Abstract
Ngari and hentaak are the two most preferred traditional salt-free fermented fish products of North-Eastern (NE) states of India. Chemical and microbial composition, antioxidative potential, fatty acid profile and electrophoretic pattern of protein in ngari and hentaak were studied. pH and total titratable acidity (TTA) of the products justified their stability at ambient temperature. Both ngari and hentaak showed higher contents of calcium (362.79 ± 26.89, 472.11 ± 62.7); sodium (199.66 ± 24.92, 94.0 ± 12.78); potassium (58.20 ± 7.36, 75.74 ± 6.62) and magnesium (16.056 ± 3.89, 21.125 ± 3.78) respectively. Iron, copper and zinc were found in lesser amount. DPPH· radical scavenging activity was close to 87 % in both the products and the ferric chloride reducing power assay was dose dependent in both the products. Both omega-3 and omega-6 fatty acids were found in ngari; whereas, only omega-3 fatty acids were observed in hentaak. Linoleic acid (11.68 %) and arachidonic acid (0.65 %) were the n-6 PUFA in ngari; while, in hentaak, it was only arachidonic acid (8.54 %). Apart from essential fatty acids, essential amino acids were also found in considerable quantity in both the products. Micrococcus sp. and Staphylococcus sp. were found to be the dominant bacterial genus in both the products; while Ngari also had lactic acid bacteria group. The nutritional properties afforded by these products justify their preference by the population.
Keywords: Ngari, Hentaak, Manipur state, Fermented fish, Northeast India, Antioxidants, Fatty acid profile
Introduction
Fish are products of high nutritional value that give several benefits to human health being important sources of proteins, lipids, minerals and vitamins. Further, fish lipids are also the richest sources of highly unsaturated fatty acids (HUFA) in the human diet (Bhaskar et al 2006). Besides being protein rich foods, fish are highly perishable items and hence preservation becomes very much necessary. Traditionally, smoking, drying, salting, fermentation, marinating etc. have been practiced for preservation of fish besides host of newer technologies. Most of these processes have extension of shelf life as their objective either through moisture or pH reduction thereby ensuring retardation of microbial activity. The North-eastern (NE) states of India, being the highst rainfall area of the world, do not provide congenial environment for any traditional methods that involve drying. Hence, fermentation and/or salting have evolved as the preferred processes of preservation by the local populace.
Fermented fish products are akin to staple foods in most parts of south-east Asia, generally as a condiment for rice dishes (Ruddle and Ishige 2010). They have even become popular in the developed countries owing to their high nutritive value and organoleptic characteristics (Sanjeev et al. 1990). Fermentation is well known for being a method that is least destructive in terms of nutritive value and is often known to produce different bioactive peptides with anti-oxidative and anti-microbial properties as well as probiotic functions that add to the health benefits (Farhad et al. 2010). In addition, fermentation foods also enhance flavor, digestibility and therapeutic value of the food (Jeyaram et al. 2009). Besides this, many peptides released from food proteins, especially fish proteins, are known to exhibit biological activities, such as antimicrobial properties, blood-pressure lowering effects, cholesterol-lowering ability, antithrombotic and antioxidative activities (Hartmann and Meisel 2007). The NE region of India is bestowed with many fermented fish products like shidal, ngari, hentaak, lona ilish, tungtap, numsing etc. Shidal, ngari and hentaak are the major and popular traditional fermented fish products of NE India. Traditionally, shidal is believed to have anti-malarial effect and even today people consume it during fever, despite its unpleasant smell, for its therapeutic properties (Muzaddadi and Basu 2012).
Ngari is a popular fermented fish product of Manipur which is prepared by using sundried salt-free punti fish (Puntius sophore), locally known as phoubu usually imported from Brahmaputra valley of Assam (India) and Bangladesh. It is a compulsory condiment in the daily diets of NE populace, especially in the state of Manipur. Similarly, hentaak is an indigenous fermented fish paste product (small ball shaped) of the state of Manipur (India) prepared from dry fish Puntius spp., Amblypharyngodan mola, Esomus denricus etc. along with vegetable like Colocasia spp. It is noteworthy that no information especially in relation to lipid profile and antioxidant properties exists, regarding these traditional fermented fish products, besides proximate composition and dominant microbes. The objective of this study therefore was to document the nutritional profile in relation to lipids and proteins in order to provide impetus for further research on these traditional products of NE India.
Materials and methods
Materials
Fresh ngari and hentaak samples were purchased from different producers in local markets of Manipur, India. All the samples were packaged in sterile pouches and aseptically brought to the laboratory for chemical and microbial analysis. Tricholoroacetic acid (TCA), sulphuric acid (H2SO4), hydrochloric acid (HCl) potassium ferric cyanide, ferric chloride and sodium hydroxide (NaOH) was procured from M/s Thermo Fisher Scientific India Pvt. Ltd. (Mumbai, India). 2, 2-diphenyl-1-picrylhydrazyl (DPPH), tris–HCl, thio-barbituric acid (TBA), Luria Bertani broth (LB broth), nutrient agar, de-Man Rogosa and Sharpe (MRS) agar and Mueller-Hinton (MH) agar were purchased from M/s Hi-media Laboratories (Mumbai, India).
