Abstract
This research was carried out to determine biochemical properties of β-glucosidase (β-D-glucoside glucohydrolase, EC 3.2.1.21) isolated from Turkish tea leaves. Two protein peaks containing β-glucosidase activity were recovered and characterized, which were denoted as isoenzyme A and isoenzyme B. Their pH optimum, thermal resistances, affinity towards p-nitrophenyl-β-D-glucopyranoside differed markedly. They both displayed maximal activity at pH 5.0. The effects of the inhibitors tested varied in a dose dependent manner.
Keywords: β-glucosidase, Purification, Tea leaves, Kinetics, Thermal inactivation, Thermal stability
Introduction
The tea plant, commonly grown for beverage production, is of great economic importance (Yang et al. 2013). It is one of the most widely consumed beverages in the world today second only to water (Khan et al. 2007). According to the Food and Agriculture Organization, world tea production is 3.2 million tons per annum, and Turkey with an annual tea production of 200,100 tonnes which constitutes 6 % of world tea production is an important tea producer along with are India, China, Sri Lanka, Kenya. Turkey with 2.3 kg tea consumption per capita is the 4th biggest tea consumer in the world (Yang et al. 2013; Demir 2002). Three varieties of cultivated tea Camellia sinensis (L.) O. Kuntze are known: var. China, var. Cambod and var. Assam. The teas grown in Turkey are hybrids of these varieties although var. China features are predominant (Yılmaz et al. 2004).
Tea quality is important for its market value and is defined by color, freshness, strength, and aroma. While phenolic compounds are responsible for the color and the taste, volatile compounds are fundamental for tea aroma. Therefore, it is not surprising that volatile compounds in tea have been investigated since the 1930s (Yang et al. 2013). Tea aroma is one of the most important factors in determining tea quality. Among more than 500 kinds of tea aroma constituents, monoterpene alcohols and aromatic alcohols, which have floral or fruity (fruit-like) smell, are known to contribute to the floral aroma of tea β-glucosidases (EC 3.2.1.21) are widely distributed throghout the plant kingdom. The hydrolyze β-D-glycoside bounds to release non-reducing β-D-glucose residues and terminal aglycone. According to recent studies, glycosides with monoterpene alcohols and aryl alcohols as aglycone are abundant in fresh tea leaves. The alcoholic tea aroma is generated by enzymatic hydrolysis of this kind of glucoside during the manufacture of tea, which is especially important for the quality of black tea, Oolong tea and green tea (Li et al. 2005).
β-glucosidase constitutes a major group among glycoside hydrolases occurring universally in all three domains of living organisms. They have been investigated intensively because of their key roles in a variety of fundamental biological and biotechnological processes. In plants, β-glucosidases play an important role in diverse fundamental biological processes including defense against pathogens and herbivores, scent release, phytohormone conjugate activation and the degradation of cell wall oligosaccharides during germination (Esen 2003; Chen et al. 2012).
There are several hundred different β-glucosidic flavor precursors identified from plants which indicate the presence of β-glucosidases in plant tissues that hydrolyze these flavor precursors. Thus, in each case there is a need for isolating and characterizing the specific enzyme that hydrolyzes a β-glucoside whose aglycon moiety is of interest to food quality and processing. Such biochemical data are crucial to making practical decisions as to whether or not enzymes from host plants or other sources should be added to drinks and beverages before, during, or after processing to enhance flavor, aroma, and other quality factors (Esen 2003).
Flavor compounds in many fruit and plant tissues are glycolyted and accumulate as nonvolatile and flavorless glycoconjugates which can be released either by acid or enzyme hydrolysis. Some aglycones are already odorous when released from glycosides. They can contribute to the floral aroma of some wines, grapes, apricots, peaches and tea. Volatile compounds (monoterpens, vanillin) can be liberated from β–D-glycosides through the action of β–glucosidase. Aglycone moiety can be released through sequential hydrolysis or one-step enzyme catalyzed reactions. In the sequential hydrolysis the inter-sugar linkage is cleaved by either an α-arabinofuranosidase, an α-rhamnopyranosidase, or a β-apiofuranosidase depending on the dissaccharidic moiety. The one-step reaction occurs through the cleavage of the agylconic linkage, yielding a disachharide and aglycone (Günata 2003).
This paper investigates partial purification and determination of biochemical properties of β-glucosidase of tea leaves. To the best of my knowledge, this is the first report on β-glucosidase from Turkish tea leaves.
