Abstract
Reduction of the AFs produced by Aspergillus parasiticus CECT 2681 in wheat tortillas by isothiocyanates (ITCs) from oriental and yellow mustard flours was evaluated in this study. Polyethylene plastic bags were introduced with wheat tortillas contaminated with A. parasiticus and treated with 0, 0.1, 0.5 or 0.1 g of either oriental or yellow mustard flour added with 2 ml of water. The wheat tortillas were stored at room temperature during 1 month. The quantification of the AFs produced was analyzed by liquid chromatography (LC) coupled to the mass spectrometry detection in tandem (MS/MS). Gaseous allyl isothiocyanate (AITC) from oriental mustard was more effective than p-hydroxybenzyl isothiocyanate (p-HBITC) from yellow mustard to inhibit the production of AFs. More importantly, 1 g of AITC was able to reduce >90 % of AFs B1, B2, G1 and G2. p-HBITC is less stable and volatile than AITC, leading to a much lower AFs (average of 17.7 to 45.2 %). Further studies should investigate the use of active packaging using oriental mustard flour and water to reduce the production of AFs by Aspergillus species in bakery goods.
Keywords: Aflatoxins, Glucosinolates, Isothiocyanates, Mustard flours, Mycotoxin reduction, LC-MS/MS
Introduction
Aflatoxins (AFs, Fig. 1) are naturally occurring mycotoxins produced by many species of Aspergillus, most notably A. flavus and A. parasiticus, during their growth on foods and animal feed (Williams et al. 2004). They are listed as group I carcinogens by the International Agency for Research on Cancer (IARC), which primarily affect the liver (Lee et al. 2004). Although more than 20 AFs have been identified, the major AFs of concern are known as B1 (AFB1), B2, G1 and G2. Among them, AFB1 is normally the most prevalent toxin and the most toxic (Delmulle et al. 2005; Zhang et al. 2009). As a result, many countries have regulatory limits for AFB1 levels in agricultural products. The European Union (EU) sets the limits for AFB1 and for total aflatoxins (B1, B2, G1 and G2) in nuts, dried fruits, cereals and spices. These limits vary according to the product, but range from 2 to 8 μg/kg for AFB1 and from 4 to 15 μg/kg for total aflatoxins (Van Egmond 1995).
Fig. 1.
General structure of aflatoxins B1, B2, G1 and G2
Human exposure to AFs can occur directly from ingestion of contaminated foods or indirectly by the consumption of meat/dairy products from animals previously exposed to AFs. AFs are extremely toxic, mutagenic, teratogenic and carcinogenic compounds that have been implicated in human hepatic and extra hepatic carcinogenesis (Iqbal et al. 2012).
Isothiocyanates (ITCs) consist of aliphatic and aromatic compounds generated from the hydrolysis of glucosinolates (GLCs) by myrosinase in cruciferous vegetables such as cauliflower, broccoli, cabbage, horseradish and mustard (Ciska and Pathak 2004; Delaquis and Mazza 1995; Whitmore and Naidu 2000). Myrosinase and GLCs are physically separated within plant cells. Hydrolysis of GLCs occurs when plant tissues are disrupted in the presence of moisture, forming ITCs, nitriles, thiocyanates or epithionitriles depending on environmental conditions (Al-Gendy et al. 2010; Delaquis and Mazza 1995; Mari et al. 2008). Studies have shown that ITCs exhibit biocidal activity against microorganisms including fungi, bacteria, nematodes and insects. In particular, it has been demonstrated that allyl ITC (AITC), which is formed from sinigrin (2-propenyl glucosinolate), effectively inhibits a variety of pathogenic microorganisms, even at low concentrations (Lin et al. 2000; Luciano and Holley 2009). The potential of AITC as a natural antimicrobial has been shown in different foods, including chicken breast (Shin et al. 2010), ground beef (Chacon et al. 2006a; Nadarajah et al. 2005), dry-cured ham (Graumann and Holley 2007), fermented dry sausages (Chacon et al. 2006b) and tuna meat (Hasegawa et al. 1999). The goals of this study were to study a) the quali-quantitative GLCs composition of yellow and oriental mustard flours and b) the reduction of AFs production by A. parasiticus in wheat tortilla treated with ITCs generated from mustard flours.
