Abstract
Carrot (Daucus carota) peels, local agricultural waste product, is rich in lignocellulolytic material, including pectin which can act as an inducer of pectinase production. Pectinolytic enzymes production by Bacillus mojavensis I4 was studied in liquid state fermentation using carrot peel as a substrate. Medium composition and culture conditions for the pectinase production by I4 were optimized using two statistical methods: Taguchi design was applied to find the key ingredients and conditions for the best yield of enzyme production and The Box-Behnken design was used to optimize the value of the four significant variables: carrot peels powder, NH4Cl, inoculum size and incubation time. The optimal conditions for higher production of pectinase were carrot peels powder 6.5 %, NH4Cl 0.3 %, inoculum level 3 % and cultivation time 32 h. Under these conditions, the pectinase experimental yield (64.8 U/ml) closely matched the yield predicted by the statistical model (63.55 U/ml) with R2 = 0.963. The best pectinase activity was observed at the temperature of 60 °C and at pH 8.0. The enzyme retained more than 90 % of its activity after 24 h at pH ranging from 6.0 to 10.0. The enzyme preserved more than 85 % of its initial activity after 60 min of pre-incubation at 30–40 °C and more than 67 % at 50 °C. The extracellular juice of I4 was applied in the process of sesame seeds oil extraction. An improvement of 3 % on the oil yield was obtained. The findings demonstrated that the B. mojavensis I4 has a promising potential for future use in a wide range of industrial and biotechnological applications.
Keywords: Bacillus mojavensis I4, Alkaline thermostable pectinase, Carrot peels powder, Culture medium optimization, Oil extraction
Introduction
Pectinolytic enzymes that hydrolyze pectins have important applications in various industries. Based on pH requirement for optimum enzymatic activity, pectinases can be broadly classified into acidic and alkaline pectinases. Acidic pectinases are useful for the extraction, clarification, and liquefaction of fruit juices (Kaur et al. 2004) and wines (Favela-Torres et al. 2005). Alkaline pectinases are widely used the fabric industry, for the retting of plant fibers, biopreparation of cotton, and enzymatic polishing of jute/cotton blended fabrics, and in the pulp and paper industry, for solving problems pertaining to waste treatment, retention, and recovery processes (Favela-Torres et al. 2005; Sharma and Satyanarayana 2004). Bioprep 3000L (Novozymes), an alkaline pectinase with a standard activity of at least 3000 APSU (Alkaline Pectinase Standard Unit), currently represents one of the most commonly commercialized pectinases.
The statistical design experiments have often been applied for the optimization and development of technically and economically viable bioprocesses. In this context, the wide scale industrial application of pectinases requires their cost-effective production to make the process economically viable. This can be achieved through the use of cheaply available agroindustrial residues, including wheat bran, oat bran, rice straw, and orange peels (Heerd et al. 2012; Kapoor et al. 2008; Sun et al. 2008). Large amounts of lignocellulosic wastes are annually generated from agro-industrial processing operations (Anuradha et al. 2007). The by-products from the fruit and vegetable industries may offer inexpensive and readily available resources from which several useful biological products can be derived (Chantaro et al. 2008). Carrot (Daucus carota) is a good source of natural antioxidants, especially carotenoids and phenolic compounds (Prakash et al. 2004). After processing, carrot residues, such as peels and pomace, are usually discarded or used as animal feed. Carrot by-products have, however high contents of beneficial substances, especially bioactive compounds with antioxidant activities (Zhang and Hamauzu 2004).
The present study aimed to investigate the feasibility of using carrot peels, a by-product from a ready-to-eat vegetable industry, as a carbon source to produce high-level of pectinase under submerged fermentation from a newly isolated strain of Bacillus majavensis I4. To the authors’ knowledge, no data is currently available in the literature on the use of carrot peels powder in the fermentation medium for the production of pectinase. The Taguchi approach and Box-Behnken design were employed to identify and optimize the critical variables for enzyme production. The biochemical properties of the extracellular pectinases were determined, and their potential gain effects on sesame seeds oil extraction were also evaluated.
Materials and methods
Bacterial strain
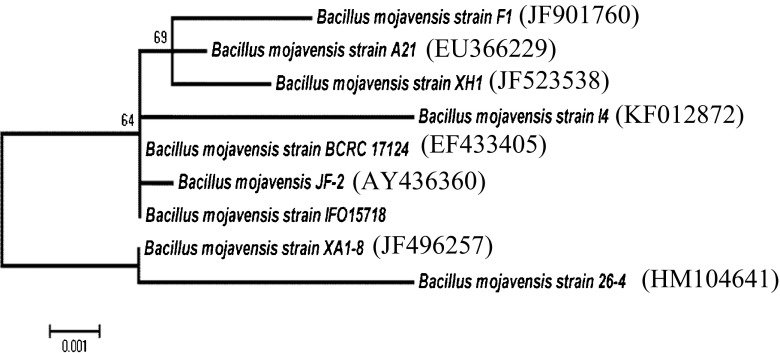
The bacterial strain was isolated from a soil sample collected from Sfax city, Tunisia, and maintained on nutrient agar slants at 4 °C and also stored as glycerol stocks at −20 °C. The isolate was identified as B. mojavensis according to the methods described in Bergey’s Manual of determinative Bacteriology and on the basis of the 16S rDNA sequence analysis. A phylogenetic tree was constructed and edited using Molecular Evolutionary Genetics Analysis version 5. Genetic relationships were inferred from neighbor-joining nucleotide alignment after 1000 bootstrap replicates using the Tajima-Nei model.
Pectinase activity
Pectinase activity was determined at 60 °C in 50 mM Tris–HCl buffer, pH 8.0, using the modified method of Bailey et al. (1992). A 0.5 ml aliquot of suitably diluted enzyme solution was incubated in the presence of 0.5 ml of 1 % (w/v) citrus pectin in 50 mM Tris–HCl, pH 8.0 buffer for 30 min. Next, 3 ml of dinitrosalicylate reagent was added to the solution, and the reaction tubes were boiled for 10 min, cooled and the absorbance values were read at 550 nm. The reducing sugars that were released were quantified by reference to a galacturonic acid standard curve. All of the enzyme activities are expressed in Units (U) where 1 U is defined as the amount of enzyme that releases 1 μmol of reducing sugar per minute.
Culture media and growth conditions
The initial medium (M1) used for pectinase production was composed of (g, %): wheat bran, 1; yeast extract, 0.2 and NaCl, 0.5; pH 8.0. Inocula were routinely grown in Luria-Bertani (LB) broth medium composed of (g, %): peptone, 1; yeast extract, 0.5; and NaCl, 0.5. Media were autoclaved at 120 °C for 20 min. Cultivations were conducted on a rotator shaker (200 rpm/min) in 250 ml Erlenmeyer flasks with a working volume of 25 ml for 24 h at 37 °C. The culture medium was centrifuged at 4000 rpm for 15 min at 4 °C and the cell-free supernatant was used for estimation of pectinolytic activity.
Selection of the best carbon and nitrogen sources by one-variable-at-a-time approach
Initial screening of the most significant carbon and nitrogen sources allowing the maximum pectinolytic enzyme production was performed by the one-variable-at-a-time approach. Nine complex carbon sources (carrot peels powder, potato peels powder, wheat bran, prickly pear powder, barley bran, grapefruit bark powder, hay, grenade bark powder and carob powder) were individually evaluated at a concentration of 1 % in the basal culture medium M1. In the investigation of nitrogen sources, cultivations were carried out with media containing (g, %): carrot peels powder, 1; NaCl, 0.5; pH 8.0, supplemented with different organic nitrogen sources (tryptone, yeast extract, sardinella peptone, gelatine and urea) and inorganic nitrogen sources (ammonium sulfate and ammonium chloride) at a concentration of 0.2 %. All experiments were carried out in triplicate.
