Abstract
Milk proteins were hydrolyzed by papain and their effect on the rheological, textural and sensory properties of bread were investigated. Water absorption capacity, emulsification capacity, foam volume, foam stability and solubility of Whey and casein protein concentrates and their hydrolysates were determined. The farinograph parameters of wheat flour and blends of wheat flour with casein and whey protein and their hydrolysates were determined to evaluate changes in water absorption capacity, dough development time, dough stability time and mixing tolerance index. The incorporation of WPC, casein and their hydrolysates up to the level of 5 % showed dough properties comparable to control. It was also found that 5 % level incorporation of milk proteins and their hydrolysates have no drastic effect on physical and sensory attributes of bread. The pasting properties showed significant decrease (p ≤ 0.05) when compared with wheat flour at all levels of addition of whey and casein protein concentrates and hydrolysates. Scanning electron microscopy of bread samples shows disruption in the well-defined protein – starch complex of wheat flour bread and the structure of gluten was weak as the concentration of whey protein increases in the wheat flour bread.
Keywords: Milk proteins, Functional properties, Bread, Rheological properties, Sensory properties
Introduction
Bakery products are an important source of nutrients viz. energy, protein, iron, calcium and several vitamins. Commercial bread and biscuits contain around 7–8 % protein, which is low. Bread, cookies, and parotta are the widely consumed products in the Indian subcontinent. Protein-calorie malnutrition (PCM) is a serious problem for people whose diets consist mainly of cereal or starchy food. PCM is a major nutritional syndrome affecting more than 170 million pre-school children and nursing mothers in developing Afro–Asian countries (Iqbal et al. 2006). Protein supplementation is one way to meet the demand for nutritious foods, particularly baked products. Milk proteins are the best quality proteins available and have high Protein efficiency ratio (3.6) and possess almost all the essential amino acids (Gani et al. 2014). Dairy proteins are being used in Bakery products both for nutritional benefits including increasing calcium content and protein efficiency ratio as well as functional benefits including flavor and texture enhancement and storage improvement. Whey protein concentrate improves the nutritional value of bread by supplying essential amino acids such as lysine, methionine and tryptophan (Vrignaud 1977; Warren et al. 1983). Besides nutritional value, WPC has desirable functional properties like emulsifying, foaming, gelling, water binding and viscosity development (Kinsella and Whitehead 1989). Higher scores of odor, taste and overall acceptability for bread with whey protein concentrate powder compared to control was observed by Madenci and Bilgiçli (2014). However information on the effect of protein concentrates and hydrolysates on bread quality is lacking in the literature. Hence, the present work was undertaken to study the effect of supplementation of milk protein concentrates and hydrolysates on the rheological characteristics of wheat flour and quality of bread.
Materials and methods
Materials
Ultrafiltered whey protein and casein concentrates, having a protein content of 80 and 88.03 % respectively, were obtained from Mahaan proteins, New Delhi. All the chemicals used in this study were of analytical grade, from E. Merck, India, Ltd., Mumbai. Papain having activity of 450 TU/mg was procured from Enzochem Company, Nasik, India. For manufacturing of bakery products, the wheat variety used (HD-2888) was got from Samastipur (IARI-RS) research station Bihar. Bakery ingredients such as fat, sugar and compressed yeast were procured locally.
Enzymatic modification of milk proteins
Enzymatic hydrolysis was carried out using papain enzyme; this enzyme works under mild conditions of pH (6–8) and temperature (40–60 °C). WPC and casein were mixed with water in the ratio of 1:10 (w/v) to obtain slurry. The pH of one batch of slurry was adjusted to 6.2 with 1 N HCl (55 °C). The slurry was allowed to stand for 1 h and then enzyme was added to the slurries at 1:60 ratio of enzyme to substrate. Hydrolysis experiments were carried out in a 1500 L reaction vessel maintained at 50 °C with the solution being agitated by an over-head stirrer. The hydrolysis process was adequately controlled by monitoring the degree of hydrolysis (DH) using the pH-stat technique (Adler-Nissen 1986). Aliquots (250 ml) were taken at 5, 10, 15 and 20 % DH. Enzyme was inactivated by placing in a boiling water bath for 10 min. The hydrolysates were centrifuged at 6000 rpm for 20 min and the supernatants collected, lyophilized and used for determination of functional properties.
Calculation of the degree of hydrolysis (DH)
The hydrolysis was carried out using the pH-stat method described by Adler-Nissen (1986) and the DH (%) was calculated from the volume and the molarity of alkali used to maintain constant pH.
Wheat flour milling
The wheat grains were cleaned manually and stored at room temperature prior to milling. The grains were milled on a Chopin (Model, CD 1) and Promylogram (Model, M3 CE) Laboratory mills into flour of different extraction rates after tempering for 24 h. Wheat grains were tempered at 14.5 % before milling. Extra 0.5 % moisture was added 30 min before milling to facilitate the separation process. The flour samples were stored at −20 °C before their analysis.
Chemical analysis of wheat flour
Moisture, protein (Kjeldal N × 5.75) and ash contents were determined by standard AACC methods (AACC 1983). The sodium dodecyl sulphate (SDS) sedimentation volumes of flour samples were estimated according to the method of Axford et al. (1978). Flour 5 g (14 % moisture basis) were added to water (50 ml) in a cylinder, a stopcock was started and the material dispersed by rapid shaking for 15 s, at 2 min and 4 min immediately after the last shake, SDS-lactic acid reagent (50 ml) was added, and mixed by inverting the cylinder four times before re-starting the clock from zero time. The SDS-lactic acid reagent was prepared by dissolving SDS (20 g) in 1 litre distilled water and then adding a stock diluted lactic acid solution (20 ml; 1 part lactic acid plus 8 part distilled water by volume). Inversion (four times) was repeated at 2, 4 and 6 min before finally starting the onces again from zero time. The continents of the cylinder were allowed to settle for 40 min before reading the sedimentation volume.
Functional properties of whey protein and sodium caseinate concentrates and hyrolysates
Water absorption capacity
Water absorption capacity was determined according to the method described by Ashwar et al. (2014) with some modifications. A 1.0 g of sample was weighed into a pre-weighed centrifuge tube, 10 ml of distilled water was added and stirred with a glass rod. After 30 min the suspension was centrifuged at 3000 rpm for 25 min. The supernatant was discarded and the tube inverted at an angle of 45° in an oven at 50 °C for 25 min, then transferred to a desiccator, cooled and weighed. The difference in the two weights gave the amount of water absorbed by the material.
Foaming capacity and stability
Foam capacity and foam stability were measured according to the method described by Ahmad et al. (2014) with some modifications. An equivalent weight of 3 g protein in the sample was accurately weighed out and mixed with 100 ml of distilled water. This was quantitatively transferred into a blender and whipped for 3 min at high speed. The slurry was poured immediately into a 250 ml measuring cylinder and the total volume of the liquid was measured immediately after 30 s. The difference in the volume was expressed as the volume of the foam. The foam stability was determined by measuring the fall in volume of the foam after 30 min. All the experiments were performed in triplicate and the results are the average of three values.
Emulsion capacity
Emulsion capacity was determined by the methods described by Beuchat et al. (1975) and Beuchat (1977). To a known amount of sample (3 g), 50 ml of distilled water was added. The slurry was transferred to a blender and blended for 30 s at low speed. Refined groundnut oil was slowly added from a burette while the blending continued. The addition of oil was continued until there was a phase separation. Emulsion capacity was expressed as the amount of oil required to emulsify 1 g of protein.
Protein solubility
Protein solubility was determined by the method of Bera and Mukherjee (1989). Two hundred milligrams of proteins were dispersed in 10 ml of deionized water. The pH of suspensions was adjusted to different levels (2.0 to 10.0) by using 1 mol/L HCl or 1 mol/L NaOH. The suspensions were stirred at room temperature for 30 min and then centrifuged at 10000 rpm for 30 min. Protein contents in supernatants were determined by Kjeldahl method (Ceirwyn 1995). The percentage of protein solubility in each suspension was calculated by the ratio of protein in the supernate to protein in 200 mg sample.
Supplementation of whey protein and sodium caseinate concentrates and hydrolysates in bakery products
Blends containing 0, 5, 10, and 15 % each of whey and casein protein concentrates and hydrolysates (with 15 % DH) replacing wheat flour were prepared.
