Abstract
Wines can be modified by microorganisms during the ageing process, by producing off-flavours like volatile phenols (VP), leading to their deterioration, with great economic losses. The development of methods to recover wines affected by unwanted VP became an important target. Molecular imprinted polymers (MIPs) are synthetic materials with artificially-generated recognition sites for selective extraction of organic compounds from different matrices. In this work, two MIPs to remove unwanted VP from wines were developed and their effects were evaluated. Volatile compounds were determined by GC-FID and GC-IT/MS and phenolic compounds (non-coloured and anthocyanins) by HPLC-DAD. The treatment with MIP-4EG and MIP-4EP significantly reduced the content of 4-ethylguaiacol and 4-ethylphenol, respectively. Nevertheless, the changes observed in wine non-coloured and coloured phenolics and sensorial analysis indicate that their specificity and selectivity regarding off-flavours still needs to be improved.
Keywords: Wine, Molecular imprinted polymers, Volatile phenols, Phenolic compounds
Introduction
Aroma is one of the most important parameters in wine quality and, hence, for consumer’s acceptance. The complex sensory quality of wine is determined by different compounds, partly derived from grapes and others resulting from yeasts. Under certain conditions, yeasts can spoil wine by producing off-flavours, turbidity, sediment, acidity and by forming biofilms. The unpleasant odours may appear in wine at different stages; however, organoleptic alterations usually occur during ageing, prior to bottling, especially when wines are kept in old barrels (Silva et al. 2005; Valentão et al. 2007).
Contaminating yeasts of the genus Dekkera/Brettanomyces, particularly D. bruxellensis, are able to produce large amounts of unwanted volatile phenols (VP) [4-vinylphenol (4VP), 4-vinylguaiacol (4VG), 4-ethylphenol (4EP) and 4-ethylguaiacol (4EG)], leading to wine deterioration, higher amounts of vinyl phenols being found in white wines and ethylphenols in the red ones (Chatonnet et al. 1997). Their presence in red wine at high concentrations (up to 400 μg/L) is associated with unpleasant aromas, often described as “phenolic”, “leather”, “horse sweat”, “stable” or “varnish” (Dias et al. 2003). Minimal quantities may also be due to various species of Lactobacillus (Chatonnet et al. 1995; Couto et al. 2006) and Pichia guillermondii (Barata et al. 2006).
The origin of VP involves the sequential action of enzymes on hydroxycinnamic acids (ferulic, p-coumaric or caffeic acids), which are naturally found in grape musts (Suárez et al. 2007). The link between Dekkera spp. and the appearance of the “phenolic smell” in red wines renewed the interest in studying these compounds. Appropriate knowledge on wine-making methods can efficiently prevent contamination by Dekkera spp. However, some modern tendencies to reduce the use of sulphur dioxide and the trend for red wines with a higher pH, along with the increasing use of old barrels, led to the presence of the undesirable “Brett character” in wines produced in several countries, causing significant economic damage (Garde-Cerdán et al. 2008).
Several procedures have been proposed for reducing 4EP and 4EG contents in wine, such as reverse osmosis and adsorption (Ugarte et al. 2005), sorption on yeast lees and cell walls (Chassagne et al. 2005; Pradelles et al. 2008; Pradelles et al. 2009), use of esterified cellulose (Larcher et al. 2012) or application of remedial treatments [activated charcoal, polyvinylpolypyrrolidone (PVPP), zeolite and bentonite] (Lisanti et al. 2013). All of these methods present limitations like price, lack of “curative” efficiency and difficulty of industrial application. For these reasons, the development of new methods for the easy and cheap recovery of wines affected by the off-flavours resulting from the presence of VP became an important target.
Molecular imprinted polymers (MIPs) have revealed ability for liquid–liquid extraction, solid-phase extraction and clean-up of complex matrices, such as biological, environmental and food samples, which require highly selective extraction techniques (Molinelli et al. 2002). MIPs are used in food and agricultural sectors as they constitute a fast, reliable, robust, and cost-effective method, being imprinted for food additives, food components and contaminants, like herbicides, pesticides and trace metals (Molinelli et al. 2002).
MIPs are synthetic polymers with recognition sites, able to specifically bind to a target molecule (template). In general, they are obtained by mixing the template with the complementary functional monomers and cross linkers in a suitable solvent. After polymerization, the template must be extracted from the synthesized polymer (Andersson 2000). The main advantage of MIPs is their high selectivity and affinity for the target molecule used in the imprinting procedure. They have high physical robustness, strength, resistance to elevated temperature and pressure, and inertness towards acids, bases, metal ions and organic solvents. In addition, their synthesis is inexpensive and they have a long storage life, keeping their recognition capacity for several years at room temperature (Vasapollo et al. 2011).
In this work we developed MIPs to remove 4EP and 4EG from red wines, since, as above mentioned, ethylphenols are found in higher amounts in these wines. The effects of their application to aged red wines were evaluated. Following these purposes, volatiles were determined by GC-IT/MS and GC/FID, and phenolic compounds (non-coloured and anthocyaninns) by HPLC-DAD. This methodology may contribute to the recovery of spoiled wines, allowing their valorisation, promoting their consumption, thus resulting in an economic benefit to the wine industry.
Materials and methods
Standards and reagents
All chemicals used were of analytical grade. The standard compounds were purchased from various suppliers: β-linalool, nerol, nerolidol, eugenol, isoamyl acetate, ethyl hexanoate, phenylethyl alcohol, 4EG, 4EP, trans-caffeoyltartaric acid (t-CAFTA), vanillic acid, caffeic acid, syringic acid, ferulic acid, p-coumaric acid, cinnamic acid, hexanoic acid, octanoic acid, decanoic acid and palmitic acid were from Sigma-Aldrich (St. Louis, MO, USA). Malvidin-3,5-O-diglucoside, cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, peonidin-3-O-glucoside, malvidin-3-O-glucoside, delphinidin, petunidin, pelargonidin, malvidin, catechin, epicatechin, epigallocatechin-3-O-gallate, epicatechin-3-O-gallate, myricetin, isorhamnetin-3-O-glucoside and quercetin were from Extrasynthèse (Genay, France). Ethyl butanoate, hexyl acetate, diethyl succinate and chloroform were from Merck (Darmstadt, Germany), (Merck). A hydrocarbon mixture C6–C20 was obtained from Fluka (Buchs, Switzerland). 1-Heptanol, 1-octanol and (E)-2-octenal were from SAFC (St. Louis, USA). Methanol and acetonitrile were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetic acid was supplied by Prolabo (Fontenay-Sous Bois, France). Water was deionized using a Milli-Q water purification system (Millipore, Bedford, MA).
For the synthesis of MIPs 2,20-azobis-(2-methylpropionitrile) (AIBN) and ethylene glycol dimethacrylate (EDMA) from Fluka, Sigma-Aldrich (Steinheim, Germany) and 4-vinylpyridine from Aldrich (St. Louis, USA) were used.
Synthesis of MIPs
MIPs were prepared by non-covalent approach, using either 4EP or 4EG as template, following a procedure adapted from previous references (Garde-Cerdán et al. 2008; Castro-López et al. 2012). 1 mmol of the template was mixed with the monomer (4-vinylpyridine, 4 mmol), crosslinker (EDMA, 20 mmol) and azo initiator (AIBN, 0.5 mmol) in 40 mL of porogen. The employed porogen was 40 mL of an acetonitrile:chloroform (3:1) solution, degassed under nitrogen atmosphere during, at least, 15 min. The mixture was sonicated for 10 min, degassed under nitrogen atmosphere for 5 min and sealed under vacuum. Polymerization was carried out in a water bath at 60 °C for 24 h. MIPs were then dried until constant weight and the template was removed by using methanol:acetic acid (4:1).
The corresponding non-imprinted polymers (NIPs) were also prepared using the same experimental conditions, but in the absence of the template.