Identification of the significant variables using Taguchi design
According to Taguchi’s OA, a standard orthogonal array L27 (310) 27 experiments were used to evaluate the effect of ten factors on the pectinase production by B. mojavensis I4. In all experiments, the three levels of factor variations were assigned with the numbers 1, 2, and 3, as shown in Table 1. They were as the following; carrot peels powder, NH4Cl, NaCl, CaCl2, K2HPO4, MgSO4, temperature, inoculum level, incubation time and agitation. Experiments were performed according to an experimental plan given in Table 2. In this selected 27 runs, in combination with ten factors at three levels (Table 1), the results were shown as pectinase activity (U/ml). The pectinase activities were the averages of three individual determinations.
Table 1.
Selected fermentation factors and their assigned levels for pectinase production with B. mojavensis I4
| Serial no. | Factors | Level 1 | Level 2 | Level 3 |
|---|---|---|---|---|
| X1 | Carrot peels (g, %) | 1 | 3 | 5 |
| X2 | NH4Cl (g, %) | 0.05 | 0.125 | 0.2 |
| X3 | NaCl (g, %) | 0.05 | 0.125 | 0.2 |
| X4 | CaCl2 (g, %) | 0.05 | 0.125 | 0.2 |
| X5 | K2HPO4 (g, %) | 0.05 | 0.125 | 0.2 |
| X6 | MgSO4 (g, %) | 0.05 | 0.125 | 0.2 |
| X7 | Inoculum level (ml, %) | 2 | 4 | 6 |
| X8 | Incubation time (h) | 24 | 48 | 72 |
| X9 | Temperature (°C) | 30 | 37.5 | 45 |
| X10 | Agitation (rpm) | 100 | 150 | 200 |
Table 2.
L27 (310) orthogonal array of Taguchi experimental design for the pectinase production by B. mojavensis I4
| Exp no. | Factor levels | Pectinase production (U/ml) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | Experimental | Predicted | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9.20 | 8.667 |
| 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 6.60 | 7.633 |
| 3 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 2.20 | 1.700 |
| 4 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 16.30 | 15.800 |
| 5 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 7.30 | 6.767 |
| 6 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 8.40 | 9.433 |
| 7 | 1 | 3 | 3 | 3 | 1 | 1 | 1 | 3 | 3 | 3 | 14.20 | 15.233 |
| 8 | 1 | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 15.30 | 14.800 |
| 9 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 17.40 | 16.867 |
| 10 | 2 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 14.40 | 17.433 |
| 11 | 2 | 1 | 2 | 3 | 2 | 3 | 1 | 2 | 3 | 1 | 22.00 | 18.900 |
| 12 | 2 | 1 | 2 | 3 | 3 | 1 | 2 | 3 | 1 | 2 | 7.00 | 7.067 |
| 13 | 2 | 2 | 3 | 1 | 1 | 2 | 3 | 2 | 3 | 1 | 15.00 | 15.067 |
| 14 | 2 | 2 | 3 | 1 | 2 | 3 | 1 | 3 | 1 | 2 | 13.70 | 16.733 |
| 15 | 2 | 2 | 3 | 1 | 3 | 1 | 2 | 1 | 2 | 3 | 20.60 | 17.500 |
| 16 | 2 | 3 | 1 | 2 | 1 | 2 | 3 | 3 | 1 | 2 | 18.90 | 15.800 |
| 17 | 2 | 3 | 1 | 2 | 2 | 3 | 1 | 1 | 2 | 3 | 30.00 | 30.067 |
| 18 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 2 | 3 | 1 | 15.00 | 18.033 |
| 19 | 3 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 21.50 | 21.011 |
| 20 | 3 | 1 | 3 | 2 | 2 | 1 | 3 | 2 | 1 | 3 | 12.50 | 10.478 |
| 21 | 3 | 1 | 3 | 2 | 3 | 2 | 1 | 3 | 2 | 1 | 10.50 | 13.011 |
| 22 | 3 | 2 | 1 | 3 | 1 | 3 | 2 | 2 | 1 | 3 | 24.00 | 26.511 |
| 23 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 3 | 2 | 1 | 21.00 | 20.511 |
| 24 | 3 | 2 | 1 | 3 | 3 | 2 | 1 | 1 | 3 | 2 | 30.50 | 28.478 |
| 25 | 3 | 3 | 2 | 1 | 1 | 3 | 2 | 3 | 2 | 1 | 27.30 | 25.278 |
| 26 | 3 | 3 | 2 | 1 | 2 | 1 | 3 | 1 | 3 | 2 | 22.20 | 24.711 |
| 27 | 3 | 3 | 2 | 1 | 3 | 2 | 1 | 2 | 1 | 3 | 23.20 | 22.711 |
Box-Behnken design and response surface analysis
Levels of the significant variables for the pectinase production must be optimized. For this purpose, response surface methodology (RSM) was adopted to increase the total pectinase production using a Box-Behnken design. The most significant variables examined were as follows: carrot peels powder, NH4Cl, inoculum level and fermentation time, each of variables was assessed at three coded levels (−1, 0 and +1), as shown in Table 3. The number of experiments suggested by the four variables Box-Behnken design is 24. The 24 experiments were uniformly scattered in the space of the coded variables. The level of the coded variables at each experiment is shown in Table 4. The experimental and predicted responses for pectinase production have also been reported in Table 4. The 24 experiments were augmented with three replications at the center point (runs 25–27) to evaluate the pure error. To those 27 experiments, 5 experiments (runs 28–32) were also added in order to subsequently check the validity of the fitted model before a predictive use of it in the studied domain. Once the experiments were performed, the experimental results were fitted with the following second order polynomial equation:
where Y is the response (pectinase activity), b0 is the constant term; b1, b2, b3 and b4 are the coefficient of linear terms; b11, b22, b33 and b44 are the coefficient of quadratic terms; b12, b13, b23, b14, b24 and b34 are coefficient of cross product terms respectively.
Table 3.
Levels of the factors tested in Box-Behnken design
| Factors | Units | Symbol code | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Carrot peels | g,% | X1 | 5.0 | 6.5 | 8.0 |
| NH4Cl | g,% | X2 | 0.2 | 0.3 | 0.4 |
| Inoculum level | % | X3 | 1 | 2 | 3 |
| Fermentation time | h | X4 | 16 | 24 | 32 |
Table 4.