Material and methods
Material
The tea leaves used in this study were obtained from Black sea region of Turkey and frozen at −25 °C until used. p-nitrophenyl-β-D-glucopyranoside (pNPG), p-nitrophenol, triton X-100, sodium metabisulfite, polyvinylpyrolidone (PVPP), ascorbic acid, ammonium sulphate, glucose, ethanol, disodiumhydrogen phosphate, bovine serum albumin were purchased from Merck (Darmstadt, Germany). Polyethylene glycol (PEG), phenylmethylsulfonyl fluoride (PMSF), citric acid, acetone, sodium carbonate and cellulose membrane (76 × 49 mm) were purchased from Sigma-Aldrich (St. Louis, USA). Toyopearl DEAE-650 M was purchased from Supelco (Montgomeryville, USA). All chemicals were of analytical grade.
Enzyme extraction
Frozen tea leaves (300 g) were homogenized in 400 mL of cold acetone (−25 °C) containing 3.33 g of polyethylene glycol, using a pre-chilled Waring blender (Model HGBTWTS3, Torrington, Connecticut, USA) for 2 min at low speed. The slurry was vacuum filtered through filter paper. The residue was re-extracted with 200 mL of cold acetone. This procedure was repeated until a white powder was obtained. The resultant acetone powder was dried overnight at room temperature and stored at −25 °C (Lecas et al. 1991).
Thirty grams of acetone powder was stirred for 4 h in 300 mL of 0.1 M phosphate-citrate buffer (pH 6.8) containing 10 mM ascorbic acid, 4.0 % polyvinylpyrolidene, 1.0 % Triton X-100 and 1 mM PMSF, using a magnetic stirrer after which the homogenate was centrifuged at 10,000×g for 45 min at 4 °C. The resulting supernatant was subjected to ammonium sulfate precipitation. The fraction precipitated at 85 % saturation was separated by centrifugation at 10,000×g for 45 min at 4 °C. The precipitate was dissolved in a small amount of 10 mM phosphate-citrate buffer, pH 6.8, and dialyzed against the same buffer overnight at 4 °C (Riou et al. 1998; Garcia-Palazon et al. 2004; Mazzuca et al. 2006).
Ion exchange chromatography
For further purification of β-glucosidase, a dialyzed enzyme aliquot was fractioned by ion exchange chromatography. In ion exchange chromatography, a column (2.5 × 30 cm) filled with DEAE-Toyopearl 650 M was equilibrated with 10 mM phosphate-citrate buffer, pH 6.8. The column was eluted with same buffer at a flow rate of 0.5 mL/min and linear gradient of phosphate-citrate buffer concentration from 10 to 200 mM. 4.5 mL fractions were collected in which the protein level and β-glucosidase activity towards pNPG as substrate were monitored (Riou et al. 1998). The fractions which showed β-glucosidase activity were combined and used as the enzyme source in the present study.
Protein determination
Protein contents of the enzyme extracts were determined according to Bradford method using bovine serum albumin as a standard (Bradford 1976).
Assay of enzyme activity
β-glucosidase activity was determined in 1.0 mL assay mixtures in a spectrophotometer (Shimadzu UV-1700, Kyoto, Japan) fitted with a thermostatted cuvette by measuring the absorbance at 400 nm. Unless otherwise stated, the standard reaction mixture consisted of 0.1 mL of enzyme solution and 0.9 mL of pNPG in 0.1 M phosphate-citrate buffer (pH 5.0). After the reaction mixture was kept in water bath at 50 °C for 15 min, the reaction was stopped by adding 1.0 mL of 1.0 M sodium carbonate. The p-nitrophenol released in the reaction was determined by the yellow colour in spectrophotometer at 400 nm. pNPG was used at a final concentration of 5.0 mM. In all experiments control experiments without enzyme were also conducted. A p-nitrophenol (20–160 μM) calibration curve was previously prepared to determine the enzyme activity. A unit of enzyme activity (U) was defined as the amount of β-glucosidases which would liberate 1 μM p-nitrophenol/min under the experimental conditions (Lecas et al. 1991).
pH optima
β-glucosidase activity was determined in a pH range of 3.0–8.0 in 0.1 M phosphate-citrate buffer. β-glucosidase activity was assayed, using the standard reaction mixture but changing the buffer. β-glucosidase activity was calculated in the form of percent relative β-glucosidase activity at the optimum pH. The optimum pH obtained for this enzyme was used in the experiments listed below.
Temperature optima
The activity of β-glucosidase was determined at temperatures ranging from 30 °C to 70 °C. The standard reaction mixture was incubated at different temperatures (30–70 °C) in a water bath. After incubation of the reaction mixture at the selected temperature, 1.0 mL of sodium carbonate (1.0 M) was added and the enzyme activity was measured. β-glucosidase activity was calculated in the form of percent relative β-glucosidase activity at the optimum temperature.