Materials and methods
Chemicals
AFs B1, B2, G1, G2, sinalbin and sinigrin (98 % purity), phosphate buffer saline (PBS) at pH 7, formic acid (HCOOH), allyl isothiocyanate (AITC), para-hydroxybenzylisothiocyanate (PHBITC), tetrabutylammonium hydrogen sulfate (TBA), ammonium formate, and sodium chloride (NaCl) were obtained from Sigma–Aldrich (St Louis MO., USA). Oriental and yellow mustard flours were provided by G.S. Dunn dry mustard millers (Hamilton, ON, Canada). Methanol was purchased from Fisher Scientific (Hudson, NH, USA). Deionized water (<18 MX cm resistivity) was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA). Chromatographic solvents and water were degassed for 20 min using a Branson 5200 (Branson Ultrasonic Corp., CT, USA) ultrasonic bath. Aspergillus parasiticus CECT 2681, was obtained from the Spanish Type Culture Collection (CECT, Valencia, Spain).
Glucosinolates extraction and determination from oriental and yellow mustard flours
GLCs from oriental and yellow mustard flours were extracted using the method described by Prestera et al. (1996) with few modifications. Twenty grams of either mustard flour was introduced in a 50 mL glass tube and autoclaved at 115 °C during 15 min to inactivate the endogenous myrosinase. Samples were added to different Erlenmeyers containing 200 ml of boiling distilled water (100 °C) and the mixtures were stirred for 10 min at 350 rpm. Then, the suspensions were cooled until they reached room temperature, centrifuged at 2500 rpm for 5 min at 4 °C and filtered through filter paper (Whatman no. 4) into 50 ml screw-capped tubes. The extracts were filtered through a 0.22 μM filter and injected into liquid chromatography diode array detector apparatus (LC-DAD). Separation and quantification of glucosinolates was performed using a Shimadzu LC system (Shimadzu, Japan), equipped with a C18 column Gemini (4.6 × 150 mm i.d. 5 μm; Phenomenex, Palo alto, CA). Elution was carried out isocratically for 20 min at a flow rate of 1 ml/min, using a solvent system containing 20 % (v/v) acetonitrile and 80 % water +0.02 M tetrabutylammonium hydrogen sulfate (pH 5.5). The injection volume used was 20 μl. A diode array detector was used to measure the absorbance at 227 nm in order to verify and quantify the presence of GLCs.
Wheat tortillas inoculation and storage
Single wheat tortillas were introduced in multilayer polyethylene plastic bags (Saplex, Barcelona, Spain). Then, they were treated with three different quantities of oriental or yellow mustard flour (0.1, 0.5 and 1 g). The flours were introduced in a 50-mm petri dish bottom and added with 2 mL of water to promote the activation of the myrosinase and, consequently, the formation of ITCs (Fig. 2). The wheat tortillas were contaminated with 1 mL of Aspergillus parasiticus CECT 2681 grown in potato dextrose broth (PDB, Oxoid, UK) medium containing 106 conidia/ml. Conidial concentration was measured by optical density at 600 nm in sterile water and adjusted to 106 conidia/ml in PDB as reported Kelly et al. (2006). The control group did not receive any treatment with mustard flour+water. The plastic bag were thermally sealed and incubated at 23 °C during 15 days. Then, bags were opened and the tortillas were autoclaved (121 °C) and extracted for AFs quantification.
Fig. 2.
Schematization of AFs reduction experiments carried out on wheat tortilla using gaseous dispersion of ITCs generated by enzymatic hydrolysis of GLCs contained in oriental and yellow mustard flours. a Control wheat tortilla contaminated by Aspergillus parasiticus CECT 2681 and wheat tortillas treated with a 0.1 b 0.5 and c 1 g of oriental mustard flours at 8 days incubation. Visible the green spots (fungal growth) are noticeable in the control group and absent in the treated groups
Aflatoxins extraction
The aflatoxins extraction was carried out using the method described by Liu et al. (2013) with modifications. Briefly, an aliquot of 5 g of each finely ground wheat tortilla (Oster Classic grinder, Oster, Valencia, Spain) was weighed in a 50 mL plastic tube. Then, 0.5 g of sodium chloride (NaCl) and 25 mL of a mixture methanol/water (80:20, V/V) were added and the samples were extracted using Ultra Ika T18 basic Ultraturrax (Staufen, Germany) for 3 min. The mixture was centrifuged at 4500×g for 5 min and the supernatant was evaporated to dryness with a Büchi Rotavapor R-200 (Postfach, Switzerland). The residue was re-dissolved in 1 mL of the extraction solvent, filtered through a 0.22 μM filter and injected into LC-MS/MS.
