Abstract
Goshtaba is a restructured meat product of Kashmiri wazwan prepared from meat emulsion with added fat (20 %), salt, spices and condiments and cooked in the curd. The present study was undertaken for the development of low fat goshtaba with the addition of xanthan gum as a fat replacer and was evaluated for proximate composition, pH, colour, lipid and protein oxidation, texture, microstructure and sensory properties. Low fat goshtaba formulations containing xanthan gum were higher in protein and moisture contents but, lower in fat content and pH value than the high fat control (p < 0.05). Colour evaluation revealed that high fat goshtaba had significantly higher L* value, but lower a* value than its low fat counterparts (p < 0.05). The significant decrease of TBARS values, protein carbonyls and loss of protein sulphydryl groups in low fat goshtaba formulations reflects the potential antioxidant activity of xanthan gum (p < 0.05). Hardness was significantly higher in high fat control but, cohesiveness, gumminess, and chewiness did not show any significant difference. Springiness increased with the increasing concentration of xanthan gum (0.5–1.5 %) and was higher in low fat product containing 1.5 % xanthan gum. SEM results indicate that xanthan gum lead to formation of an additional gel network which holds more water. Sensory evaluation revealed that goshtaba product with 0.5 % xanthan gum had quality characteristics that were similar to the control product containing 20 % fat.
Keywords: Goshtaba, High fat, Low fat, Xanthan gum, Quality
Introduction
Present day consumers are very much conscious about their nutrition and health. Thus demand healthier meat products that are low in fat, cholesterol and calories. In restructured meat products fat plays an important role in stabilizing meat emulsions, reducing cooking loss, improving water holding capacity and providing organoleptic quality (Yoo et al. 2007). However, high animal fat used in restructured meat products provides higher amounts of saturated fatty acids and cholesterol (Pappa et al. 2000). High saturated fat intake increases risk of obesity and some types of cancer, and is also closely related to high blood cholesterol and coronary heart disease (AHA 1986; Gök et al. 2011; WHO 2003). In addition to serious health concerns of animal fat, oxidation of lipids is a major threat to meat quality. The onset of oxidative reactions in muscle foods during handling, processing and storage leads to undesirable sensory changes and deterioration of nutritive value (Ganhão et al. 2010; Hęś et al. 2012). A deterioration of nutritive value may be a consequence of interactions between lipid oxidation products and proteins (Hęś et al. 2012). Hence fat reduction has generally been seen as an important strategy to improve the fat content of meat products and produce healthier products because some traditional meat products contain high proportions of animal fat (Huda et al. 2014). But in many cases, low fat meat products have been rejected by the consumers because they were considered less juicy, firmer, more rubbery, darker in color and overall less acceptable than traditional counterparts (Keeton 1994).On the other hand, consumers expect that these low fat meat products with altered formulations to taste, look and smell in the same way as their traditionally formulated and processed counterparts. In this regard, manufacturers have introduced several modifications in an attempt to offset the detrimental effects of reducing the fat levels in restructured meat products. These modifications include the use of fat replacers such as hydrocolloids, vegetable and connective tissue proteins and vegetable oils (Candogan and Kolsarici 2003b; Ako 1998).
The state of Jammu and Kashmir in India is widely known for wazwan which is a combination of restructured traditional meat products like kabab, rista, goshtaba, etc. Goshtaba forms the essential item of Kashmiri wazwan. This is an emulsion type ground meat product and is usually prepared by using mutton or beef. Considerable amount of animal fat (20 %) is used in its formulation to achieve a stable emulsion, and also to impart a special taste and flavour to the product (Rather et al. 2015; Samoon and Sharma 1991).Thus there is a great scope and need for improvements over the traditional practices followed in its formulation, preparation and preservation, so as to enhance its quality as well as shelf life and thus safeguard the health of consumers.
One of the most promising fat substitutes is Xanthan gum. Xanthan gum is an anionic microbial hydrocolloid produced by aerobic fermentation of Xanthomonas campestris. The primary structure of xanthan gum consist of linear (1→4) β-D-glucopyranosyl unit, which is substituted at C-3 on every other glucose residue with a charged trisaccharide side chain. The trisaccharide chain consists of a D-glucuronic acid unit between two D-mannose units. Approximately half of the terminal D-mannose unit contains a pyruvic acid residue linked via a keto group to the 4 and 6 positions, while the D-mannose linked to the main chain contains an acetyl group at position O-6 (Garcia-Ochoa et al. 2000). Xanthan gum is soluble in hot and cold water and even when used in low concentration; it forms a pseudoplastic and viscous solution (Pettit 1979). Its viscosity increases when salt is added to the formulation and this solution is stable although a variation in pH and temperature occurs. Pseudoplasticity is important for flavour, mouthfeel and outward appearance of the product (Luruena-Martınez and Vivar-Quintana 2004; Glicksman 1982). Recently, the potential antioxidant activity of xanthan gum attracted more and more attention for using in lipid model system (Trommer and Reinhard 2005). The objective of this study was to evaluate the effect of xanthan gum as a fat replacer on some important quality parameters of goshtaba, a traditional meat product of Jammu and Kashmir, India.
Materials and methods
Materials
Fresh mutton from hind leg portion and mutton fat (moisture 12.59 %, fat 86.23 %) from male sheep mean age 16 months at 12 h postmortem, slaughtered according to the traditional halal method was procured from a selected retail meat shop located at Hazratbal Srinagar, India. All subcutaneous fat and visible connective tissues were removed from fresh muscles by a butcher knife. The meat was initially analyzed for fat content (2.08 %) prior to the manufacture of product. Non meat ingredients such as salt, curd, oil and spices were procured from the local market. Xanthan gum used in this study was procured from Hi-Media Pvt. Ltd., Mumbai, India.