Kinetic parameters
In order to determine Michaelis constant (Km) and maximum velocity (Vm), β-glucosidase activities were measured using pNPG as substrate at various concentrations (1.25–40 mM). Km and Vm values of the enzyme were calculated from a plot of 1/V vs. 1/S by the method of Lineweaver and Burk.
Thermal inactivation kinetics
Thermal inactivation of β-glucosidase was studied at the selected temperatures (55, 60 and 65 °C) for various times (2, 4, 6 min) using screw-cap tubes. The screw-cap tubes were pre-heated to the selected temperature to prevent temperature lag before the addition of a 0.4 mL aliquot of enzyme solution. The enzyme samples were removed from the water bath after pre-set times and were immediately transferred to ice bath to stop thermal inactivation. After the sample was cooled in ice bath and brought to room temperature, 0.1 mL of the heated enzyme solution was mixed with 0.9 mL of pNPG at a final concentration of 5.0 mM in 0.1 M phosphate-citrate buffer (pH 5.0). The residual activity (A) was determined spectrophotometrically. A non-heated enzyme sample was used as blank (Ao). The percentage residual activity was calculated by comparison with the non-heated sample. First order inactivation constant (kD) was calculated from the slope of the natural logarithm (ln) of A/Ao vs. time graph. Half-lives of the enzymes (t1/2) were calculated by using the following equation: t1/2 = 0.693/ kD.
Decimal reduction time (D value) was estimated from the relationship between kD and D value: D = ln (10)/kD. The Z value, which is the temperature increase required for a one-log10 reduction (90 % decrease) in D value was determined from a plot of log10D versus temperature. The slope of the graph is equal to 1/Z value. The energy of activation of denaturation (Ea) was calculated by multiplying the slope of Arrhenius plot (i.e. natural logarithm of kD values vs. reciprocal of absolute temperatures (1/T)) with universal gas constant, R (kJ/mol K) (Ünal 2007).
Effects of inhibitors
Inhibitors examined were glucose, ethanol and CaCl2. The reaction mixture contained 0.8 mL of pNPG at a final concentration of 5.0 mM in 0.1 M phosphate-citrate buffer (pH 5.0), 0.1 mL inhibitor at a final concentration of 0.5, 1.0, and 2.0 % for glucose, 4.0, 6.0, and 8.0 % for ethanol and 0.5, 1.0, and 2.0 % for calcium chloride and 0.1 mL enzyme solution. Percentage inhibition was calculated using the following equation: Inhibition (%) = [(Ao – Ai)/Ao)].100, where, Ao is the initial β-glucosidase activity (without inhibitor) and Ai is the β-glucosidase activity with inhibitor.
Results
Extraction and purification
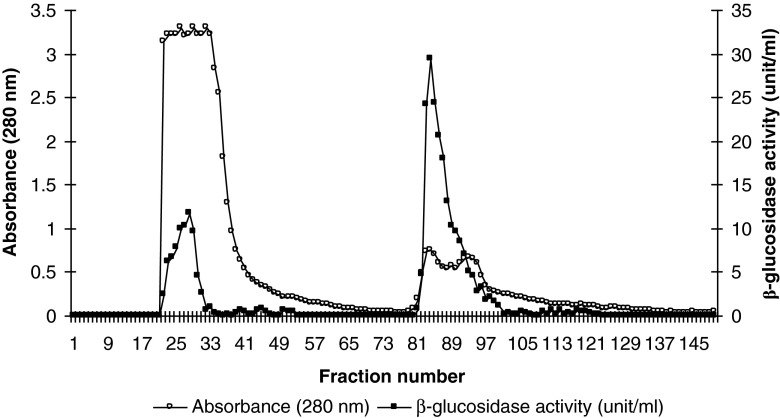
Tea leaves β-glucosidase was purified from crude extract by ammonium sulfate fractionation and ion exchange chromatography using DEAE-Toyopearl 650 M. Figure 1 shows the elution process of Tea leaves β-glucosidase. Ion-exchange chromatography is widely used in enzyme preparation. Two protein peaks containing β-glucosidase activity were recovered (isoenzyme A and isoenzyme B) (Fig. 1). A 5.95 fold purification of isoenzyme A with a recovery of 13.7 % and 14.16 fold purification of isoenzyme B with a recovery of 23.8 % were achieved. Similar finding was reported by Lecas et al. (1991) who studied partial purification and characterization of a grape β-glucosidase. They reported that when the desalted protein solution was passed through Ultrogel AcA 44 and DEAE-Sepharose CL-6B columns, two distinct peaks with β-glucosidase activity were reported wit a 4.04 fold purification of β-glucosidase-1 and 6.87 fold purification of β-glucosidase-2.
Fig. 1.
