± 0.11 | 4.7 ± 0.26 | +2.1 |
| Fructan content (FW) mg/100 g | 1494.7 ± 22.5 | 1158.0 ± 9.0 | −22.5 |
| Fructan content (DW) g/100 g | 3.7 ± 0.05 | 2.7 ± 0.01 | −25.7 |
FW FreshWeight, DW Dry Weight
* Mean ± S.D (n = 3)
Effect of puree processing in Nendran
Moisture content of Nendran decreased from 51.8 to 43.8 %, titratable acidity increased slightly, and pH decreased on pureeing. Total fructan and inulin increased in puree due to its concentration/loss of water (Table 5). The increase in fructan content observed in the present study is different from the result reported by Homme et al. (2003), who observed that fructan loss was insignificant at 80–100 °C for 30 min in apple dessert, apple puree, banana puree and stewed apple-banana fruits.
Table 5.
Analysis of fresh fruits and fruit puree (Nendran)
| Fresh fruit | Blanched fruit | Fruit puree | ||
|---|---|---|---|---|
| Moisture content (%) | 51.87 ± 0.49 | 54.83 ± 0.59 | 43.86 ± 0.09 | |
| Titratable acidity (% anhydrous citric acid) | 0.64 ± 0.03 | 0.68 ± 0.02 | 0.68 ± 0.01 | |
| pH | 4.2 ± 0.14 | 4.19 ± 0.26 | 4.17 ± 0.21 | |
| Fructan content (FW) mg/100 g | 1493.7 ± 0.29 | 1287.6 ± 0.51 | 2471.1 ± 0.77 | |
| Inulin mg/100 g | 870.4 ± 0.68 | 976.6 ± 0.39 | 1025.2 ± 0.78 | |
| Colour | L* | 62.5 ± 0.03 | 64.2 ± 0.17 | 58.9 ± 0.09 |
| A* | 10.8 ± 0.02 | 10.5 ± 0.14 | 10.2 ± 0.21 | |
| B* | 31.5 ± 0.08 | 25.4 ± 0.12 | 28.4 ± 0.03 | |
| Texture | Firmness (g) | 308.6 ± 0.27 | 301.5 ± 0.16 | 342.6 ± 0.19 |
| Consistency (gs) | 9224.0 ± 0.15 | 9237.3 ± 0.17 | 9382.6 ± 0.18 | |
| Cohesiveness (g) | 182.0 ± 0.17 | 180.9 ± 0.06 | 198.2 ± 0.15 | |
| Index of viscosity (gs) | 7320.0 ± 0.09 | 7314.7 ± 0.04 | 8010.4 ± 0.13 | |
FW FreshWeight
* Mean ± S.D (n = 3)
L*, a* and b* value showed that colour was brighter in fresh fruit compared to puree. Nendran puree showed more firmness, consistency and cohesiveness compared to fresh fruits.
Conclusion
Fructan composition in banana is influenced by the cultivar and varies in the levels of low molecular forms and inulin. Low temperature ripening (16 °C) of Nendran banana resulted in higher fructan distribution indicating accumulation of these carbohydrates in cold conditions. Treatment of Nendran banana with pectinase enhanced fructan extraction significantly. The relatively simple enzymatic method developed could provide a basis to optimize extraction from other food sources. Fructan loss during steaming of Nendran banana may be due to thermal degradation. In contrast, fructan content increased during puree preparation because of the concentration resulting from loss of moisture. The fructan data documented in this study will facilitate incorporation of high-fructan natural foods in the diet and nutritional formulation and gourmet selection for optimal fructan consumption.
Acknowledgments
This work was supported by the Senior Research Fellowship (SRF) from Council of Scientific & Industrial Research, New Delhi, India.
Contributor Information
R. Shalini, Email: shalinifoodtech@gmail.com
Usha Antony, Email: usha.antony@gmail.com.