Formulation and processing of goshtaba
Lean meat and mutton fat were initially ground separately in a mincer (SIRMAN, TC 22, Italy) through an 8-mm plate and divided into five batches for various formulations, which differed in composition with respect to fat level and the addition of xanthan gum (Table 1). The first batch was used as high fat control (T0) and the fat content was adjusted to 20 % by the addition of mutton fat. The second batch was used as low fat control (TC) and the fat content was adjusted to 10 %. The other batches were supplemented with various levels of xanthan gum 0.5 % (T1), 1 % (T2), and 1.5 % (T3) and the fat content was adjusted to 10 %. The lean mutton, fat and xanthan gum were homogenized in a blender for 2 min. The remaining non meat ingredients (salt 2.5 %, large cardamom seeds 0.2 % and cumin 0.1 %) were then added to the mixture and mixed for eight additional min to make homogenous viscoelastic mass. All batches were reground in a mincer (SIRMAN, TC 22, Italy) through a 6-mm plate. The meat emulsion mixtures were then shaped into compact balls of 60 mm diameter and 50–60 g weight. The meat balls from each formulation were then processed in yakhni (gravy) separately to get goshtaba as the finished product.
Table 1.
Product formulation (g/100 g) with varying fat and xanthan gum levels
| Ingredient | Treatment: percentage of fat and xanthan gum | ||||
|---|---|---|---|---|---|
| T0 | TC | T1 | T2 | T3 | |
| Sheep meat | 67.2 | 67.2 | 67.2 | 67.2 | 67.2 |
| Mutton fat | 20 | 10 | 10 | 10 | 10 |
| Ice water | 10 | 20 | 19.5 | 19.0 | 18.5 |
| Xanthan gum | 0 | 0 | 0.5 | 1.0 | 1.5 |
| Common salt (NaCl) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Large cardamom seeds | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Cumin | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
T0: control containing fat (20 %) + xanthan gum (0 %); TC: fat (10 %) + xanthan gum (0 %); T1 = fat (10 %) + xanthan gum (0.5 %); T2 = fat (10 %) + xanthan gum (1.0 %); T3 = fat (10 %) + xanthan gum (1.5 %)
Yakhni is the gravy, in which the meat balls are processed. The yakhni formulation is given in Table 2. For preparing yakhni two parts of fresh curd was homogenized with one part of water with a stirrer, transferred to a thick bottomed stainless steel vessel and heated rapidly on a gas stove for 10–15 min. During heating curd was constantly stirred until it reached the boiling point. Hydrogenated mustard oil was added to it and boiling continued for 10–15 min. Then garlic paste was added followed by spice mixture. Fried onion paste was added at the end. Boiling was continued until the added oil floated back. At this stage, the remaining water was added and yakhni was cooked further for 10–15 min, to obtain a desirable consistency. Salt was added towards the end of cooking. The meat balls was then transferred to the boiling yakhni and cooked for 30 min to get the goshtaba in its final form. After cooking the goshtaba samples were drained and subjected to analysis.
Table 2.
Standardized recipe for Yakhni (Gravy) preparation
| Ingredient | Quantity (%) |
|---|---|
| Water | 45.0 |
| Curd (Dahi) | 45.0 |
| Vegetable oil | 4.50 |
| Large cardamom | 0.15 |
| Small cardamom | 0.08 |
| Cinnamon | 0.22 |
| Cloves | 0.05 |
| Dried ginger powder | 0.3 |
| Aniseed powder | 0.4 |
| Garlic paste | 0.5 |
| Fried onion paste | 2.25 |
| Common salt | 1.55 |
Proximate composition
Moisture (925.10), protein (960.10), fat (920.85) and ash (923.03) contents were determined according to Association of Official Analytical Chemists (AOAC 1990). The samples were analyzed in triplicate for each component.
pH value
The pH value of goshtaba samples were measured in a homogenate prepared with 5 g of sample and distilled water (20 ml) using a pH meter (HANNA-HI 2215 pH/ORP Meter). All determinations were performed in triplicate.
Colour
The colour values of the goshtaba samples were determined using a Hunter Colour Lab (Mini Scan XE Plus, Hunter Associate Laboratory Reston, USA). The instrument was calibrated with black and white tiles before colour measurement. The ‘L*’ value indicates the lightness, 0–100 representing dark to light. The ‘a*’ value gives the degree of the red–green colour, with a higher positive ‘a’ value indicating more red. The ‘b*’ value indicates the degree of the yellow–blue colour, with a higher positive ‘b’ value indicating more yellow. The average value of three replicates was reported.
Lipid oxidation (TBARS)
Oxidative stability was evaluated by changes in thiobarbituric acid reactive substances (TBARS). The procedure for measurement of TBARS was based on methods used by Serrano et al. (2006). Five gram sample was homogenized in 35 ml of 7.5 % trichloroacetic acid. The homogenized sample was centrifuged (3000 g, 2 min) and 5 ml of the supernatant was mixed with 5 ml of 20 mM thiobarbituric acid and kept in dark for 20 h at 20 ± 1.5 °C. The pink colour that formed was measured spectrophotometrically (UV- Spectrophotometer, Model U-2900 2JI-0003, Hitachi, Japan) at 532 nm. A calibration curve was plotted with 1, 1, 3, 3-tetraethoxypropane to obtain the malonaldehyde (MDA) concentration and results were expressed as mg MDA/kg of sample. TBARS determinations for each sample were performed in triplicate.
Protein oxidation measurement
Protein carbonyls
Protein carbonyls were measured by estimation of total carbonyl groups according to the method of Levine et al. (1990) with some modifications as described by Srinivasan and Hultin (1995). From two fractions of 50 μl protein samples, one aliquot was treated with 2 ml of 2.0 N HCl (control) and the other was treated with 2.0 ml of 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2.0 N HCl for 1 h at room temperature. After incubation, the two fractions were then precipitated with 2.0 ml of 20 % trichloroacetic acid. The precipitate was washed twice with 4.0 ml of ethanol: ethylacetate (1:1, v/v) solution to remove unreacted DNPH and blow-dried. The pellet was then dissolved in 1.5 ml of 6.0 M guanidine hydrochloride with 20 mM potassium phosphate buffer (pH 2.3). Absorbance was measured spectrophotometrically at 370 nm. The amount of protein carbonyl content was expressed as nmol of mg protein using an absorption coefficient of 2.2 × 104 M−1 cm−1 for protein hydrazones.