Elution pattern of tea leaves β-glucosidase on Toyopearl 650 M. In order to remove unadsorbed proteins, the column was washed with 10 mM pH, 6.8 phosphate-citrate buffer until the 60th fraction after which gradient elution was started
The overall purification protocol for β-glucosidase is summarized in Table 1. A 5.95 fold purification with a recovery of 13.7 % was achieved for isoenzyme A and 14.16 fold purification with a recovery of 23.8 % was achieved after ammonium sulphate precipitation and ion exchange chromatography of crude extract. Chen et al. (2012) who studied purification and characterization of β-glucosidase from Prunus domestica seeds achieved 59-fold purification with a yield of 5.4 %, using ammonium sulphate precipitation, hydrophobic interaction chromatography on a Phenyl Sepharose CL-4B column and anion exchange chromatography on a Mono QTM 5/50 GL column. The apple seed β-glucosidase was purified from crude extract by ammonium sulfate fractionation, anion-exchange, hydrophobic chromatography and gel filtration with a 46-fold purification with a yield of 12.8 % (Yu et al. 2007). Zhang et al. (2005) used affinity chromatography for the purification of β-glucosidase from Camellia sinensis var. sinensis cv. Yabukita. They obtained two β-glucosidases which were found to be monomers. Hsieh and Graham (2001) reported a 20 fold purification with a recovery of 20 % soybean β-glucosidase after ammonium sulphate precipitation and DEAE-Sephadex and CM-Sephadex chromatography of crude extract.
Table 1.
Purification of β-glucosidase from tea leaves
| Purification step | Volume (ml) | Total protein (mg) | Total activity (units) | Specific activity (unit/mg protein) | Purification fold | Recovery (%) |
|---|---|---|---|---|---|---|
| Crude extract | 280 | 35.08 | 4952.70 | 141.18 | 1.00 | 100.00 |
| (NH4)2SO4 precipitation and dialysis | 35 | 4.06 | 1532.59 | 377.49 | 2.67 | 30.94 |
| DEAE-Toyopearl 650M | ||||||
| Isoenzyme A | 49 | 0.81 | 680.37 | 839.96 | 5.95 | 13.74 |
| Isoenzyme B | 54 | 0.59 | 1179.86 | 1999.76 | 14.16 | 23.82 |
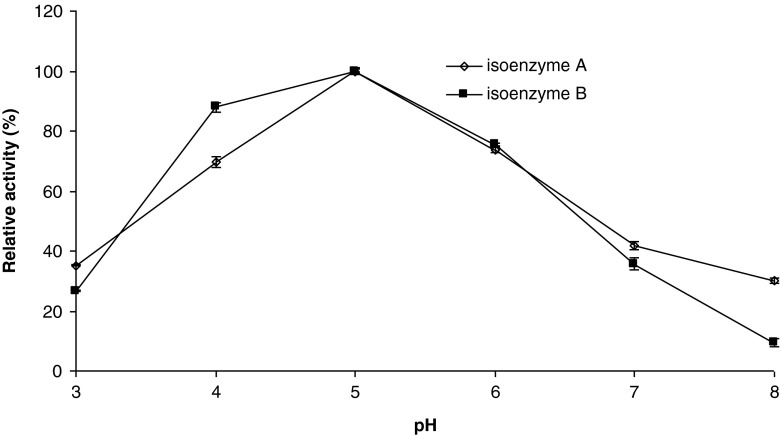
pH optima
The changes in ionization of prototropic groups in the active site of an enzyme at lower acid an higher alkali pH values may prevent proper conformation of the active site, binding of substrates, and/or catalysis of the reaction (Yoruk and Marshall 2003). β-glucosidase activity as a function of pH was determined in a pH range of 3.0–8.0, and the results are depicted in Fig. 2. As seen in Fig. 2, both isoenzyme A and B had an optimum pH at 5.0, after which enzyme activity started to decline. The isoenzymes retained over 60 % of the original activities between pH 4.0 and 6.0. The isoenzymes activities at pH 3.0 were less than 40 %. Lecas et al. (1991) reported a pH optimum of 5.0 for Muscat of Alexandria grapes. It was reported that the optimum pH for β-glucosidase activitiy in plants and endoglycosidase (5.0–6.0) is similar to that for intracellular yeast β-glucosidase, whereas that for extracellular yeast and Aspergillus is generally lower (4.0–5.0) (Günata 2003). Reported optimum pH values for β-glucosidase from different sources are 5.0 for papaya (Hartmann-Schreier and Schreier 1987), gingseng (Zhang et al. 2001), and tea (Li et al. 2005), 6.0 for apple (Yu et al. 2007) and soybean (Hsieh and Graham 2001). The pH optimum of 5.0 found in this study is in accord with these values.