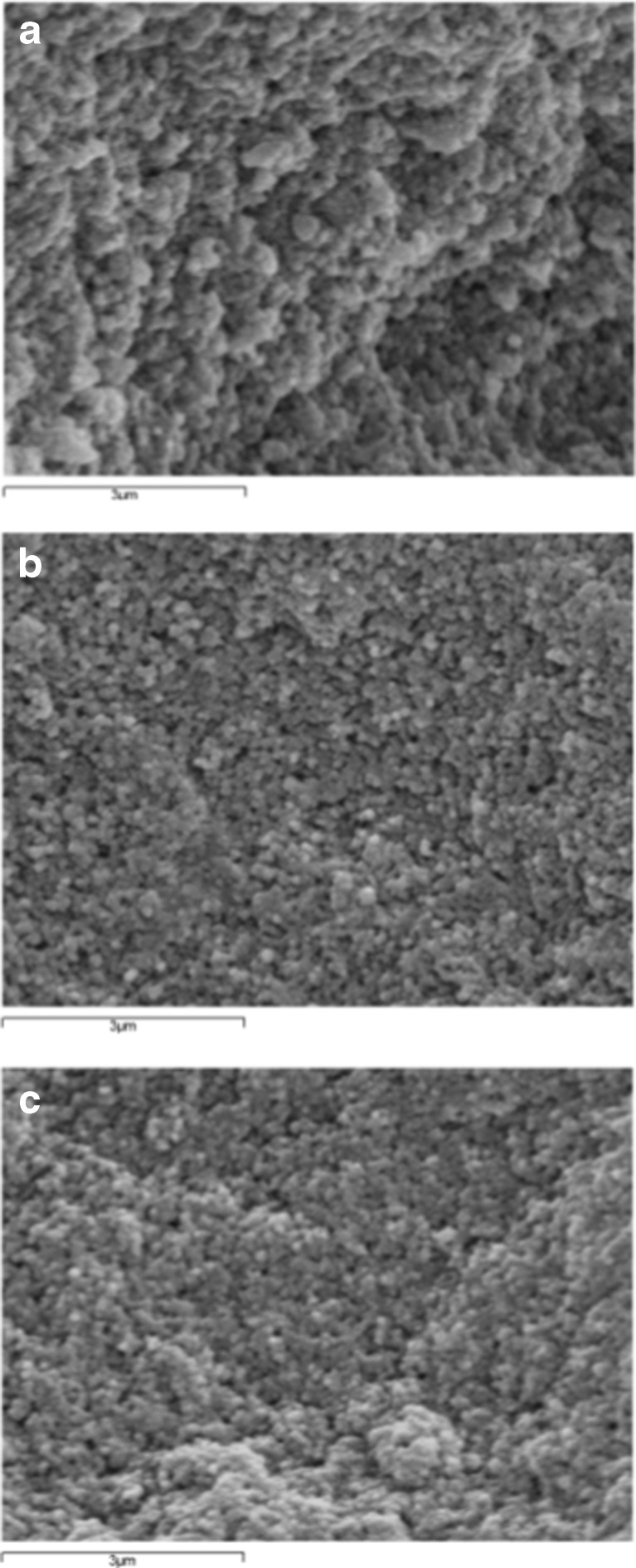
The surface morphology of MIPs and NIPs was characterized by scanning electron microscopy (SEM), using a JEOL JSM-6400 apparatus (Michigan, USA). The samples were sputter-coated with gold prior to the observation (Fig. 1).
Fig. 1.
SEM images of a NIP, b MIP-4EG and c MIP-4EP; magnification 15000×
Rebinding study
A standard solution of 4EP and 4EG in synthetic wine was used. Synthetic wine consisted on an aqueous solution of ethanol 15 % (v/v) and tartaric acid 3 g/L at pH 3. For binding evaluation of MIPs two procedures were carried out based on previous studies: rebinding study by solid phase extraction (SPE) (Castro-López et al. 2012) and batch rebinding method (Cela-Pérez et al. 2011).
For SPE procedure, 200 mg of dried polymers were packed into 6 mL polypropylene SPE cartridges (Supelco, Bellefonte, PA, USA) and capped with fritted polytetrafluoroethylene (PTFE) disks at the bottom and the top of the cartridges. The outlet tips were connected to a vacuum pump (VISIPREP Solid Phase Extraction Vacuum Manifold, Sigma-Aldrich Co., St. Louis, MO, USA). The volume of the samples corresponded to 25 or 100 mL. Each assay was performed twice.
For batch rebinding dried polymer and sample were placed together into 5 mL vessels in the ratio 24 mg/3 mL or 200 mg/25 mL, at room temperature; incubation time was 4 h, the first one under stirring (100 rpm). Each assay was performed twice.
Wine samples preparation
The samples consisted on commercial red wine produced in a winery (Sogrape, Portugal) from Alicante Bouschet (84 %) and Aragonês (16 %) grapes growing in the Alentejo region (Portugal) and collected in September 2011. The wine sample used had the following characteristics: pH =3.69, volatile acidity =0.68 g/L, reducer sugars =2.8 g/L, total acidity =3.4 g/L, SO2 free =22.0 mg/L. Wine (10 L) was removed from an oak barrel, filtered through a 0.45 μm membrane filter (Millipore, USA) and divided by four glass flasks (500 mL each); this process was repeated three times independently. Sample control did not receive any treatment. The three aliquots were extracted and analysed separately.
Wine samples were subjected to a clean-up step by using MIPs as batch rebinding method; the ratio 100 mL of sample/200 mg of polymer was kept throughout the assays. The percentage of each compound retained by the polymer was calculated by difference from the initial concentration and expressed as % of depletion.
Sensorial analysis
A panel composed of six people (professors, laboratory personnel and winemakers) was engaged in sensorial evaluation of control and treated samples, concerning colour, odour and flavour. The samples were ordered from 1 to 4, being 1 = less intense and 4 = more intense. A constant volume of 30 mL of each wine was evaluated in wine-taster glasses at 12 °C in accordance with ISO 3591, in an adequate room (without sensory odours). Control proved to be the sample with greater intensity of colour, odour and flavour (4 ± 0.0 for all parameters). The colour of NIP, MIP-4EG and MIP-4EP was 2.5 ± 0.83, 2.0 ± 0.89 and 1.67 ± 0.82, respectively. MIP-4EP showed the best results concerning odour and flavour (1.33 ± 0.51 and 1.5 ± 0.55, respectively), followed by MIP-4EG (2.00 ± 0.89 and 1.66 ± 0.81, respectively) and NIP (2.66 ± 0.51 and 2.83 ± 0.41, respectively). These results revealed the importance of MIPs in the depletion of volatile phenols and off-flavours.
Volatile phenols
Compounds were removed according to a described procedure (Valentão et al. 2007): 50 mL of a mixture containing the wine sample and 50 μL of 4-decanol (1 g/L in 60 % ethanol, internal standard) were extracted with 4 ml of ethyl ether:n-hexane (1:1) for 5 min, followed by extraction with 2 ml of the same solution (twice). The volatile fractions were gathered and 2 μL were analysed by GC-FID.
The analysis was carried out according to a previous method (Valentão et al. 2007). A Focus GC Thermo Finnigan (Milan, Italy), equipped with a flame ionisation detector (FID) and a CPWax 57 (WCOT Fused Silica; 25 m × 0.25 mm × 0.20 μm) column was used. The injection port was a split–splitless one, working at 200 °C, in splitless mode for 0.5 min and split ratio 30:1. The carrier gas was hydrogen, at a flow rate of 2.8 mL/min. The oven temperature was 40 °C (5 min), then increasing 3 °C/min to 200 °C (20 min), and the detector temperature was set at 250 °C. The detector signals were recorded and processed by Chrom-Card for Windows software (Fisions, USA). The compounds were identified by comparing their retention times with those from standards. Quantification was achieved by the external standard method.