Sulphydryls
Sulphydryl groups (thiol content) were determined according to the method described by Srinivasan and Hultin (1997). Total free sulphydryl groups were determined by reacting with 5, 5′-dithiobis (2-nitrobenzoic acid) (DTNB). One gram of meat was blended with 50 ml of cold distilled water and homogenized. Protein concentration of homogenate was diluted to 2 mg/ml with 0.1 M phosphate buffer (pH 7.4) and protein content was determined using the Biuret method. A 0.5 ml of homogenate was transferred to a tube and dissolved in urea buffer (1:1). After incubation with 0.5 ml DTNB reagent at room temperature for 15 min, absorbance was measured at 412 nm. Sample blanks with 0.5 ml phosphate buffer without DTNB and reagent blanks with only water were prepared. Sulphydryl content was calculated using a molar extinction coefficient of 11,400 M−1 cm−1 for 5, 5′-dithiobis at this wavelength. Results were expressed as nmol of total free sulphydryl groups per milligram of protein.
Texture profile analysis
Texture profile analysis (TPA) of goshtaba samples was performed in triplicate on each sample at room temperature (20 °C) with a texture analyzer (TA.HD. Plus, Stable Micro Systems, Godalming, Surrey, UK) using P/75 probe. The capacity of the load cell used was 50 kg and sample was compressed (at three different locations) to 80 % of its original height at a crosshead speed of 100 mm/min twice through a two cycle sequence. Values for hardness (Kg), cohesiveness, springiness, chewiness (Kg mm), and gumminess (Kg) were determined as described by Bourne (1978).
Microstructure
Microstructure was analyzed by scanning electron microscopy (SEM) as reported by Jiménez-Colmenero et al. (1995). The goshtaba samples were fixed with a mixture (1:1 v/v) of paraformaldehyde (4 g/100 g) and glutaraldehyde (0.2 g/100 g) in 0.1 M phosphate buffer pH 7.2, post-fixed with OsO4, washed, dehydrated in increasing concentrations of acetone, critical-point-dried, sputter-coated with gold/palladium in a metallizer (Blazer, SCD004) and scanned by SEM (S-3000 H, Hitachi, Japan) at 5.0 kV. A large number of micrographs were taken in order to select the most representative ones.
Sensory evaluation
Sensory evaluation was conducted according to the testing procedures of AMSA (1978) and IFT (1985). Samples were served in random order at temperature of approximately 60 °C. A selected ten member panel consisting of researchers and faculty members from the Department of Food Science and Technology, University of Kashmir, whose ages ranged from 24 to 35 years were selected and asked to express their opinion of the product. Samples were evaluated for firmness, flavour intensity, juiciness and overall palatability using a 9-point hedonic scale. Each attribute was discussed and tests were initiated after panelists were familiarized with the scales. Each sample was coded with a randomly selected 3-digit number and served in a white paper plate. Panelists were instructed to cleanse their palates between samples using cold water.
Statistical analysis
Mean values, standard deviation, analysis of variance (ANOVA) were computed using a commercial statistical package SPSS 16 (USA). The data were then compared using Duncan’s multiple range tests at 5 % significance level.
Results and discussion
Proximate composition
As expected, the partial replacement of fat by xanthan gum affected the proximate composition of the goshtaba formulations (Table 3). Moisture content ranged between 63.96 and 73.10 % and differed significantly between samples (P < 0.05). The moisture content of the high fat control was significantly lower than the low fat formulations (p < 0.05), because the high fat control was prepared with more fat and less water. The moisture content of the low fat formulations increased concomitantly with the increased level of xanthan gum that could be due to fat substituted by moisture in the low fat products (Pietrasik and Janz 2010), and higher moisture retention capacity of xanthan gum. Protein content ranged between 15.40 and 20.22 % and was significantly lower in high fat control and low fat product containing 1.5 % xanthan gum (p < 0.05). Though there is no variation in the initial meat protein content of the formulations. The decrease in protein content of the high fat control could be due to the higher fat content (20 %) used in the formulation and more water retained during cooking of the product. Instead of the lower water content used in the formulation of high fat control, higher moisture was retained during cooking compared to low fat products. Similar results were reported by Dzudie et al. (2002) in ground beef formulations with increasing levels of fat. However, the decreased protein content of the low fat product containing 1.5 % xanthan gum could be due to the more water used in the formulation and retained during cooking of the product, resulting in dilution of solid components. Fat content ranged between 8.86 and 17.03 % and was significantly higher in high fat control (p < 0.05). In spite of high fat content of the high fat control product, higher fat loss was observed in it compared to the low fat counterparts. This indicated that xanthan gum has the ability to retain fat during heating that resulted in the lower cooking loss. For the reason, the increased loss of fat in high fat control goshtaba with higher initial fat content can create larger fat pools, which help fat to migrate out of the inner to the outer part of the goshtaba and results in higher fat loss during cooking (Piñero et al. 2008). The ash content of the goshtaba samples ranged from 3.45 to 3.81 % and did not show any significant difference between high fat control and low fat counterparts (p > 0.05).
Table 3.