Effect of whey protein and casein concentrates and hydrolysates on dough characteristics of wheat flour
Farinograph
A Farinograph is a recording dough mixer. It measures and records the resistance offered by the dough against mixing blades operating at a constant speed and temperature. Sample (50 g on 14 % moisture basis) was taken in the mixing bowl. The mixing bowl and distilled water were kept at 30 °C for 60 min to maintain uniform temperature. Preliminary titration was carried out by running the machine for 1 min until zero min line on the scale was reached. Water was added to the sample from a burette equal to its expected water absorption capacity and allowed to mix. Quantity of water was added such that maximum consistency of the dough was attained at the centre of farinogram band [500 Brabender Unit (BU)]. For final titration, whole of the water was added within 25 s and sample run for 25 min. The information was recorded:
Where, x = water added (ml) and y = Flour used (g)
- Mixing tolerance index
It is the difference in BU from top of the curve measured at 5 min after the peak is reached.
- Degree of softening
t is the difference in BU between the centre of the curve at the peak and obtained at 10 min after the peak, and reported nearest to 5 BU.
- Arrival time
It is the time (min) required for top of the curve to reach 500 BU line after the addition of water
- Dough development time
It is the time (min) to the nearest half min from the first addition of water to the development of maximum consistency of the dough.
- Dough stability
It is defined as the difference in time to the nearest half min between the points where top of the curve first intercepts (arrival time).
Pasting properties
The effect of the whey protein concentrates and hydrolysates on wheat flour pasting properties was determined with the use of a Starch Master (Newport Scientific Pvt. Ltd., Warrie-wood, Australia). Triplicate measurements using a 13 min controlled heating and cooling profile with constant shear were used, wherein the sample was held for 1 min at 50 °C, heated at 12 °C per min from 50 to 95 °C, held for 2.5 min at 95 °C, cooled at 12 °C per min to 50 °C, and held for 2 min at 50 °C. In each case, 2.5 g (db) wheat flour supplemented with protein isolates and concentrates and 25 g accurately weighed distilled water, were added to the sample canister. The analysis used the standard temperature profile and followed hold at 50 °C; 0–1 min at 50 °C, 1–4:45 min a ramp up to 95 °C; 4:45–7:15 min hold at 95 °C; 7:15–11 min cooling (set at 50 °C); hold at 50 °C to 13 min. Parameters recorded were pasting point, peak viscosity (PV), Final paste viscosity (FV), breakdown (BD), and set back (SB). All measurements were replicated thrice.
Dough extensibility
Dough extensibility was determined on TATX2 Texture Analyser (Stable Microsystems Ltd, Godalming, UK) according to the method of Verbruggen et al. (2001). Small amount of oil was applied to both sides of the dough to avoid sample adhesion. Dough sample (15 g) was clamped onto the grooved base of the form and cut into strips by pushing down the upper block of Kieffer Dough of the texture analyser. The dough strips were placed onto the grooved region of the sample plate and, holding down the spring loaded clamp lever, and the plate was inserted into the gluten extensibility rig. The handle was released slowly and the tensile test was performed. The pre test speed was 2 mm/s, test speed 3.3 mm/s, post test speed 10.0 mm/s, distance 75 mm, trigger force 5 g with a data acquisition rate of 200 pps.
Baking properties
Bread making
Blends containing 0, 5, 10, and 15 % whey and casein protein concentrate and hydrolysate replacing wheat flour were prepared by gradual mixing in a rotary mixer. The bread making procedure described by Finney (1984) was fallowed. For 30 g bread making method the test sample formula was: flour (30 g, 14 % moisture basis), compressed yeast (1.59 g), salt (0.45 g), sugar (1.8 g), fat (0.45 g), malted barley flour (0.075 g), ascorbic acid (100 ppm, flour basis). For 100 g bread making method, formulation amounts for 30 g method were multiplied by the factor 3.33. Salt, sugar, ascorbic acid and yeast were added in solution form. Yeast was added as a suspension, which was mixed well each time before dispensing. Dough was prepared in a farinograph mixer. The mixing time in the baking test was optimized using the farinograph as a guide as practiced by Finney and his coworkers (Finney 1984) using mixograph. Similarly, water absorbtion was also optimized. After mixing, doughs were placed in bowls and coverd with a wet muslin cloth and fermented for 90 min at 30 °C and 98 % RH. Doughs were punched after 52, 77 and 90 min in a machine moulder (Nagpal, New Delhi) by passing through a set of rollers with a gape setting of 9 min. After the final punch the doughs were placed in lightly greased tins. Dough’s were proved at 30 °C and 98 % RH. The baking process was carried out for 25 min at 230 °C in Mono universal bake-off oven, England. The water container was placed in the oven to provide adequate moisture conditions.
Loaf volume
Loaf volume was measured by bean displacement method (Greene and Bovell-Benjamin 2004). The beans were poured into a container of known volume until the bottom was covered. The test bread was then placed inside the container, followed by more beans, which were leveled across the top with a spatula. The displacement of the beans that were not required to fill the container were measured in a graduated cylinder and used to express volume of the loaf. Samples were measured in triplicate, and the average was recorded.
Total colour difference
The colour of breads at different levels of protein fortification was determined using Hunter colour lab (Hunter Lab D25, Hunter associates Lab, Reston, USA). Calibration with black and white tiles was performed before colour measurement. Total colour difference (ΔE) was calculated as:
Where,
- ΔL
(L sample - L std)
- Δa
(a sample - a std)
- Δb
(b sample - b std)
Texture analysis of bread
The bread firmness was determined using AACC (74–09) standard method. The texture analyzer TA-XT2 was used with 25 mm cylindrical probe (p/36 R). The pre test speed, test speed and post test speed was 1, 1.7 and 10 mm/s, respectively with data acquisition rate of 250 pps.
Microstructure
Dried bread samples (1.27 cm × 1.27 cm) were mounted on specimen stub using conducting silver paint and sputter coated using gold–palladium target prior to the examination. The specimen stub was then mounted on a specimen holder and put in the machine. The microstructures of the samples were viewed on a Leo scanning electron microscope Model 435 VP (Leo Electronic Systems, Cambridge, UK).
Sensory evaluation
The trained panel consisted of nine members (average age mid-40s) selected randomly from laboratory staff and lecturers of the Food Science and Technology Department. They were trained and instructed to rate the score of crust colour, crumb colour, crumb texture, flavour and overall quality of the breads. A rating scale of l–7 points (1 = dislike very much; 7 = like very much) was used (Peryam and Pilgrim 1957). Bread was evaluated 3 h after baking, when loaves were sliced into l-cm thick slices by a bread slicing machine. Panellists evaluated one slice of different bread systems, which were offered at the same time in an open area without special lighting. Water was provided for rinsing purposes.
Statistical analysis
Mean values, standard deviation, analysis of variance (ANOVA) were computed using a commercial statistical package SPSS 10.1 (USA). These data were then compared using Duncan’s multiple range tests at 5 % significance level.
Results and discussions
Proximate composition of whey and casein protein concentrates and their hydrolysates
The protein, moisture and ash content of whey and casein protein concentrates and their freeze-dried hydrolysates at four different DH levels were compared (Table 1). In general, protein content decreased after hydrolysis but no relationship between DH and protein content was observed. The differences in protein content of hydrolysates at different DH were due to the corresponding difference in non-protein nitrogen (NPN). The produced peptides can interact with unhydrolyzed protein (WPC or casein) via hydrophobic interactions resulting in increase of the insoluble protein fraction (Sindayikengera and Xia 2005). In addition, some protein/peptides were lost during the centrifugation prior to freeze-drying. The apparent decrease in protein content of freeze-dried hydrolysates is also related to higher ash levels in the samples. In general, protein was lost and moisture increased with increasing hydrolysis. The differences in moisture could arise from varying efficiency of freeze-drying or storage conditions. Ash content of the hydrolysates increased with increasing DH with all hydrolysates having higher ash content than the unhydrolyzed protein (WPC or casein). Increase in ash content corresponded to the increase in base (NaOH) consumption with DH and the adjustment of pH before enzymatic hydrolysis (Sindayikengera and Xia 2005). 1). Significant difference was found in all hydrolysates produced at various DH levels when compared with Parental protein (WPC or casein) in protein, moisture and ash content.
Table 1.