AFs identification and quantification by LC-MS/MS
The liquid-chromatography analysis system consisted of a binary LC-20AD pump, a SIL-20AC homoeothermic auto sampler, a CTO-20A column oven, a CMB-20A controller and Analyst Software 1.5.2 was used for data acquisition and processing. The separation of AFs was performed on a Gemini NX C18 column (150 × 2.0 mm I.D, 3.0 μm, Phenomenex, Palo Alto, CA) at room temperature (20 °C). The mobile phase was composed of solvents A (0.1 % formic acid in water) and B (0.1 % formic acid in acetonitrile) at a flow rate of 0.2 mL/min. After a hold time of 0.6 min, 10 % of B reached 95 % in 1.6 min and was kept constant for 0.3 min. Afterwards, the column was re-equilibrated with 10 % solvent A until the end of the run at 4.0 min. An API-4000 triple-quadruple MS/MS system (Applied Biosystems, Foster City, CA, USA) equipped with an ESI interface in positive mode was used for detection in multiple reactions monitoring (MRM) mode. The main MS parameters were optimized and finally set as follows: nebulizer gas (GS1), 55 psi; auxiliary gas (GS2), 50 psi; curtain gas (CUR) 15 psi; capillary temperature 550 °C; ion spray voltage (IS) 5500 V. Nitrogen was used as the nebulizer, heater, curtain and collision gas. The precursor-to-product ion transitions were m/z 313.3/241.3–228.5, m/z 315.3/259.0–288.4, m/z 329.7/243.3–200.5, m/z 331.9/189.3–217.1 for AFB1, AFB2, AFG1 and AFG2 respectively.
Results and discussion
Glucosinolates in oriental and yellow mustard flour
The GLCs present in the yellow and oriental mustard flours were analytically characterized to predict the total amount of the GLCs that can be converted in ITCs through the action of myrosinase. Sinigrin was detected in the oriental mustard flour at 5.2 %, whereas sinalbin was 4.6 % of the yellow mustard flour. Sinigrin and sinalbin are the precursors of the antimicrobial compounds allyl (AITC) and parahydroxybenzyl isothiocyanate (p-HBITC), respectively.
The addition antimicrobials that can act during the storage of bakery products to control the presence of the mycotoxigenic fungi is of great interest. Most bakery goods are added with propionate, sorbate or benzoate salts to avoid mold growth, but consumers have become more reluctant to the use of synthetic preservatives in their foods. All plants in the Brassicaceae family contain GLCs as secondary metabolites, and oriental and yellow mustard contains the GLCs sinigrin and sinalbin. Upon physical damage of the plant tissue, hydrolysis of GLCs is catalyzed by the endogenous enzyme myrosinase in the presence of moisture to produce the antimicrobials compounds AITC and p-HBITC (Delaquis and Mazza 1995; Ekanayake et al. 2006). The mechanism of action of these antimicrobial compounds is uncertain, but they may inhibit essential enzymes and cause membrane damage (Lin et al. 2000).
AFs reduction in wheat tortilla
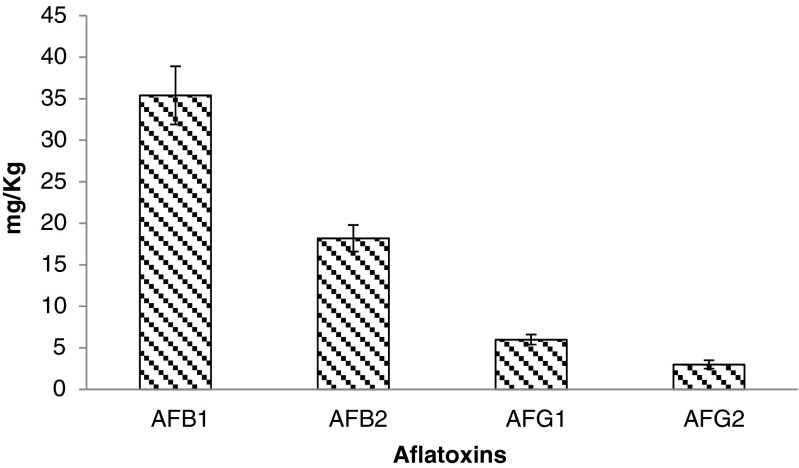
The simulation of AFs contamination was carried out by inoculating the wheat tortillas with Aspergillus parasiticus CECT 2681 (AFs producer). As presented on Fig. 3, the control wheat tortilla contained 35.4 mg/kg of the AFB1, 18.2 mg/kg of AFB2, 6 mg/kg of AFG1 and 3 mg/kg of AFG2.