Proximate composition of low fat goshtaba formulated with varying levels of xanthan gum
| Treatment | Protein (%) | Moisture (%) | Fat (%) | Ash (%) |
|---|---|---|---|---|
| T0 | 15.40 ± 0.40a | 63.96 ± 0.06a | 17.03 ± 0.23b | 3.61 ± 0.31a |
| TC | 20.22 ± 1.02c | 67.01 ± 0.01b | 8.86 ± 0.15a | 3.81 ± 0.06a |
| T1 | 17.01 ± 0.20b | 70.69 ± 0.38c | 9.16 ± 0.19a | 3.68 ± 0.19a |
| T2 | 16.48 ± 0.50ab | 71.56 ± 1.04c | 9.01 ± 0.07a | 3.67 ± 0.11a |
| T3 | 15.46 ± 0.53a | 73.10 ± 0.30d | 8.97 ± 0.11a | 3.45 ± 0.22a |
All values are mean ± standard deviation of three replicates
Means in the same column with different superscripts differ significantly: *P < 0.05
T0: control containing fat (20 %) + xanthan gum (0 %); TC: fat (10 %) + xanthan gum (0 %); T1 = fat (10 %) + xanthan gum (0.5 %); T2 = fat (10 %) + xanthan gum (1.0 %); T3 = fat (10 %) + xanthan gum (1.5 %)
pH and colour
The pH value of the goshtaba formulations ranged from 5.0 to 5.31 and decreased significantly with an increase in xanthan gum concentration from 0.5 to 1.5 % (Table 4). The highest pH value (5.31) was obtained from the low fat control (TC) and lowest value (5.0) was obtained from low fat goshtaba containing 1.5 % xanthan gum (T3). The decrease in pH of the low fat formulations containing xanthan gum might be due to the presence of pyruvic acid residue in xanthan polysaccharide (Khouryieh et al. 2015; Sharma et al. 2006). The ultimate pH of the meat and meat products increases from 5.5 to above 6 when subjected to heated treatments, because imidazolium, which is base in histidine amino acids, was exposed due to the denaturation of proteins (Choi et al. 2007), but reverse was observed in the present study. This might be because the goshtaba treatments were cooked in the fermented milk (curd) having low pH due to lactic acid. The low pH of the goshtaba samples is a desirable characteristic for extending the shelf life of meat products (Rather et al. 2015).
Table 4.
pH and Colour analysis of low fat goshtaba formulated with varying levels of xanthan gum
| Treatments | pH | Colour | ||
|---|---|---|---|---|
| L* | a* | b* | ||
| C | 5.28 ± 0.08c | 51.79 ± 0.65c | 0.21 ± 0.14a | 14.41 ± 0.98ab |
| TC | 5.31 ± 0.09c | 43.74 ± 0.92a | 3.50 ± 1.16c | 14.55 ± 0.84b |
| T1 | 5.21 ± 0.08bc | 47.13 ± 1.94b | 2.23 ± 0.12b | 13.68 ± 0.63ab |
| T2 | 5.10 ± 0.05ab | 42.41 ± 2.01a | 2.45 ± 0.10b | 13.43 ± 0.64ab |
| T3 | 5.00 ± 0.03a | 41.46 ± 1.97a | 2.83 ± 0.08bc | 13.07 ± 0.15a |
All values are mean ± standard deviation of three replicates
Means in the same column with different superscripts differ significantly: *P < 0.05
T0: control containing fat (20 %) + xanthan gum (0 %); TC: fat (10 %) + xanthan gum (0 %); T1 = fat (10 %) + xanthan gum (0.5 %); T2 = fat (10 %) + xanthan gum (1.0 %); T3 = fat (10 %) + xanthan gum (1.5 %)
The Hunter L*, a* and b* values of goshtaba formulations was significantly affected with partial replacement of fat by different concentrations of xanthan gum (Table 4). The lightness (L*) value of the high fat control was significantly higher than low fat formulations and decreased with the increasing concentration of xanthan gum (0.5–1.5 %) (p < 0.05). The decrease in lightness (L*) values of the low fat formulations might be due to the reduction in fat content (Salcedo-Sandoval et al. 2013). The redness (a*) value of the low fat formulations were significantly higher than high fat control containing 20 % fat (p < 0.05). The results are similar to those reported by Ruiz-Capillas et al. (2012), who found an increase in redness in low fat dry fermented sausages than a high fat product. The low fat control showed significantly higher yellowness (b*) than that of high fat control and formulations containing varying concentrations of xanthan gum. Significant decrease was observed in yellowness (b*) with increasing concentration of xanthan gum from 1 % - 1.5 % (p < 0.05).
Lipid oxidation (TBARS)
The results of the TBARS values are shown in Table 5. The high fat control (T0) had exhibited significantly higher TBARS value than low fat formulations (p < 0.05). The higher TBARS value in high fat goshtaba might be a result of high fat content (20 %) used in the formulation (Candogan and Kolsarici 2003a). The TBARS values of low fat formulations decreased significantly with increasing concentration of xanthan gum (p < 0.05) and low fat goshtaba containing 1.5 % xanthan gum showed lower TBARS value. The lower TBARS values of the low fat formulations containing xanthan gum could be due to the suppression of lipid oxidation by chelation of iron between two side chains with a pyruvate residue of xanthan gum and therefore inactivating the peroxyl radicals in the products (Sun et al. 2007).
Table 5.
