Abstract
Liposomes were developed with bioactive constituents (omega-3, omega-6, tocopherol) incorporated in acid food. They were made of soy phosphatidylcholine (SPC) allowing the encapsulation of antioxidant vitamin C (VC) and tocopherol. Stearic acid (SA) or calcium stearate (CaS) was added as a bilayer stabilizer. The structural and oxidative stability of the liposomes were studied considering the heat effect of pasteurization. Size was analyzed by light scattering; shape and structure were studied by optical and transmission electron microscopy, respectively. Membrane packing was studied with merocyanine 540. Surface charge and oxidative stability were analyzed by zeta potential and ORAC method, respectively. The liposomes showed significant stability in all of the parameters mentioned above and an important protective effect over thermolabile VC. To confirm their applicability in food, the rheological behavior and a sensory evaluation of liposomes with vitamin C and bioactive constituents were studied. The sensory evaluation of liposomes in orange juice was performed by the overall acceptability and triangular tests with 40 and 78 potential consumers, respectively. The incorporation of all liposomal formulation did not change the acceptability of orange juice. Noteworthy, SPC and SPC:SA systems had rheological behavior similar to a Newtonian fluid whereas that SPC:CaS presented a pseudoplastic one, both considered excellent for larger scale production. From all the obtained results, we can conclude that these liposomal formulations are suitable for food industry applications, incorporating bioactive constituents and generating functional orange juice that conserves its bioactivity after pasteurization.
Keywords: Bioactive-constituents, Liposomes, Thermal-stability, Rheological, Sensory-evaluation
Introduction
Bioactive substances present as natural constituents in food and provide health benefits beyond the basic nutritional value of the product (Biesalski et al. 2009). For example, it is important to consume essential fatty acids that are not naturally produced by the human body (López and Suárez 2003). Also, they have proven benefits in preventing cardiovascular diseases (Lee and Lip 2003) and cancer (Jenski et al. 1995).
Besides, vitamins have important functions in certain metabolic processes. Vitamin E (VE) or α-tocopherol is the major fat soluble antioxidant in the body. It protects the lipids against oxidative damage (Atkinson et al. 2008). Also, it is related to the decrease of blood cholesterol, having a positive effect on the incidence of atherosclerosis and the cardio-circulatory system. Another antioxidant vitamin is the ascorbic acid or vitamin C (VC), whose major biological activity is related to the maintenance of the oxidation-reduction potential, inducing free radical inactivation. Another function of this hydrosoluble vitamin is the inhibition of nitrosamine formation and the participation in the synthesis of collagen (Primo Yúfera 1998).
However, most of the bioactive compounds: e.g. fatty acids, carotenoids, tocopherols, flavonoids, polyphenols, phytosterols, fat soluble vitamins have hydrophobic nature (Kris-Etherton et al. 2002). Besides, it is important the stability of vitamins: VE is liposoluble and destroyed by UV light (Primo Yúfera 1998), while the VC is dramatically reduced by a different heat treatment process (Abioye et al. 2013).
Liposomes are microscopic spherical vesicles composed of polar lipids which enclose liquid compartments within their structure and enable the encapsulation of both hydrophilic and lipophilic materials (Keller 2001). Liposomes can be obtained with soy phosphatidylcholine (SPC) which contains linolenic acid (ω-3) and linoleic acid (ω-6) as essential fatty acids. Besides, the liposomes allow the incorporation of VE and VC in functional food. Typically, a food marketed as functional contains added, technologically developed ingredients with a specific health benefit (Niva 2007).
In the food industry, for a given industrial application, membrane stability and structure are important factors when designing liposomes (Keller 2001), considering that phospholipids can be oxidized and limiting their life. It is very important that liposomes remain stable after pasteurization because the higher the stability, the higher the protection of both vitamins (Marsanasco et al. 2011) and bioactive constituents (BC).
The aim of this work was the structural study, oxidative stability and the application in food of different liposomal formulations with BC (omega-3, omega-6, VE) and VC to develop a functional food in pasteurized orange juice.