Calibrations were carried out for each compound from a stock solution (100 mg/L), by dilution in synthetic wine to concentrations between 0.25 and 20 mg/L. These solutions were then subjected to the above described process, with the prior addition of the internal standard. The correlation coefficient for the standard curves is 0.9998 for both studied compounds. The regression equations for 4EP and 4EG are y = 148323×-6263.5 and y = 110321×-429.21, respectively. The limit of detection (LOD) and limit of quantification (LOQ) were lower than their known olfactory thresholds in red wines (110 and 605 μg/L for 4EG and 4EP, respectively) (Chatonnet et al. 1992). The LODs were 15 and 25 μg.L−1, and the LOQs were 32 and 84 μg.L−1 for 4EP and 4EG, respectively.
Other volatile compounds
The quantification of the other volatile compounds was performed according to Barros et al. (2012) by HS-SPME-GC-IT/MS.
The HS-SPME procedures were performed using a Combi-PAL autosampler (Varian Pal Autosampler, Switzerland) and the Cycle Composer software (CTC Analytics System Software, Switzerland). Wine samples (5 mL) were stirred (250 rpm) with 0.5 g of NaCl for 20 min, at 45 °C. Afterwards, a DVB/CAR/PDMS fibre (Supelco, Bellefonte, PA, USA) was pulled into the needle sheath and the SPME device was removed from the vial and inserted into the injection port of the GC system for thermal desorption, for 2 min.
GC-IT/MS analyses were performed on a Varian CP-3800 gas chromatographer (USA) equipped with a Varian Saturn 4000 ion trap mass detector (USA) and a Saturn GC-IT/MS workstation software version 6.8. Chromatographic separation was achieved using a capillary column VF−5 ms (30 m × 0.25 mm × 0.25 μm) from Varian and high purity helium C-60 (Gasin, Portugal) as carrier gas, at a constant flow of 1.0 mL/min, in splitless mode. The initial oven temperature of 40 °C was held for 1 min, then increasing 5 °C/min to 250 °C (5 min), followed by increase of 5 °C/min to 300 °C (0 min). The ion trap detector was set as follows: the transfer line, manifold and trap temperatures were 280, 50 and 180 °C, respectively. All mass spectra were acquired by electron impact (EI). The mass range was 35–600 m/z, with a scan rate of 6 scan/s. The emission current was 50 μA and the electron multiplier was set in relative mode to auto-tune procedure. The maximum ionization time was 25,000 μs, with an ionization storage level of 35 m/z. Analysis was performed in full scan mode.
Non-coloured phenolic compounds
Extracts’ preparation was adapted from Silva et al. (2005). Wine samples (20 mL) were extracted three times with 30 mL of diethyl ether for 5 min, with agitation. The ether fraction was then separated and concentrated to dryness using a rotary evaporator. The residue obtained was redissolved in 1 mL of methanol.
The phenolic compounds were analysed on an analytical HPLC unit (Gilson), with an ODS-Hypersil reversed phase column (20 × 0.21 cm, 5 μm particle size), using a previously described procedure (Silva et al. 2005). Elution solvents were water:formic acid (19:1) (A) and methanol (B), at a flow rate of 0.3 mL/min. A linear gradient starting at 2 % B, increasing to 62 % B after 60 min, was employed and detection was achieved with a Gilson DAD. The compounds in each sample were identified by comparing their retention times and UV–Vis spectra in the range of 200–400 nm and chromatograms were recorded at 280, 320 and 350 nm. Data were processed on Unipoint system Software (Gilson Medical Electronics, Villiers le Bel, France). Peak purity was checked by the software contrast facilities. Phenolic compounds quantification was achieved by the absorbance recorded in the chromatograms relative to external standards. Trans-p-Coumaroyltartaric acid (t-COUTA) was quantified as p-coumaric acid. Quantification of hydroxybenzoic acids and catechin derivatives was performed at 280 nm, hydroxycinnamic acids at 320 nm and flavonols at 350 nm.
Anthocyanins
Sample preparation consisted on the acidification of 5 ml of wine with 20 μL HCl. 20 μL of the acidified samples were analysed by HPLC-DAD.
The extracts were analysed on an analytical HPLC unit (Gilson), using a Spherisorb ODS2 (25.0 × 0.46 cm; 5 μm, particle size) column (Valentão et al. 2007). The solvent system used was a gradient of water:formic acid (19:1) (A) and methanol (B), starting with 5 % methanol and installing a gradient to obtain 15 % B at 3 min, 25 % B at 13 min, 30 % B at 25 min, 35 % B at 35 min, 45 % B at 39 min, 45 % B at 42 min, 50 % B at 44 min, 55 % B at 47 min, 70 % B at 50 min, 75 % B at 56 min and 100 % B at 60 min, at a solvent flow rate of 0.9 ml/min. Detection was achieved with a Gilson DAD. Spectral data from all peaks were accumulated in the range of 200–600 nm. The compounds in each sample were identified by comparing their retention times and UV–Vis spectra in the 200–600 nm range with the library of spectra previously compiled by the authors. Anthocyanin quantification was achieved by the absorbance recorded in the chromatograms at 500 nm, relative to external standards.
Calibrations were carried out for each compound from a stock solution (100 mg/L), by dilution in methanol acidified with HCl. The correlation coefficient for the standard curves invariably exceeded 0.99 for all studied compounds.
The repeatability and reproducibility of the chromatographic method was evaluated by measuring the peak chromatographic area of each compound six times on the same standard solution in the same day and in different days, respectively. The chromatographic methods are precise: the repeatability study showed relative standard deviation (R.S.D.) less than 5 %. The reproducibility study showed the R.S.D. lower than 10 % for anthocyanins.
Statistical analysis
Data were analysed by using Graph Pad Prism Version 5.00, Inc. (San Diego, CA) using the t-test (Wilcoxon matched pairs test). The significance was calculated for p < 0.05.
Results and discussion
Rebinding study
Before using the MIPs to clean up wine samples, a preliminary rebinding study was carried out to test their performance to retain unwanted volatile compounds. With this purpose the adsorption of 4EP and 4EG from standard solutions prepared in synthetic wine was investigated. Two variables, namely the ratio amount of MIP/volume of solution, and the conditions of contact between the MIP and the sample (SPE or batch rebinding) were checked.
The influence of the ratio amount of MIP/volume of solution on the retention of the unwanted VP was tested by SPE; sample volumes of 25 and 100 mL of synthetic wine were used to study the retention of 0.25 mg of unwanted VP by 200 mg of MIPs. The increase of volume sample caused a reduction of the depletion from the range 51–60 % obtained for 25 mL to the 29–59 % achieved for the samples of 100 mL, so that the ratio 200 mg of MIPs/25 mL of sample was selected for further assays.
SPE and batch rebinding methods were compared. For batch rebinding method the MIP was put into contact with the sample under magnetic stirring. Batch rebinding led to enhanced retention of the VP by the MIP. The best results (62–74 % retention) were achieved with 2 h of contact time under magnetic stirring.
MIP-4EP and MIP-4EG were then applied to the clean-up of wine samples. The morphology of the synthetized MIPs and NIP was studied by SEM. The scanning electron micrographs revealed particles with spherical shapes. Homogeneous size distribution with diameter values in the range 100–300 nm was observed (Fig. 1).
Sensorial analysis
The treated samples showed a loss of colour compared with the untreated sample, the one treated with NIP displaying the minor colorant intensity. Concerning odour and flavour, a beneficial effect was observed in treated samples, the MIP-4EP-treated being selected as the preferred of the panel, mainly due to the lowest intensity in volatile phenols off-flavours.
Volatile compounds
In this work, 40 volatile compounds were identified by HS-SPME/GC-IT/MS in red wine sample, being distributed by distinct chemical classes. A similar qualitative profile was observed after the clean-up procedure using the developed MIPs, but quantitative differences were found (Tables 1 and 2).
Table 1.