Proximate composition of WPC, CPC and their hydrolysates
| Protein (%) | Moisture (%) | Ash (%) | ||||
|---|---|---|---|---|---|---|
| Sample | WPC | CPC | WPC | CPC | WPC | CPC |
| 0 % DH | 79.44e ± 1.10 | 88.14e ± 0.31 | 3.97a ± 0.06 | 4.78a ± 0.19 | 3.52a ± 0.50 | 5.10a ± 0.18 |
| 5 % DH | 60.36a* ± 1.90 | 84.95d* ± 0.08 | 6.51c* ± 0.01 | 5.30b* ± 0.10 | 9.24b* ± 0.28 | 8.68b* ± 0.17 |
| 10 % DH | 66.25c* ± 0.25 | 80.83b* ± 0.76 | 6.10b* ± 0.01 | 6.53d* ± 0.05 | 10.43c* ± 0.37 | 10.13c* ± 0.17 |
| 15 % DH | 64.66c* ± 0.35 | 76.21a* ± 0.20 | 6.22b* ± 0.02 | 6.30c* ± 0.07 | 12.0d* ± 0.35 | 11.90d* ± 0.17 |
| 20 % DH | 62.36b* ± 0.85 | 76.83a* ± 0.02 | 7.28d* ± 0.25 | 7.34e* ± 0.19 | 12.83e* ± 0.28 | 13.34e* ± 0.30 |
Means in the same column with different letters were significantly different at p < 0.05
*Denotes significant differences from control (P < 0.05)
Effect of enzymatic modification of whey and casein protein concentrates on functional properties
The functional properties of enzymatically hydrolyzed whey and casein protein concentrates are presented in Table 2. The water absorption capacity of WPC and casein significantly increased (p < 0.05) on enzymatic hydrolysis with papain from 10.4 to 32.23 ml/g and 18.47 to 35.37 ml/g respectively, with highest water absorption capacity at 20 % DH (degree of hydrolysis) for both the proteins. This can be attributed to dissociation of proteins into smaller subunits, which have more water binding sites (Castimopoolas et al. 1970). The results obtained are in accordance with Rhicha et al. (2007) in which they reported increase in water absorbtion capacity of whey protein concentrate at increased hydrolysis periods. The emulsification capacity of WPC and casein, in contrast, reduced significantly (p < 0.05) from 39.3 to 30.3 ml oil/g protein and 45.13 to 34.17 ml oil/g protein respectively on enzyme hydrolysis in both samples. The emulsifying capacity of proteins is related to their capacity to lower interfacial tension between the hydrophobic and hydrophilic components in foods. Similar results on emulsifying capacity were also obtained by other researchers and have been attributed to dry heat treatment (Rahma and Mostafa 1998), partial hydrolysis (Sekul et al. 1978), and enzyme modification (Bhagya and Srinivasan 1989).
Table 2.
Functional properties of whey protein and casein protein concentrates and hydrolysates
| Sample | Water absorption (ml/100 g) | Emulsion capacity (ml oil/g protein) | Foam volume (ml/g) | Foam stability (ml/g) | ||||
|---|---|---|---|---|---|---|---|---|
| Whey protein | Casein protein | Whey protein | Casein protein | Whey protein | Casein protein | Whey protein | Casein protein | |
| 0 % DH | 10.4a ± 0.36 | 18.47a ± 0.45 | 39.3e ± 0.26 | 45.13e ± 0.15 | 25.2a ± 0.20 | 19.47a ± 0.50 | 20.16e ± 0.29 | 15.27e ± 0.25 |
| 5 % DH | 16.43b* ± 0.40 | 26.4b* ± 0.10 | 38.4d* ± 0.15 | 40.23d* ± 0.25 | 35.3d* ± 0.26 | 29.83e* ± 0.76 | 10.23d* ± 0.20 | 8.26d* ± 0.16 |
| 10 % DH | 24.77c* ± 0.25 | 28.7c* ± 0.10 | 35.27c* ± 0.25 | 36.57c* ± 0.15 | 35.0d* ± 0.15 | 27.33d* ± 0.35 | 5.43c* ± 0.44 | 4.27c* ± 0.25 |
| 15 % DH | 29.23d* ± 0.20 | 33.3d* ± 0.26 | 32.27b* ± 0.15 | 35.06b* ± 0.20 | 34.43c* ± 0.25 | 25.00c* ± 0.50 | 3.70b* ± 0.16 | 2.51b* ± 0.49 |
| 20 % DH | 32.23e* ± 0.25 | 35.37e* ± 0.32 | 30.3a* ± 0.20 | 34.17a* ± 0.20 | 28.27b* ± 0.55 | 20.50b* ± 0.43 | 2.06a* ± 0.11 | 1.56a* ± 0.19 |
Means in the same column with different letters were significantly different at p < 0.05
*Denotes significant differences from control (P < 0.05)
The foam volume of the control was found to be less than that of the treated samples in both the proteins. Enzymatic hydrolysis of whey proteins and casein caused an increase in the foam volume initially and then a decrease with time. Hydrolysis of whey protein generally resulted in increased foam-forming ability of the hydrolysates compared to the parental proteins (Britten et al. 1994; Ludwig et al. 1995; Lieske and Konrad 1996; Caessens et al. 1999). In case of WPC treated with papain no significant difference was found in foam volume at 5 % (35.3 ml/g) and 10 % DH (35 ml/g), while in case of casein gradual decrease in foam volume with increase in hydrolysis time was found.
The foam stability of the control was greater than that of the treated samples in case of both whey and casein concentrates. Samples treated with papain showed gradual decrease in stability with an increase in proteolysis in both the cases. The foam stability at 20 % DH was almost negligible. The above results show that a limited amount of hydrolysis is desirable to increase foaming but foam stability is greatly decreased because of such hydrolysis. This is probably due to an initial increase in the polypeptide content, which allows more air to be incorporated. However, the polypeptides do not have the strength required to give stable foam. The decrease in foam stability manifests itself primarily in the initial 30 min of reaction (Keuhler and Stine 1974). Further hydrolysis is likely to result in peptides, which lack the ability to stabilize the air cells of the foam.
Protein solubility
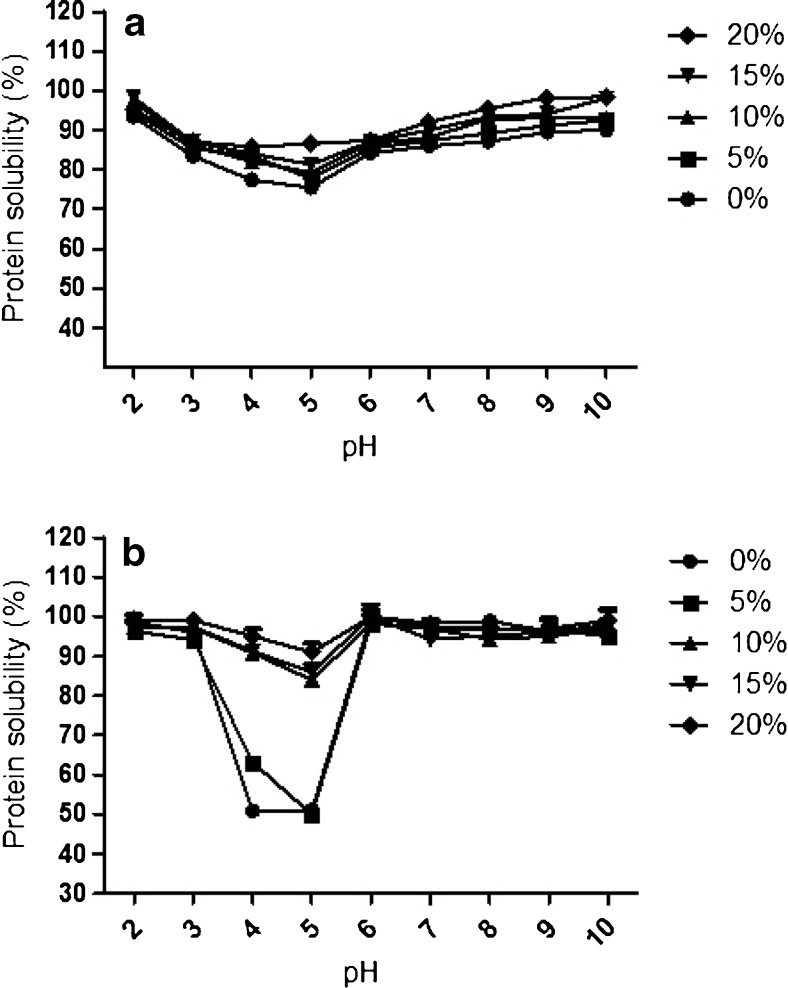
The protein solubility was measured in the pH range of 2 to 10. The pH-protein solubility profiles of whey and casein protein concentrates and their hydrolysates are shown in Fig. 1. Whey and casein protein concentrates had minimum solubility at pH 4.0 to 5.0. They had the highest solubility values at alkaline pH and in the pH range between 2.0 and 3.0. Solubility in the pI (isoelectric point) range increased from 75.5 to 77.8, 79.3, 81.3 and 86.5 % for WPC and its hydrolysates at 5, 10, 15 and 20 % DH, respectively. At 5, 10, 15 and 20 % DH, the casein hydrolysates were 50, 84.8, 86 and 91 % soluble at pH = 5.0, respectively. These results accorded with those of Slattery and Fitzgerald (1998), Chobert et al. (1988a) and Wani et al. (2014). The results indicated that the enzymatic hydrolysis of whey and casein protein concentrates by Papain improved the solubility of their hydrolysates. This enzymatic hydrolysis of whey and casein protein concentrates increased the number of ionizable groups (NH4+, COO−) with concomitant increase in hydrophilicity and net charge of the resulting hydrolysates, promoting hydrolysate-water interaction and enhancing their solubility. It altered their structure and exposed previously buried hydrophobic regions to the aqueous environment. The enhanced solubility of casein and WPC hydrolysates was also due to their smaller molecular size as confirmed by Chobert et al. (1988b) and Mutilangi et al. (1996).