TBARS, Carbonyl content, and Sulphydryl content of low fat goshtaba formulated with varying levels of xanthan gum
| Treatment | TBARS (mg MDA/kg) |
Carbonyl content (nmol/mg protein) |
Sulphydryl content (nmol/mg protein) |
|---|---|---|---|
| T0 | 0.62 ± 0.03d | 3.98 ± 0.20e | 25.74 ± 0.86a |
| TC | 0.53 ± 0.04c | 2.95 ± 0.08d | 28.70 ± 0.69b |
| T1 | 0.43 ± 0.02b | 2.63 ± 0.08c | 33.89 ± 1.00c |
| T2 | 0.40 ± 0.02ab | 2.40 ± 0.06b | 37.39 ± 0.81d |
| T3 | 0.36 ± 0.01a | 2.06 ± 0.07a | 39.88 ± 1.4e |
All values are mean ± standard deviation of three replicates
Means in the same column with different superscripts differ significantly: *P < 0.05
T0: control containing fat (20 %) + xanthan gum (0 %); TC: fat (10 %) + xanthan gum (0 %); T1 = fat (10 %) + xanthan gum (0.5 %); T2 = fat (10 %) + xanthan gum (1.0 %); T3 = fat (10 %) + xanthan gum (1.5 %)
Protein oxidation
Determination of the carbonyl groups by the DNPH assay showed that high fat control recorded significantly (p < 0.05) higher carbonyl group content than its low fat counterparts with or without xanthan gum (Table 5). The higher carbonyl content in high fat control might be due to the high lipid content (20 %) used in the formulation. The protein carbonylation is correlated to the occurrence of other biochemical changes such as the increase of lipid oxidation (Estévez and Cava 2004). According to Soyer et al. (2010) the primary and secondary lipid oxidation products can act as substrates for protein oxidation, so once the oxidation of lipids starts, the oxidation of proteins will also occur. Among the low fat formulations the carbonyl groups decreased with the increased concentration of xanthan gum (p < 0.05). The decrease in carbonyl content with increasing levels of xanthan gum concentration could be due to the antioxidant activity of xanthan gum. Xanthan gum suppresses the oxidation by chelation of iron between two side chains with a pyruvate residue because initiation of protein oxidation in meat proteins occurs due to heme and non-heme iron (Ganhão et al. 2010; Khouryieh et al. 2015; Kroger-Ohlsen et al. 2003).
The protein oxidation measurements by loss of sulphydryls of goshtaba formulations are presented in Table 5. The high fat control had significantly higher loss of sulphydryls (p < 0.05), which can be due to more reactive oxygen species generation due to high fat content (20 %), and proteins are targeted by reactive oxygen species. This interaction leads to the loss of sulphydryl groups from the proteins (Soyer and Hultin 2000). Loss of total sulphydryl groups decreased significantly with the increased levels of xanthan gum (0.5–1.5 %) and the goshtaba formulated with 1.5 % xanthan gum showed lower loss of sulphydryl groups. The decrease in loss of sulphydryl groups with the addition of xanthan gum can be due to the potential antioxidant activity of xanthan gum (Xiong et al. 2013).
Texture profile analysis
The texture attributes of the goshtaba formulations with or without xanthan gum is shown in Table 6. The hardness of control goshtaba with regular fat level (20 %) was significantly higher than low fat goshtaba formulations (p < 0.05). There was a significant decrease in hardness with increasing concentration of xanthan gum however, low fat product formulated with 0.5 % xanthan gum resulted higher hardness value than low fat control without xanthan gum. The decreased hardness of the low fat formulations may be due to the reduction in fat content and increasing water content while keeping protein level virtually constant (Pietrasik and Janz 2010). Similar results were obtained by Ulu (2006) indicated that meatballs made with the addition of carrageenan and guar gum had characteristics similar to those of the present study. No significant differences were observed in cohesiveness, gumminess and chewiness values between high fat control and the low fat formulations with or without xanthan gum (P > 0.05). The springiness increased with the decrease in fat content and increase in xanthan gum concentration (0.5–1.5 %) and was highest for the sample containing 1.5 % xanthan gum. The increase in springiness might be due to the gel network formed and increased water held by xanthan gum (Rongrong et al. 1998).
Table 6.
Texture profile analysis of low fat goshtaba formulated with varying levels of xanthan gum
| Treatment | Hard (Kg) | Cohe | Sprin | Gumm (Kg) | Chew (Kg mm) |
|---|---|---|---|---|---|
| T0 | 20.50 ± 0.90c | 0.41 ± 0.11a | 6.70 ± 0.04a | 8.04 ± 1.96a | 53.98 ± 1.50a |
| TC | 18.91 ± 1.01b | 0.39 ± 0.10a | 6.72 ± 0.13a | 7.45 ± 0.49a | 50.06 ± 2.34a |
| T1 | 19.46 ± 0.13bc | 0.39 ± 0.06a | 6.78 ± 0.29ab | 8.11 ± 0.34a | 55.09 ± 4.08a |
| T2 | 17.60 ± 0.32a | 0.32 ± 0.02a | 7.07 ± 0.11bc | 8.40 ± 0.29a | 59.41 ± 2.29a |
| T3 | 16.73 ± 0.64a | 0.32 ± 0.02a | 7.23 ± 0.15c | 8.60 ± 0.33a | 62.19 ± 2.54a |
All values are mean ± standard deviation of three replicates
Means in the same column with different superscripts differ significantly: *P < 0.05
T0: control containing fat (20 %) + xanthan gum (0 %); TC: fat (10 %) + xanthan gum (0 %); T1 = fat (10 %) + xanthan gum (0.5 %); T2 = fat (10 %) + xanthan gum (1.0 %); T3 = fat (10 %) + xanthan gum (1.5 %)
Microstructure
Scanning electron microscopy (SEM) provides high resolution imaging of fine surface morphology, and was used as a qualitative tool to distinguish differences in three dimensional structures (Fig. 1). The morphology of the high fat control sample (20 % fat) (Fig. 1a) showed the formation of numerous cavities, producing structures with a spongy (honeycomb like) appearance. The formation of these cavities may have been due to the expansion of a number of constituents, mainly fat, water or air (Salcedo-Sandoval et al. 2013). The scanning electron microscopy micrographs of the low fat control goshtaba (10 % fat) presented less spongy and more compact appearance than high fat control, so that the goshtaba was firmer than high fat control. The Fig. 1c, d and e shows that xanthan gum forms gel network and this gel network is formed by developing connections with existing proteins gel network. Such an evolution of microstructure can explain the change in the water holding capacity, textural and sensory parameters. As the concentration of gum increases (0.5–1.5 %) the gel network increased and this resulted increase in the water holding capacity of low fat goshtaba formulations (Ayadi et al. 2009). Significant increase in juiciness was observed at high added levels of xanthan gum (1.5 %) due to the high water holding capacity.