Unwanted volatile phenols (VP) of red wine sample and depletion caused by MIP-4EP, MIP-4EG and NIP treatment1
| VP | Samples | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | MIP-4EP | MIP-4EG | NIP | |||||
| Treated | Depletion (%) | Treated | Depletion (%) | Treated | Depletion (%) | |||
| 24 | 4-EP | 1658.9 ± 55.06a | 737.0 ± 41.04b | 55.6 ± 1.64 | 785.0 ± 116.46b | 55.1 ± 7.19 | 887.0 ± 75.50b | 46.4 ± 6.31 |
| 29 | 4-EG | 149.4 ± 0.94a | 68.3 ± 0.77b | 54.3 ± 0.59 | 89.2 ± 0.86b | 40.3 ± 0.89 | 85.1 ± 1.77b | 43.0 ± 1.00 |
| ∑ | 1808.3 | 805.3 | 873.2 | 855.0 | ||||
1Values are expressed as mean ± standard deviation of three assays; ∑ sum of the determined compounds in the same line, different superscript letters represent significant differences compared with the respective control (p < 0.05). 4-EP, 4-ethylphenol; 4-EG, 4-ethylguayacol
Table 2.
Volatile compounds of red wine samples treated with MIP-4EP, MIP-4EG and NIP
| Compounds | RI | RI1 | ID2 | QI3 | A4/1000 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | MIP-4EP | MIP-4EG | NIP | |||||||||||
| Esters | ||||||||||||||
| 1 | Ethyl isobutyrate | 741 | 751 | MS (73/79) | 43/71/161 | 146.3(4.11)a | 59.0(0.76)b | 85.5(0.30)b | 45.6(0.33)b | |||||
| 2 | Isobutyl acetate | 757 | 763 | MS (76/81) | 43/56/73/101 | 153.4(1.57)a | 39.6(0.19)b | 65.2(0.16)b | 56.4(0.49)b | |||||
| 3 | Ethyl butanoate | 784 | 778 | S,MS (85/87) | 43/71/88/116 | 494.6(45.59)a | 172.1(1.19)b | 194.6(15.91)b | 205.4(3.17)b | |||||
| 4 | Methyl butyrate | 837 | 820 | MS (79/80) | 57/102 | 33.7(0.72)a | 9.5(0.05)b | 10.1(0.12)b | 7.8(0.03)b | |||||
| 5 | Ethyl isovalerate | 842 | 854 | MS (78/80) | 55/88 | 45.9(0.78)a | 5.3(0.04)b | 7.5(0.12)b | 6.1(0.02)b | |||||
| 7 | Isoamyl acetate | 865 | 876 | S,MS (84/85) | 43/70 | 1644.0(37.51)a | 568.9(13.90)b | 625.1(13.92)b | 730.7(49.77)b | |||||
| 11 | Ethyl hexanoate | 982 | 998 | S,MS (83/84) | 43/88 | 598.4(6.28)a | 297.6(0.29)b | 303.2(19.05)b | 419.2(0.83)b | |||||
| 12 | Hexyl acetate | 994 | 1007 | S,MS (74/80) | 43/55/56 | 38.3(1.67)a | 10.7(0.19)b | 11.7(0.37)b | 11.7(0.22)b | |||||
| 15 | Ethyl heptanoate | 1030 | 1043 | MS (76/79) | 97/55/56 | 29.6(0.34)a | 6.7(0.02)b | 7.4(0.09)b | 10.9(0.02)b | |||||
| 16 | Ethyl-2-furoate | 1039 | 1047 | MS (77/87) | 95/112/140 | 24.4(0.20)a | 9.5(0.50)b | 20.7(0.09)b | 24.2(0.10)a | |||||
| 17 | Isoamyl lactate | 1054 | 1047 | MS (74/82) | 43/45/70 | 101.6(0.87)a | 116.5(0.79)b | 138.1(1.12)b | 159.6(0.57)b | |||||
| 19 | Ethyl heptanoate | 1081 | 1096 | MS (70/84) | 88/113 | 39.3(1.28)a | 2.6(0.00)b | 2.6(0.01)b | 4.3(0.04)b | |||||
| 22 | Methyl octanoate | 1105 | 1128 | MS (82/87) | 74,787/158 | 123.2(3.52)a | 5.6(0.02)b | 7.3(0.01)b | 10.5(0.02)b | |||||
| 23 | Linalyl butyrate | 1149 | 1168 | MS (72/75) | 93/121 | 21.4(1.15)a | 3.1(0.07)b | 3.7(0.08)b | 4.5(0.05)b | |||||
| 26 | Isopentyl hexanoate | 1202 | 1244 | MS (76/79) | 43/70/99 | 76.7(1.86)a | 4.0(0.03)b | 6.5(0.05)b | 4.5(0.07)b | |||||
| 28 | Phenylethyl acetate | 1226 | 1255 | MS (87/90) | 43/104 | 433.6(20.26)a | 192.8(12.59)b | 194.3(3.99)b | 246.0(4.23)b | |||||
| 30 | Methyl decanoate | 1269 | 1300 | MS (70/83) | 74/87 | 104.1(3.22)a | 11.5(0.30)b | 10.9(0.17)b | 14.6(0.22)b | |||||
| 34 | Ethyl trans-4-decenoate | 1358 | 1296 | MS (80/80) | 88/110/69 | 278.4(1.51)a | 16.4(0.32)b | 17.3(0.16)b | 20.7(0.03)b | |||||
| 35 | Ethyl decanoate | 1367 | 1394 | MS (77/78) | 88/157 | 1535.7(64.17)a | 178.6(8.21)b | 242.1(24.98)b | 220.7(16.21)b | |||||
| 37 | Ethyl dodecanoate | 1567 | 1592 | MS (77/77) | 88/101/ | 546.9(54.64)a | 97.7(0.13)b | 113.6(2.71)b | 90.2(0.11)b | |||||
| 38 | Ethyl tetradecanoate | 1762 | 1792 | MS (80/82) | 88/101 | 27.2(7.77)a | 172.7(7.84)b | 258.4(29.69)b | 236.8(19.81)b | |||||
| 40 | Ethyl hexadecanoate | 1957 | 1990 | MS (80/80) | 88/157/384 | 50.8(11.03)a | 454.7(27.19)b | 515.3(0.53)b | 573.9(5.04)b | |||||
| ∑ | 6547.1 | 2435.3 | 2841.1 | 3104.3 | ||||||||||
| Depletion (%) | 62.8 | 56.6 | 52.6 | |||||||||||
| Alcohols | ||||||||||||||
| 6 | 1-Hexanol | 858 | 865 | MS (78/80) | 56/69 | 478.4(12.29)a | 425.0(3.87)b | 442.3(18.81)b | 455.0(3.41)b | |||||
| 9 | 1-Heptanol | 957 | 962 | S,MS (85/87) | 41/56/70/116 | 162.2(3.48)a | 112.3(0.27)b | 110.9(0.60)b | 137.6(0.22)b | |||||
| 14 | Benzyl alcohol | 1023 | 1031 | MS (86/89) | 79/108 | 142.3(13.72)a | 91.5(7.30)b | 125.3(2.62)a | 143.0(4.39)a | |||||
| 18 | 1-Octanol | 1057 | 1068 | S,MS (82/85) | 41/84 | 226.0(6.57)a | 70.9(0.42)b | 69.0(0.41)b | 88.7(0.38)b | |||||
| 21 | Phenylethyl alcohol | 1097 | 1111 | S,MS (90/92) | 91/122 | 8194.7(751.12)a | 8199.7(716.05)a | 7787.7(191.45)a | 8666.5(311.83)a | |||||
| ∑ | 9203.6 | 8899.3 | 8535.3 | 9490.9 | ||||||||||
| Depletion (%) | 3.3 | 7.3 | – | |||||||||||
| Sulphur Compounds | ||||||||||||||
| 8 | 2-Furfurylthiol | 885 | 905 | MS (76/85) | 53/81 | 63.7(1.38)a | 49.4(0.40)b | 52.1(2.34)b | 57.6(3.78)b | |||||
| ∑ | 63.7 | 49.4 | 52.1 | 57.6 | ||||||||||
| Depletion (%) | 22.4 | 18.2 | 9.6 | |||||||||||
| Acids | ||||||||||||||
| 10 | Hexanoic acid | 972 | 984 | S,MS (75/81) | 60/73 | 473.3(7.30)a | 261.8(1.10)b | 290.4(1.07)b | 294.5(3.83)b | |||||
| 25 | Octanoic acid | 1171 | 1201 | S,MS (76/80) | 60/73 | 576.3(2.66)a | 247.5(2.06)b | 215.2(0.57)b | 142.6(0.40)b | |||||
| 33 | Decanoic acid | 1336 | 1347 | S,MS (86/88) | 60/73 | 1924.5(2.12)a | 204.4(4.65)b | 224.7(4.63)b | 274.3(11.77)b | |||||
| 39 | Palmitic acid | 1925 | 1951 | S,MS (72/78) | 60/73 | 101.8(1.88)a | 125.5(2.46)b | 134.9(1.32)b | 166.6(0.63)b | |||||
| ∑ | 3075.9 | 839.2 | 865.2 | 394.5 | ||||||||||
| Depletion (%) | 72.2 | 71.9 | 87.2 | |||||||||||
| Aldehydes | ||||||||||||||
| 13 | (E)-2-Octenal | 1014 | 1034 | S,MS (67/77) | 57/70/83 | 18.3(0.38)a | 32.5(0.16)b | 26.4(0.18)b | 29.4(0.10)b | |||||
| ∑ | 18.3 | 32.5 | 26.4 | 29.4 | ||||||||||