Materials and methods
Chemicals
SPC was purchased from Avanti Polar Lipids (Alabaster, AL, USA). SPC had purity higher than 99 % and with respect to the fatty acid composition, it contains palmitic (14.9 %), stearic (3.7 %), oleic (11.4 %), linoleic (63 %), and linolenic (5.7 %) acids, according to the company’s specifications. SA and CaS were purchased from Vitalquim (Buenos Aires, Argentina). All these components comply with local food regulations (Mercosur Resolution No. 31 of 1992). VE was obtained from Parafarm (Buenos Aires, Argentina) and VC was obtained from Baker (New Jersey, NJ, USA).
Preparation of liposomes with BC and VC
Assays were performed in acetic acid 3 % w/v which simulate an aqueous food having a pH lower than 4.6 (Mercosur Resolution No. 30 of 1992), such as orange juice.
Multilamellar liposomes were prepared by the dehydration-rehydration method (Bangham 1972). Briefly, 40 μmol of lipids were dissolved in 500 μL ethanol in a round bottom flask, solvent was dried in a rotary evaporator at 37 °C. Dry lipid film composed by SPC, SPC:SA (1:0.25, mol ratio), and SPC:CaS (1:0.25, mol ratio) was rehydrated with 2 mL of acetic acid 3 % w/v to a final 50 mM lipid concentration.
In order to prepare liposomes with VE, a stock solution of this vitamin dissolved in ethanol was prepared. Stock concentration was 22.4 mM. Then, 0.445 mL of this stock was taken and mixed with a proper amount of lipids. Solvent was evaporated and lipid film was obtained. When the film was rehydrated in 2 mL of acetic acid 3 % w/v, a final concentration of 5 mM was reached.
In the case of VC, fresh solutions of this vitamin were prepared at the moment of rehydration. VC was weighted and dissolved with acetic acid 3 % w/v to reach a 90 mM concentration.
Structure and morphology determination
Optical microscopy
Micrographs of liposomes with BC and VC were obtained with an optical microscope operating at 400× magnification and using an adapted digital camera [Canon™ A570 IS; Tokyo, Japan] at 4× optical zoom.
Transmission electron microscopy (TEM)
Negative stain micrographs were prepared on copper grids covered with a formvar/carbon film, 300 mesh, (Ted Pella, Inc., Altadena, CA, USA). A 1 μL drop of the liposomal with BC and VC dispersion was set onto the copper grid and, after 1 min, liquid was adsorbed with filter paper down to a thin film. Negative staining was performed with a drop of a 1 % uranyl acetate solution. After 1 min this drop was removed with filter paper and the resulting stained film was viewed and photographed with a Zeiss™ EM 109 Turbo transmission electron microscope [Göttingen, Germany], at an accelerating voltage of 80 kV (Marsanasco et al. 2011).
Particle size distribution
Particle size distributions were determined ranging 0.1–1000 μm by laser scattering using a Particle Analyzer [Malvern Mastersizer 2000E, Malvern Instruments Ltd.™, Worcestershire, UK]. Liposomal suspensions were diluted in 500 mL of acetic acid 3 % w/v. The dispersion was carried out at 2000 rpm and the degree of obscuration was between 10 and 15 %. Sauter mean diameter (D3,2) and De Brouker mean diameter (D4,3) were used as simultaneous parameters. D3,2 and D4,3 are the mean diameters from the surface and volume distributions, respectively, and they are defined as:
| 1 |
| 2 |
where ni is the number, Si the surface, and Vi the volume corresponding to all particles with diameter di (Marsanasco et al. 2011).
Determination of surface charge
For the determinations of zeta potential (ZP), liposomal suspensions were used at 1/100 dilution of the formulations at 5 mM concentration. Measurements were made on a Nanosizer [Malvern Mastersizer 2000E, Malvern Instruments Ltd.™, Worcestershire, UK] with 1 mL cuvette. Each measurement was performed in quintuplicate, and each event was recorded by accumulation of 10 consecutive scans, which were processed to obtain an average value. Analysis software provided by the manufacturer was used.
Membrane packing
The merocyanine 540 (MC540) interacting with the interfacial region of the polar head group is situated at the glycerol area of the membrane phospholipids, with its polar sulphonated group towards the more polar outer surface of the head group region. The rest of the rodlike dye is ranging through the ester bonds and anchored with the two butyl groups in the hydrocarbon chain region (Lelkes and Miller 1980).