Methods
Preparation of sample extract
Both ngari and hentaak sample (5 g) was mixed with distilled water (25 ml) and the mixture was homogenised at a speed of 12,000 rpm for 1 min. The mixture was shaken at 110 rpm on an orbital shaker for 40 min at room temperature. The mixtures were then centrifuged at 7000 × g for 15 min at 4 °C to remove undesired debris. The collected supernatant was used for analysis of ferric chloride reducing power assay and antimicrobial assay.
Biochemical analysis
Proximate composition
Moisture, ash, crude protein, fat and total titratable acidity (TTA) contents of samples were determined according to AOAC methods (AOAC 2000). Total volatile basic nitrogen (TVBN) was determined by distillation method (AOAC 2000). Thiobarbituric acid (TBA) value was determined by using the method given by Buege and Aust (1978). Sample was homogenised with TBA-TCA-HCl solution followed by heating and subsequent cooling in tape water. The supernatant was collected and absorbance was taken at 532 nm. The pH of samples were determined by a pH meter (Sartorius, Goetingen, Germany), as described by Benjakul et al. (1997). The mineral contents of all fermented fish products were determined employing atomic absorption Spectrometer (AAS; Analyst A700, M/s Perkin-Elmer, USA) as per AOAC methods (AOAC 2000).
Amino acid analysis
Amion acid analysis was accomplished by employing the EZ:faast™ amino acid analysis kit (M/s Phenomenex-India, Hyderabad) that is based on solid-phase extraction (SPE) followed by derivatization procedure and a liquid/liquid extraction step. The derivatized amino acids were analysed on the column [ZB-AAA-10 m (10 m × 0.25 mm × 0.25 μm)] supplied along with the kit. The GC analysis conditions were - split ratio of 1:15, injection temperature of 300 °C and detection temperature of 320 °C. The column was subjected to a temperature gradient programme of 100 to 320 °C at a gradient rate of 35 °C per min with nitrogen as the carrier gas at 50 kPa. The amino acids were identified by comparing with authentic standards.
Fatty acid analysis
Total lipid was extracted from muscle tissues of threadfin breams by Folch method and the fatty acid methyl ester (FAME) was prepared from the isolated lipids by heating with the methanolic NaOH first and then with BF3 methanol for esterification. 5 ml n-heptane was added to recover the methyl esters in organic phase. The mixture was washed with saturated NaCl solution and two phases were separated using a separating funnel. The upper n-heptane phase was pipette out and stored in 10 ml all glass vials until further analysis.
Fatty acid methyl esters were separated by using gas Chromatography-mass spectrometer (GCMS; Shimadzu-QP2010 quadrupleMS, M/s Shimadzu, Kyoto, Japan) equipped with a Carbowax (30 m × 0.25 mm ID; 0.25 μm film thickness) capillary column (M/s Cromlab, USA). Helium was used as the carrier gas. Injector and detector temperatures were set at 250 °C. Injection was performed in split mode (1:15). The column temperature was programmed initially at 50 °C for 2 min and then ramped at a rate of 10 °C per min to a final temperature of 230 °C. FAME was separated at constant pressure (23.1 kpa) and peaks were identified by comparing standard mass spectra with the relative abundances of m/z ranging from 40.00 to 550. The values of fatty acids are presented in area percentage of total identified fatty acids.
In-vitro antioxidant assays
Total antioxidant activity and DPPH radical scavenging ability of the samples were determined as per methods described in Ganesan et al (2008). Antioxidant activity was expressed in μg ascorbic acid equivalent per mg of protein; while, DPPH radical scavenging was expressed as percentage. ABTS radical scavenging was determined as described in Balakrishnan et al (2011) and scavenging was expressed as percentage. The reducing power of both the samples were determined as per the method of Oyaizu (1986) and expressed as change in absorbance at 700 nm. Appropriate blanks as described in the respective procedures were also used for calculation.
Microbiological analysis
Total plate count (TPC) of each sample was determined using standard methods (APHA 2001). Counts were expressed in log colony forming units (CFU) per gram of sample. Enumeration of lactic acid bacteria (LAB) in the samples was done by employing MRS agar. The dominant bacterial colonies were randomly selected by their similarity in morphological characteristics from the TPC plates and bio-chemically characterized as per the protocol outlined in Bergey’s manual of determinative bacteriology (Bergey et al 2002).