| Depletion (%) | – | – | – | |||||||||||
| Monoterpenes | ||||||||||||||
| 20 | β-Linalool | 1084 | 1097 | S,MS (82/87) | 73/91/121 | 50.9(0.68)a | 27.6(0.41)b | 25.6(1.73)b | 31.3(0.03)b | |||||
| 27 | Nerol | 1223 | 1234 | S,MS (74/78) | 69/93 | 77.8(1.29)a | 28.1(1.88)b | 28.1(0.05)b | 37.7(0.07)b | |||||
| ∑ | 128.6 | 55.7 | 53.7 | 69.0 | ||||||||||
| Depletion (%) | 56.7 | 58.2 | 46.3 | |||||||||||
| Phenols | ||||||||||||||
| 31 | Eugenol | S,MS (85/93) | 38.4(0.46)a | 13.1(0.58)b | 11.5(0.31)b | 16.6(0.12)b | ||||||||
| ∑ | 38.4 | 13.1 | 11.5 | 16.6 | ||||||||||
| Depletion (%) | 65.9 | 70.1 | 56.8 | |||||||||||
| Norisoprenoids | ||||||||||||||
| 32 | TDN5 | 1288 | 1333 | MS (74/85) | 142/157/172 | 62.1(2.80)a | 5.3(0.04)b | 5.7(0.45)b | 7.6(0.09)b | |||||
| ∑ | 62.1 | 5.3 | 5.7 | 7.6 | ||||||||||
| Depletion (%) | 91.5 | 90.8 | 87.8 | |||||||||||
| Sesquiterpen alcohols | ||||||||||||||
| 36 | Nerolidol | 1536 | 1537 | S,MS (82/86) | 69/93 | 285.4(7.17)a | 22.4(0.30)b | 26.5(0.28)b | 26.3(0.26)b | |||||
| ∑ | 285.4 | 22.4 | 26.5 | 26.3 | ||||||||||
| Depletion (%) | 92.2 | 90.7 | 90.8 | |||||||||||
| Total | 19,423.1 | 12,352.2 | 12,417.5 | 13,196.2 | ||||||||||
| Depletion (%) | 36.4 | 36.1 | 32.1 | |||||||||||
1Retention index from NIST Chemistry WebBook (http://webbook.nist.gov/chemistry/name-ser.html)
2ID = identification; S = identified by comparison with reference compounds; MS = tentatively identified by NIST05
3QI = quantification ions
4Area expressed as arbitrary units (standard deviation of three assays); ∑, sum of the determined compounds; in the same line, different superscript letters represent significant differences compared with the respective control (p < 0.05)
5TDN: 1,1,6-Trimethyl-1,2-dihydronaphthalene
The unwanted VP contents in treated and non-treated wine are shown in Table 1. The imprinted polymers showed a similar ability to reduce both 4EP and 4EG, MIP-4EP being slightly more effective and specific (54–56 % reduction) than MIP-4EG (40–55 %) (Table 1).
These retention values were higher than those previously obtained by Larcher et al. (2012) using esterified cellulose, who reported averages of depletion of around 38 % for both 4EP and 4EG. Additionally, the results obtained herein are not as good as those described by Garde-Cerdán et al. (2008), but those authors did not evaluate the effect on sensorial characteristics, coloured or non-coloured phenolics. Nevertheless, our results cannot be considered as negative, since some oenologists suggest that moderate concentrations of the negative-associated aromas (4EP and 4EG) add complexity to high-quality wines (Ugarte et al. 2005). Furthermore, MIPs treatment of low contaminated red wines could be sensorial effective, as observed in our study with samples treated with MIPs, which showed better characteristics in terms of flavour.
Eugenol (31) is typically associated with oak barrel maturation, predominantly deriving from the thermal degradation of oak lignin during the toasting process of cooperage, although there are significant levels of this compound in untoasted oak (Kennison et al. 2008). The levels of eugenol were significantly reduced in samples treated with MIP-4EP and MIP-4EG (ca. 66 and 70 %, respectively) (Table 2).
Esters are flavour compounds widely occurring in a variety of food products; they are the primary source of fruit aroma, having a strong influence on wine aroma, mainly ethyl esters. Esters constituted the class with higher number of compounds in red wine samples, isoamyl acetate (7) being the major one in all samples (Table 2). In a general way, treated samples showed a lower esters levels, a depletion of 63 and 57 % being noticed with MIP-4EP and MIP-4EG, respectively (Table 2). For all treatments, depletion tended to increase with the ester hydrophobicity.
Elevated alcohol levels clearly increase the perceived warmth of hotness of wine and might mask some wine aroma and flavour attributes. Five alcohols were detected, phenylethyl alcohol (21) being clearly the main one in all analysed samples. Treatments with MIPs and NIP had no influence in its contents (Table 2).
Concerning to sulphur-containing compounds, like thiols and disulphides, they play an important role in wine aroma (Moreira et al. 2010). 2-Furfurylthiol (8) was the only compound identified in this work. Its content decreased 22, 18 and 10 % with MIP-4EP, MIP-4EG and NIP treatments, respectively (Table 2). The results showed a significant affinity between sulphur compounds and polymers.
Considering fatty acids, they are formed during alcoholic fermentation and can be released by yeasts, contributing to wine flavour: volatile fatty acids and fruity ethyl esters directly, unsaturated fatty acids indirectly, as precursors of aldehydes and alcohols of six carbons with herbaceous flavour (Gallart et al.Gallart et al. 1997). Additionally, fatty acids influence foam formation and stability and some of them (medium-chain fatty acids) are toxic to both yeasts and malolactic bacteria (Gallart et al. 1997). Four fatty acids were identified in this work, decanoic acid (33) being the main one in control sample, while hexanoic acid was the one found at highest amounts in the three treated samples (Table 2). All treatments resulted in a great depletion of this class of compounds (72 % with either MIP-4EP or MIP-4EG) (Table 2).
Many volatile aldehydes have remarkable odour properties, being formed from alcohols and other precursors by oxidation processes. Aldehydes have been reported to be normal constituents of the volatile fraction of wines, being important in flavour development and deterioration (Culleré et al. 2007). One aldehyde (13) was detected in the analysed samples, the treated ones showing a higher content compared with control (Table 2), thus suggesting a concentration induced by the treatments.