Fig. 3.
AFs B1, B2, G1 and G2 produced by Aspergillus parasiticus CECT 268 on wheat tortillas in the absence of oriental and yellow mustard (control group)
As shown on Table 1, the AITC generated from oriental mustard flour was more efficient that p-HBITC to avoid the production of AFs. The highest reduction of the AFs (>90 %) was obtained employing 1 g of the oriental mustard flour. AFBs production were the most sensitive to AITC, where 0.1 g of oriental flour already produced a >74.8 % of these toxins. Aflatoxin production was affected quite evenly when 0.5 or 1 g of oriental mustard flour was used, with the exception of AFG1 that was reduced by 54.17 ± 3.6 % with 0.5 g of the AITC-producing flour.
Table 1.
AFB1, AFB2, AFG1 and AFG2 reduction rates produced by the gaseous dispersion of ITCs produced by the enzymatic hydrolysis of the glucosinolates present in a) oriental and b) yellow mustard flour. The plastic bag were thermally sealed and incubated at 23 °C during 15 days
| % of reduction | ||||
|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | |
| a) Oriental mustard flour (g) | ||||
| Control | 0.00 | 0.00 | 0.00 | 0.00 |
| 0.1 | 74.86 ± 3.5 | 85.88 ± 4.8 | 18.33 ± 2.0 | 5.66 ± 0.6 |
| 0.5 | 84.29 ± 4.2 | 87.06 ± 3.9 | 54.17 ± 3.6 | 87.17 ± 2.9 |
| 1.0 | 94.00 ± 5.0 | 93.82 ± 3.8 | 90.00 ± 4.0 | 96.23 ± 3.6 |
| b) Yellow mustard flour (g) | ||||
| Control | 0.00 | 0.00 | 0.00 | 0.00 |
| 0.1 | 37.14 ± 2.2 | 19.12 ± 2.5 | 16.67 ± 0.6 | 16.98 ± 1.0 |
| 0.5 | 71.43 ± 3.8 | 67.65 ± 3.7 | 18.33 ± 0.7 | 60.38 ± 3.4 |
| 1.0 | 72.29 ± 4.1 | 82.35 ± 1.9 | 35.83 ± 2.7 | 77.36 ± 2.9 |
AFs reduction was much lower with the use of yellow mustard. The mean reduction found ranged from 17.7 to 45.2 % for each aflatoxin. Again, % reduction was higher for AFBs than for AFGs. However, it is noteworthy that AFBs were produced in much higher doses that AFGs in the control group (Fig. 3). Figure 4 shows a LC-MS/MS chromatogram of the AFs present in wheat tortillas treated with yellow mustard flour. The mean AFs reduction acquired with yellow mustard was 1.5 fold lower than those found for oriental mustard. This phenomenon can be related to the stability of the isothiocyanates generated by the hydrolysis of the glucosinolates present in both flours used. In particular, p-hydroxybenzyl isothiocyanate is less stable in aqueous media and presents less volatility than allyl isothiocyanate (Luciano et al. 2011).
Fig. 4.
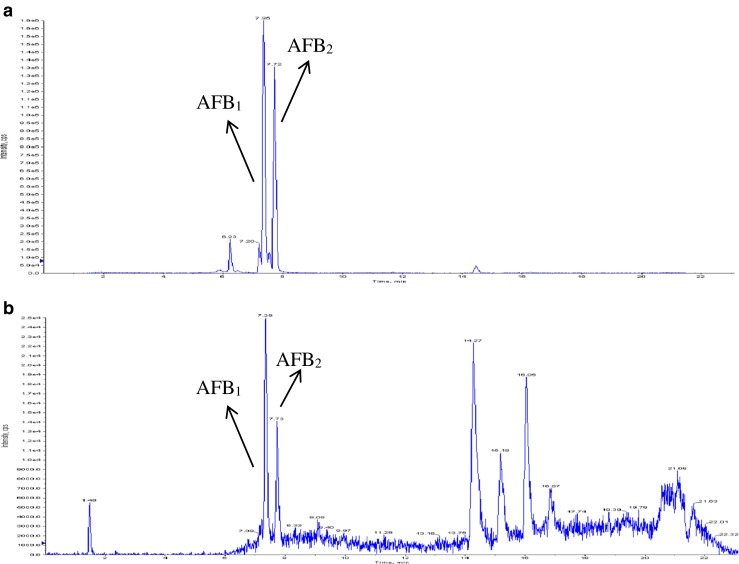
LC-MS/MS chromatograms of the AFB1 and AFB2 detected in wheat tortillas; a control and b treated with 1 g of yellow mustard flour
This article can be considered the first where the application of isothiocyanates has been applied in wheat tortilla to reduce the Aspergillus parasiticus growth and consequently the content of AFs. Soher and Amal (2011) studied the effect of hydrochloric acid (HCl) on AFB1 degradation in contaminated corn gluten under different HCl concentrations, hydrolysis temperatures and hydrolysis times. During the wet milling process the highest AFB1 level (37.86 %) was found in corn gluten fraction. Treatment with 1 mol/L HCL at 110 °C produced a degradation of AFB1 from 27.6 to 42.5 %.