Fig. 1.
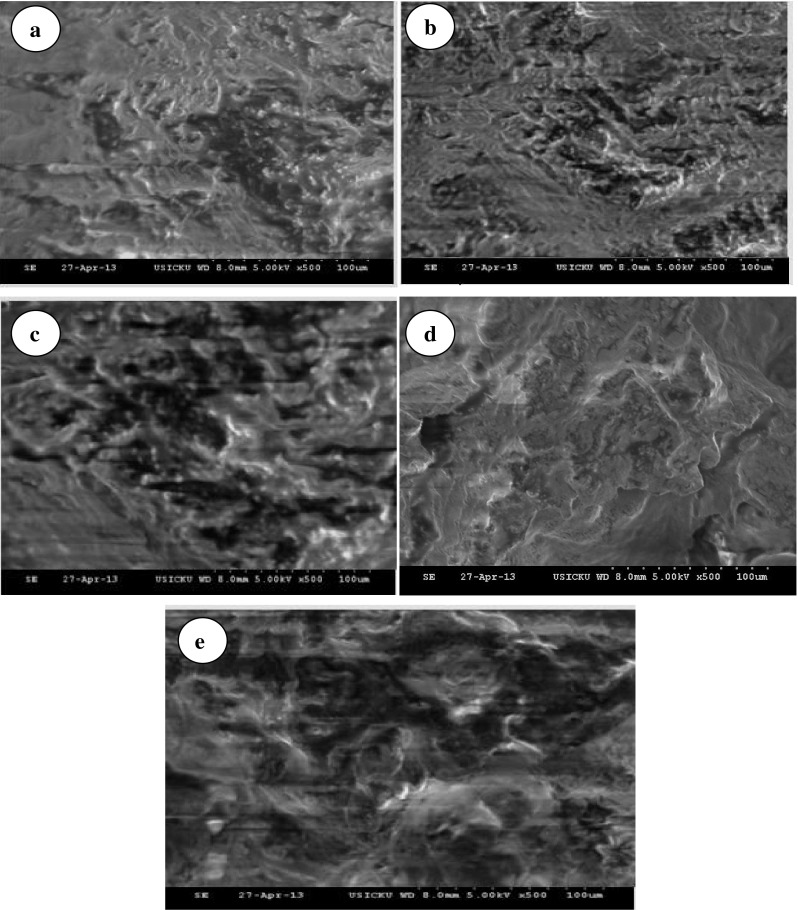
Scanning electron micrographs (SEM) of control and low fat Goshtaba formulated with varying xanthan gum concentrations: a T0: control containing fat (20 %) + xanthan gum (0 %); b TC: fat (10 %) + xanthan gum (0 %); c T1 = fat (10 %) + xanthan gum (0.5 %); d T2 = fat (10 %) + xanthan gum (1.0 %); e) T3 = fat (10 %) + xanthan gum (1.5 %)
Sensory evaluation
The effect of xanthan gum addition on the sensory properties of goshtaba formulations is shown in Table 7. The mean values of the firmness, flavor and juiciness were evaluated to determine overall acceptability. The firmness score decreased significantly with the increasing concentration of xanthan gum from 0.5 to 1.5 % (p < 0.05). No significant difference was recorded in the firmness score between control products (T0 and TC) and low fat product containing 0.5 % xanthan gum (p > 0.05). The lower firmness score of the low fat goshtaba containing 1.5 % xanthan gum may be related to the effects of both fat reduction and higher concentration of gum addition. The flavour intensity was significantly highest for the high fat control and increasing the concentration of xanthan gum from 0.5–1.5 % decreased the flavour scores of the low fat products (p < 0.05). There was no significant difference in the flavour intensity between the low fat control and the goshtaba containing 1.0 % xanthan gum. Similarly non-significant difference was observed in juiciness score between the high fat control and low fat formulations containing varying concentrations of xanthan gum (p > 0.05). This comparable juiciness score between control and low fat formulations could be attributed to higher moisture retention by xanthan gum (Piñero et al. 2008). The difference in the juiciness scores of the samples does not seem to be linked to the moisture level (Table 3); the level of water release in chewing may be a determinant factor for it (Ruiz-Capillas et al. 2012). Several studies have found similar results for low fat meat products being higher in juiciness (Desmond et al. 1998). The results indicate that better sensory score for overall acceptability was observed for high fat control (20 % fat) and low fat goshtaba containing 0.5 % xanthan gum.
Table 7.
Sensory analysis of low fat goshtaba formulated with varying levels of xanthan gum
| Treatment | Firmness | Flavour intensity | Juiciness | Overall acceptability |
|---|---|---|---|---|
| T0 | 6.9 ± 0.23c | 7.9 ± 0.50d | 7.0 ± 0.30b | 7.3 ± 0.08c |
| TC | 7.1 ± 0.10c | 6.3 ± 0.20b | 6.2 ± 0.30a | 6.3 ± 0.17b |
| T1 | 6.8 ± 0.25c | 7.5 ± 0.11c | 6.8 ± 0.11b | 7.0 ± 0.15c |
| T2 | 6.0 ± 0.11b | 6.1 ± 0.15b | 7.1 ± 0.30b | 6.4 ± 1.08b |
| T3 | 5.1 ± 0.28a | 4.8 ± 0.28a | 7.1 ± 0.11b | 5.6 ± 0.20a |
All values are mean ± standard deviation of three replicates
Means in the same column with different superscripts differ significantly: *P < 0.05
T0: control containing fat (20 %) + xanthan gum (0 %); TC: fat (10 %) + xanthan gum (0 %); T1 = fat (10 %) + xanthan gum (0.5 %); T2 = fat (10 %) + xanthan gum (1.0 %); T3 = fat (10 %) + xanthan gum (1.5 %)
Conclusion
It is concluded from the study that xanthan gum can be a suitable fat replacer in goshtaba as it does not result in any significant decline in quality and acceptability of the product as perceived by consumer. Low fat formulations containing xanthan gum had significantly lower TBARS values, protein carbonyls and loss of protein sulphydryl groups than the conventional high fat goshtaba (p < 0.05). That corresponds to potential antioxidant activity of xanthan gum to suppress oxidation of fats and proteins in meat products. In addition to this, xanthan gum retain more water in low fat formulations, as revealed by SEM. Replacing fat with 0.5 % xanthan gum did not show any significant difference in overall acceptability from that of high fat goshtaba containing 20 % fat. Thus the addition of xanthan gum could be a valuable alternative to improve the quality of low fat goshtaba.