Terpenes play an important role in wine aroma, being a group of flavour compounds characteristic of specific grapes used for wine production. They can undergo several reactions during wine production and storage, as result of the duration of storage, relatively low pH and of the presence of compounds that can interact with them (Dziadas and Jelén 2010). Monoterpenes like β-linalool (20) and nerol (27) were detected in wine samples. Treated samples showed a significant depletion: ca. 57 and 58 % for MIP-4EP and MIP-4EG, respectively.
Sesquiterpenoids represent an important chemical group in Vitis vinifera L. due to their aroma properties and also bioactive effects as anti-bacterial (Tamemoto et al. 2001), or the ability to enhance bacterial permeability and susceptibility to exogenous antimicrobial compounds (Brehm-Stecher and Johnson 2003). Nerolidol (36) was the only sesquiterpen alcohol identified in wine samples. Treatments led to depletion rates higher than 90 % (Table 2).
Some C13 norisoprenoids have been frequently found in wines and are very important contributors to the wine aroma, due to their pleasant odour descriptors, usually presenting low odour threshold. These compounds are products of direct degradation of carotenoid molecules, such as β-carotene, lutein, neoxanthin and violaxanthin, or they can also be released after hydrolysis of glycoside molecules during wine making or aging processes (Baumes et al. 2002). 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) (32), that has been described as resulting from the direct degradation of β-carotene (Vinholes et al. 2009), was the only norisoprenoid found in the analysed samples. A great affinity of MIPs for this kind of compounds was observed (92 and 91 % for MIP-4EP and MIP-4EG, respectively) (Table 2).
Non-coloured phenolic compounds
Phenolic compounds constitute one of the most important quality parameters of wine, since they contribute markedly to the colour, flavour, bitterness and astringency of the final product (Rice-Evans et al. 1996). Interest in phenolic compounds in wine has increased in recent years because of their potential beneficial effects on human health (Zafrilla et al. 2003).
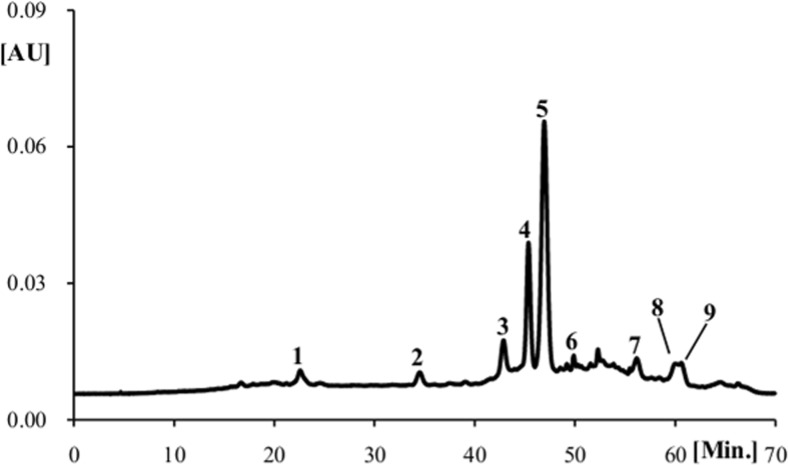
The analysis of wine samples by HPLC-DAD allowed the identification of 17 non-coloured phenolic compounds (Fig. 2, Table 3). Catechin (4) and epicatechin (8) were the major ones (Table 3). Both compounds exhibit antioxidant activity and may act as inhibitors of low density lipoprotein oxidation (Hayek et al. 1997; Silva et al. 2005).
Fig. 2.
HPLC-DAD profile of non-coloured phenolic compounds identified in red wine control sample. Detection at a 280 and b 350 nm: (1) gallic acid; (2) trans-caffeoyltartaric acid; (3) trans p-coumaroyltartaric acid; (4) catechin; (5) vanillic acid; (6) caffeic acid; (7) syringic acid; (8) epicatechin; (9) epigallocatechin-3-O-gallate; (10) p-coumaric acid; (11) ferulic acid; (12) epicatechin-3-O-gallate; (13) ellagic acid; (14) cinnamic acid; (15) myricetin; (16) isorhamnetin-3-O-glucoside; (17) quercetin
Table 3.
Phenolics compounds in red wine samples treated with MIP-4EP, MIP-4EG and NIP1
| Phenolic compounds | Samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (mg/L) |
MIP-4EP (mg/L) |
MIP-4EG (mg/L) |
NIP (mg/L) |
||||||||
| Hydroxybenzoic acids | |||||||||||
| 1 | 22.4 ± 0.03a | 19.1 ± 0.01b | 19.0 ± 0.00b | 20.5 ± 0.02b | |||||||
| 5 | 8.1 ± 0.01a | 5.9 ± 0.02b | 5.1 ± 0.12b | 7.0 ± 0.04b | |||||||
| 7 | 7,0 ± 0,21a | 4.4 ± 0.00b | 4.4 ± 0.03b | 5.5 ± 0.02b | |||||||
| 13 | 6.5 ± 0.04a | 0.8 ± 0.03b | 1.0 ± 0.00b | 1.3 ± 0.00b | |||||||
| ∑ | 44.0 | 30.2 | 29.5 | 34.3 | |||||||
| Depletion (%) | 31.4 | 33.0 | 22.1 | ||||||||
| Hydroxycinnamic acids | |||||||||||
| 2 | 8.6 ± 0.03a | 4.8 ± 0.01b | 5.1 ± 0.01b | 5.9 ± 0.02b | |||||||
| 3 | 3.5 ± 0.04a | 1.6 ± 0.04b | 1.9 ± 0.00b | 2.3 ± 0.00b | |||||||
| 6 | 9,2 ± 0,05a | 4.2 ± 0.03b | 4.4 ± 0.00b | 5.5 ± 0.05b | |||||||
| Non-coloured2 | 10 | 7.5 ± 0.07a | 2.5 ± 0.01b | 2.7 ± 0.00b | 3.7 ± 0.02b | ||||||
| 11 | 0.4 ± 0.00a | 0.1 ± 0.00a | 0.1 ± 0.01b | 0.2 ± 0.00b | |||||||
| 14 | 0.6 ± 0.01a | nq | 0.1 ± 0.00b | 0.1 ± 0.00b | |||||||
| ∑ | 29.8 | 13.2 | 14.3 | 17.7 | |||||||
| Depletion (%) | 55.7 | 52.0 | 40.6 | ||||||||
| Flavonols | |||||||||||
| 15 | 2.1 ± 0.01a | 0.2 ± 0.00b | 0.3 ± 0.00b | 0.3 ± 0.00b | |||||||
| 16 | 0.2 ± 0.00 | nd | nd | nd | |||||||
| 17 | 3.3 ± 0.04 | nd | nd | nd | |||||||
| ∑ | 5.6 | 0.2 | 0.3 | 0.3 | |||||||
| Depletion (%) | 90.5 | 85.7 | 85.7 | ||||||||
| Flavanols | |||||||||||
| 4 | 45.8 ± 1.45a | 27.3 ± 0.5b | 25.5 ± 0.52b | 35.8 ± 0.09b | |||||||
| 8 | 36.1 ± 0.23a | 20.2 ± 0.78b | 21.7 ± 0.28b | 27.2 ± 0.46b | |||||||
| 9 | 1.1 ± 0.01a | 0.6 ± 0.00b | 0.7 ± 0.00b | 0.9 ± 0.01b | |||||||
| 12 | 3.1 ± 0.05a | 1.0 ± 0.02b | 1.3 ± 0.00b | 1.5 ± 0.02b | |||||||
| ∑ | 86.1 | 49.1 | 49.2 | 65.4 | |||||||
| Depletion (%) | 43.0 | 41.9 | 24.0 | ||||||||
| Total | 165.5 | 92.7 | 93.3 | 117.7 | |||||||
| Depletion (%) | 44.0 | 43.6 | 28.9 | ||||||||
| 1 | 6.3 ± 0.02a | 5.1 ± 0.03b | 5.3 ± 0.01b | 5.7 ± 0.05b | |||||||
| 2 | 0.6 ± 0.01a | 0.8 ± 0.00b | 0.6 ± 0.00a | 0.4 ± 0.00b | |||||||
| 3 | 6.4 ± 0.02a | 5.7 ± 0.01b | 5.5 ± 0.01b | 5.8 ± 0.02b | |||||||
| Coloured3 | 4 | 15.1 ± 0.05a | 11.5 ± 0.02a | 11.6 ± 0.07b | 12.6 ± 0.00b | ||||||
| 5 | 118.1 ± 0.42a | 97.8 ± 1.33b | 99.3 ± 1.04b | 106.9 ± 0.03b | |||||||
| 6 | 0.3 ± 0.00a | 0.1 ± 0.00b | 0.1 ± 0.00b | 0.1 ± 0.03b | |||||||
| 7 | 5.1 ± 0.02a | 3.5 ± 0.01b | 3.7 ± 0.04b | 3.9 ± 0.03b | |||||||
| 8 | 2.3 ± 1.30a | 0.3 ± 0.00a | 0.5 ± 0.00b | 0.7 ± 0.00b | |||||||
| 9 | 16.2 ± 0.33a | 3.8 ± 0.02b | 2.5 ± 0.02b | 3.3 ± 0.00b | |||||||
| Total | 170.4 | 128.6 | 129.1 | 139.4 | |||||||
| Depletion (%) | 24.5 | 24.2 | 18.2 | ||||||||
1Results are expressed as mean ± standard deviation of three determinations; nd, not detected; nq, not quantified; ∑, sum of the determined compounds from each class. 2Identity of compounds as in Fig. 2. 3Identity of compounds as in Fig. 3. In the same line, different superscript letters represent significant differences compared with the respective control (p < 0.05)