Antibiotic sensitivity
Antibiotic sensitivity of isolated dominant groups of bacteria was tested against 5 antibiotic discs namely Chloramphenicol (C) (30 mcg), Erythromycin (E) (15 mcg), Norfloxacin (NX) (10 mcg), Co.-Trimoxazole (COT) (25 mcg) and Ofloxacin (OF) (5 mcg). Young culture of bacteria from Luria Bertani broth (LB broth) was swabbed on Mueller Hinton Agar (MH agar) and a disc of particular antibiotic was placed at centre of the plate. The diameter of clear zone was measured after 24 h of incubation.
Antibacterial assay
The antibacterial activity was carried out using agar-well diffusion method (Denton and Kerr 1998). Antimicrobial assay was conducted following agar-well diffusion method. Microorganisms used in this experiment were Gram-positive bacteria (Staphylococcus aureus and Bacillus cereus) and Gram negative bacteria (E. coli and Salmonella typhimurium). The tested strains were grown for 18–20 h in LB broth at 30 °C. Wells were bored on MH agar plate with a sterile cork borer no.2 (with 5 mm diameter). The sample extracts were firstly filtered through a 0.45 μm sterile nylon membrane filter and then loaded to the agar well at a volume of 15 μl. The plates were incubated in an upright position at 37 °C for 24 h. Tetracycline (10 μl/ml) was used as positive control. The zone of growth inhibition was expressed in mm.
Results and discussion
Bio-chemical and microbial quality of ngari and hentaak
The biochemical parameters including proximate composition and microbial quality indices of both ngari and hentaak are presented in Table 1. Moisture content of ngari and hentaak was 29.72 and 35.0 %, respectively. The lower moisture content is due to the fact that sun-dried fish (moisture content <10 %) are usually employed in the preparation of the products. The differences in lipid and protein content between the products are mainly due to the protein and lipid contents of the raw material used for the preparation. Our results corroborate with similar proximate composition in related products like fermented Setipinna sp. from Manipur (Sarojnalini and Suchitra 2009) and Punti shidal of Assam (Kakati and Goswami 2013). The pH and total titratable acidity (TTA) have been found as 6.14, 0.415 g% and 6.11, 0.386 g% in ngari and hentaak, respectively. The higher pH in these products as compared to other fermented fish products of the region may possibly be due to higher amounts of volatile nitrogenous compounds produced and accumulated in the product as a result of fermentation. Similar pH values have also reported by several researchers in different fermented fish products (Thapa and Pal 2007; Kakati and Goswami 2013; Yatsunami and Takenaka 1996). Both ngari and hentaak had relatively higher TVBN content (Table 1) which may be attributed to biochemical and microbial changes in that proceed in the fish muscle as a result of fermentation (Kakati and Goswami 2013). However, such high concentration of TVBN usually does not manifest any ammonia-like odour in the product. Similar higher TVBN content has also been reported by other researchers in various fermented fish products (Anihouvi et al. 2006; Kakati and Goswami 2013; Roy et al. 2014). Degree of lipid oxidation as measured by estimating thiobarbituric acid (TBA) value was found 1.27 ± 0.12 and 0.84 ± 0.15 mg malonaldehyde per kg meat respectively. The TBA values represent the degree of rancidity in the products and the values above 3–4 indicate quality loss (Karacam and Boran 1996). The lower TBA values indicate that the secondary lipid oxidation is limited in these product possibly due to the micro-aerobic condition as well as absence of pro-oxidants such as metallic container, salt etc. almost similar TBA value was also reported by several authors in related fermented fish products (Sarojnalini and Suchitra 2009; Priyadarshini et al. 2014).
Table 1.
Biochemical and microbial quality composition of ngari and hentaak (n = 6)
| Ngari | Hentaak | |
|---|---|---|
| pH | 6.14 ± 0.10 | 6.11 ± 0.06 |
| TTA (g %) | 0.415 ± 0.06 | 0.386 ± 0.11 |
| Moisture (%) | 29.72 ± 0.94 | 35.0 ± 1.04 |
| Crude protein (%) | 42.87 ± 0.35 | 37.63 ± 0.89 |
| Total lipid (%) | 13.51 ± 1.01 | 9.91 ± 0.17 |
| TVBN (mg %) | 100.17 ± 1.14 | 95.23 ± 1.24 |
| TBA (mg malonaldehyde/kg meat) | 1.27 ± 0.12 | 0.84 ± 0.15 |
| TPC (log cfu/g) | 6.65 ± 1.00 | 7.81 ± 0.09 |
| LAB (log cfu/g) | 6.2 ± 0.08 | 4.9 ± 0.11 |
Mineral composition (mg per 100 g dry weight) of Ngari and Hentaak is presented in Table 2. Both ngari and hentaak showed higher contents of calcium (362.79 ± 26.89, 472.11 ± 62.7); sodium (199.66 ± 24.92, 94.0 ± 12.78); potassium (58.20 ± 7.36, 75.74 ± 6.62) and magnesium (16.056 ± 3.89, 21.125 ± 3.78) respectively. Iron, copper and zinc were found in lesser amount. The values are in corroboration with mineral contents of a related fermented fish product (Phasa shidal) which had calcium, magnesium, iron and copper content of 176.09, 25.42, 7.78 and 0.16 mg/100 g respectively (Roy et al. 2014). The higher calcium in case of ngari and hentaak could possibly be due to the presence of pin bones which could not be separated during collection of flesh from the product during analysis.