Fig. 1.
Protein solubility of a whey protein concentrate and its hydrolysates and b casein protein concentrate and its hydrolysates at PH range 2 to 10
Wheat flour quality
The data pertaining to the wheat flour quality was determined. The protein content was 11.0 % whereas gluten content was 21.29 and 8.78 % as wet and dry gluten respectively. Flour contained ash content (0.48 %), SDS, sedimentation value (30.19 %) and diastatic activity of 265 mg/10 g flour. Singh et al.(1993) reported similar protein content of wheat flour from four Indian cultivars whereas it was lower than those reported several researchers (Lorenz et al. 1979; Giami 2001; Giami and Barber 2004). Previous reports on ash content of wheat flour are in the range of 0.44–0.7 %, for wet and dry gluten (Lorenz et al. 1979; Giami 2001).
Effect of whey protein and casein concentrates and hydrolysates on dough rheology of wheat flour
Farinograph
The farinograph parameters of wheat flour and blends of wheat flour and whey protein, casein and their hydrolysates were determined to evaluate changes in water absorption capacity, dough development time, dough stability time, mixing tolerance index and degree of softening (Table 3). Water absorption (%) decreased significantly (p ≤ 0.05) as the amount of milk protein concentrates and hydrolysates increased up to a level of 15 % in the wheat flour. The water absorption of wheat flour (control) was 58.4 %, however, it was in the range of 51.7–54.4 % for WPC, 49.8–50.7 % for WPH, 53.3–56.4 % for casein and 51.5–53.5 % for casein hydrolysate. The relative lower percentage of water absorption in wheat flour blend with protein concentrates may be attributed to lower water binding ability of milk proteins than wheat flour. Indrani et al. (2007) reported water absorption decreased as the amount of whey protein concentrate in the wheat flour blend increased up to 10 %.
Table 3.
Effect of whey and casein protein concentrates and hydrolysates on Farinograph and extensibility characteristics of wheat flour dough (n = 3)
| Protein % added | Water absorption, (%). | Arrival time | Dough development time, min. | Dough stability time, min | Mixing tolerance index after5min (BU*) | Degree of softening (BU*) | Dough extensibility | ||
|---|---|---|---|---|---|---|---|---|---|
| Force (KG) | Distance (CM) | ||||||||
| WPC | 0 | 58.4d ± 0.10 | 1.1a ± 0.05 | 5.3k ± 0.03 | 14.2b ± 0.20 | 30.2a ± 0.80 | 40.2l ± 0.20 | 0.05g | 7.2f |
| 5 | 54.4c* ± 0.05 | 1.2b* ± 0.05 | 5.9l* ± 0.15 | 14.8c* ± 0.01 | 47.2b* ± 0.20 | 30.2k* ± 0.25 | 0.04f* | 5.4d* | |
| 10 | 53.5b* ± 0.08 | 2.1c* ± 0.02 | 4.9j* ± 0.15 | 14.8c* ± 0.08 | 59.1c* ± 0.11 | 20.1j* ± 0.11 | 0.03e* | 5.6e* | |
| 15 | 51.7a* ± 0.24 | 3.2d* ± 0.05 | 3.1i* ± 0.10 | 13.3a* ± 0.02 | 67.4d* ± 0.36 | 10.0i* ± 0.17 | 0.02e* | 4.0c* | |
| WPH | 0 | 58.4c ± 0.01 | 1.1e ± 0.05 | 5.3b ± 0.03 | 14.2f ± 0.20 | 30.2e ± 0.80 | 40.2a ± 0.20 | 0.05c | 7.2j |
| 5 | 50.7b* ± 0.60 | 1.5f* ± 0.05 | 6.3c* ± 0.03 | 14.8g* ± 0.02 | 40.0f* ± 0.80 | 40.2a ± 0.48 | 0.04b* | 4.3i* | |
| 10 | 50.3ab* ± 0.01 | 2.7g* ± 0.04 | 5.2b ± 0.01 | 14.7g* ± 1.20 | 53.2g* ± 0.07 | 40.3b* ± 0.11 | 0.03a* | 3.7h* | |
| 15 | 49.8a* ± 0.11 | 3.4h* ± 0.06 | 4.5a* ± 0.50 | 13.6e* ± 0.32 | 61.0h* ± 0.18 | 60.0c* ± 0.07 | 0.02a* | 3.3g* | |
| CPC | 0 | 58.4d ± 0.10 | 1.1a ± 0.05 | 5.3j ± 0.03 | 14.2b ± 0.20 | 30.2a ± 0.80 | 40.2l ± 0.20 | 0.05h | 7.2e |
| 5 | 56.4c* ± 0.10 | 1.2a ± 0.07 | 2.1i* ± 0.11 | 14.8c* ± 0.01 | 49.4b* ± 0.36 | 32.2k* ± 0.21 | 0.03g* | 5.1d* | |
| 10 | 54.3b* ± 0.05 | 1.6b* ± 0.20 | 2.0i* ± 0.10 | 14.7c* ± 0.15 | 60.1c* ± 0.04 | 25.2j* ± 0.25 | 0.02f* | 5.14d* | |
| 15 | 53.3a* ± 0.01 | 2.4d* ± 0.02 | 2.2i* ± 0.10 | 12.8a* ± 0.01 | 69.2d* ± 0.25 | 14.3i* ± 0.35 | 0.01e* | 4.1c* | |
| CPH | 0 | 58.4d ± 0.10 | 1.1e ± 0.05 | 5.3c ± 0.03 | 14.2f ± 0.20 | 30.2e ± 0.80 | 40.2a ± 0.20 | 0.05c | 7.2j |
| 5 | 53.5c* ± 0.07 | 1.4f* ± 0.02 | 4.0b* ± 0.21 | 14.7g* ± 0.04 | 44.4f* ± 0.40 | 45.0b* ± 0.08 | 0.03b* | 4.5i* | |
| 10 | 52.5b* ± 0.15 | 2.3g* ± 0.02 | 3.0a* ± 0.08 | 14.7g* ± 0.02 | 55.3g* ± 0.33 | 66.2c* ± 0.22 | 0.03a* | 4.0h* | |
| 15 | 51.5a* ± 0.07 | 3.2h* ± 0.04 | 3.4a* ± 0.36 | 12.8e* ± 0.04 | 64.5h* ± 0.48 | 87.2d* ± 0.22 | 0.03a* | 3.7g* | |
Data are mean value of three replicates
Means for the same blend and variable with unlike superscripts indicate significant differences using Duncan’s multiple range test (P < 0.05)
*Denotes significant differences from control (P < 0.05)
Arrival times and mixing tolerance increased significantly (p ≤ 0.05) by replacement of wheat flour with whey protein, casein and their hydrolysates, while dough development time showed significant (p ≤ 0.05) increase on 5 % level of whey protein but significantly decreased on 10 and 15 % addition when compared with control. Same trend was shown by the addition of whey protein hydrolysate, except at 10 % level which shows no significant difference with control. Casein and its hydrolysate showed significant decrease in dough development time when compared with control but no significant difference was found between 5, 10 and 15 % in case of casein and 10 and 15 % in casein hydrolysates.