Polymers were able to adsorb non-coloured phenolics, reducing their contents in 44 % for both MIP-4EP and MIP-4EG (Table 3). Very few reports exists about the application of MIPs to remove individual non-coloured phenolics from red wines. Molinelli et al. (2002) developed a MIP as a sorbent material for SPE of quercetin from a complex red wine sample, with recovery rates of 98 %. Additionally, Chen et al. (2013) developed a MIP for selective recognition of resveratrol in wine, having obtained recoveries near 90 %. As far as we know, this is the first report about the influence of MIPs treatment directed to the non-coloured phenolic composition of wine and food matrices and not just to a single compound.
Anthocyanins
The colour of red wine is the first attribute to be perceived by consumers and hence, one of the most important sensorial characteristic. Anthocyanins are the main pigments responsible for the red/violet colour presented by red wines, especially by young ones (Oliveira et al. 2013).
HPLC-DAD analysis allowed the identification of nine anthocyanic compounds in all samples (Fig. 3), the total amounts ranging between ca. 129 and 170 mg/l (Table 3). Despite the difference observed in the content of each anthocyanin, there are common characteristics in the profile obtained. All of the samples exhibited malvidin-3-O-glucoside (5) as the major compound (69 to 77 % of determined compounds), which is in accordance with previous works that reported this as the main compound in other red wines (Mateus et al. 2002; Valentão et al. 2007).
Fig. 3.
HPLC-DAD anthocyanins profile of control red wine sample. Detection at 500 nm: (1) malvidin-3,5-O-diglucoside; (2) cyanidin-3-O-glucoside; (3) cyanidin-3-O-rutinoside; (4) peonidin-3-O-glucoside; (5) malvidin-3-O-glucoside; (6) delphinidin; (7) petunidin; (8) pelargonidin; (9) malvidin
Concerning the effects of treatment, significant reductions were observed (Table 3), being obtained average reductions of around 24 % for MIP-4EP and MIP-4EG.
Conclusions
MIPs are a novel approach for sample preparation and pre-concentration, gaining increased interest in the fields of environmental, clinical and food analysis. MIPs revealed to be a promising alternative, not only because of the reduction of off-flavours from wine, like unwanted VP, but also due to the possibility of wash and reuse them. However, the changes observed in the red wine profile in terms of phenolic compounds indicate that their specificity and selectivity regarding off-flavours needs to be improved. This study opens up good prospects for study possible industrial applications of polymers in wine affected by volatile phenols and other drinks affected by off-flavours.
Acknowledgments
The authors are grateful to the financial support from the European Union (FEDER funds through COMPETE) and National Funds (FCT, Fundação para a Ciência e Tecnologia) through project Pest-C/EQB/LA0006/2013 and from the European Union (FEDER funds) under the framework of QREN through Project NORTE-07–0124-FEDER-000069. Luís R. Silva acknowledges FCT the financial support for the Post-doc grant (SFRH/BPD/105263/2014). Rafaela Teixeira is indebted to Doutor António Graça from Sogrape, for supplying samples.
Contributor Information
Paula B. Andrade, Email: pandrade@ff.up.pt
Luís R. Silva, Phone: + 351 220 428 654, Email: luisfarmacognosia@gmail.com
References
- Andersson LI. Molecular imprinting for drug bioanalysis. A review on the application of imprinted polymers to solid-phase extraction and binding assay. J Chromatogr B Biomed Sci Appl. 2000;739:163–173. doi: 10.1016/S0378-4347(99)00432-6. [DOI] [PubMed] [Google Scholar]
- Barata A, Nobre A, Correia P, Malfeiro-Ferreira M, Loureiro V. Growth and 4-ethylphenol production by the yeast Pichia guillermondii in grape juices. Am J Enol Vitic. 2006;57:133–138. [Google Scholar]
- Barros EB, Moreira N, Pereira GE, Leite SGF, Rezende CM, Guedes de Pinho P. Development and validation of automatic HS-SPME with a gas chromatography-ion trap/mass spectrometry method for analysis of volatiles in wines. Talanta. 2012;101:177–186. doi: 10.1016/j.talanta.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Baumes R, Wirth J, Bureau S, Gunata Y, Razungles A. Biogeneration of C13-norisoprenoid compounds: experiments supportive for an apo-carotenoid pathway in grapevines. Am J Enol Vitic. 2002;458:3–14. [Google Scholar]
- Brehm-Stecher BF, Johnson EA. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. J Antimicrob Chemother. 2003;47:3357–3360. doi: 10.1128/AAC.47.10.3357-3360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-López M.M, Cela-Pérez MC, Dopico-Garcia M.S, López-Vilariño JM, González-Rodríguez MV, Barral-Losada LF. Preparation, evaluation and characterization of quercetin-molecularly imprinted polymer for preconcentration and clean-up of catechins. Anal Chim Acta. 2012;721:68–78. doi: 10.1016/j.aca.2012.01.049. [DOI] [PubMed] [Google Scholar]
- Chassagne D, Guilloux-Benatier M, Alexandre H, Voilley A. Sorption of wine volatile phenols by yeast lees. Food Chem. 2005;91:39–44. doi: 10.1016/j.foodchem.2004.05.044. [DOI] [Google Scholar]
- Chatonnet P, Dubourdieu D, Boidron JN. The influence of brettanomyces/dekkera sp. Yeasts and lactic acid bacteria on the ethylphenol content of red wine. Am J Enol Vitic. 1995;46:463–468. [Google Scholar]
- Chatonnet P, Dubourdieu D, Boidron JN, Pons M. The origin of ethylphenols in wines. J Sci Food Agric. 1992;60:165–178. doi: 10.1002/jsfa.2740600205. [DOI] [Google Scholar]
- Chatonnet P, Viala C, Dubourdieu D. Influence of polyphenolic components of red wines on the microbial synthesis of volatile phenols. Am J Enol Vitic. 1997;48:443–448. [Google Scholar]
- Cela-Pérez MC, Castro-López MM, Lasagabáster-Latorre A, López-Vilariño JM, González-Rodríguez MV, Barral-Losada LF. Synthesis and characterization of bisphenol - a imprinted polymer as a selective recognition receptor. Anal Chim Acta. 2011;706:275–284. doi: 10.1016/j.aca.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Chen FF, Xie XY, Shi YP. Preparation of magnetic molecularly imprinted polymer for selective recognition of resveratrol in wine. J Chromatogr A. 2013;1300:112–118. doi: 10.1016/j.chroma.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Couto A, Campos FM, Figueiredo AR, Hogg TA. Ability of lactic acid bacteria to produce volatile phenols. Am J Enol Vitic. 2006;57:166–171. [Google Scholar]