Liposomes formulations, before and after pasteurization, were dissolved with acetic acid 3 % w/v until a concentration of 0.868 mM was reached. MC540 stock concentration was 4.344 × 10−3 mM and it was incorporated into the vesicles; probe/lipid ratio was 1/200 (Bernik and Disalvo 1993).
A scan of each sample between 400 and 600 nm was obtained with a UV–VIS spectrophotometer [Shimadzu™ UV–Vis 160A, Kyoto, Japan], at room temperature as previously described (Disalvo et al. 2003). The partition coefficient (PC) is the concentration of monomer in a non-polar phase to monomer in an aqueous phase and it was calculated as: PC = A570/A530 (Disalvo et al. 2003).
Oxidative stability of liposomes: ORAC method
For each determination, an aliquot of a sample dilution (1/250) with acetic acid 3 % w/v was prepared at room temperature. Each aliquot was incubated for 2 min at 37 °C with 75 mM Buffer Phosphate (pH = 7) and 10 μM fluorescein. After the addition of 275 mM 2,2-azo-bis(2-amidinopropane)-dihydrochloride (AAPH), fluorescence intensity was determined at 37 °C every 60 s for 15 min. The consumption of fluorescein was assessed from the decrease in the sample fluorescence intensity (exCitation at 493 nm and emission at 515 nm) employing an F-3010 Fluorescence Spectrophotometer [HITACHI™, Tokyo, Japan]. Calculated values of f/f0 were plotted related to time. To obtain f0, acetic acid 3 % w/v was used. The area under the curve (AUC) was calculated up to 5 % of the initial value and was used to obtain the ORAC values according to the equation proposed by Atala et al. (2009).
Rheology
Liposomal dispersions behavior was studied using an AR-G2 rheometer [TA Instrument™; New Castle, DE, USA] with a cone-and-plate geometry (gap, 55 μm; cone diameter, 40 mm; cone angle, 2). Temperature (21 °C) was controlled with a water bath [Julabo ACW100, Julabo Labortechnik™; Seelbach, Germany] associated with the rheometer. Flow behavior using 3 mL of formulation was analyzed by increasing the shear rate from 0.1 to 100 s−1 over 312 s, then keeping it constant at 100 s−1 for 60 s, and finally decreasing it from 100 to 0.1 s−1 over 312 s.
Besides, n (flow behavior index) and apparent viscosity at 100 s−1 values were obtained using the model of power law. Ostwald’s equation or power law is calculated as follows: τ = k.Dn, where τ is the shear stress (MPa) and D is the shear rate (sec−1). If n = 1, the fluid is Newtonian; if n < 1, the fluid is pseudoplastic; and if n > 1, the fluid is dilatant (Sharma et al. 2003).
Pasteurization process
To analyze the effects of increasing temperature on liposomal systems they were incubated at 65 °C for 30 min to simulate the process of low temperature for a long time (LTLT). This LTLT process is employed as a type of pasteurization of orange juice. Besides, this pasteurization favors the preservation of the liposomal formulations and the maintenance of low microbiology flora.
Sensory evaluation
A commercial orange juice of an Argentinean trademark (Citric® of El Carmen S. A.) was selected. The day before the sensory evaluation the liposomes with vitamins were obtained, and pasteurized; then incorporated to orange juice (1/100 ratio). Samples were kept at 4 °C until the evaluation.
Commercial orange juice keeps always the same physicochemical, microbiological and sensory characteristics. If variation in the flavor exists, it would be induced by the addition of liposomes.
For both tests, samples were given to each evaluator in disposable cups of 200 mL; each cup had 30 mL of product. Unsalted crackers were given as neutralizers and mineralized water was given for mouth rinsing (Meilgaard et al. 1999).
Triangular test (discrimination) was performed to compare the differences between commercial orange juice with and without liposomes. For analyzed similarities between samples, 78 evaluators were selected and the following hypotheses were taken into account:
H0: there were no significant differences between samples.
Ha: samples had significant differences between each other.
It was used a 0.10 value of the significance level. This value represents the highest risk, equivalent to say that the products are different when, in fact, they are similar (Meilgaard et al. 1999).