Fig. 2.
Activity of tea leaves β-glucosidase as a function of pH. Each data point is the mean of three determinations. The vertical bars represent standard deviations
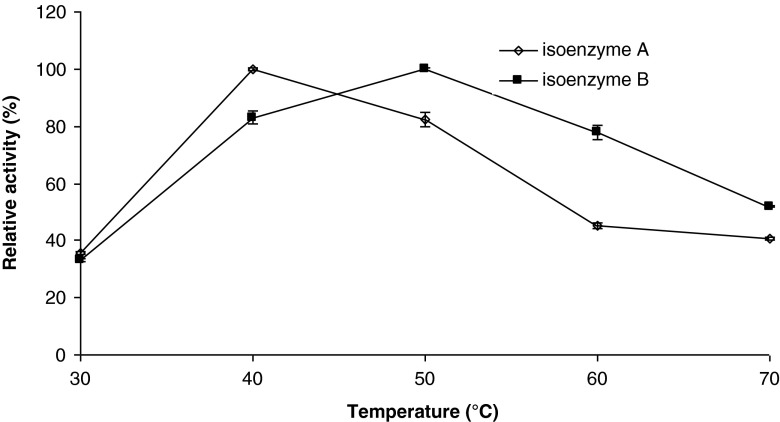
Temperature optima
Temperature significantly influences the catalytic activity of the enzymes. It is well known that a decrease in the kinetic energy of the reactant molecules at low temperatures corresponds to a slower reaction. In addition, integrity of the delicate three-dimensional structure of the enzyme is subjected to disruption and denaturation at high temperatures (Yoruk and Marshall 2003). The effect of temperature on β-glucosidase activity was investigated in the range 30–70 °C and the results are depicted in Fig. 3. Thermal-activity profiles of the isoenzyme A and isoenzyme B were similar in that both enzymes had high activities between 40 and 50 °C, with maximum activity occurring at 40 °C for isoenzyme A and 50 °C for isoenzyme B. After the optimum temperatures, enzyme activities started to decline rapidly. The drop in enzyme activity after optimum temperature was more severe in the isoenzyme A.
Fig. 3.
Activity of tea leaves β-glucosidase as a function of temperature. Each data point is the mean of three determinations. The vertical bars represent standard deviations
Several research groups reported an optimum temperature of 50 °C for β-glucosidase from different sources, e.g. papaya (Hartmann-Schreier and Schreier 1987), honey bee (Pontoh and Low 2002), tea (Li et al. 2005), apple (Yu et al. 2007), Aspergillus oryzae (Riou et al. 1998). However, a lower and higher temperature optimums were also reported, e.g. 30 °C for soybean β-glucosidase (Hsieh and Graham 2001), and 75 °C for Paecilomyces thermophila (Yang et al. 2008).
Kinetic parameters
Affinity of β-glucosidases toward β-glucosides varies depending on the source. The Km values for good substrates, including natural substrates, are typically 1 mM or less. It is believed that the physiological function of these enzymes for the cell dictates slow hydrolysis of the subtrates (Li et al. 2005). The Michaelis-Menten constant (Km) is a measure of affinity of the enzyme for the substrate, with smaller values representing greater affinity. Km and the maximum rate (Vmax) of the partially purified tea leaves β-glucosidase was determined from Lineweaver–Burk plot with artificial substrate p-nitrofenol-β-D-glucopiranozide at a range of 1.25–20 mM. Km and Vmax values for tea β-glucosidase using pNPG as substrate were found to be 5.4 mM and 22.5 Unit for isoenzyme A and 3.1 mM and 30.9 Unit for isoenzyme B, respectively. The affinity of the isoenzyme B for pNPG was higher than that for the isoenzyme A. Kara et al. (2011) who studied purification and characterization of β-glucosidase from olive fruit tissue reported a Km value of 2.22 mM using pNPG as substrate. Km value for β-glucosidase shows great variability depending on the source, e.g. 0.27 mM for blood orange (Barbagallo et al. 2007), 1.20 mM for apple (Yu et al. 2007), 2.60 mM for Clostridium thermocellum (Patchett et al. 1987), 3.30 mM for vanilla (Dignum et al. 2004), 3.90 mM for soybean (Hsieh and Graham 2001).