Table 2.
Elemental composition (mg per 100 g dry weight) of ngari and hentaak (n = 6)
| Element | Ngari | Hentaak |
|---|---|---|
| Calcium | 362.79 ± 26.89 | 472.11 ± 62.7 |
| Potassium | 58.20 ± 7.36 | 75.74 ± 6.62 |
| Iron | 0.020 ± 0.0062 | 0.017 ± 0.004 |
| Sodium | 199.66 ± 24.92 | 94.0 ± 12.78 |
| Manganese | 0.51 ± 0.016 | 0.36 ± 0.043 |
| Copper | 0.021 ± 0.003 | 0.02 ± 0.0064 |
| Zinc | 0.86 ± 0.092 | 1.03 ± 0.16 |
| Magnesium | 16.056 ± 3.89 | 21.125 ± 3.78 |
In-vitro antioxidant activity of aqueous extracts of ngari and hentaak
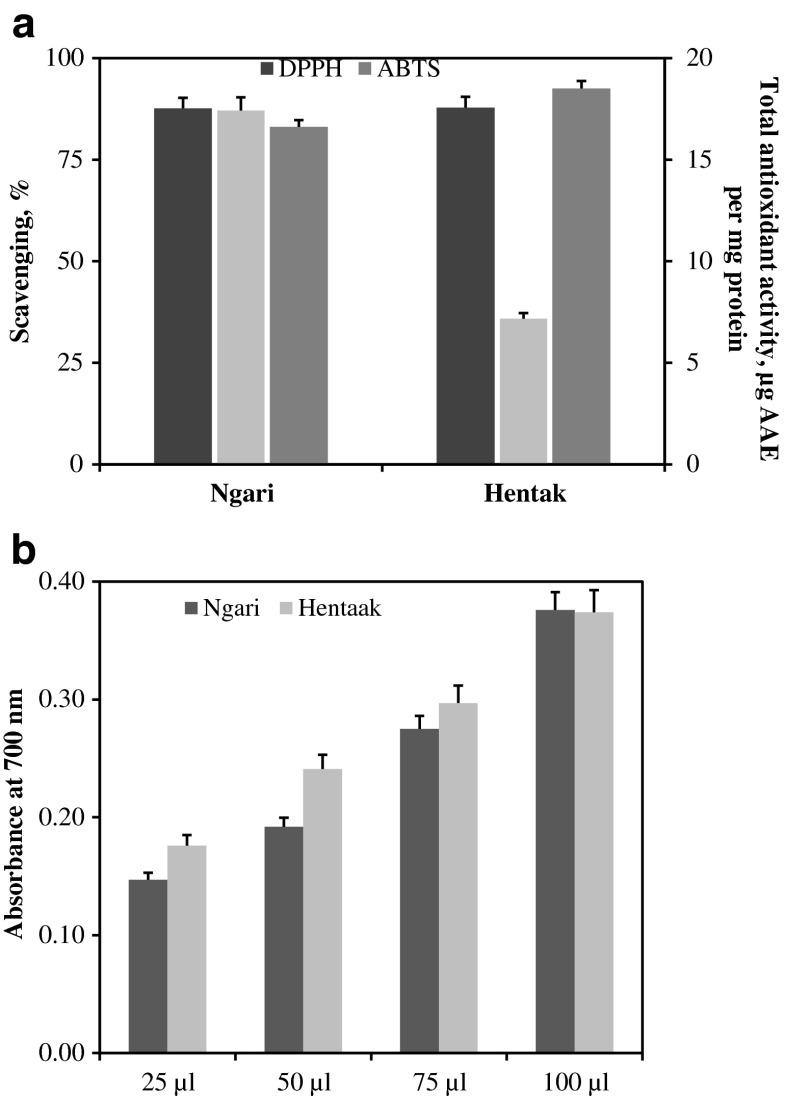
Water-soluble fractions from the samples possessed the ability to quench DPPH· and ABTS radicals. Total antioxidant activity, DPPH· and ATBS radical scavenging (%) activity of aqueous extract of ngari and hentaak is presented in Fig. 1a. Total antioxidant activity (expressed as μg ascorbic acid equivalent per mg protein) was found to be 17.41 and 7.17 in respect of ngari and hentaak, respectively. DPPH· radical scavenging activity of ngari and hentaak was about 87 %. Ngari showed slightly lower ABTS radical scavenging activity than that of hentaak (Fig. 1a). Both the products exhibited dose dependent increase in the reducing power (Fig. 1b). At lower concentration (25 to 75 μl), the reducing power of ngari was found to be less than hentaak, whereas at higher concentration (100 μl), both the products showed similar reducing power. Similar scavenging of DPPH· radical is reported in respect of some of the related fermented fish products like squid miso prepared with koji which exhibited close to 90 % scavenging (Giri et al. 2011) and water-soluble fraction from kapi, a fermented fish product of Thailand showed antioxidative activity (Faithong et al. 2010). Usually peptides from fermented fish products have been reported to act as antioxidants (Faithong et al. 2010; Giri et al 2011). The observed DPPH· radical scavenging activity indicated that peptides or free amino acids in ngari and hentaak could possibly be responsible for the observed activity.