Box-Behnken design along with the experimental and predicted values of pectinase activity for optimization of pectinase production
| Exp no | Experimental conditions | Pectinase activity (U/ml) | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Experimental | Predicted | |
| 1 | −1 | −1 | 0 | 0 | 52.220 | 51.537 |
| 2 | 1 | −1 | 0 | 0 | 40.170 | 40.726 |
| 3 | −1 | 1 | 0 | 0 | 43.870 | 42.328 |
| 4 | 1 | 1 | 0 | 0 | 50.400 | 50.336 |
| 5 | −1 | 0 | −1 | 0 | 52.950 | 53.549 |
| 6 | 1 | 0 | −1 | 0 | 41.250 | 42.489 |
| 7 | −1 | 0 | 1 | 0 | 46.500 | 47.097 |
| 8 | 1 | 0 | 1 | 0 | 54.360 | 55.353 |
| 9 | −1 | 0 | 0 | −1 | 52.870 | 53.369 |
| 10 | 1 | 0 | 0 | −1 | 52.660 | 53.010 |
| 11 | −1 | 0 | 0 | 1 | 57.000 | 57.814 |
| 12 | 1 | 0 | 0 | 1 | 55.300 | 55.370 |
| 13 | 0 | −1 | −1 | 0 | 52.470 | 51.853 |
| 14 | 0 | 1 | −1 | 0 | 51.200 | 50.423 |
| 15 | 0 | −1 | 1 | 0 | 53.080 | 53.429 |
| 16 | 0 | 1 | 1 | 0 | 54.000 | 55.259 |
| 17 | 0 | −1 | 0 | −1 | 56.320 | 56.195 |
| 18 | 0 | 1 | 0 | −1 | 55.610 | 56.422 |
| 19 | 0 | −1 | 0 | 1 | 58.410 | 59.625 |
| 20 | 0 | 1 | 0 | 1 | 59.120 | 59.797 |
| 21 | 0 | 0 | −1 | −1 | 56.780 | 56.948 |
| 22 | 0 | 0 | 1 | −1 | 62.200 | 62.450 |
| 23 | 0 | 0 | −1 | 1 | 63.650 | 62.647 |
| 24 | 0 | 0 | 1 | 1 | 64.800 | 63.556 |
| 25 | 0 | 0 | 0 | 0 | 55.170 | 54.660 |
| 26 | 0 | 0 | 0 | 0 | 55.200 | 54.660 |
| 27 | 0 | 0 | 0 | 0 | 53.360 | 54.660 |
| 28 | 0.5590 | −0.3227 | −0.2282 | −0.1768 | 53.370 | 50.315 |
| 29 | 0.5590 | −0.3227 | −0.2282 | −0.1768 | 53.370 | 56.128 |
| 30 | 0.0000 | 0.6455 | −0.2282 | −0.1768 | 53.990 | 53.013 |
| 31 | 0.0000 | 0.0000 | 0.6847 | −0.1768 | 55.830 | 56.128 |
| 32 | 0.0000 | 0.0000 | 0.0000 | 0.7071 | 59.130 | 58.865 |
The goodness of fit of the polynomial equation was expressed by coefficient of determination R2 and its statistical significance level was checked by F-test.
Time course of pectinase production by B. mojavensis I4
To study the relation between pectinase production and the growth profile of the bacterium, 100 ml of the optimized production medium was inoculated in 1 l flask, and the growth was measured at regular intervals by viable count determination. The pectinase production at different time intervals was determined using the standard pectinase assay. The growth of the microorganism was estimated using the total plate count method on nutrient agar. This procedure involves making decimal serial dilutions of the sample in sterile physiological water. Nutrient agar plates were then incubated at 37 °C for 24 h.
Detection of pectinase activity on polyacrylamide gel (citrus pectin-zymography)
Pectin-zymography was performed to test the pectinolytic activity of the crude supernatant. Zymography was carried out in conjunction with SDS-PAGE as described by Laemmli (1970). The methods used for SDS-PAGE and zymogram analyses were modified, which resulted in improved protein resolution and staining, as well as improved zymograms. The sample was not heated before electrophoresis. After electrophoresis, the gel was submerged in 50 mM Tris–HCl buffer (pH 8.0) containing 2.5 % Triton X-100 for 60 min, with constant agitation to remove SDS. TritonX-100 was then removed by washing the gel three times with 50 mM Tris–HCl buffer (pH 8.0). A replicate gel containing 1 % citrus pectin was incubated for 30 min at 50 °C to detect pectinase activity. After incubation, the gel was stained with Ruthenium red (0.03 %), and the bands of pectinase activity appeared as clear areas in a red gel background.
Biochemical properties
Effect of temperature on pectinase activity and stability
The effect of temperature on pectinase activity was studied from 30 to 80 °C in 50 mM Tris–HCl buffer, pH 8.0 and using citrus pectin (1 % w/v) as a substrate. The thermal stability of the crude enzyme was determined by incubating the enzyme for 60 min at different temperatures. Aliquots were withdrawn at desired time intervals to test the remaining activity at pH 8.0 and 60 °C. The unheated enzyme was used to define 100 % activity.
Effect of pH on pectinase activity and stability
The optimal pH of the pectinase was studied over a pH range of 5.0–10.0 at 60 °C with 1 % (w/v) citrus pectin as a substrate. To measure the pH stability, the enzyme was incubated at 4 °C for 24 h in different buffers, and then the residual pectinase activity was determined under standard assay conditions. The following buffer systems were used: 50 mM sodium acetate buffer, pH 5.0–6.0; 50 mM phosphate buffer, pH 7.0; 50 mM Tris–HCl buffer, pH 8.0 and 50 mM glycine-NaOH buffer, pH 9.0–11.0.
Extraction of sesame seeds oil
The sesame seeds used were obtained from the local market. Milling was carried out on a domestic coffee mill and the powder was used for the extraction of oil. Ten grams of milled seeds were treated with enzymatic extract of pectinase (150 U) in 40 ml distilled water. The reaction proceeded under continuous stirring at 150 rpm at 37 °C for 1 h. Then, the water was removed by centrifugation for 20 min at 4000 rpm.
Data analysis and software
Software “NemrodW” (Mathieu et al. 2000) was used for the experimental design and statistical analysis of the experimental data. The analysis of variance (ANOVA) was used to estimate the statistical parameters.
Results
Identification of the strain
16S rDNA gene was sequenced and submitted to NCBI GenBank (accession no. KF012872). Based on the nucleotide sequence of 16S rDNA gene, the isolate I4 belonged to the genus of Bacillus and showed closest relationship with B. mojavensis BCRC 17124 (Fig. 1).
Fig. 1.