Thermal inactivation
Thermal inactivation of tea leaves β-glucosidase between 55 °C and 65 °C followed first order kinetics. The first order inactivation constants (kD values) are presented in Table 2. From the table, it is clear that the enzyme is less thermostable at higher temperatures since a higher rate constant means that the enzyme is less thermostable (Marangoni 2003). The half-life (t1/2) is another important parameter used in the characterization of enzyme stability. Increasing the temperature from 55 °C to 65 °C resulted in a decrease in t1/2 values (Table 2). The half-life values of the isoenzyme A ranged between 2.1–7.4 mins, and those for the isoenzyme B ranged between 3.9 and 10.9 mins. Patchett et al. (1987) reported half life values of β-glucosidase from thermophilic anaerobic bacteria at 70, 75 and 85 °C as 330, 47, 1.4 min, respectively. It was reported that rapid inactivation of glucosidases occurs at temperatures above 50 °C (Günata 2003). The thermal inactivation of Sulfolobus solfataricus β-glucosidase followed first-order kinetics with halflives of 136, 80, 51, 31, 18, and 10 h at 70, 75, 80, 85, 90, and 95 °C, respectively (Kim et al. 2012). As seen, thermostability of β-glucosidases show considerable variation. The decimal reduction time (D value) is the time, at a given temperature and pressure, needed for 90 % reduction of the initial activity. D values ranged between 7.0 and 36.0 min at the temperatures studied (Table 2). Barbagallo et al. (2007) who studied characterization of β-glucosidase from sicilian blood oranges reported a D value of 3 min at 75 °C.
Table 2.
Thermal inactivation parameters of tea leaves β-glucosidase
| Temperature (°C) | Isoenzyme A | Isoenzyme B | ||||||
|---|---|---|---|---|---|---|---|---|
| k D (min−1) | R 2 | t 1/2 (min) | D (min) | k D (min−1) | r 2 | t 1/2 (min) | D (min) | |
| 55 | 0.0940 | 1.00 | 7.4 | 24 | 0.0636 | 0.99 | 10.9 | 36 |
| 60 | 0.1572 | 0.99 | 4.4 | 15 | 0.0964 | 0.95 | 7.2 | 24 |
| 65 | 0.3359 | 0.97 | 2.1 | 7 | 0.1733 | 0.99 | 3.9 | 13 |
The Z value obtained in this study was 18.1 °C (r2 = 0.9878) for the isoenzyme A and 23.0 °C (r2 = 0.9904) for the isoenzyme B. Ea values for thermal inactivation of the isoenzyme A and B were found to be 117.3 kJ/mol (r2 = 0.9858) and 92.3 kJ/mol (r2 = 0.9887) respectively. Low Z-values are thought to indicate greater sensitivity to heat and a low Ea values are thought to indicate less sensitivity to heat. Therefore, the isoenzyme B is more thermostable than the isoenzyme A. Srivastava et al. (1984) investigated thermal inactivation kinetics of β-glucosidase from Aspergillus wentii (Pt 2804), using pNPG and cellobiose as substrate. They reported Ea values of 111.30 kj/mol and 63.70 kj/mol, respectively. In a study carried out by Patchett et al. (1987) on β-glucosidase from thermophilic anaerobic bacteria, the calculated Ea value was 60 kj/mol. The calculated Z value for β-glucosidase from sicilian blood oranges was about 9.5 °C (Barbagallo et al. 2007).
Effect of inhibitors
Inhibition of β-glucosidase by glucose poses a problem not only in flavour release in fruit juice processing but also enzymatic debittering of citrus juices by hydrolysis of naringin and efficient enzymatic saccharification of cellulosic material. This inhibition is often competitive type and a common characteristic of these enzymes. β-glucosidase activity is not influenced by other sugars such as fructose, galactose, arabinose, mannose, lactose and sucrose at concentrations occurring in food products (Günata 2003).
Effects of D-glucose, ethanol and calcium chloride on tea leaves β-glucosidase activity were studied at various concentrations using pNPG as substrate. The results were reported as percentage inhibition in Table 3. The effects of inhibitors varied in a dose dependent manner. As seen in Table 3 neither of the inhibitors inhibited tea leaves β-glucosidase 100 %. The effects of inhibitors varied in a dose dependent manner. The inhibition of the isoenzyme A and B differed from each other. The inhibitory effect of ethanol on the isoenzyme B was less than that on enzyme A. However the inhibitory effects of glucose and calcium chloride on the isoenzyme A were less than those on the enzyme B. Sripunya (2005) reported 80 % inhibition of β-D-glucosidase from Saccharomyces cerevisiae 71B-1122 by ethanol at 10–15 %. In a study carried out by Hemingway et al. (1999) on commercially availabe β-glucosidases from brewers yeast, almonds, and Aspergillus niger, it was found that the α and β-glucosidases from yeast and almonds were inhibited to a similar degree with 50–70 % inhibition at 5 % ethanol, rising to around 80 % inhibition at 15 % ethanol. In contrast, the A. niger β-glucosidase was inhibited to a lesser degree with only 35 % inhibition at 15 % ethanol. They also stated that it is impossible to extrapolate from the activities measured in model systems using p-nitrophenol substrates, as the activity of glucosidases varies significantly with the type of aglycone present.