In case of whey protein concentrate and hydrolysate dough stability time significantly (p ≤ 0.05) increased except at 15 % addition when compared with control but no significant difference (p ≤ 0.05) was found between 5 and 10 % level of addition, while casein protein concentrate and hydrolysate addition showed increase in dough stability when compared with control, except at the level of 15 % which showed decrease in dough stability time than control but no significant difference was found between 5 and 15 % level. Indrani et al. (2007) also reported that addition of milk proteins increased dough stability up to 10 % level but beyond it dough stability decreased. As the level of flour blends in composite doughs increased, farinograph mixing tolerance index increased significantly from 5 to 15 % addition of whey and casein proteins and their hydrolysates. Degree of softening (BU) decreased significantly with the addition of whey and casein protein and their hydrolysates, except at 5 % level no significant difference was found with control. Farinograph helps in determining the amount of water in a flour to achieve dough of fixed consistency during mixing, to measure the mixing characteristics of flour and to predict the baking performance. Baking performance is associated with high resistance of dough to extension, which is measured with texture analyser or extensograph. Dough extensibility (Force (Kg) required to break the dough) decreased significantly (p ≤ 0.05) with increase in parental proteins and their hydrolysates in wheat flour (Tables 3). This could be due to dilution of gluten content as well as interaction of whey protein with wheat protein fractions, which resulted in short dough. Zadow (1981) also reported that addition of WPC in the preparation of bread resulted in a weaker and less elastic dough. He further opined that the weakening of the wheat flour dough is due to interference of WPC sulphydryl groups in the normal sulphydryl/disulphide interchange reactions occurring during wheat flour dough development. This could also be applied to the present study. Giami (2001) reported that dough extensibility remained unchanged upto 5 % level substitution with fluted pumpkin flour but decreased with further increase in substitution level.
Pasting properties
The pasting point, peak viscosity, final viscosity, hold paste viscosity, breakdown viscosity and set back viscosity showed significant decrease (p ≤ 0.05) when compared with wheat flour at all levels of addition of whey and casein protein concentrates and hydrolysates in the wheat flour (Table 4). Peak viscosity decreased significantly (p ≤ 0.05) with increase in concentration of protein concentrates and hydrolysates in the wheat flour. The peak viscosity of wheat flour with whey protein concentrates and hydrolysates at 5–15 % was in the range of 1328 to 2243 cP and 1047 to 2078 cP respectively, while in case of casein protein concentrate and hydrolysate it was in the range of 1773 to 2498 cP and 985 to 2345 cP respectively. Same trend was found in case of final viscosity, which also showed significant decrease (p ≤ 0.05) with increase in concentration of protein concentrates, and hydrolysates in the wheat flour. The final viscosity of wheat flour with whey protein concentrates and hydrolysates at 5–15 % was in the range of 2020 to 3048 cP and 1483 to 2570 cP respectively, while in case of casein proteins and hydrolysates it was in the range of 2025 to 2462 cP and 1360 to 2674 cP respectively. Pasting point showed significant (p ≤ 0.05) decrease with the fortification of milk protein concentrates and hydrolysates when compared with control, while in case of casein concentrates and hydrolysates no significant difference (p ≤ 0.05) was found between 5 and 15 and 5–10 % level of incorporation respectively. Indrani et al. (2007) reported decrease in peak viscosity values with increased level of WPC in the wheat flour WPC blend. Bimlesh and Malik (1996) in their study on ultrafiltered whey protein concentrate stated that there was a decrease in viscosity of WPC initially and then (at 50 °C and above) an increase due to denaturation of whey proteins. Lorenz et al. (1979) reported that with the increase in percentage of faba bean protein concentrate, viscosity at each of the reference points decreased in faba protein-wheat flour blend.
Table 4.
Effect of whey, casein protein concentrates and their hydrolysates on pasting properties wheat flour (n = 3)
| Protein Added (%) | Peak viscosity (*cP) | Final viscosity (*cP) | Paste point (*cP) | Hold viscosity (*cP) | Breakdown viscosity (*cP) | Setback viscosity (*cP) | |
|---|---|---|---|---|---|---|---|
| WPC | 0 | 2925d ± 7.7 | 3432l ± 3.5 | 334d ± 4.9 | 2094d ± 5.6 | 831d ± 2.1 | 1338d ± 8.4 |
| 5 | 2243c* ± 2.5 | 3048k* ± 2.0 | 263c* ± 7.6 | 1861c* ± 3.6 | 383c* ± 2.5 | 1187a* ± 7.0 | |
| 10 | 1832b* ± 1.5 | 2448j* ± 2.5 | 191b* ± 1.7 | 1563b* ± 2.5 | 271b* ± 3.0 | 1033c* ± 2.5 | |
| 15 | 1328a* ± 4.5 | 2020i* ± 0.57 | 184a* ± 3.6 | 1208a* ± 2.6 | 123a* ± 3.0 | 817b* ± 5.6 | |
| WPH | 0 | 2925h ± 7.07 | 3415d ± 3.5 | 334d ± 4.9 | 2094d ± 5.6 | 831d ± 2.1 | 1338d ± 8.4 |
| 5 | 2078g* ± 2.5 | 2570c* ± 2.08 | 243c* ± 2.5 | 1800c* ± 4.5 | 278c* ± 1.0 | 771c* ± 2.3 | |
| 10 | 1513f* ± 2.0 | 2005b* ± 2.08 | 204b* ± 3.6 | 1276b* ± 2.0 | 234b* ± 3.6 | 725b* ± 4.7 | |
| 15 | 1047e* ± 2.0 | 1483a* ± 3.0 | 178a* ± 1.0 | 868a* ± 2.5 | 180a* ± 1.5 | 616a* ± 4.0 | |
| CPC | 0 | 2925d ± 7.7 | 3432l ± 3.5 | 334c ± 4.9 | 2094d ± 5.6 | 831d ± 2.1 | 1338d ± 8.4 |
| 5 | 2498c* ± 1.0 | 2462k* ± 2.0 | 75b* ± 1.0 | 1787c* ± 1.5 | 712a* ± 2.0 | 977a* ± 3.0 | |
| 10 | 2133b* ± 3.5 | 2361j* ± 3.0 | 48a* ± 1.0 | 1466b* ± 1.0 | 667c* ± 2.6 | 895c* ± 1.5 | |
| 15 | 1773a* ± 3.0 | 2025i* ± 5.0 | 75b* ± 2.5 | 1335a* ± 2.5 | 438b* ± 5.0 | 690b* ± 1.0 | |
| CPH | 0 | 2925h ± 7.07 | 3432d ± 3.5 | 334c ± 4.9 | 2094d ± 5.6 | 831d ± 2.1 | 1338d ± 8.4 |
| 5 | 2345g* ± 4.5 | 2674c* ± 3.0 | 87b* ± 2.0 | 1847c* ± 7.6 | 498c* ± 1.5 | 827b* ± 2.5 | |
| 10 | 1854f* ± 2.0 | 2143b* ± 3.0 | 86b* ± 2.0 | 1497b* ± 1.5 | 357a* ± 1.5 | 646c* ± 5.0 | |
| 15 | 985e* ± 3.0 | 1360a* ± 5.0 | 69a* ± 1.0 | 763a* ± 2.6 | 222b* ± 5.1 | 597a* ± 4.0 |
WPC Whey protein concentrate, WPH Whey protein hydrolysates, CPC Casein protein concentrate, CPH Casein protein hydrolysate
Means for the same blend and variable with unlike superscripts indicate significant differences using Duncan’s multiple range test (P < 0.05)
*Denotes significant differences from control (P < 0.05)
Effect of milk protein concentrates and hydrolysates on physical attributes of bread
Loaf volume is regarded as the most important bread characteristic since it provides a quantitative measurement of baking performance (Tronsmo et al. 2003). Loaf volume is also extremely important to consumers because they desire breads that appear to be light and not so dense. Infinite loaf volume is not so desirable, but consumers associate a certain amount of lightness and high loaf volume with certain breads, and low loaf volumes with others. For example, flat bread such as chapatti is not expected to have high loaf volume. Loaf volume for control bread was 520 ml that showed significant decrease (p ≤ 0.05) in the range of 447–289 ml, 548–535 ml, 450–290 ml, 552–531 ml when whey proteins, whey protein hydrolysates, casein and casein hydrolysates were added respectively (Table 5). This is similar to the findings of Erdogdu-Arnoczky et al. (1996); Gelinas et al. (1995), and Kadharmestan et al. (1998).
Table 5.