Fig. 1.
a Total antioxidant actitivy (expressed as μg ascorbic acid equivalent per mg protein), DPPH and ATBS radical scavenging (%) and b Reducing power (Absorbance at 700 nm) of aqueous extacts of ngari and hentaak samples. TBHQ at 1 mg per ml concentration showed 86.6 % and 99 % of DPPH and ABTS radical scavenging activity, respectively. The extracts had a protein content (mg per ml) of 1.89 and 3.27 in case of ngari and hentaak, respectively. All the values are mean ± SD of atleast 4 replicates
Amino acid and fatty acid composition
Analysis of amino acid composition (% dry weight) of the fermented fish products (Table 3) revealed that ngari was rich in glycine, proline, aspartic acid and the essential amino acids phenylalanine, leucine, lysine (4.95, 3.15, 3.64, 3.23, 2.46 and 3.00 % dry weight respectively); while, the hentaak was found to be rich source of glycine, alanine, proline, aspartic acids, glutamic acid and the essential amino acids phenylalanine, lysine, leucine (5.72, 4.09, 4.45, 3.84, 3.35, 4.91, 3.81 and 4.79 % dry weight respectively). Essential amino acids such as arginine and tryptophan could not be detected, whereas, threonine and histidine were found in very low amount. On the other hand, amount of lysine was found to be satisfactory. The total amount of essential amino acids was 39.6 and 44.1 % of total amino acids in respect of ngari and hentaak respectively. In a related product, shidal, Majumdar et al (2009) reported that it contained more of non-essential amino acids such as aspartic acid, glutamic acid, alanine (7.71, 14.15, 7.55 g/100 g protein respectively) as compared to essential amino acids like valine, isoleucine, leucine, phenylalanine and lysine (4.51, 4.19, 6.81, 4.14 and 6.16 g/100 g protein respectively). Lower amount as well as absence of some amino acids indicates the possibility of formation of derivatives of amino acids such as amines and gluconeogenic substances during fermentation. Higher amount of some non-essential amino acids like glutamic acid, aspartic acid, and glycine, were reported to contribute to the taste attributes of fermented fish and shellfish products (Jung et al. 2004).
Table 3.
Amino acid Composition of ngari and hentaak (g/100 g dry weight)
| Amino acid | Ngari | Hentaak |
|---|---|---|
| Essential | ||
| Valine | 1.52 | 2.55 |
| Leucine | 2.46 | 4.79 |
| Isoleucine | 1.03 | 1.87 |
| Threonine | 0.48 | 0.47 |
| Phenylalanine | 3.23 | 4.91 |
| Lysine | 3.00 | 3.81 |
| Histidine | 0.80 | 1.00 |
| Methionine | 1.12 | 1.86 |
| Non-essential | ||
| Tyrosine | 2.58 | 3.65 |
| Alanine | 2.64 | 4.09 |
| Glycine | 4.95 | 5.72 |
| Serine | 0.59 | 0.72 |
| Proline | 3.15 | 4.45 |
| Aspartic acid | 3.64 | 3.84 |
| Hydroxyproline | 0.98 | 1.11 |
| Glutamic acid | 2.28 | 3.35 |
Fatty acid profiles of both the products is presented in Table 4. Amongst the saturated fatty acids, palmitic acid (C16:0) was found to be dominant in ngari and contributed about 7 % of the total fatty acids; whereas, stearic acid (C18:0) was dominant in case of hentaak contributing to 25 % of the total fatty acids. Amongst the monoenoic fatty acids, vaccenic acid (C18:1n-7) contributed about one third (29.23 %) of the total fatty acids followed by oleic acid (23.58 %) in ngari; while, in case of hentaak, monoenoic fatty acids were dominated by C16:1n-5 which contributed 11.75 % of total fatty acid followed by oleic acid (11.40 %). Some odd carbon number fatty acids were also found in both the products. There is possibility that such odd carbon chain fatty acids might be from the microbes involved in fermentation. Very few works on the fatty acid profile of fermented fish products has been reported but there are possibilities of loss of PUFAs during fermentation as well as post fermentation exposure of the product out of fermenting containers. Linoleic acid (11.68 %) and arachidonic acid (0.65 %) were the n-6 PUFA in ngari; while, in hentaak, it was only arachidonic acid (8.54 %). EPA and DHA content in ngari and hentaak were 0.74 % & 0.86 % and 1.17 % & 2.07 %, respectively. Presence of both omega-3 and omega-6 fatty acids indicate the nutritional significance of these two traditional fermented fish products.