Phylogenetic tree of B. Mojavensis I4 associated with the other members of the Bacillus genus
Selection of the most suitable carbon and nitrogen sources by the one-variable-at-a-time approach
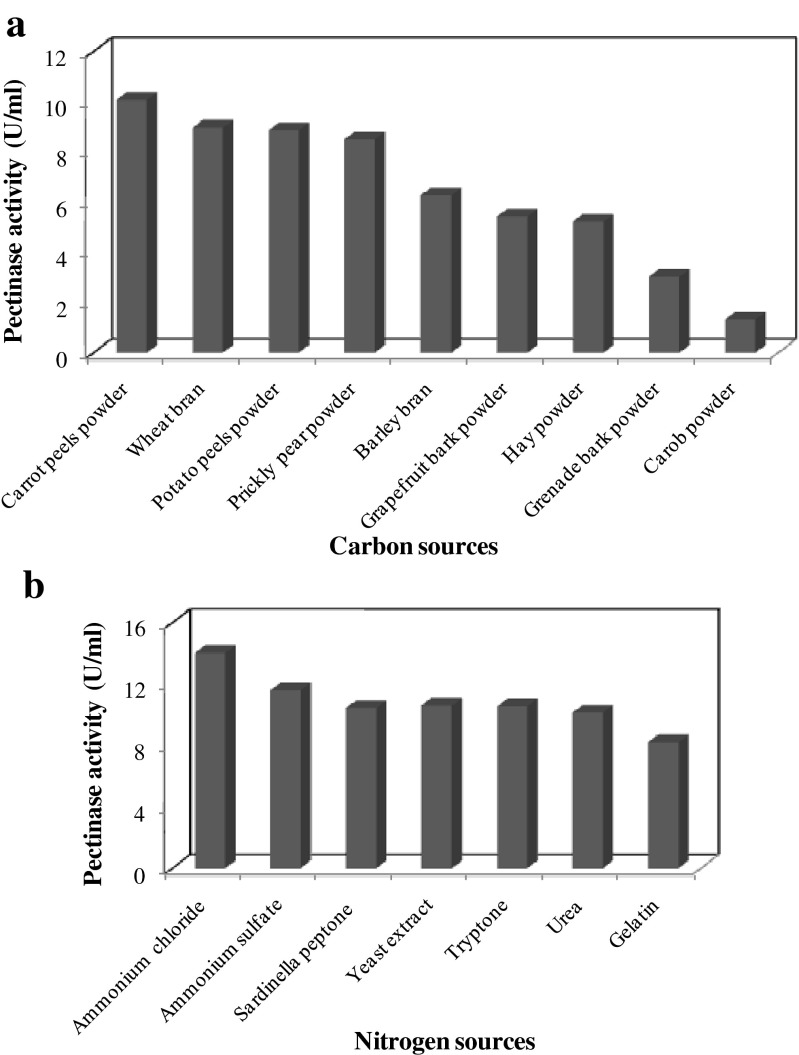
Pectinase production was assayed in M1 medium containing different carbon sources at a concentration of 1.0 %. The highest levels of pectinase activity were obtained with carrot peels powder (10.5 U/ml), followed by wheat bran (8.96 U/ml), potato peels powder (8.86 U/ml), prickly pear powder (8.49 U/ml), barley bran (6.26 U/ml), grapefruit bark (5.43 U/ml), and hay powder (5.22 U/ml). Grenade bark powder (3 U/ml) and carob powder (1.32 U/ml) resulted in low enzyme production yields (Fig. 2a).
Fig. 2.
Effects of different carbon (a) and nitrogen substrates (b) on the production of pectinase by B. mojavensis I4
In a second experiment, pectinase production was performed in M1 media containing carrot peels powder at 1.0 % as a carbon source and various nitrogen sources, each added at 0.2 % (Fig. 2b). The highest pectinase production yield was obtained with NH4Cl (14 U/ml), followed by (NH4)2S04 (11.59 U/ml) and yeast extract (10.6 U/ml). Among the various carbon and nitrogen sources tested, carrot peels powder and ammonium chloride were noted to be the most suitable substrates for pectinase production, respectively. They were, therefore, selected for further optimization assays.
Selection of significant variables by Taguchi design
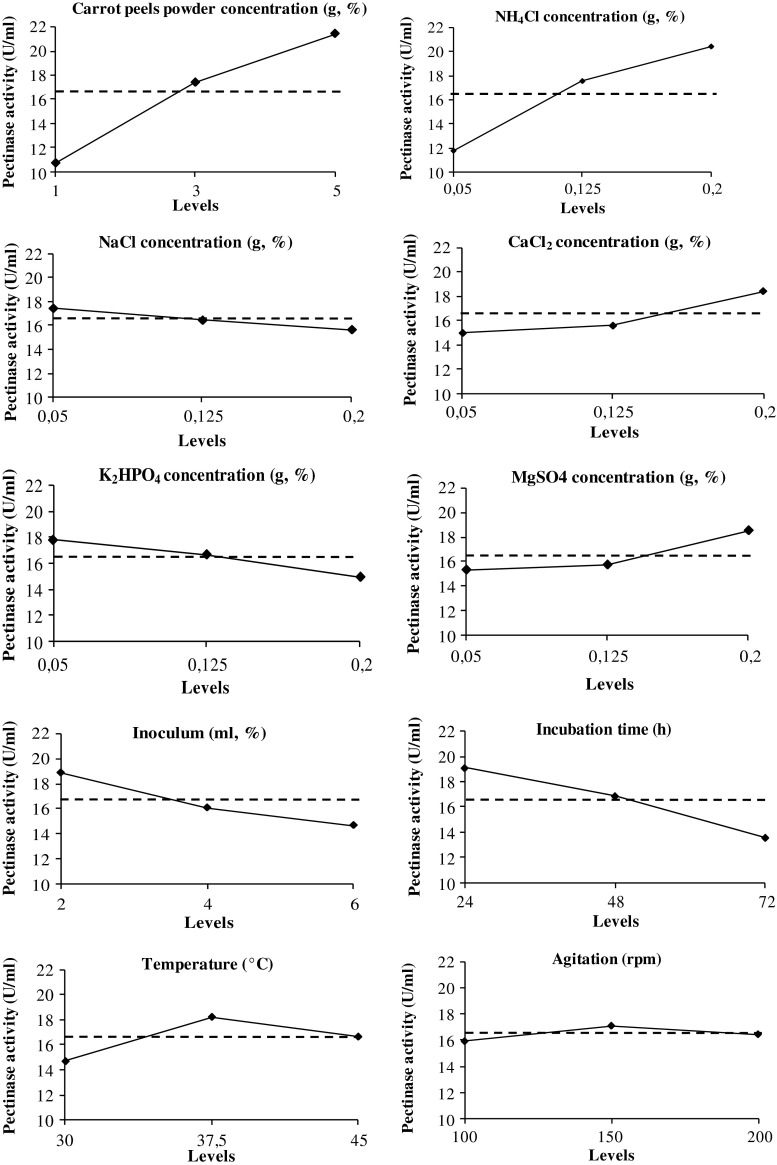
A total of ten variables were analyzed with regard to their effects on pectinase production using the Taguchi design. The design matrix selected for screening the significant variables for pectinase production and corresponding responses are shown in Table 2. The maximum experimental value recorded for pectinase production was 30.5 U/ml, with a significant improvement in enzyme yields as compared to the conventional one-at-a-time experimentation. The variations observed in terms of pectinase production values at given experimental conditions revealed that the enzyme production titers were regulated by the selected concentration factor (Fig. 3). The increase in the concentration of carrot peels powder was noted to induce positive effects on enzyme production yields. In fact, the variation of the concentration of carrot peels powder in the submerged fermentation medium from 1 to 5 % was noted to induce an increase in enzyme production, indicating that the concentration of the carbon substrate mediated the regulation of pectinase production. Such carbon source concentration-dependent regulation of enzyme production is in agreement with previous reports involving different pectinase-producing microbial strains (Ahlawat et al. 2009; Debing et al. 2006).
Fig. 3.
Impact of selected factor levels on pectinase production by B. mojavensis I4 under submerged fermentation
Furthermore, the selected inorganic nitrogen source was noted to exhibit maximum and minimum enzyme production yields at levels 3 (20.38 U/ml) and 1 (11.76 U/ml), respectively. Such variation in productivity could presumably be attributed to nitrogen source mediated microbial growth and metabolism, which is consistent with previous reports describing nitrogen as a key element for enzyme production and growth since it is an important cellular component of the protein (Kulkarni et al. 1999).
The findings also revealed that enzyme production was influenced by the initial inoculum level. The maximum enzyme yield (18.84 U/ml) was recorded with 2 % of inoculum, and variation was noted to bring a decrease in pectinase production. The observed reduction in enzyme yields at inoculum levels 2 and 3 could be attributed to the limitation in other fermentation medium components. The results showed that 24 h of incubation gave the maximum pectinase activity. The optimal temperature and agitation rates for B. mojavensis I4 to secrete pectinase were 37.5 °C and 150 rpm, respectively. The change of NaCl and K2HPO4 concentration from level 1 (0.05 %) to level 2 (0.125 %) showed no effect on pectinase production yield. Concentration level 3 (0.2 %) was, however, the optimum concentration for CaCl2 and MgSO4 to produce pectinase.