Jubeen et al. (2012) investigated the effect of ultra violet irradiation on aflatoxins in ground and tree nuts. Samples of nuts were randomly selected from the retail market of Faisalabad with a moisture content of the nuts artificially increased to 10 ± 3 % and 16 ± 3 % to facilitate the mold growth. The samples were stored at a room temperature of 25–30 °C for 12 weeks. The stored nut samples were checked after 12 weeks and the fungi were found growing in all nuts along with considerable AFs production. AFs and mold contaminated samples were exposed to UV radiations of 265 nm for 15, 30 and 45 min. The fungicidal activity of UV radiation was more pronounced in nuts adjusted at high moisture level. The order of sensitivity for fungal disinfection by UV irradiation was walnut > almond = pistachio > peanuts. There was a proportional decrease in AFs levels with increased exposure time. Complete elimination of AFG2 was achieved in all nut samples after 15 min exposure, while AFG1 showed 100 % degradation only in almond and pistachio. After 45 min exposure to UV, AFB1 showed maximum reduction of 96.5 % in almond and pistachio. The degradation of total AFs as well as that of AFB1 by UV irradiation was found to follow first order kinetics.
Méndez-Albores et al. (2013) studied the effect of roasting and alkalization processes on the stability of AFB1 and AFB2, on cocoa samples contaminated with AFs at a concentration of 220.7 μg/kg and roasted at 250 °C for 15 min. Roasting conditions caused a notable reduction in the AFs content (up to 71 %). The resulting cocoa liquors contaminated with 63.9 ng/g were thermal-alkaline treated with sodium, potassium, and calcium hydroxide at three different concentrations (10, 20, and 30 g/kg). At a concentration of 10 g/kg, the AFs reduction was more effective when using NaOH and Ca(OH)2 (up to 94 %) than when using KOH (up to 88 %). However, at concentrations of 20 and 30 g/kg, all of the three chemicals were almost equally effective for AFs degradation (up to 98 %).
Azaiez et al. (2013) studied the reduction of the fumonisins (FBs) present in loaf bread contaminated with Gibberella moniliformis CECT 2987 by allyl (AITC), phenyl (PITC) and benzyl isothiocyanates (BITC). In addition, the antifungal activity of these ITCs toward Fusarium mycotoxigenic strains was also evaluated. The ITCs employed inhibited the growth of three mycotoxigenic Fusarium strains, reducing the mycelium size by 2.1 to 89.7 %, depending on the type of ITC and dose used. The ITCs used also reacted with the FB2 produced by G. moniliformis in the bread, reducing its levels by 73–100 % depending on the dose and time of exposure.
Similarly to the present study, Hontanaya et al. (2015) studied the effect of isothiocyanates (ITCs) generated by the enzymatic hydrolysis of the glucosinolates (GLCs) present in oriental and yellow mustard flours in liquid media and in nuts (peanut, cashew, walnut, almond, hazelnut and pistachio) contaminated by an aflatoxinogenic A. parasiticus. The ITCs reduced the A. parasiticus growth in both liquid media, where AFs reduction in ranged from 83.1 to 87.2 % using oriental mustard flour and 27.0 to 32.5 % with yellow flour. Nuts were only treated with oriental mustard flour and the mean AFs reduction ranged from 88 to 89 %.