Table 4.
Fatty acid composition (% w/w of total lipids) of ngari and hentaak
| Fatty acid | Ngari | Hentaak |
|---|---|---|
| C12:0 | 0.30 | 0.28 |
| C13:0 | 0.16 | 0.19 |
| C14:0 | 1.23 | 2.30 |
| C15:0 | 1.70 | 2.06 |
| C16:0 | 7.15 | 5.75 |
| C16:1(n-7) | 4.99 | 3.38 |
| C16:1(n-5) | 11.36 | 11.75 |
| C17:0 | 1.84 | 4.14 |
| C18:0 | 0.36 | 24.77 |
| C18:1(n-9) | 23.58 | 11.40 |
| C18:1(n-7) | 29.23 | – |
| C18:2(n-6) | 11.68 | 10.46 |
| C18:3(n-6) | 0.54 | – |
| C18:3(n-3) | 1.58 | 1.06 |
| C19:0 | – | 1.92 |
| C19:1(n-9) | – | 1.39 |
| C20:0 | 0.19 | – |
| C20:1(n-9) | 1.45 | 2.98 |
| C20:2(n-9) | 0.09 | – |
| C20:3(n-9) | 0.05 | 2.06 |
| C20:4(n-6) | 0.65 | 8.54 |
| C20:5(n-3) | 0.74 | 1.17 |
| C22:1(n-9) | 0.27 | 2.30 |
| C22:6(n-3) | 0.86 | 2.07 |
Microbial characteristics of ngari and hentaak
Overall, microbiological indices of ngari and hentaak are given in Table 1. The TPC and LAB of both products were found as 6.65 ± 1.00, 6.2 ± 0.08 log cfu/g and 7.81 ± 0.09, 4.9 ± 0.11 log cfu/g respectively. The higher count of microbes may be due to keeping pattern i.e., in open condition for longer time and unhygienic handling. Similar microbial load for different fermented fish products is also reported (Anihouvi et al. 2006; Roy et al. 2014). The dominant bacterial colonies were randomly selected by their similarity in morphological characters from the TPC plates; and, the details of bio-chemical characteristics of dominant bacterial groups isolated from both the products is presented in Table 5. It was found that lactic acid bacteria, Micrococcus and Staphylococcus were the dominant bacterial genus in ngari, whereas the dominant genera in hentaak was found to be Micrococcus and Staphylococcus. In case of antibiotic sensitivity test (Table 6), the zone of inhibition ranged from 1.8 to 4.0 cm for the isolates. Microflora plays an important role at initial period as well as continuing the fermentation process. The presence of microorganisms during fermentation contributes to the degradation of proteins and development of flavour and aroma (Salampessy et al. 2010).
Table 5.
Colony and biochemical characteristics of dominant bacterial groups isolated from ngari and hentaak
| 1. Product | Ngari | Hentaak | ||||||
| 2. Colony code | Ng-1 | Ng-2 | Ng-3 | Ng-4 | Hn-1 | Hn-2 | Hn-3 | |
| 3. Color & size* | Y, B | Y, S | W, B | W, S | Y, S | W, B | W, S | |
| 4. Cell morphology | - All were coccus - | |||||||
| 5. Gram’s stain | + | + | + | + | + | + | + | |
| 6. Catalase | − | + | − | + | + | + | + | |
| 7. Oxidase | + | + | − | − | + | − | − | |
| 8. Growth at pH | 4.0 | − | − | + | − | − | − | + |
| 7.0 | + | + | + | + | + | + | + | |
| 9.0 | + | + | + | + | + | + | + | |
| 9. O/F test | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | |
| 10. MR/VP | +/− | +/− | +/− | +/− | +/− | +/− | +/− | |
| 11. Citrate test | − | − | − | − | − | − | − | |
| 12. Indole | − | − | − | − | − | − | − | |
| 13. Amylase | − | − | − | − | − | − | − | |
| 14. Lipolysis | − | − | − | − | + | − | + | |
| 15. Deaminase | + | − | − | − | − | − | − | |
| 16. Salt (%) Tolerance | 0 | + | + | + | + | + | + | + |
| 2 | + | + | + | + | + | + | + | |
| 4 | + | + | + | + | + | + | + | |
| 6 | − | − | + | + | + | + | + | |
| 8 | − | − | + | − | + | − | + | |
| 17. Sugars Fermented | Maltose | + | + | + | − | + | + | + |
| Lactose | + | + | − | + | + | − | − | |
| Mannitol | + | + | − | + | − | − | + | |
| Sucrose | − | + | − | + | − | − | − | |
| Sorbitol | − | + | + | + | + | + | + | |
| Glucose | + | + | − | − | − | − | − | |
| Dextrose | + | + | + | + | + | + | + | |
| 18. Suspected bacterial genus# | LAB | Mic. | LAB | Staph | Mic | Staph | Staph | |
*Colour codes W & Y represent white and yellow respectively; size codes S & B represent small and big respectively
#LAB – lactic acid bacteria (either Lactococcus or Streptococcus); Mic : Micrococcus; Staph : Staphylococcus
Table 6.