Effect of whey and casein protein concentrates and hydrolysates on the physical attributes of bread, (n = 4)
| Protein (%) added | Loaf Vol. (ML) | Color difference (ΔE) | Texture (firmness) (KG) | |
|---|---|---|---|---|
| Control | 0 | 520d ± 18.0 | 20.3e ± 0.01 | 0.8a ± 0.01 |
| WPC | 5 | 447c* ± 2.51 | 27.3f* ± 0.02 | 1.4b* ± 0.02 |
| 10 | 315b* ± 5.00 | 29.3g* ± 0.04 | 1.5c* ± 0.05 | |
| 15 | 289a* ± 9.50 | 31.4h* ± 0.04 | 1.9d* ± 0.05 | |
| WPH | 5 | 548b* ± 4.72 | 27.3f* ± 0.02 | 1.1b* ± 0.1 |
| 10 | 546b* ± 3.60 | 29.2g* ± 0.04 | 1.2b* ± 0.04 | |
| 15 | 535ab ± 5.03 | 31.3h* ± 0.05 | 1.5c* ± 0.04 | |
| CPC | 5 | 450c* ± 5.00 | 27.3f* ± 0.09 | 1.6b* ± 0.03 |
| 10 | 314b* ± 3.60 | 29.2 g* ± 0.05 | 1.8c* ± 0.08 | |
| 15 | 290a* ± 8.32 | 31.3 h* ± 0.03 | 1.9d* ± 0.02 | |
| CPH | 5 | 552b* ± 5.29 | 27.3f* ± 0.01 | 1.4f* ± 0.03 |
| 10 | 535ab ± 7.50 | 29.2 g* ± 0.01 | 1.3f* ± 0.32 | |
| 15 | 531a ± 1.00 | 31.5 h* ± 0.11 | 1.9g* ± 0.06 |
Data are mean value of three replicates
Means for the same blend and variable with unlike superscripts indicate significant differences using Duncan’s multiple range test (P < 0.05)
*Denotes significant differences from control (P < 0.05)
Breads containing the added milk protein and its hydrolysates were generally darker when compared to control. The color difference of breads supplemented with milk protein concentrates and hydrolysates were significantly (p ≤ 0.05) higher than breads prepared from control (Table 5). However, the color difference increased significantly (p ≤ 0.05) with increasing levels of concentrates and hydrolysates. This was attributed to higher degree of Maillard browning and caramelisation, which are influenced by the distribution of water and the reaction of reducing sugars and amino acids (Kent and Evers 1994).
Measuring texture allows bakers to consistently and objectively monitor their bread while maintaining their own concept of quality (Day and Rogers 1996). Texture analysis is one of the main tests used to measure firmness of bread (Sidhu et al. 1997). The firmness of the Control breads (peak force) was 0.8 Kg, which significantly (p ≤ 0.05) increased on addition of milk proteins and its hydrolysates. The highest firmness in terms of peak force was found with the addition of 15 % whey and casein proteins and their hydrolysates. Our findings were in accordance with the findings of Crowley et al. (2002), in which they reported that bread Crumb firmness was significantly affected by the type of powder and the level of addition. Addition of sodium caseinate at 1 % resulted in a significant increase (P < 0.05) in crumb firmness and increasing the level to 4 % resulted in a further increase. Using sodium caseinate hydrolysate at the same levels had no effect on crumb firmness compared with the parent powder.
Effect of whey proten fortification on microstructure of bread
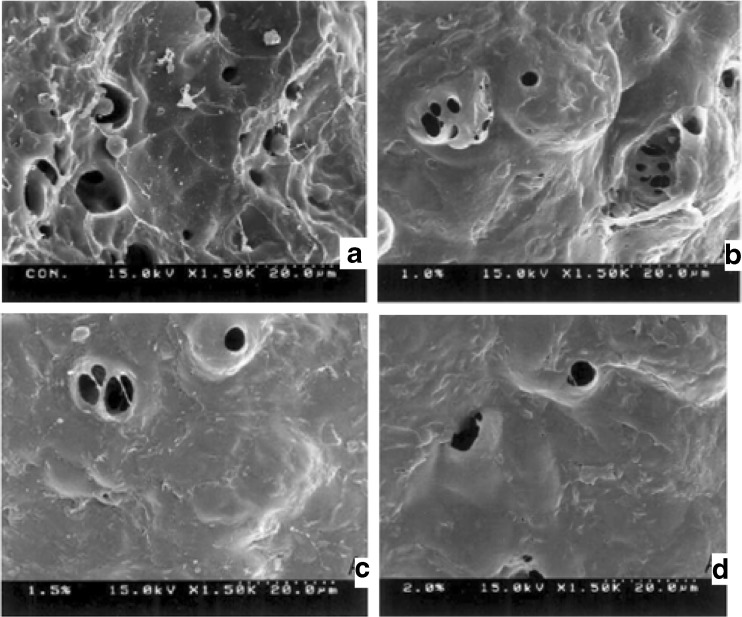
The structures of the bread samples at various levels of whey protein incorporation are shown in Fig. 2. Micrograph of the control bread (0 % added whey protein) showed small starch granules on the surface of the crumb. Gelatinization resulted in coarse surface with a few small gas vacuoles. When whey protein concentrates are added from 5 to 15 % starch granules are in the processes of distortion and degradation that appear in the protein matrix, and remains unchanged in structure during baking (Khoo et al. 1975). Scanning electron microscopy of bread samples shows disruption in the well defined protein – starch complex and shape of the starch granules changes as the concentration of whey protein increases in the wheat flour bread. Fleming and Sosulski (1978) reported that microstructure of bread replaced by soy flour, sunflower concentrate, faba bean concentrate and field pea concentrate showed disruption in the well-defined protein – starch complex of wheat flour bread and the structure of gluten was weak.
Fig. 2.
Scanning electron micrographs of bread crumbs (magnification = ×1500);. a The control bread b 5 % WP c 10 % WP d 15 % WP
Effect of milk protein concentrates and hydrolysates on sensory analysis of bread
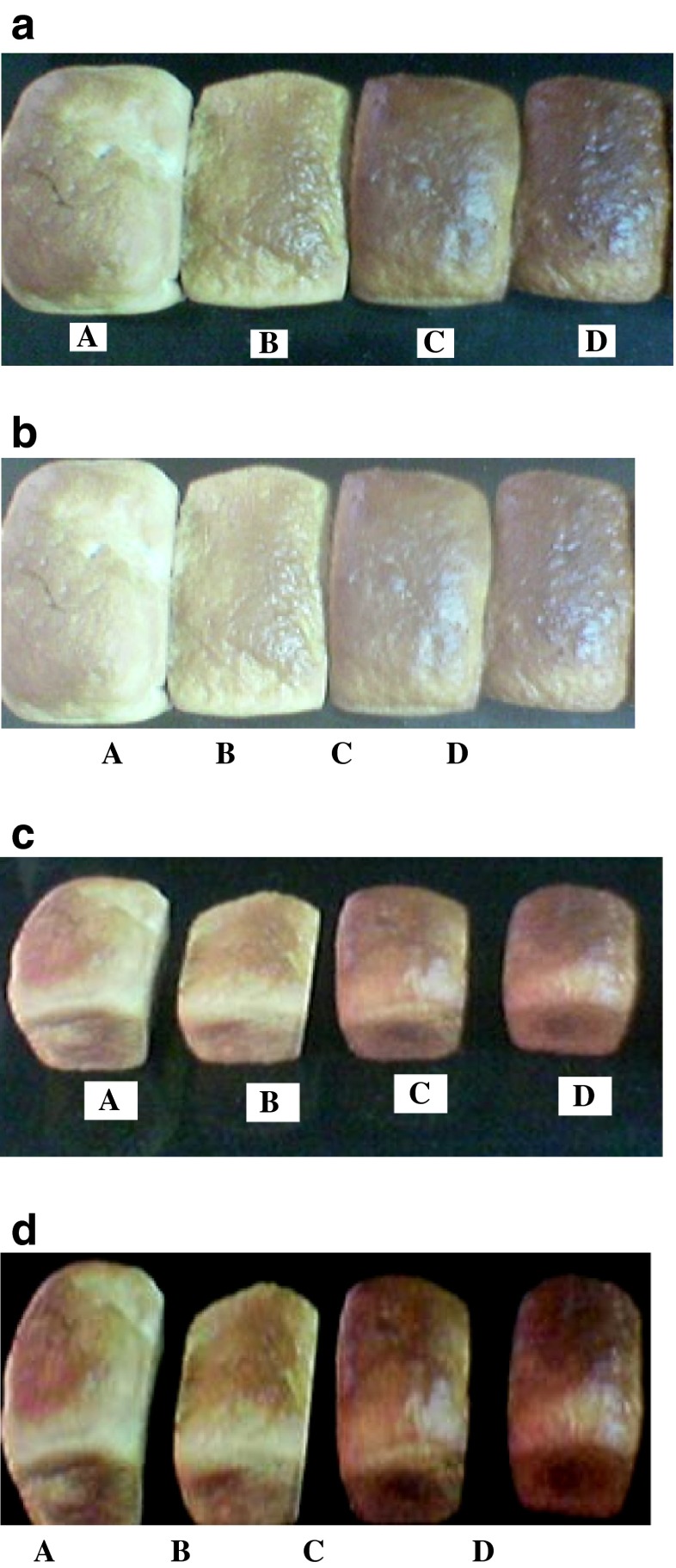
A sensory evaluation panel judged the crust color, crumb color, crumb texture, flavor and overall eating characteristics of the breads fortified with milk protein concentrates and hydrolysates. Sensory scores of bread showed significant decrease in all the parameters when compared with control, which is also evident from the Fig. 3a–d. Lowest points were given to bread fortified with 15 % of both whey protein, casein and their hydrolysates (Table 6). Low score for crust and crumb color given by panelists was because of darkening which may be because of Millard reaction, which obviously will increase with increase in milk proteins and its hydrolysates in the bread. 5 % fortification of breads by milk protein and its hydrolysates were found satisfactory by the panelists and 15 % fortified bread was totally unaccepted by the judges. Matthews et al. (1970) mentioned that substituting high levels of sunflower flour resulted in deterioration of crumb color, grain and texture of the bread. The results of sensory analysis showed that scores assigned by the judges for texture, color and loaf volume were in good agreement with the measurements derived from the physical tests.