Conclusions
The present study showed the capacity of ITCs generated by oriental and yellow mustard flours to reduce AFs naturally produced in wheat tortilla by Aspergillus parasiticus. This study shows that antimicrobial devices containing mustard flour and water can be used as natural preservatives for bakery products that are commonly contaminated by Aspergillus species. Further investigation will be focused on use of active packaging, through an antimicrobial sachet or patch, as a form to introduce the production of ITCs in wheat tortilla bags.
Acknowledgments
This research was supported by the Ministry of Economy and Competitiveness (AGL2013-43194-P).
References
- Al-Gendy AA, El-gindi OD, Hafez AS, Ateya AM. Glucosinolates, volatile constituents and biological activities of Erysimum corinthium Boiss. (Brassicaceae) Food Chem. 2010;118:519–524. doi: 10.1016/j.foodchem.2009.05.009. [DOI] [Google Scholar]
- Azaiez I, Meca G, Manyes L, Fernández-Franzón M. Antifungal activity of gaseous allyl, benzyl and phenyl isothiocyanate in vitro and their use for fumonisins reduction in bread. Food Control. 2013;32:428–434. doi: 10.1016/j.foodcont.2013.01.020. [DOI] [Google Scholar]
- Chacon PA, Buffo RA, Holley RA. Inhibitory effects of microencapsulated allyl isothiocyanate (AIT) against Escherichia coli O157:H7 in refrigerated, nitrogen packed, finely chopped beef. Int J Food Microbiol. 2006;107:231–237. doi: 10.1016/j.ijfoodmicro.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Chacon PA, Muthukumarasamy P, Holley RA. Elimination of Escherichia coli O157:H7 from fermented dry sausages at an organoleptically acceptable level of microencapsulated allyl isothiocyanate. Appl Environ Microbiol. 2006;72:3096–3102. doi: 10.1128/AEM.72.5.3096-3102.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciska E, Pathak DR. Glucosinolate derivatives in stored fermented cabbage. J Agric Food Chem. 2004;52:7938–7943. doi: 10.1021/jf048986+. [DOI] [PubMed] [Google Scholar]
- Delaquis PJ, Mazza G. Antimicrobial properties of isothiocyanates in food preservation. Food Technol. 1995;49:73–84. [Google Scholar]
- Delmulle BS, De Saeger SMDG, Sibanda L, Barna-Vetro I, Van Peteghem CH. Development of an immuno-based lateral flow dipstick for the rapid detection of aflatoxin B1 in pig feed. J Agric Food Chem. 2005;53:3364–3368. doi: 10.1021/jf0404804. [DOI] [PubMed] [Google Scholar]
- Ekanayake A, Kester JJ, Li JJ, Zehentbauer GN, Bunke PR, Zent JB. Isoguard TM: a natural anti-microbial agent derived from white mustard seed. Acta Horticult. 2006;709:101–108. doi: 10.17660/ActaHortic.2006.709.11. [DOI] [Google Scholar]
- Graumann GH, Holley RA. Survival of Escherichia coli O157:H7 in needle-tenderized dry cured Westphalian ham. Int J Food Microbiol. 2007;118:173–179. doi: 10.1016/j.ijfoodmicro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Hasegawa N, Matsumoto Y, Hoshino A, Iwashita K. Comparison of effects of Wasabia japonica and allyl isothiocyanate on the growth of four strains of Vibrio parahaemolyticus in lean and fatty tuna meat suspensions. Int J Food Microbiol. 1999;49:27–34. doi: 10.1016/S0168-1605(99)00043-4. [DOI] [PubMed] [Google Scholar]
- Hontanaya C, Meca G, Luciano FB, Mañes J, Font G. Inhibition of the aflatoxins B1, B2, G1, and G2, production by Aspergillus parasiticus in nuts using yellow and oriental mustard flours. Food Control. 2015;47:154–160. doi: 10.1016/j.foodcont.2014.07.008. [DOI] [Google Scholar]
- Iqbal SZ, Asi MR, Ariño A, Akram N, Zuber M. Aflatoxin contamination in different fractions of rice from Pakistan and estimation of dietary intakes. Mycotoxin Res. 2012;28:175–180. doi: 10.1007/s12550-012-0131-1. [DOI] [PubMed] [Google Scholar]