Antibiotic sensitivity test of dominant bacterial groups isolated from ngari and hentaak samples
| Sl. no. | Colony code# | Diameter of clear zone (cm) | ||||
|---|---|---|---|---|---|---|
| C* (30 mcg) | E* (15 mcg) | NX* (10 mcg) | COT* (25 mcg) | OF* (5 mcg) | ||
| 1 | Ng-1 | 2.6 | 1.8 | 2.6 | 2.4 | 2.6 |
| 2 | Ng-2 | 4.0 | 3.9 | 3.4 | 3.3 | 2.7 |
| 3 | Ng-3 | 2.6 | 2.3 | 2.6 | 2.6 | 2.4 |
| 4 | Ng-5 | 2.9 | 2.8 | 3.0 | 2.6 | 2.6 |
| 5 | Hn-1 | 1.8 | 2.6 | 3.1 | 3.0 | 2.8 |
| 6 | Hn-2 | 2.6 | 2.4 | 2.6 | 2.1 | 2.4 |
| 7 | Hn-3 | 2.9 | 2.7 | 2.8 | 2.7 | 2.5 |
*Antibiotic discs : C - Chloramphenicol (SD006-5CT); E - Erythromycin (SD013-5CT); NX - Norfloxacin (SD057-5CT); COT - Co.-Trimoxazole (SD010-5CT); OF - Ofloxacin (SD087-5CT)
#Colony codes as in Table 5
Conclusion
Traditionally processed fish products are very popular among the populace of North-eastern India. Ngari and Hentaak plays a very important role in the nutrition of people of North East India, especially amongst the ethnic people of Manipur. The present study revealed that ngari and hentaak are having immense nutritional value in respect of essential fatty acids and antioxidative potential. These biofunctionalities could possibly be the reason for their use in daily diets as well as for some therapeutic purposes by the local populace. This work is hoped to provide a base line information regarding these fermented fish products and further studies to decipher mechanisms involved in the therapeutic properties are required to ascertain their value.
Acknowledgments
RKM and BN thank Department of Biotechnology, Govt. of India, New Delhi for partial funding of the project (Grant #BT/307/NE/TBP/2012) under the TWINNING programme for the North-East. The technical assistance provided by all technical staff of the Dept. of Fish Processing Technology & Engineering, College of Fisheries (CAU), Lembucherra, Tripura (India) is gratefully acknowledged. Authors thank Dean, College of Fisheries, Lembucherra and Director, CSIR-CFTRI, Mysore for according permission to the collaborative project.
Footnotes
Ranendra K. Majumdar and Sandeep K. Bejjanki equal contribution.
Contributor Information
Ranendra K. Majumdar, Phone: +919862441057, Email: drrkmcof@gmail.com
Bhaskar Narayan, Phone: +91-821-2514840, Email: bhasg3@yahoo.co.in.
References
- Anihouvi VB, Ayernor GS, Hounhouigan JD, Sakyi-Dawson E (2006) Quality characteristics of lanhouin: a traditionally processed fermented fish product in the Republic of Benin. African J Food Agri Nutri Dev 6(1): 1–15
- AOAC . Official methods of analysis, association of official analytical chemists, AOAC. Virginia, USA: Arlington; 2000. [Google Scholar]
- APHA (2001) In: Compendium of Methods for Microbiological Examination of Foods. In Speak ML (ed) American Public Health Assn. Washington, USA
- Benjakul S, Seymour TA, Morrissey MT, An H. Physico-chemical changes in pacific whiting muscle proteins during iced storage. J Food Sci. 1997;62:729–733. doi: 10.1111/j.1365-2621.1997.tb15445.x. [DOI] [Google Scholar]
- Bergey DH, Holt JG, Kreig NR, Sneath PHA. Bergey’s manual of determinative bacteriology. New York: Springer-Verlag; 2002. [Google Scholar]
- Bhaskar N, Miyashita K, Hosokawa M. Physiological effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—a review. Food Rev Intl. 2006;22:291–307. doi: 10.1080/87559120600694622. [DOI] [Google Scholar]
- Balakrishnan B, Prasad B, Rai AK, Suresh PV, Mahendrakar NS, Bhaskar N. In vitro antioxidant and antibacterial properties of hydrolysed proteins of delimed tannery fleshings: comparison of acid hydrolysis and fermentation methods. Biodegradation. 2011;22:287–295. doi: 10.1007/s10532-010-9398-0. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Denton M, Kerr KG. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin Microbiol Rev. 1998;11:57–80. doi: 10.1128/cmr.11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faithong N, Benjakul S, Phatcharat S, Binsan W. Chemical composition and antioxidative activity of Thai traditional fermented shrimp and krill products. Food Chem. 2010;119(1):133–140. doi: 10.1016/j.foodchem.2009.06.056. [DOI] [Google Scholar]