Fig. 3.
a Physical appearance of Bread (A) control; (B) 5 % WPC; (C) 10 % WPC; (D) 15 % WPC. b: Physical appearance of Bread (A) control; (B) 5 % WPH; (C) 10 % WPH; (D) 15 % WPH. c: Physical appearance of Bread (A) control; (B) 5 % CPC; (C) 10 % CPC; (D) 15 % CPC. d: Physical appearance of Bread (A) control; (B) 5 % CPH; (C) 10 % CPH; (D) 15 % CPH
Table 6.
Sensory characteristics of bread supplemented with whey protein and casein concentrate and hydrolysates
| Protein (%) added | crust color | Crumb color | crumb texture | flavor | Overall acceptability | |
|---|---|---|---|---|---|---|
| Control | 0 | 6.8d ± 0.04 | 6.8h ± 0.06 | 6.7l ± 0.04 | 6.6d ± 0.03 | 6.7d ± 0.03 |
| WPC | 5 | 6.0c* ± 0.08 | 6.1g* ± 0.06 | 6.2k* ± 0.01 | 6.2c* ± 0.05 | 6.1c* ± 0.05 |
| 10 | 5.7b* ± 0.03 | 5.6f* ± 0.04 | 5.5j* ± 0.03 | 5.1b* ± 0.02 | 5.5b* ± 0.03 | |
| 15 | 4.7a* ± 0.04 | 4.9e* ± 0.03 | 4.9i* ± 0.03 | 4.8a* ± 0.04 | 4.8a* ± 0.03 | |
| Control | 0 | 6.8d ± 0.04 | 6.8h ± 0.06 | 6.7l ± 0.04 | 6.6d ± 0.03 | 6.7d ± 0.03 |
| WPH | 5 | 6.2c* ± 0.16 | 6.5g* ± 0.02 | 6.4k* ± 0.02 | 6.0c* ± 0.07 | 6.3c* ± 0.01 |
| 10 | 5.2b* ± 0.03 | 5.8f* ± 0.02 | 5.7j* ± 0.04 | 5.5b* ± 0.04 | 5.5b* ± 0.02 | |
| 15 | 4.8a* ± 0.03 | 4.2e* ± 0.03 | 4.1i* ± 0.09 | 4.1a* ± 0.09 | 4.3a* ± 0.05 | |
| Control | 0 | 6.8d ± 0.04 | 6.8h ± 0.06 | 6.7l ± 0.04 | 6.6d ± 0.03 | 6.7d ± 0.03 |
| CPC | 5 | 6.1c* ± 0.02 | 6.2g* ± 0.01 | 6.5k* ± 0.05 | 6.1c* ± 0.05 | 6.2c* ± 0.05 |
| 10 | 5.8b* ± 0.03 | 5.6f* ± 0.04 | 5.5j* ± 0.02 | 5.2b* ± 0.40 | 5.5b* ± 0.12 | |
| 15 | 4.7a* ± 0.04 | 4.7e* ± 0.02 | 4.6i* ± 0.04 | 4.2a* ± 0.08 | 4.5a* ± 0.04 | |
| Control | 0 | 6.8d* ± 0.04 | 6.8h ± 0.06 | 6.7l ± 0.04 | 6.6d ± 0.03 | 6.7d ± 0.03 |
| CPH | 5 | 6.1c* ± 0.02 | 6.3g* ± 0.04 | 6.4k* ± 0.02 | 6.3c* ± 0.26 | 6.2c* ± 0.08 |
| 10 | 5.7b* ± 0.03 | 5.1f* ± 0.11 | 5.7j* ± 0.03 | 5.9b* ± 0.04 | 5.6b* ± 0.05 | |
| 15 | 4.1a* ± 0.11 | 4.8e* ± 0.04 | 4.5i* ± 0.04 | 4.3a* ± 0.08 | 4.5a* ± 0.09 |
Data are mean value of three replicates
Means for the same blend and variable with unlike superscripts indicate significant differences using Duncan’s multiple range test (P < 0:05)
*Denotes significant differences from control (P < 0.05)
Conclusion
The results revealed that highly nutritious bread can be prepared by supplementing wheat flour with milk protein concentrates and hydrolysates at 5–15 % levels. The incorporation of WPC, casein and their hydrolysates up to the level of 5 % showed dough properties comparable to control. It was also found that 5 % level incorporation of milk proteins and their hydrolysates have no drastic effect on physical and sensory attributes of bread. Scanning electron microscopy of bread samples shows disruption in the well-defined protein – starch complex of wheat flour bread and the structure of gluten was weak as the concentration of whey protein increases in the wheat flour bread.
Acknowledgments
The authors would like to thank Department of Biotechnology, Govt. of India for financial support.
References
- AACC . Approved methods of analysis. 8. St. Paul: American Association of Cereal Chemists; 1983. [Google Scholar]
- Adler-Nissen J. Enzymic hydrolysis of food proteins. New York: Elsevier 521 Applied Science Publishers; 1986. [Google Scholar]
- Ahmad A, Baba WN, Wani TA, Gani A et al (2014) Effect of green tea powder on thermal, rheological and functional properties of wheat of wheat flour and physical, nutraceutical and sensory analysis of cookies. J Food Sci Technol. doi:10.1007/s13197-014-1701-3 [DOI] [PMC free article] [PubMed]
- Ashwar BA, Shah A, Gani A, Rather SA, et al. Effect of gamma irradiation on the physicochemical properties of alkali extracted rice starch. Radiat Phys Chem. 2014;99:37–44. doi: 10.1016/j.radphyschem.2014.02.002. [DOI] [Google Scholar]
- Axford DWE, Mc Dermott EE, Redman DG. Small scale tests of bread making quality. Milling Feed Fertil. 1978;66:18–2. [Google Scholar]
- Bera MB, Mukherjee RK. Solubility, emulsifying, and foaming properties of rice bran protein concentrates. J Food Sci. 1989;54:142–145. doi: 10.1111/j.1365-2621.1989.tb08587.x. [DOI] [Google Scholar]
- Beuchat LR. Functional and electrophoretic characteristics of succinylated peanut flour protein. J Agric Food Chem. 1977;25:258–261. doi: 10.1021/jf60210a044. [DOI] [Google Scholar]
- Beuchat LR, Cherry JP, Quinn MR. Physicochemical properties of peanut flour as affected by proteolysis. J Agric Food Chem. 1975;23:616–620. doi: 10.1021/jf60200a045. [DOI] [PubMed] [Google Scholar]
- Bhagya S, Srinivasan KS. Effect of different methods of drying on functional properties of enzyme treated ground flour. J Food Sci Technol. 1989;22:329. [Google Scholar]
- Bimlesh M, Malik RC. Studies on some functional characteristics of whey protein polysaccharide complex. J Food Sci Technol. 1996;33:202–206. [Google Scholar]
- Britten M, Giroux HJ, Gaudin V. Effect of pH during heat processing of partially hydrolyzed whey protein. J Dairy Sci. 1994;77:676–684. doi: 10.3168/jds.S0022-0302(94)76999-X. [DOI] [PubMed] [Google Scholar]
- Caessens PWJR, Visser S, Gruppen H, Voragen AGJ. β-Lactoglobulin hydrolysis. I. Peptide composition and functional properties of hydrolysates obtained by the action of plasmin, trypsin, and Staphylococcus aureus V8 protease. J Agric Food Chem. 1999;47:2973–2979. doi: 10.1021/jf981229p. [DOI] [PubMed] [Google Scholar]
- Castimopoolas N, Funk SK, Meyer EW. Thermal aggregation of glycinin subunits. Cereal Chem. 1970;47:331. [Google Scholar]
- Ceirwyn SJ. Analytical chemistry of foods. London: Chapman & Hall; 1995. pp. 88–89. [Google Scholar]
- Chobert JM, Sitohy MZ, Whitaker JR. Solubility and emulsifying properties of caseins modified enzymatically by Staphylococcus aureus V8 protease. J Agric Food Chem. 1988;36:220–224. doi: 10.1021/jf00079a055. [DOI] [Google Scholar]
- Chobert JM, Bertrand HC, Nicolas MG. Solubility and emulsifying properties of caseins and whey proteins modified enzymatically by trypsin. J Agric Food Chem. 1988;36(5):883–892. doi: 10.1021/jf00083a002. [DOI] [Google Scholar]
- Crowley PO, Brien CM, Slattery H, Chapman D, Arendt EK, Stanton C. Functional properties of casein hydrolysates in bakery applications. Eur Food Res Technol. 2002;215(2):131–137. doi: 10.1007/s00217-002-0510-5. [DOI] [Google Scholar]
- Day DD, Rogers D. Fourier-based texture measures with application to the analysis of the cell structure of baked products. Digital Signal Process. 1996;6:138–144. doi: 10.1006/dspr.1996.0014. [DOI] [Google Scholar]