Acknowledgments
The authors are thankful to Department of Biotechnology, Govt. of India, for their financial support.
References
- AHA (1986) Dietary guidelines for healthy adult Americans. American Heart Association, 74(Circulation) 1465–1475 [PubMed]
- Ako C. Fat replacers. Food Technol. 1998;52:47–53. [Google Scholar]
- AMSA . Guidelines for cookery and evaluation of meat. IL: American meat association Chicago; 1978. [Google Scholar]
- AOAC . Official Methods of Analysis of the Association of Official Analytical Chemist. 18th. Washington, DC: Horwitz William Publication; 1990. [Google Scholar]
- Ayadi MA, Kechaou A, Makni I, Attia H. Influence of carrageenan addition on turkey meat sausages properties. J Food Eng. 2009;93:278–283. doi: 10.1016/j.jfoodeng.2009.01.033. [DOI] [Google Scholar]
- Bourne MC. Texture profile analysis. Food Technol. 1978;33:62–66. [Google Scholar]
- Candogan K, Kolsarici N. Storage stability of low-fat beef frankfurters formulated with carrageenan or carrageenan with pectins. Meat Sci. 2003;64:207–214. doi: 10.1016/S0309-1740(02)00182-1. [DOI] [PubMed] [Google Scholar]
- Candogan K., Kolsarici N. The effects of carrageenan and pectin on some quality characteristics of low-fat beef frankfurters. Meat Sci. 2003;64(2):199–206. doi: 10.1016/S0309-1740(02)00181-X. [DOI] [PubMed] [Google Scholar]
- Choi YS, Lee MA, Jeong JY, Choi JH, Han DJ, Kim HY. Effects of wheat fiber on the quality of meat batter. J Korean Soc Food Sci Animal Res. 2007;27(1):22–28. doi: 10.5851/kosfa.2007.27.1.22. [DOI] [Google Scholar]
- Desmond E, Troy D, Buckley D. The effects of tapioca starch, oat fibre and whey protein on the physical and sensory properties of low-fat beef burgers. Lebensm Wiss Technol. 1998;31(7&8):653–657. doi: 10.1006/fstl.1998.0415. [DOI] [Google Scholar]
- Dzudie T, Scher J, Hardy J. Common bean flour as an extender in beef sausages. J Food Eng. 2002;52:143–147. doi: 10.1016/S0260-8774(01)00096-6. [DOI] [Google Scholar]
- Estévez M, Cava R. Lipid and protein oxidation, release of iron from heme molecule and colour deterioration during refrigerated storage of liver pâté. Meat Sci. 2004;68:551–558. doi: 10.1016/j.meatsci.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Ganhão R, Morcuende D, Estévez M. Protein oxidation in emulsified cooked burger patties with added fruit extracts: influence on colour and texture deterioration during chill storage. Meat Sci. 2010;85:402–409. doi: 10.1016/j.meatsci.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Ochoa F, Santos VE, Casas E, Gomez E. Xanthan gum: production, recovery, and properties. Biotechnol Adv. 2000;18:549–579. doi: 10.1016/S0734-9750(00)00050-1. [DOI] [PubMed] [Google Scholar]
- Glicksman M. Food hydrocolloids (Vol. 1) Boca Raton: CRC Press; 1982. [Google Scholar]
- Gök V, Akkaya L, Obuz E, Bulut S. Effect of ground poppy seed as a fat replacer on meat burgers. Meat Sci. 2011;89:400–404. doi: 10.1016/j.meatsci.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Hęś M, Waszkowiak K, Szymandera-Buszka K. The effect of iodine salts on lipid oxidation and changes in nutritive value of protein in stored processed meats. Meat Sci. 2012;92:139–143. doi: 10.1016/j.meatsci.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Huda AB, Parveen S, Rather SA, Akhter R, Hassan M. Effect of incorporation of apple pomace on the physico-chemical, sensory and textural properties of mutton nuggets. Int J Adv Res. 2014;2(4):974–983. [Google Scholar]
- IFT Guidelines for the preparation and review of papers reporting sensory evaluation data. J Food Sci. 1985;60:210–211. [Google Scholar]
- Jiménez-Colmenero F, Carballo J, Solas MT. The effect of use of freeze thawed pork on the properties of Bologna sausages with two fat levels. Int J Food Sci Technol. 1995;30:345–355. [Google Scholar]
- Keeton JT. Low-fat meat products—technological problems with processing. Meat Sci. 1994;36(1–2):261–276. doi: 10.1016/0309-1740(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Khouryieh H, Puli G, Williams K, Aramouni F. Effects of xanthan–locust bean gum mixtures on the physicochemical properties and oxidative stability of whey protein stabilised oil-in-water emulsions. Food Chem. 2015;167:340–348. doi: 10.1016/j.foodchem.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Kroger-Ohlsen MV, Ostdal H, Andersen ML. The effect of pH on the oxidation of bovin serum albumin by hypervalent myoglobin species. Arch Biochem Biophys. 2003;146:202–208. doi: 10.1016/S0003-9861(03)00317-5. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl groups in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-H. [DOI] [PubMed] [Google Scholar]