- Farhad M, Kailasapathy K, Tamang JP. Health aspect of fermented foods. In: Tamang JP, Kailasapathy K, editors. Fermented foods and beverages of the world. New York: CRC Press, Taylor & Francis Group; 2010. pp. 391–414. [Google Scholar]
- Ganesan P, Chandini SK, Bhaskar N. Antioxidant properties of methanol extract and other solvent fractions obtained from selected Indian red seaweed. Biores Technol. 2008;99:2717–2723. doi: 10.1016/j.biortech.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Giri A, Osako K, Okamoto A, Okazaki E, Ohshima T. Antioxidative properties of aqueous and aroma extracts of squid miso prepared with Aspergillus oryzae inoculated Koji. Food Res Int. 2011;44:317–325. doi: 10.1016/j.foodres.2010.10.013. [DOI] [Google Scholar]
- Hartmann R, Meisel H. Food-derived peptides with biological activity: from research to food applications. Curr Opin Biotech. 2007;18:163–169. doi: 10.1016/j.copbio.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Jeyaram K, Singh TH, Romi W, Devi AR, Singh WM, Dayanidhi H, Singh NR, Tamang JP. Traditional fermented foods of Manipur. Indian J Trad Know. 2009;8(1):115–121. [Google Scholar]
- Jung WK, Rajapakse N, Kim SK. Antioxidative activity of low molecular weight peptide derived from the sauce of fermented blue mussel, Mytilus edulis. Eur Food Res Technol. 2004;220:535–539. doi: 10.1007/s00217-004-1074-3. [DOI] [Google Scholar]
- Kakati BK, Goswami UC (2013) Characterization of the traditional fermented fish product Shidol of North East India prepared from Puntius sophore and Setipinna phasa. Ind J Trad Know 12(1):85–90
- Karacam H, Boran M. Quality changes in frozen whole and gutted anchovies during storage at −18 °C. Int J Food Sci Tech. 1996;31:527–531. doi: 10.1046/j.1365-2621.1996.00355.x. [DOI] [Google Scholar]
- Majumdar RK, Basu S, Nayak BB. Assessment of nutritional quality of 'Shidal' a fermented fish product of northeast India. J Ind Fish Assoc. 2009;36:25–34. [Google Scholar]
- Muzaddadi AU, Basu S. Shidal- a traditional fermented fishery product of North East India. Indian J Trad Know. 2012;11(2):323–328. [Google Scholar]
- Oyaizu M. Studies on products of browning reaction prepared from glucosamine. Japan J Nutr. 1986;44:307–314. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Priyadarshini BM, Roy D, Majumdar RK, Kanasi S. Quality characterization of market available Tungtap- a fermented fish product of Meghalaya, India. Asian J Micro Biotechnol Env Sci. 2014;16(4):931–934. [Google Scholar]
- Roy D, Majumdar RK, Maurya SK, Tripathi HH, Dhar B, Priyadarshini BM. Understanding of traditional knowledge and characterization of telesech- a fermented fish product of Tripura state. Indian J Nat Prod Res. 2014;5(4):351–358. [Google Scholar]
- Ruddle K, Ishige N (2010) On the origins, diffusion and cultural context of fermented fish products in Southeast Asia. In : Farrer J (Ed.) Globalization, Food and Social Identities in the Asia Pacific Region, Sophia University Institute of Comparative Culture, Tokyo, 18p (http://icc.fla.sophia.ac.jp/global%20food%20papers/html/ruddle_ishige.html)
- Salampessy J, Kailasapathy K, Thapa N. Fermented fish products. In: Tamang JP, Kailasapathy K, editors. Fermented foods and beverages of the world. New York: CRC Press, Taylor & Francis Group; 2010. pp. 289–307. [Google Scholar]
- Sanjeev K, Soni Dhanwant K. Indian fermented foods; microbiological and biochemical aspects. Ind J Microbiol. 1990;30(2):135–157. [Google Scholar]
- Sarojnalini C, Suchitra T. Microbial and nutritional evaluation of fermented Setipinna species. Fish Tech. 2009;46(2):165–170. [Google Scholar]
- Thapa N, Pal J. Proximate composition of traditionally processed fish products of eastern Himalayas. J Hill Res. 2007;20(2):75–77. [Google Scholar]
- Yatsunami K, Takenaka T. Changes in nitrogenous components and protease activity of fermented sardine with rice bran. J Fish Sci. 1996;62:790–795. [Google Scholar]