- Erdogdu-Arnoczky N, Czuchzjowska Z, Pomeranz Y. Functionality of whey and casein in bread making by fixed and optimized procedures. Cereal Chem. 1996;73:309–316. [Google Scholar]
- Finney KF. Optimized, straight dough, bread-making method ofter 44 years. Cereal Chem. 1984;61:20–27. [Google Scholar]
- Fleming SE, Sosulski FW. Evaluation of bread fortified with concentrated plant proteins. Cereal Chem. 1978;55:373–382. [Google Scholar]
- Gani A, Broadway AA, Mudasir A, Ashwar BA, Wani AA, Wani SM, Masoodi FA, Khatkar BS. Effect of whey and casein protein hydrolysates on rheological, textural and sensory properties of cookies. J Food Sci Technol. 2014 doi: 10.1007/s13197-014-1649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas P, Audet J, Lachance O, Vachon M. Fermented dairy ingredients for bread: effects on dough rheology and bread characteristics. Cereal Chem. 1995;72(2):151–154. [Google Scholar]
- Giami SY. Rheological and bread-making properties of wheat fluted pumpkin seed flour blends. J Dairying Foods Home Sci. 2001;20:41–45. [Google Scholar]
- Giami SY, Barber LI. Utilization of protein concentrates from ungerminated and germinated fluted pumpkin (Telfairia occidentalis Hook) seeds in cookie formulations. J Sci Food Agric. 2004;38:56–60. [Google Scholar]
- Greene JL, Bovell-Benjamin AC. Macroscopic and sensory evaluation of bread supplemented with sweet potato flour. J Food Sci. 2004;69:167–173. [Google Scholar]
- Indrani D, Prabhasankar P, Rajiv J, Venkateswara RG. Influence of whey protein concentrate on the rheological characteristics of dough, microstructure and quality of unleavened flat bread (parotta) Food Res Int. 2007;40:1254–1260. doi: 10.1016/j.foodres.2007.08.005. [DOI] [Google Scholar]
- Iqbal A, Khalil IA, Ateeq N, Khan MS. Nutritional quality of important food legumes. Food Chem. 2006;97:331–335. doi: 10.1016/j.foodchem.2005.05.011. [DOI] [Google Scholar]
- Kadharmestan C, Baik BK, Czuchajowska Z. Whey protein concentrated with high heat or hydrostatic pressure in wheat-based products. Cereal Chem. 1998;75:762–766. doi: 10.1094/CCHEM.1998.75.5.762. [DOI] [Google Scholar]
- Kent NL, Evers AD. Bread made with gluten substitutes. Technol cereal. Oxford: Pergamon Press; 1994. [Google Scholar]
- Keuhler CA, Stine CM. Effect of enzymatic hydrolysis on some functional properties of whey protein. J Food Sci. 1974;39:379–382. doi: 10.1111/j.1365-2621.1974.tb02899.x. [DOI] [Google Scholar]
- Khoo U, Christianson DD, Inglett GE. Scanning and transmission microscopy of dough and bread. Bakers Digest. 1975;49(4):24–26. [Google Scholar]
- Kinsella JE, Whitehead DM. Proteins in whey: chemical, physical, and functional properties. Adv Food Nutr Res. 1989;33:437–438. doi: 10.1016/s1043-4526(08)60130-8. [DOI] [PubMed] [Google Scholar]
- Lieske B, Konrad G. Physico-chemical and functional properties of whey protein as affected by limited papain proteolysis and selective ultrafiltration. Int Dairy J. 1996;6:13–31. doi: 10.1016/0958-6946(94)00049-2. [DOI] [Google Scholar]
- Lorenz K, Dilsaver W, Wolt N (1979) Faba bean flour and protein concentrate in baked goods and in pasta products. Bakers Digest 39–49
- Ludwig I, Krause W, Hajos G. Functional properties of enzymatically modified milk proteins. Acta Aliment. 1995;24:289–296. [Google Scholar]
- Madenci AB, Bilgiçli N. Effect of whey protein concentrate and buttermilk powders on rheological properties of dough and bread quality. J Food Qual. 2014;37:117–124. doi: 10.1111/jfq.12077. [DOI] [Google Scholar]
- Matthews RH, Sharpe EJ, Clark WM. The use of some oilseed flours in bread. Cereal Chem. 1970;47:181–185. [Google Scholar]
- Mutilangi WAM, Panyam D, Kilara A. Functional properties of hydrolyzates from proteolysis of heat denatured whey protein isolate. J Food Sci. 1996;61:270–274. doi: 10.1111/j.1365-2621.1996.tb14174.x. [DOI] [Google Scholar]
- Peryam DR, Pilgrim FJ. Hedonic scale method of measuring food preferences. Food Technol. 1957;11:9–13. [Google Scholar]
- Rahma EH, Mostafa MM. Functional properties of peanut fruit as affected by different heat treatment. J Food Sci Technol. 1998;25:11–15. [Google Scholar]
- Rhicha S, Radha C, Prakash J, Kaul P. Whey protein hydrolysate: functional properties, nutritional quality and utilization in beverage formulation. Food Chem. 2007;101:1484–1491. doi: 10.1016/j.foodchem.2006.04.021. [DOI] [Google Scholar]
- Sekul AA, Vinnett CH, Ory RL. Some functional properties of peanut proteins partially hydrolyzed with papain. J Agric Food Chem. 1978;26:855–858. doi: 10.1021/jf60218a035. [DOI] [Google Scholar]
- Sidhu JS, Al-Saqer J, Al-Zenki S. Comparison of methods for the assessment of the extent of staling in bread. Food Chem. 1997;58:161–167. doi: 10.1016/S0308-8146(96)00196-3. [DOI] [Google Scholar]
- Sindayikengera S, Xia W. Nutritional evaluation of caseins and whey proteins and their hydrolysates from Protamex. J Zhejiang Univ. 2005;7:90–98. doi: 10.1631/jzus.2006.B0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Bajaj M, Sharma S, Sidhu JS. Studies on the development of high-protein biscuits from composite flours. Plant Foods Hum Nutr. 1993;43:181–189. doi: 10.1007/BF01087922. [DOI] [PubMed] [Google Scholar]
- Slattery H, Fitzgerald RJ. Functional properties and bitterness of sodium caseinate hydrolysates prepared with a bacillus proteinase. J Food Sci. 1998;63(3):418–422. doi: 10.1111/j.1365-2621.1998.tb15755.x. [DOI] [Google Scholar]
- Tronsmo KM, Faergestad EM, Schofield JD, Magnus S. Wheat protein quality in relation to baking performance evaluated by the Chorleywood bread process and a hearth bread baking test. Cereal Sci. 2003;38:205–215. doi: 10.1016/S0733-5210(03)00027-4. [DOI] [Google Scholar]
- Verbruggen IM, Veraverbeke WS, Delcour JA. Significance of LMW-GS and HMW-GS for dough extensibility: addition versus incorporation protocols. J Cereal Sci. 2001;33:253–260. doi: 10.1006/jcrs.2000.0353. [DOI] [Google Scholar]
- Vrignaud MY. Milk proteins, their properties and applications. CCB Rev Chocolate Confectionery Bak. 1977;2:11–13. [Google Scholar]
- Wani IA, Sogi DS, Shivhare US, Gill BS (2014) Physico-chemical and functional properties of native and hydrolyzed kidney bean (Phaseolus vulgaris L.) protein isolates. http://dx.doi.org/10.1016/j.foodres.2014.08.027
- Warren AB, Hnat DL, Michnowski J. Protein fortification of cookies, crackers, and snack bars: uses and needs. Cereal Foods World. 1983;28:441–444. [Google Scholar]
- Zadow JG. Measurement of the effect of whey protein concentrates on fermenting doughs by the instron tester. Aust J Dairy Technol. 1981;36:56–59. [Google Scholar]