Table 3.
Effect of inhibitors on tea leaves β-glucosidase activity
| Inhibitor | Concentration | Inhibitiona
(%) |
|
|---|---|---|---|
| Isoenzyme A | Isoenzyme B | ||
| Glucose | % 2.0 | 29.9 ± 0.4 | 32.6 ± 0.7 |
| % 1.0 | 16.0 ± 0.6 | 25.1 ± 0.2 | |
| % 0.5 | 7.7 ± 0.6 | 17.3 ± 0.2 | |
| Ethanol | % 8.0 | 46.6 ± 0.7 | 27.2 ± 0.3 |
| % 6.0 | 38.3 ± 0.4 | 21.4 ± 0.3 | |
| % 4.0 | 34.8 ± 0.6 | 16.8 ± 0.5 | |
| CaCl2 | 2.0 mM | 25.0 ± 0.8 | 30.7 ± 0.3 |
| 1.0 mM | 13.5 ± 0.3 | 23.4 ± 0.3 | |
| 0.5 mM | 7.2 ± 0.4 | 14.8 ± 0.7 | |
aEach value is the mean of three determinations ± standard deviations
References
- Barbagallo RN, Palmeri R, Fabiano S, Rapisarda P, Spagna G. Characteristic of β-glucosidase from sicilian blood oranges in relation to anthocyanin degradation. Enzym Microb Technol. 2007;41:570–575. doi: 10.1016/j.enzmictec.2007.05.006. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen L, Li N, Zong M-H. A glucose-tolerant β-glucosidase from Prunus domestica seeds: purification and characterization. Process Biochem. 2012;47:127–132. doi: 10.1016/j.procbio.2011.10.023. [DOI] [Google Scholar]
- Demir A (2002) Çay, TEAE 1:1–4 (In Turkish)
- Dignum MJW, Heijden R, Kerler J, Winkel C, Verpoorte R. Identification of glucosides in green beans of (Vanilla Planifolia) andrews and kinetics of vanilla β-glucosidase. Food Chem. 2004;85:199–205. doi: 10.1016/S0308-8146(03)00293-0. [DOI] [Google Scholar]
- Esen A. β-Glucosidase. In: Whitakeer JR, Voragen AGJ, Wong DWS, editors. Handbook of food enzymology. New York: Marcel Dekker; 2003. pp. 791–803. [Google Scholar]
- Garcia-Palazon A, Suthanthangjai W, Kajda P, Zabetakis I. The effects of high hydrostatic pressure on β-glucosidase, peroxidase and polyphenoloxidase in red rasperry (Rubus idaeus) and strawberry (Fragaria x ananassa) Food Chem. 2004;88:7–10. doi: 10.1016/j.foodchem.2004.01.019. [DOI] [Google Scholar]
- Günata Z. Flavor enhancement in fruit juices and derived beverages by exogenous glycosidases and consequences of the use of enzyme preparations. In: Whitakeer JR, Voragen AGJ, Wong DWS, editors. Handbook of food enzymology. New York: Marcel Dekker; 2003. pp. 303–329. [Google Scholar]
- Hartmann-Schreier J, Schreier P. Properties of β-glucosidase from Carica Papaya fruit. Food Chem. 1987;26:201–212. doi: 10.1016/0308-8146(87)90035-5. [DOI] [Google Scholar]
- Hemingway KM, Alston MJ, Colin G, Chappell CG, Taylor AJ. Carbohydrate-flavour conjugates in wine. Carbohydr Polym. 1999;38:283–286. doi: 10.1016/S0144-8617(98)00103-9. [DOI] [Google Scholar]
- Hsieh MC, Graham TL. Partial purification and characterization of a soybean β-glucosidase with high specific activity towards isoflavone conjugates. Phytochemistry. 2001;58:995–1005. doi: 10.1016/S0031-9422(01)00380-6. [DOI] [PubMed] [Google Scholar]
- Kara HE, Sinan S, Turan T. Purification of beta-glucosidase from olive (Olea europaea L.) fruit tissue with specifically designed hydrophobic interaction chromatography and characterization of the purified enzyme. J Chromatogr B. 2011;879:1507–1512. doi: 10.1016/j.jchromb.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Khan SA, Priyamvada S, Arivarasu NA, Khan S, Yusufi ANK. Influence of green tea on enzymes of carbohydrate metabolism, antioxidant defense, and plasma membrane in rat tissues. Nutrition. 2007;23:687–695. doi: 10.1016/j.nut.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Kim BN, Yeom SJ, Kim YS, Oh DK. Characterization of a β-glucosidase from Sulfolobus solfataricus for isoflavone glycosides. Biotechnol Lett. 2012;34:125–129. doi: 10.1007/s10529-011-0739-9. [DOI] [PubMed] [Google Scholar]