- Luruena-Martınez MA, Vivar-Quintana AMI. Revilla effect of locust bean/xanthan gum addition and replacement of pork fat with olive oil on the quality characteristics of low-fat frankfurters. Meat Sci. 2004;68:383–389. doi: 10.1016/j.meatsci.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Pappa IC, Bloukas J G, Arvanitoyannis IS. Optimization of salt, olive oil and pectin level for low-fat frankfurters produced by replacing pork backfat with olive oil. Meat Sci. 2000;56(1):81–88. doi: 10.1016/S0309-1740(00)00024-3. [DOI] [PubMed] [Google Scholar]
- Pettit DJ. Xanthan gum. In: Blanshard J. M. V., Mitchell J. R., editors. Polysaccharides in food. London: Butterworths; 1979. pp. 263–278. [Google Scholar]
- Pietrasik Z, Janz JAM. Utilization of pea flour, starch-rich and fiber-rich fractions in low fat bologna. Food Res Int. 2010;43:602–608. doi: 10.1016/j.foodres.2009.07.017. [DOI] [Google Scholar]
- Piñero MP, Parra K, Huerta-Leidenz N, Arenas de Moreno L, Ferrer M, Araujo S, Barboza Y. Effect of oat’s soluble fibre (β-glucan) as a fat replacer on physical, chemical, microbiological and sensory properties of low-fat beef patties. Meat Sci. 2008;80:675–680. doi: 10.1016/j.meatsci.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Rather SA, Akhter R, Masoodi FA, Gani A, Wani SM (2015) Utilization of apple pomace powder as a fat replacer in goshtaba: a traditional meat product of Jammu and Kashmir, India. J Food Meas Char. doi:10.1007/s11694-015-9247-2 [DOI] [PMC free article] [PubMed]
- Rongrong L, Carpenter JA, Cheney R. Sensory and instrumental properties of smoked sausage made with technically separated poultry (MSP) meat and wheat protein. J Food Sci. 1998;63:923–929. doi: 10.1111/j.1365-2621.1998.tb17928.x. [DOI] [Google Scholar]
- Ruiz-Capillas C, Triki M, Herrero AM, Rodriguez-Salas L, Jiménez-Colmenero F. Konjac gel as pork backfat replacer in dry fermented sausages: processing and quality characteristics. Meat Sci. 2012;92:144–150. doi: 10.1016/j.meatsci.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Salcedo-Sandoval L, Cofrades S, Ruiz-Capillas PC, Solas MT, Jiménez-Colmenero F. Healthier oils stabilized in konjac matrix as fat replacers in n − 3 PUFA enriched frankfurters. Meat Sci. 2013;93:757–766. doi: 10.1016/j.meatsci.2012.11.038. [DOI] [PubMed] [Google Scholar]
- Samoon AH, Sharma N. Studies on processing and preservation of goshtaba. J Food Sci Technol. 1991;28(4):212–215. [Google Scholar]
- Serrano A, Cofrades S, Jiménez-Colmenero F. Characteristics of restructured beef steak with different proportions of walnut during frozen storage. Meat Sci. 2006;72(1):108–115. doi: 10.1016/j.meatsci.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Sharma BR, Naresh L, Dhuldhoya NC, Merchant SU, Merchant UC. Xanthan gum – A boon to food industry. Food Prom Chro1. 2006;5:27–30. [Google Scholar]
- Soyer A, Hultin HO (2000) Kinetics of oxidation of the lipids and proteins of cod sarcoplasmic reticulum. J Agri Food Chem 48:2127–2134 [DOI] [PubMed]
- Soyer A, Ozalp B, Dalmıs U, Bilgin V (2010) Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem 120:1025–1030
- Srinivasan S, Hultin HO. Hydroxyl radical modification of fish muscle proteins. J Food Biochem. 1995;18:405–425. doi: 10.1111/j.1745-4514.1994.tb00513.x. [DOI] [Google Scholar]
- Srinivasan S, Hultin HO. Chemical, physical and functional properties of cod proteins modified by a nonenzymic free-radical-generating system. J Agric Food Chem. 1997;45:310–320. doi: 10.1021/jf960367g. [DOI] [Google Scholar]
- Sun C, Sundaram G, Mark RP. Effect of xantham gum on physiochemical properties of whey protein isolate stabilized oil-in-water emulsion. Food Hydrocoll. 2007;21:555–564. doi: 10.1016/j.foodhyd.2006.06.003. [DOI] [Google Scholar]
- Trommer H, Reinhard H. The examination of polysaccharides as potential antioxidative compounds for topical administration using a lipid model system. Int J Pharm. 2005;298:153–163. doi: 10.1016/j.ijpharm.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Ulu H. Effects of carrageenan and guar gum on the cooking and textual properties of low fat meatballs. Food Chem. 2006;95:600–605. doi: 10.1016/j.foodchem.2005.01.039. [DOI] [Google Scholar]
- WHO (2003) Diet, nutrition and the prevention of chronic diseases. Report of a joint WHO/FAO expert consultation. WHO Technical Report Series, vol. 916, Geneva [PubMed]
- Xiong X., Li M, Xie J, Jin Q, Xue B, Sun T. Antioxidant activity of xanthan oligosaccharides prepared by different degradation methods. Carbohydr Polym. 2013;92:1166–1171. doi: 10.1016/j.carbpol.2012.10.069. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Kook SH, Park SY, Shim J H, Chin KB. Physicochemical characteristics, extural properties and volatile compounds in comminuted sausages as affected by various fat levels and fat replacers. Int J Food Sci Technol. 2007;42:1114–1122. doi: 10.1111/j.1365-2621.2006.01402.x. [DOI] [Google Scholar]