Taken together, the results indicated that the optimum levels for each factor in terms of achieving higher pectinase production yield were as follows: 5 % carrot peels powder, 0.2 % NH4Cl, 0.05 % NaCl, 0.05 % K2HPO4, 0.2 % CaCl2, 0.2 % MgSO4, 2 % inoculum size, 37.5 °C incubation temperature, and 24 h incubation time. Carrot peels powder, NH4Cl, inoculum level, and fermentation time were determined as the most significant factors. They were, therefore, selected for further optimization by a response surface methodology.
Optimization of significant variables by Box-Behnken design
Once the optimal region for running the process had been identified, a response surface design was developed for the four variables (carrot peels powder, NH4Cl, inoculum level and fermentation time) affecting pectinase production. A Box-Behnken design of response surface methodology based on the Taguchi design was adopted to search for the optimum combination of the major components of the production medium. The respective low and high levels with the coded levels for the four variables are defined in Table 3. A total of 32 runs were performed with different combination of carrot peels powder, NH4Cl, inoculums level, and fermentation time (Table 4). The experimental results were analyzed by standard ANOVA (Table 5) and the Box-Behnken design was fitted with the second-order polynomial equation:
Where X1, X2, X3 and, X4 refer to the coded factors of carrot peels powder, NH4Cl, inoculum level, and fermentation time, respectively.
Table 5.
Analysis of variance of the Box-Behnken design response
| Source of variation | Sum of squares | df | Mean square | Ratio | P-value (significance) |
|---|---|---|---|---|---|
| Regression | 1791.9 | 14 | 127.995 | 242.0044 | <0.01* |
| 68.9242 | 44 | 1.56646 | |||
| Lack of fit | 53.5862 | 15 | 3.57241 | 6.7544 | <0.01* |
| Error | 15.3380 | 29 | 52.8898 | ||
| Total | 1860.86 | 58 |
*Significant at the 99 % level
A variance analysis was performed for the fitted model, and the results showed that the regression sum of squares was statistically significant with the F-test and lack of fit values being statistically significant at a probability level of 99 % and confidence interval of 99 % (Table 5). The model was, therefore, in good correlation with the measured data (Sarabia and Ortiz 2009). From the variance analysis, the values of the determination coefficient (R2) and the adjusted determination coefficient (R2adj) were computed as 0.963 and 0.951, respectively, thus indicating that adequacy of this mathematical model to represent the experimental results. The response prediction quality by the fitted model was tested by comparing the experimental values obtained at the five check points (Experiments 28–32) with their theoretical counterparts predicted by the model (Table 4). The experimental and predicted values showed an average error close to zero. This indicated the absence of bias in the model predictions and further confirmed that the second order model was adequate to describe the response surface and represent the process.
The relationship between the response and the experimental variables were graphically illustrated by plotting the two dimensional isoresponse curves and the three dimensional response surface plots (Fig. 4a and b) in accordance with two factors, while the two others remained constant at their mean level. Figure 4a provides a graphical representation for the evolution of pectinase production versus carrot peels powder concentration (4.38–8.62 %) and cultivation time (12.67–35.33 h) at fixed levels of inoculum size 2 % and NH4Cl concentration (0.3 %). High enzyme yields were obtained with an incubation time and concentration of carrot peels powder of 32 h and 6.5 %, respectively. Pectinase production yields were, however, noted to decrease at a higher carrot peels powder concentrations. Figure 4b represents the effect of the inoculum size (0.58–3.42 %) and incubation time (12.66–35.34 h) on enzyme activity at fixed levels of carrot peels powder and NH4Cl (6.5 and 0.3 %, respectively). Isoresponse curves highlighted the important role of inoculum size and incubation time. In fact, pectinase production yields increased significantly at high fermentation time and a high inoculum size.
Fig. 4.
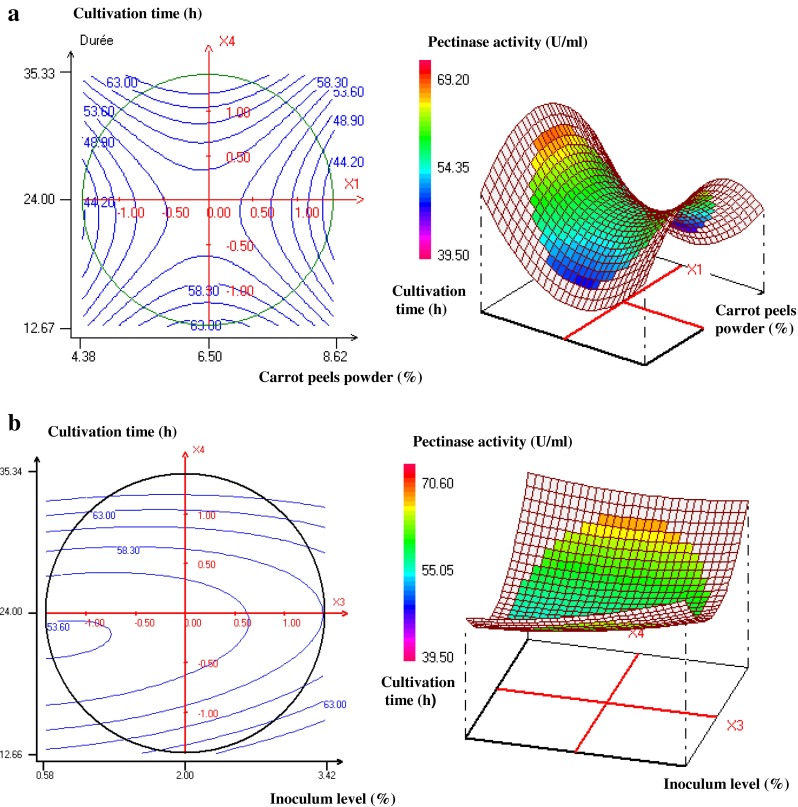
Contour plots and response surface plots
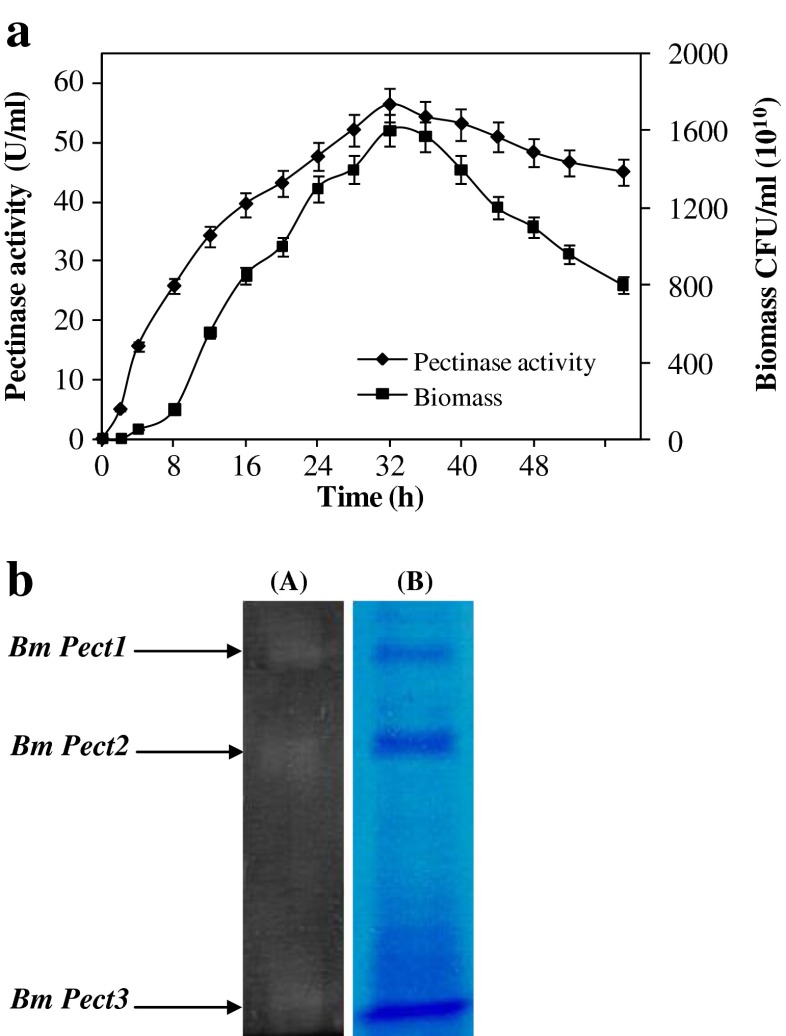
Time course of pectinase production and cell growth during fermentation
The time course of pectinase production and the cell growth of B. mojavensis I4 in an optimized medium were investigated. The results illustrated in Fig. 5a show that pectinase synthesis was strongly associated with cell growth. Maximum pectinase activity reached 57 U/ml at 32 h.