- Lecas M, Günata ZY, Sapis JC, Bayonove CL. Purification and partial characterization of β-glucosidase from grape. Phytochemistry. 1991;30:451–454. doi: 10.1016/0031-9422(91)83702-M. [DOI] [Google Scholar]
- Li YY, Jiang CJ, Wan XC, Zhang ZZ, Li DX. Purification and partial characterization of β-Glucosidase from fresh leaves of tea plants (Camellia sinensis L.) Acta Biochim Biophys Sin. 2005;37:363–370. doi: 10.1111/j.1745-7270.2005.00053.x. [DOI] [PubMed] [Google Scholar]
- Marangoni AG. Enzyme kinetics: a modern approach. Hoboken: Wiley; 2003. pp. 140–157. [Google Scholar]
- Mazzuca S, Spadafora A, Innocenti AM. Cell and tissue localization of β-glucosidase during the ripening of olive fruit (Olea europea) by in situ activity assay. Plant Sci. 2006;171:726–733. doi: 10.1016/j.plantsci.2006.07.006. [DOI] [Google Scholar]
- Patchett ML, Daniel RM, Morgan HW. Purification and properties of a stable β-glucosidase from an extremely thermophilic anaerobic bacterium. Biochemistry. 1987;243:779–787. doi: 10.1042/bj2430779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontoh J, Low NH. Purification and characterization of β-glucosidase from honey bees (Apis mellifera) Insect Biochem Mol Biol. 2002;32:679–690. doi: 10.1016/S0965-1748(01)00147-3. [DOI] [PubMed] [Google Scholar]
- Riou C, Salmon JM, Vallier MJ, Günata Z, Barre P. Purufication, characterization, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl Environ Microbiol. 1998;64:3607–3614. doi: 10.1128/aem.64.10.3607-3614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripunya P (2005) Selection of yeast strains containing β-dglucosidase for improving aroma in wine. M.Sc. Thesis, Suranaree University of Technology, Thailand ISBN 974-533-483-9, p 56.
- Srivastava SK, Gopalkrishnan KS, Ramachandran KB. Kinetic characterization of crude β-glucosidase from Aspergillus wentii Pt 2804. Enzym Microb Technol. 1984;6:508–512. doi: 10.1016/0141-0229(84)90005-X. [DOI] [Google Scholar]
- Ünal MÜ. Properties of polyphenol oxidase from Anamur banana (Musa cavendishii) Food Chem. 2007;100:909–913. doi: 10.1016/j.foodchem.2005.10.048. [DOI] [Google Scholar]
- Yang S, Jiang Z, Yan Q, Zhu H. Characterization of a thermostable extracellular β-glucosidase with activities of exoglucanase and transglycosylation from Paecilomyces thermophila. J Agric Food Chem. 2008;56:602–608. doi: 10.1021/jf072279+. [DOI] [PubMed] [Google Scholar]
- Yang Z, Baldermann S, Watanabe N. Recent studies of the volatile compounds in tea. Food Res Int. 2013;53:585–599. doi: 10.1016/j.foodres.2013.02.011. [DOI] [Google Scholar]
- Yılmaz G, Kandemir N, Kınalıoğlu K. Effects of different pruning intervals on fresh shoot yield and some quality properties of tea (Camellia sinensis (L.) O. Kuntze) in Turkey. Pak J Biol Sci. 2004;7:1208–1212. doi: 10.3923/pjbs.2004.1208.1212. [DOI] [Google Scholar]
- Yoruk R, Marshall MR. Physicochemical properties and function of plant polyphenol oxidase: a review. J Food Biochem. 2003;27:361–422. doi: 10.1111/j.1745-4514.2003.tb00289.x. [DOI] [Google Scholar]
- Yu HL, Xu JH, Lu WY, Lin GO. Identification, purification and characterization of β-glucosidase from apple seed as a novel catalyst for synthesis of O-glucosidase. Enzym Microb Technol. 2007;40:354–361. doi: 10.1016/j.enzmictec.2006.05.004. [DOI] [Google Scholar]
- Zhang C, Yu H, Bao Y, An L, Jin F. Purification and characterization of ginsenoside-β-glucosidase from ginseng. Chem Pharm Bull. 2001;49:795–798. doi: 10.1248/cpb.49.795. [DOI] [PubMed] [Google Scholar]
- Zhang Z-Z, Wan X-C, Kanzo S (2005) Purification by affinity chromatography and characterization of tea leaf β-glucosidase. J Tea Sci 01