References
- Abrams SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G, Ellis KJ. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone miner-alization in young adolescents. Am J Clin Nutr. 2005;82:471–476. doi: 10.1093/ajcn.82.2.471. [DOI] [PubMed] [Google Scholar]
- Agopian RGD, Soares CA, Purgatto E, Cordenunsi BR, Lajolo FM. Identification of fructooligosaccharides in different banana cultivars. J Agric Food Chem. 2008;56:3305–3310. doi: 10.1021/jf073011l. [DOI] [PubMed] [Google Scholar]
- Agopian RGD, Purgatto E, Cordenunsi BR, Lajolo FM. Synthesis of fructooligosaccharides in banana ‘Prata’ and its relation to inver-tase activity and sucrose accumulation. J Agric Food Chem. 2009;57:10765–10771. doi: 10.1021/jf902163f. [DOI] [PubMed] [Google Scholar]
- AOAC (Association of Official Analytical Chemists) (2000) Official method of analysis (17th Ed.). Gaithersburg, MD, USA
- AOAC (Association of Official Analytical Chemists) (2003) Official method of analysis, Method 999.03, (17th Ed.) Measurement of total fructan in foods- enzymatic/ spectrophotometric method. Gaithersburg, MD, USA
- Ashwell G (1957) Determination of inulin and fructose. In: Colowick, SJ and Kaplan NO (eds) Methods in enzymology, 3rd edn. Academic press, New York, p 75
- Ayman EM, Sania AH, Ahmed MK. Studies on production of soda crackers biscuits for diabetics. Ann Agric Sci. 2004;49:585–595. [Google Scholar]
- Beylot M. Effects of inulin-type fructans on lipid metabolism in man and in animal models. Br J Nutr. 2005;93:163–168. doi: 10.1079/BJN20041339. [DOI] [PubMed] [Google Scholar]
- Briet F, Achour L, Flourie B, Beaugerie L, Pellier P, Franchisseur C, Bornet F, Rambaud JC. Symptomatic response to varying levels of fructooligosaccharide consumed occasionally or regularly. Eur J Clin Nutr. 1995;49:501–507. [PubMed] [Google Scholar]
- Campbell JM, Bauer LL, Fahey GC, Jr, Hogarth AJCL, Wolf BW, Hunter DE. Selected fructooligosaccharide (1-kestose, nystose, and 1 F-β fructofuranosyl nystose) composition of foods and feeds. J Agric Food Chem. 1997;45:3076–3082. doi: 10.1021/jf970087g. [DOI] [Google Scholar]
- Hograrth AJCL, Hunter DE, Jacobs WA, Garleb KA, Wolf BW. Ion chromatographic determination of three fructooligosaccharide oligomers in prepared and preserved foods. J Agric Food Chem. 2000;48:5326–5330. doi: 10.1021/jf000111h. [DOI] [PubMed] [Google Scholar]
- Homme CL, Peschet JL, Puigserver A, Biagini A. Evaluation of fructans in various fresh and stewed fruits by high performance anion-exchange chromatography with pulsed amperometric detec-tion. J Chromatogr A. 2001;920:291–297. doi: 10.1016/S0021-9673(00)01262-0. [DOI] [PubMed] [Google Scholar]
- Homme CL, Puigserver A, Biagini A. Effect of food-processing on the degradation of fructooligosaccharides in fruit. Food Chem. 2003;82:533–537. doi: 10.1016/S0308-8146(03)00003-7. [DOI] [Google Scholar]
- Hond ED, Geypens B, Ghoos Y. Effect of high performance chicory inulin on constipation. Nutr Res. 2000;20:731–736. doi: 10.1016/S0271-5317(00)00162-7. [DOI] [Google Scholar]
- Indian Horticulture Database . National horticulture board, ministry of agriculture. Gurgaon: Government of India, Institutional Area; 2012. pp. 36–43. [Google Scholar]
- Kleessen B, Schwarz S, Boehm A, Fuhrmann H, Richter A, Henle T, Krueger M. Jerusalem artichoke and chicory inulin in bakery products affect faecalmicrobiota of healthy volunteers. Br J Nutr. 2007;98:540–549. doi: 10.1017/S0007114507730751. [DOI] [PubMed] [Google Scholar]
- Muir JG, Shepherd SJ, Rosella O, Rose R, Barrett JS, Gibson PR. Fructan and free fructose content of common Australian fruits and vegetables. J Agric Food Chem. 2007;55:6619–6625. doi: 10.1021/jf070623x. [DOI] [PubMed] [Google Scholar]
- O’Donoghue EM, Somerfield SD, Shaw M, Bendall M, Hedderly D, Eason J, Sims I. Evaluation of carbohydrates in Pukekohe long keeper and Grano cultivars of Allium cepa. J Agric Food Chem. 2004;52:5383–5390. doi: 10.1021/jf030832r. [DOI] [PubMed] [Google Scholar]
- Roberfroid MB. Inulin-type fructans: functional food ingredients. J Nutr. 2007;137:2493–2502. doi: 10.1093/jn/137.11.2493S. [DOI] [PubMed] [Google Scholar]
- Van Loo J, Coussement P, De Leenheer L, Hoebregs H, Smits G. On the presence of inulin and oligofructose as natural ingredients in the Western diet. Crit Rev Food Sci Nutr. 1995;35:525–552. doi: 10.1080/10408399509527714. [DOI] [PubMed] [Google Scholar]
- Vijn I, Smeekens S. Fructan: more than a reserve carbohydrate. Plant Physiol. 1999;120:351–359. doi: 10.1104/pp.120.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]