Fig. 5.
Kinetics of growth and pectinase production by B. mojavensis I4 in the optimized medium (a) and Pectin-zymography analysis of I4 crude enzyme (b)
Pectin-zymography
The pectin-zymography analysis of the crude enzyme preparation revealed the presence of at least three clear zones, suggesting the presence of at least three pectinases (Fig. 5b).
Biochemical properties
Effect of temperature on pectinase activity and stability
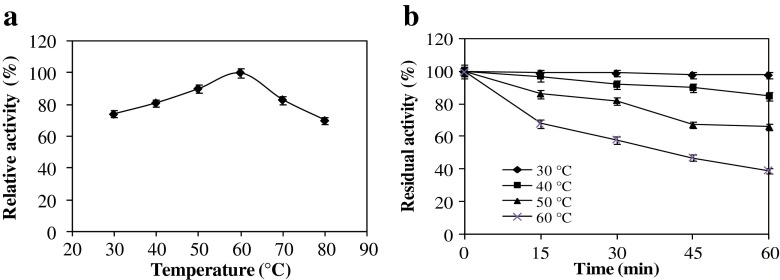
The temperature-activity profile of the enzyme is reported in Fig. 6a. The I4 crude pectinase was active at temperatures ranging from 30 to 80 °C, with an optimum at 60 °C. The relative activities recorded at 70 and 80 °C were about 83 and 70 %, respectively. Figure 6b shows the effect of temperature on enzyme stability. The pre-incubated crude enzyme retained 98, 85 and 67 % of its initial activity at 30, 40 and 50 °C for 1 h, respectively. At 60 °C, it maintained 47 % of residual activity for 45 min. The characteristics of alkaline pectinase of B. mojavensis I4 are comparable to those of B. halodurans M29 that was optimally active at pH 10.0 and 80 °C (Mei et al. 2013), the pectinase from B. pumilus dcsr1 reported to exhibits maximal activity at pH 10.5 and 50 °C (Sharma and Satyanarayana 2006) and also pectate lyase from B. tequilensis SV11 which kept maximum activity at pH 9.0 and 60 °C (Chiliveri and Linga 2014).
Fig. 6.
Effect of temperature on the activity (a) and stability (b) of the extracellular pectinase of B. mojavensis I4
Effect of pH on enzyme activity and stability
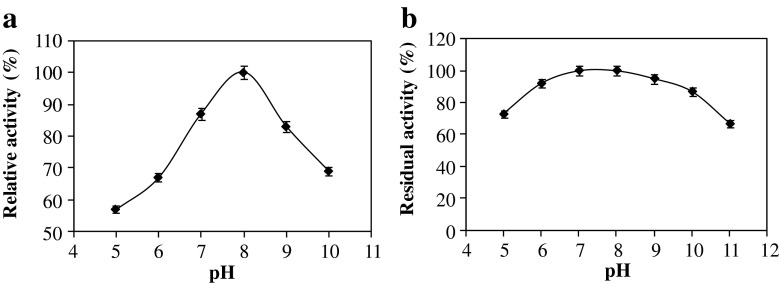
The effect of pH on the activity of the crude enzyme was determined over a pH range of 5.0–10.0 at 60 °C. Figure 7a shows that the optimum pH value was 8.0. The relative activity at pH 7.0, 9.0, and 10.0 were about 87, 83 and 70 % as compared to pH 8.0, respectively. The pH stability pattern of the pectinase illustrated in Fig. 7b shows that the crude enzyme retained more than 90 % of its initial maximum activity at a pH range of 6.0–10.0 after 24 h of pre-incubation and maintained 67 % of this activity at pH 11.0.
Fig. 7.
Effect of pH on the activity (a) and stability (b) of the extracellular pectinase of I4 strain
Improvement of sesame seeds oil extraction by the use of I4 pectinases
The extracellular juice of B. mojavensis I4 cultured on carrot peels powder was applied in the sesame seeds oil extraction process. Figure 8 clearly shows that the application of I4 pectinases improved sesame seeds oil extraction, with 3 and 6 %, extraction yields obtained without and with the enzymes, respectively. This demonstrates that an enzymatic treatment may certainly be adopted, with quantitative advantage, to enhance sesame seeds oil extraction.
Fig. 8.

Application of the extracellular pectinases produced by B. mojavensis I4 in the improvement of sesame seeds oil extraction. a: Test without enzyme, b: test with enzyme
Discussion
Pectinolytic enzymes produced by various microorganisms are useful for several industrial applications (Kaur et al 2004; Favela-Torres et al. 2005; Sharma and Satyanarayana 2004). The members of the genus Bacillus and related genera are known to produce extracellular pectinases (Kapoor et al. 2008). In this study, a bacterium belonging to the genus Bacillus was newly isolated from local soil samples at Sfax-Tunisia. The bacterium, identified as Bacillus mojavensis I4 was noted to generate a thermostable alkaline pectinase. The improvement of microbial pectinase production has attracted the attention of several researchers during the last decades (Ahlawat et al. 2009; Debing et al. 2006; Sharma and Satyanarayana 2006). However, no medium has so far been established for the production of pectinases from different microorganisms; each strain has its own specific conditions required for maximum enzyme production. The production of high titers of any enzyme by optimizing the growth parameters is of prime importance in industrial enzymology (Ahlawat et al. 2009). In addition to stringent considerations pertaining to operational conditions, the large scale application of pectinases requires that the production of those enzymes be cost-effective so as to make the process economically viable. Accordingly, the present study aimed to reduce the cost of pectinase production by optimizing the fermentation medium. Research on the selection of suitable substrates for pectinase production has mainly centered on tropical agro-industrial crops and residues. Wheat bran has often been described as one of the most effective substrates so far reported in the literature (Pandey et al. 2000). The B. mojavensis I4 strain produced 8.96 U/ml of pectinolytic activity in initial medium (M1) containing 1.0 % wheat bran as a carbon source. Several other locally available and low-cost agro-industrial waste materials were screened for their suitability for the production of pectinase by strain B. mojavensis I4 under liquid submerged fermentation. The findings from preliminary assays revealed that pectinase production varied with the type of agro-industral material used, and maximum yields of enzyme production were obtained in the presence of carrot peels powder followed by wheat bran. In fact, no previous studies have reported on the induction of pectinase production by the use of carrot peels powder in the fermentation medium. Kashyap et al. (2003) and Debing et al. (2006) reported that the maximum pectinase production yields by Bacillus sp. DT7 and Aspergillus niger were obtained in fermentation medium containing wheat bran.