- Culleré L, Cacho J, Ferreira V. An assessment of the role played by some oxidation-related aldehydes in wine aroma. J Agric Food Chem. 2007;55:876–881. doi: 10.1021/jf062432k. [DOI] [PubMed] [Google Scholar]
- Dias L, Dias S, Sancho T, Stender T, Querol H, Malfeito-Ferreira M. Identification of yeasts isolated from wine-related environments and capable of producing 4-ethylphenol. Food Microbiol. 2003;20:567–574. doi: 10.1016/S0740-0020(02)00152-1. [DOI] [Google Scholar]
- Dziadas M, Jelén HH. Analysis of terpenes in white wines using SPE-SPME-GC/MS approach. Anal Chim Acta. 2010;677:43–49. doi: 10.1016/j.aca.2010.06.035. [DOI] [PubMed] [Google Scholar]
- Gallart M, Francioli S, Viu-Marco A, López-Tamames E, Bruxaderas S. Determination of free fatty acids and their methyl esters in musts and wines. J. Chromatogr. A. 1997;776:283–291. doi: 10.1016/S0021-9673(97)00383-X. [DOI] [Google Scholar]
- Garde-Cerdán T, Zalacain A, Lorenzo C, Alonso JL, Salinas MR. Molecularly imprinted polymer-assisted simple clean-up of 2, 4, 6-trichloroanisole and ethylphenols from aged red wines. Am J Enol Vitic. 2008;59:396–400. [Google Scholar]
- Hayek T, Furhman B, Vaya J, Rosenblat M, Belinky P, Coleman R, Elis A, Aviram M. Reduced progression of atherosclerosis in the apolipoprotein E deficient mice following consumption of red wine, or its polyphenols quercetin and catechin, is associated with reduced susceptibility of LDL oxidation and aggregation. Arterioscler Thromb Vasc Biol. 1997;17:2744–2752. doi: 10.1161/01.ATV.17.11.2744. [DOI] [PubMed] [Google Scholar]
- Kennison KR, Gibberd MR, Pollnitz AP, Wilkinson KL. Smoke-derived taint in wine: the release of smoke-derived volatile phenols during fermentation of merlot juice following grapevine exposure to smoke. J Agric Food Chem. 2008;56:7379–7383. doi: 10.1021/jf800927e. [DOI] [PubMed] [Google Scholar]
- Larcher R, Puecher C, Rohregger S, Malacarne M, Nicolini G. 4-ethylphenol and 4-ethylguaiacol depletion in wine using esterified cellulose. Food Chem. 2012;132:2126–2130. doi: 10.1016/j.foodchem.2011.12.012. [DOI] [Google Scholar]
- Lisanti MT, Piombino P, Gambuti A, Genovese A, Siani VL, Moio L (2013) Analytical evaluation of remedial treatments for red and whites wines contaminated by volatile phenols. Private document http://oiv2007.hu/documents/viniculture/203_paper_oiv_lisanti.pdf (consulted in 12/03/2013)
- Mateus N, Machado JM, de Freitas V. Development changes of anthocyanins in vitis vinífera grapes grown in the Douro valley and concentration in respective wines. J Sci Food Agric. 2002;82:1689–1695. doi: 10.1002/jsfa.1237. [DOI] [Google Scholar]
- Molinelli A, Weiss R, Mizaikoff B. J advanced solid phase extraction using molecularly imprinted polymers for the determination of quercetin in red wine. J Agric Food Chem. 2002;50:1804–1808. doi: 10.1021/jf011213q. [DOI] [PubMed] [Google Scholar]
- Moreira N, Guedes de Pinho P, Santos C, Vasconcelos I. Volatile sulphur compounds composition of monovarietal white wines. Food Chem. 2010;123:1198–1203. doi: 10.1016/j.foodchem.2010.05.086. [DOI] [Google Scholar]
- Oliveira J, Silva MA, Parola AJ, Mateus N, Brás NF, Ramos MJ, Freitas V. Structural characterization of a-type linked trimeric anthocyanin derived pigment occurring in a young port wine. Food Chem. 2013;141:1987–1996. doi: 10.1016/j.foodchem.2013.04.091. [DOI] [PubMed] [Google Scholar]
- Pradelles R, Alexandre H, Ortiz-Julien A, Chassagne D. Effects of yeast cell-wall characteristics on 4-ethylphenol sorption capacity in model wine. J Agric Food Chem. 2008;56:11854–11861. doi: 10.1021/jf802170p. [DOI] [PubMed] [Google Scholar]
- Pradelles R, Vichi S, Alexandre H, Chassagne D. Influence of the drying processes of yeasts on their volatile phenol sorption capacity in model wine. Int J Food Microbiol. 2009;135:152–157. doi: 10.1016/j.ijfoodmicro.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller J, Paganga G. Structure–antioxidant activity relationship flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Silva LR, Andrade PB, Valentão P, Seabra RM, Trujillo ME, Velázquez E. Analysis of non-coloured phenolics in red wine: effect of Dekkera bruxellensis yeast. Food Chem. 2005;89:185–189. doi: 10.1016/j.foodchem.2004.02.019. [DOI] [Google Scholar]
- Suárez R, Suárez-Lepe JA, Morata A, Calderón F. The production of ethylphenols in wine by yeasts of the genera brettanomyces and dekkera: a review. Food Chem. 2007;102:10–21. doi: 10.1016/j.foodchem.2006.03.030. [DOI] [Google Scholar]
- Tamemoto K, Takaishi Y, Chen B, Kawazoe K, Shibata H, Higuti T, Honda G, Ito M, Takeda Y, Kodzhimatov OK, Ashurmetov O. Sesquiterpenoids from the fruits of ferula kuhistanica and antibacterial activity of the constituents of F. kuhistanica. Phytochemistry. 2001;58:763–767. doi: 10.1016/S0031-9422(01)00307-7. [DOI] [PubMed] [Google Scholar]
- Ugarte P, Agosin E, Bordeu E, Villalobos JI. Reduction of 4-ethylphenol and 4-ethylguaiacol concentration in red wines using reverse osmosis and adsorption. Am J Enol Vitic. 2005;56:30–36. [Google Scholar]
- Valentão P, Seabra RM, Lopes G, Silva LR, Martins V, Trujillo ME, Velázquez E, Andrade PB. Influence of Dekkera bruxellensis on the contents of anthocyanins, organic acids and volatile phenols of Dão red wine. Food Chem. 2007;100:64–70. doi: 10.1016/j.foodchem.2005.09.010. [DOI] [Google Scholar]
- Vasapollo G, Del Sole R, Mergola L, Lazzoi MR, Scardino A, Mele G. Molecularly imprinted polymers: present and future prospective. Int J Mol Med Sci. 2011;12:5908–5945. doi: 10.3390/ijms12095908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinholes J, Coimbra MA, Rocha SM. Rapid tool for assessment of C13 norisoprenoids in wines. J Chromatogr A. 2009;1216:8398–8403. doi: 10.1016/j.chroma.2009.09.061. [DOI] [PubMed] [Google Scholar]
- Zafrilla P, Morillas J, Mulero J, Cayula JM, Martínez-Cachá A, Pardo F, Nicolás JML. Changes during storage in conventional and ecological wine: phenolic content and antioxidant activity. J Agric Food Chem. 2003;51:4694–4700. doi: 10.1021/jf021251p. [DOI] [PubMed] [Google Scholar]