- Jubeen F, Bhatti IA, Khan MZ, Zahoor-Ul H, Shahid M. Effect of UVC irradiation on aflatoxins in ground nut (Arachis hypogea) and tree nuts (Juglans regia, prunus duclus and pistachio Vera) J Chem Soc Pak. 2012;34:1366–1374. [Google Scholar]
- Kelly S, Grimm LH, Bendig C, Hempel DC, Krull R. Effects of fluid dynamic induced shear stress on fungal growth and morphology. Process Biochem. 2006;41:2113–2117. doi: 10.1016/j.procbio.2006.06.007. [DOI] [Google Scholar]
- Lee NA, Wang S, Allan RD, Kennedy IR. A rapid aflatoxin B1 ELISA: development and validation with reduced matrix effects for peanuts, corn, pistachio, and soybeans. J Agric Food Chem. 2004;52:2746–2755. doi: 10.1021/jf0354038. [DOI] [PubMed] [Google Scholar]
- Lin CM, Preston JF, Wei CI. Antibacterial mechanism of allyl isothiocyanate. J Food Prot. 2000;63:727–734. doi: 10.4315/0362-028x-63.6.727. [DOI] [PubMed] [Google Scholar]
- Liu S, Qiu F, Kong W, Wei J, Xiao X, Yang M. Development and validation of an accurate and rapid LC-ESI-MS/MS method for the simultaneous quantification of aflatoxin B1, B2, G1 and G2 in lotus seeds. Food Control. 2013;29:156–161. doi: 10.1016/j.foodcont.2012.05.069. [DOI] [Google Scholar]
- Luciano FB, Holley RA. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157:H7. Int J Food Microbiol. 2009;131:240–245. doi: 10.1016/j.ijfoodmicro.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Luciano FB, Belland J, Holley RA. Microbial and chemical origins of the bactericidal activity of yellow mustard powder toward Escherichia coli O157:H7 during dry sausage ripening. Int J Food Microbiol. 2011;145:69–74. doi: 10.1016/j.ijfoodmicro.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Mari M, Leoni O, Bernardi R, Neri F, Palmieri S. Control of brown rot on stone fruit by synthetic and glucosinolate-derived isothiocyanates. Postharvest Biol Technol. 2008;47:61–67. doi: 10.1016/j.postharvbio.2007.06.003. [DOI] [Google Scholar]
- Méndez-Albores A, Campos-Aguilar AZ, Moreno-Martínez E, Vázquez-Durán A. Physical and chemical degradation of B-aflatoxins during the roasting and dutching of cocoa liquor. J Agric Sci Technol. 2013;15:557–567. [Google Scholar]
- Nadarajah D, Han JH, Holley RA. Inactivation of Escherichia coli O157:H7 in package ground beef by allyl isothiocyanate. Int J Food Microbiol. 2005;99:269–279. doi: 10.1016/j.ijfoodmicro.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Prestera T, Fahey JW, Holtzcaw WD, Abeygunawardana C, Kachinski JL, Talalay P. Comprehensive chromatographic and spectroscopic methods for the separation and identification of intact glucosinolates. Anal Biochem. 1996;239:168–179. doi: 10.1006/abio.1996.0312. [DOI] [PubMed] [Google Scholar]
- Shin JM, Harte B, Ryser E, Selke S. Active packaging of fresh chicken breast, with allyl isothiocyanate (AITC) in combination with modified atmosphere packaging (MAP) to control the growth of pathogens. J Food Sci. 2010;75:65–71. doi: 10.1111/j.1750-3841.2009.01465.x. [DOI] [PubMed] [Google Scholar]
- Soher EA, Amal SH. Fate of aflatoxin B1 in contaminated corn gluten during acid hydrolysis. J Sci Food Agric. 2011;91:421–427. doi: 10.1002/jsfa.4201. [DOI] [PubMed] [Google Scholar]
- Van Egmond HP. Mycotoxins: regulations, quality assurance and reference materials. Food Addit Contam. 1995;12:321–330. doi: 10.1080/02652039509374309. [DOI] [PubMed] [Google Scholar]
- Whitmore BB, Naidu AS. Thiosulfinates. In: Naidu A.S., editor. Natural food antimicrobial systems. Boca Raton: CRC Press; 2000. pp. 349–362. [Google Scholar]
- Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- Zhang D, Li P, Zhang Q, Zhang W, Huang Y, Ding X, Jiang J. Production of ultrasensitive generic monoclonal antibodies against major aflatoxins using a modified two-step screening procedure. Anal Chim Acta. 2009;636:63–69. doi: 10.1016/j.aca.2009.01.010. [DOI] [PubMed] [Google Scholar]