The effect of nitrogen sources was also investigated by incorporating different organic and inorganic compounds into the fermentation media. Among the various nitrogen sources tested, ammonium chloride emerged as the most effective supplement for the synthesis of pectinase, followed by ammonium sulfate. In fact, several researchers have previously reported that organic nitrogen source are most favorable for pectinase production (Ahlawat et al. 2009). Yeast extract was reported to be effective for pectinase production by Bacillus pumilus dcsr1 (Sharma and Satyanarayana 2006); Bacillus subtilis WSHB04-02 (Wang et al. 2007). However, certain microorganisms are known to produce pectinase in the presence of inorganic nitrogen sources (Kashyap et al. 2003; Debing et al. 2006). The results of the present work indicate that the use of carrot peels powder and ammonium chloride as carbon and nitrogen sources, respectively, may result in a more cost-effective process and make the process of producing pectinase by the I4 strain economically viable.
The use of statistical models to optimize medium components has increased in present day biotechnology, particularly due to their ease of applicability, reliability and validity. Statistical experimental designs have been adopted to enhance enzyme titer. The Taguchi design confirmed that carrot peels powder concentration, NH4Cl concentration, inoculum level and fermentation time significantly affected pectinase production. The Box-Behnken design applied to the optimization of pectinase production in this investigation suggested the importance of a variety of factors at different levels. Among the four significant variables selected by the Taguchi design experimentation, carrot peels powder concentration was noted to have the greatest effect on pectinase production. Maximum pectinase production (63.55 U/ml) was achieved at 6.5 % carrot peels powder, 0.3 % NH4Cl, 3 % inoculum size, and 32 h fermentation time, an agitation speed of 150 rpm, and a temperature of 37.5 °C, in addition to other salts used at suitable levels, as shown by the Taguchi design. The predicted and experimental values obtained for the responses reflected the accuracy and applicability of RSM for the optimal production of extracellular pectinases by B. mojavensis I4. As compared to pectinase production by I4 in a non-optimized medium, a 7.2-fold increase in pectinase production was recorded for the RSM optimized medium in shake flasks. A 1.5-fold increase in pectinolytic enzyme secretion by Kluyveromyces wickerhamii was previously reported when pH, temperature and incubation period were optimized by RSM (Moyo et al. 2003). Sharma and Satyanarayana (2006) reported that a 41-fold increase in pectinase production by B. pumilus dcsr1 was achieved using RSM.
Furthermore, the findings revealed that most of the I4 pectinase productions occurred within the first 32 h of culture and corresponded to the exponential growth phase of the strain. Pectinase production by B. subtilis was previously reported to start after 18 h of incubation, reach its highest at 24 h (110 U/ml), and to decrease thereafter (Ahlawat et al. 2009). Zymogram analysis was performed to obtain further information on the diversity of the extracellular pectinases secreted by the B. mojavensis I4 strain. Three pectinases were observed in the pectinolytic activity profile of the cell-free enzymatic preparation of I4 grown on the carrot peel powder medium. B. licheniformis SVD1 appeared to possess a pectin methyl esterase as well as a pectin or pectate lyase from zymogram data (van Dyk et al. 2010). Four pectate lyases and a pectin methyl esterase were reported to be present in the genome of B. licheniformis DSM 13 (Veith et al. 2004).
Recently, there has been much work on the application of alkaline pectinases in the textile industry for the retting and degumming of fiber crops, production of good quality paper, fermentation of coffee and tea, enhancement of oil extractions, and treatment of pectic waste water. These enzymes come mostly from bacterial sources. In the industrial sector, alkaline pectinases, mainly from Bacillus spp., are applied for various purposes. The results demonstrated that the B. mojavensis I4 strain is a good producer of thermostable alkaline pectinases. The pectinases produced by the I4 strain were stable over a broad range of pH (6.0 to 10.0). The optimum pH for pectinolytic activity was 8.0, and relatively significant levels of activity, reaching up to 83 % and 70 %, were detected at pH 9.0 and 10.0, respectively. The optimum temperature for the pectinolytic activity of the I4 strain was 60 °C. The pre-incubated crude enzyme preserved 98, 85 and 66 % of its initial activity at 30, 40 and 50 °C for 1 h, respectively. At 60 °C, it maintained 47 % of residual activity for 45 min.
Oils from rape seed (Canola), coconut germ, sun-flower seed, palm, kernel and olives are traditionally produced by extraction with organic solvents. Hexane is one of the most commonly used solvents but has often been considered to be potentially carcinogenic. Cell-wall-degrading enzymes, including pectinase, may be used to extract vegetable oils in an aqueous process by liquefying the structural cell wall components of the oil-containing crop. Enzyme treatment has, therefore, often been applied to induce an increase in oil extraction yields. The increase in the yield depends upon pH, temperature, and enzyme concentration (Kashyap et al. 2001). Olivex, an enzyme preparation derived from A. aculeatus, which contains pectinolytic activity as well as hemicellulase and cellulolytic activities, has been shown to induce good oil extraction yields and better stability rates when stored. The oil also shows increased content of polyphenols and vitamin E, which stabilizes oil against rancidity. In this context, the crude extract of B. mojavensis I4 was applied in the process of oil extraction from sesame seeds. By comparing the yield obtained without and with enzymes, an improvement of about 3 % was recorded for the treated sesame seeds, representing a promising gain for oil extraction. This suggests that the enzymes are highly suitable for the degradation of the pecto-cellulosic wall of sesame cells, thus enabling the recovery of more oil.
Conclusion
The findings presented in this study demonstrated that the bacterium strain B. mojavensis I4 is an excellent producer of thermostable alkaline pectinases, using a carrot peel powder by-product from a ready-to-eat vegetable industry under liquid submerged fermentation. To the authors’ knowledge, this study is the first to report on the use of carrot peel powders in the fermentation medium for the production of pectinase. Among the various nitrogen sources tested, the low-cost inorganic nitrogen chloride ammonium emerged as most efficient for pectinase production. A Box-Behnken design of response surface methodology based on the Taguchi design was employed to optimize the parameters significantly affecting pectinase production by B. mojavensis I4. Pectin-zymography analysis using pectin citrus revealed that the strain produced at least three extracellular pectinases. The crude enzyme was highly stable and active at a broad range of pH, with optimum activity at pH 8.0 and at 60 °C. The crude pectinase preparation was also noted to suitably enhance sesame seed oil extraction. Taken together, the findings demonstrated that the B. mojavensis I4 has a promising potential for future use in a wide range of industrial and biotechnological applications including extraction and clarification of juices and removal of pectic waste. Safety assessment of enzyme preparation needs to be studied.
Acknowledgments
This work was funded by the “Ministry of Higher Education, Scientific Research and Technology, Tunisia”. The authors thank Mr. Anouar Smaoui and Mrs. Hanen Ben Salem from the Faculty of Science of Sfax, Tunisia for their professional support in the proofreading, editing, and language polishing of the present manuscript.
References
- Ahlawat S, Dhiman SS, Battan B, Mandhan RP, Sharma J. Pectinase production by Bacillus subtilis and its potential application in biopreparation of cotton and micropoly fabric. Process Biochem. 2009;44:21–526. doi: 10.1016/j.procbio.2009.01.003. [DOI] [Google Scholar]
- Anuradha P, Vijayalakshmi K, Prasanna ND, Sridevi K. Production and properties of alkaline xylanases from Bacillus sp isolated from sugarcane fields. Curr Sci. 2007;92:1283–1286. [Google Scholar]
- Bailey MJ, Biely P, Poutanen K. Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol. 1992;23:257–270. doi: 10.1016/0168-1656(92)90074-J. [DOI] [Google Scholar]
- Chantaro P, Devahastin S, Chiewchan N. Production of antioxidant high dietary fiber powder from carrot peels. LWT Food Sci Technol. 2008;41:1987–1994. doi: 10.1016/j.lwt.2007.11.013. [DOI] [Google Scholar]
- Chiliveri SR, Linga VR. A novel thermostable, alkaline pectate lyase from Bacillus tequilensis SV11 with potential in textile industry. Carbohydr Polym. 2014;111:264–272. doi: 10.1016/j.carbpol.2014.04.065. [DOI] [PubMed] [Google Scholar]
- Debing J, Peijun L, Stagnitti F, Xianzhe X, Li L. Pectinase production by solid fermentation from Aspergillus niger by a new prescription experiment. Ecotoxicol Environ Saf. 2006;64:244–250. doi: 10.1016/j.ecoenv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Favela-Torres E, Aguilar C, Contreras-Esquivel JC, Viniegra-Gonzalez G. Pectinases. In: Pandey A, Webb C, Soccol CR, Larroche C, editors. Enzyme technology. New Delhi: Asiatech Publishers Inc.; 2005. pp. 273–296. [Google Scholar]
- Heerd D, Yegin S, Tari C, Fernandez-Lahore M. Pectinase enzyme-complex production by Aspergillus spp. in solid-state fermentation: a comparative study. Food Bioprod Process. 2012;90:102–110. doi: 10.1016/j.fbp.2011.08.003. [DOI] [Google Scholar]
- Kapoor M, Nair LM, Kuhad RC. Cost-effective xylanase production from free and immobilized Bacillus pumilus strain MK001 and its application in saccharification of Prosopis juliflora. Biochem Eng J. 2008;38:88–97. doi: 10.1016/j.bej.2007.06.009. [DOI] [Google Scholar]
- Kashyap DR, Vohra PK, Chopra S, Tewari R. Applications of pectinases in the commercial sector: a review. Bioresour Technol. 2001;77:215–227. doi: 10.1016/S0960-8524(00)00118-8. [DOI] [PubMed] [Google Scholar]
- Kashyap DR, Soni SK, Tewari R. Enhanced production of pectinase by Bacillus sp. DT7 using solid state fermentation. Bioresour Technol. 2003;88:251–254. doi: 10.1016/S0960-8524(02)00206-7. [DOI] [PubMed] [Google Scholar]
- Kaur G, Kumar S, Satyanarayana T. Production, characterization and application of a thermostable polygalacturonase of a thermophilic mould Sporotrichum thermophile Apinis. Bioresour Technol. 2004;94:239–243. doi: 10.1016/j.biortech.2003.05.003. [DOI] [PubMed] [Google Scholar]
- Kulkarni N, Shendye A, Rao M. Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev. 1999;23:411–456. doi: 10.1111/j.1574-6976.1999.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mathieu D, Nony J, Phan-Tan-Luu R. NEMROD-W software. Marseille: LPRAI; 2000. [Google Scholar]
- Mei YZ, Chen YR, Zhai RY, Liu Y. Cloning, purification and biochemical properties of a thermostable pectinase from Bacillus halodurans M29. J Mol Catal B Enzym. 2013;94:77–81. doi: 10.1016/j.molcatb.2013.05.004. [DOI] [Google Scholar]
- Moyo S, Gashe BA, Collison EK, Mpuchane S. Optimising growth conditions for the pectinolytic activity of Kluyveromyces wickerhamii by using response surface methodology. Int J Food Microbiol. 2003;85:87–100. doi: 10.1016/S0168-1605(02)00503-2. [DOI] [PubMed] [Google Scholar]
- Pandey A, Soccol CR, Mitchell D. New developments in solid state fermentation: I-bioprocesses and products. Process Biochem. 2000;35:1153–1169. doi: 10.1016/S0032-9592(00)00152-7. [DOI] [Google Scholar]
- Prakash S, Jha SK, Datta N. Performance evaluation of blanched carrots dried by three different driers. J Food Eng. 2004;62:305–313. doi: 10.1016/S0260-8774(03)00244-9. [DOI] [Google Scholar]
- Sarabia LA, Ortiz MC (2009) Response surface methodology. In: Editors-in-Chief: Stephen DB, Romà T, Beata W (eds) Comprehensive chemometrics. Elsevier, Oxford, pp 345–390
- Sharma DC, Satyanarayana T. Production and application of pectinolytic enzymes of Sporotrichum thermophile and Bacillus pumilus. In: Reddy MS, Khanna S, editors. Biotechnological approaches for sustainable development. India: Allied Publishers Pvt. Ltd.; 2004. pp. 164–169. [Google Scholar]
- Sharma DC, Satyanarayana T. A marked enhancement in the production of a highly alkaline and thermostable pectinase by Bacillus pumilus dcsr1 in submerged fermentation by using statistical methods. Bioresour Technol. 2006;97:727–733. doi: 10.1016/j.biortech.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Sun X, Liu Z, Qu Y, Li X. The effects of wheat bran composition on the production of biomass-hydrolyzing enzymes by Penicillium decumbens. Appl Biochem Biotechnol. 2008;146:119–128. doi: 10.1007/s12010-007-8049-3. [DOI] [PubMed] [Google Scholar]
- van Dyk JS, Sakka M, Sakka K, Pletschke BI. Characterisation of the multi-enzyme complex xylanase activity from Bacillus licheniformis SVD1. Enzym Microb Technol. 2010;47:174–177. doi: 10.1016/j.enzmictec.2010.06.004. [DOI] [Google Scholar]
- Veith B, Herzberg C, Steckel S, Feesche J, Maurer KH, Ehrenreich P, … Gottschalk G (2004) The complete genome sequence of Bacillus licheniformis DSM13, an organism with great industrial potential. J Mol Microbiol Biotechnol 7:204–211 [DOI] [PubMed]
- Wang Q, Fan XR, Hua ZZ, Chen J. Optimizing bioscouring condition of cotton knitted fabrics with an alkaline pectinase from Bacillus subtilis WSHB04-02 by using response surface methodology. Biochem Eng J. 2007;34:107–113. doi: 10.1016/j.bej.2006.11.004. [DOI] [Google Scholar]
- Zhang DL, Hamauzu Y. Phenolic compounds and their antioxidant properties in different tissues of carrots (Daucus carota L.) J Food Agric Environ. 2004;2:95–100. [Google Scholar]