Consumers of commercial orange juice (men and women over 18 years old) were selected and instructed in the test (Santa Cruz et al. 2005).
For affective test (overall acceptability), 40 consumers of the commercial orange juice were selected, men and women over 18 years old (Anzaldúa-Morales 1994). They evaluated the acceptability without knowing what they were judging. The orange juice was at room temperature and the randomness of the samples was ensured throughout the test. Hedonic rating scales associated with score was used and detailed as follows: 1) I really dislike it; 3) I dislike it; 5) I neither dislike nor like it; 7) I like it; 9) I really like it. The evaluators could use these values or intermediate ones (Meilgaard et al. 1999). Between samples, evaluators were instructed to drink water to avoid sensory fatigue.
Statistical analysis
All samples were prepared in triplicate or quadruplicate batches as stated in individual experimental session. ANOVA was carried out with GraphPad Software (version Prism 5.0, Statistical Analysis System, La Jolla, USA). Dunnett, Tukey and paired samples tests were performed for a mean comparison test at a significance level of 5 %.
Results and discussion
Liposomes were obtained with soy phosphatidylcholine which contains omega-3 and omega-6, and allow the encapsulation of antioxidant VC and VE. Stearic acid (SA) and calcium stearate (CaS) were incorporated to increase stability. CaS also provides calcium as well. The structure and stability of liposomes were determined by zeta potential, light scattering, optical and transmission electron microscopy, merocyanine 540 surface polarity and oxygen radical absorbance capacity (ORAC) method with or without vitamins, before and after pasteurization. The experiments mentioned were made in a food model system, to avoid fluctuations in data due to other components of the food product. At last, rheological behavior and a sensorial evaluation were performed for application of liposomes with BC and VC in the food. The sensory evaluation in commercial orange juice, with and without liposomes, was done by the overall acceptability and triangular tests with 40 and 78 potential consumers, respectively.
Structural and morphological characteristics of liposomes with BC and VC
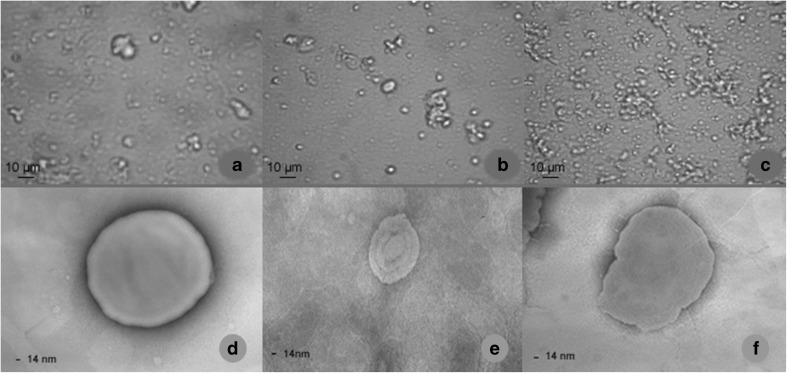
Micrographs of liposomes with BC and VC after pasteurization are shown in Fig. 1. Heterogeneity in size was observed in all cases with or without pasteurization (Fig. 1a, b and c), which is related to the preparation method and the composition of the formulation (Marsanasco et al. 2011). All formulations showed aggregation as well as isolated liposomes. Other authors like Nacka et al. (2001) reported liposomal aggregation (mainly phosphatidylcholine and phosphatidylethanolamine) including at different pH. Liposome aggregation is a physicochemical mechanism that depends on pH, heat treatment, external load and the presence of cations, among others (Marsanasco et al. 2011).
Fig. 1.
Optical micrographs (a SPC; b SPC:SA, c SPC:CaS) and negative stained TEM (d SPC, e SPC:SA, f SPC:CaS) of the liposomal formulations with both vitamins after pasteurization
The use of TEM is normally required to obtain information about the laminar structure of liposomes (Chen et al. 2013). The three liposomal formulations with BC and VC presented a lamellar structure with a central core with spherical and non-spherical shapes (Fig. 1d, e and f). On top of that, the liposomes proposed in this work, showed thermal stability maintaining their structure even after the pasteurization process in acid media.
Physico-chemical characteristics of liposomes with BC and VC
Liposomal size
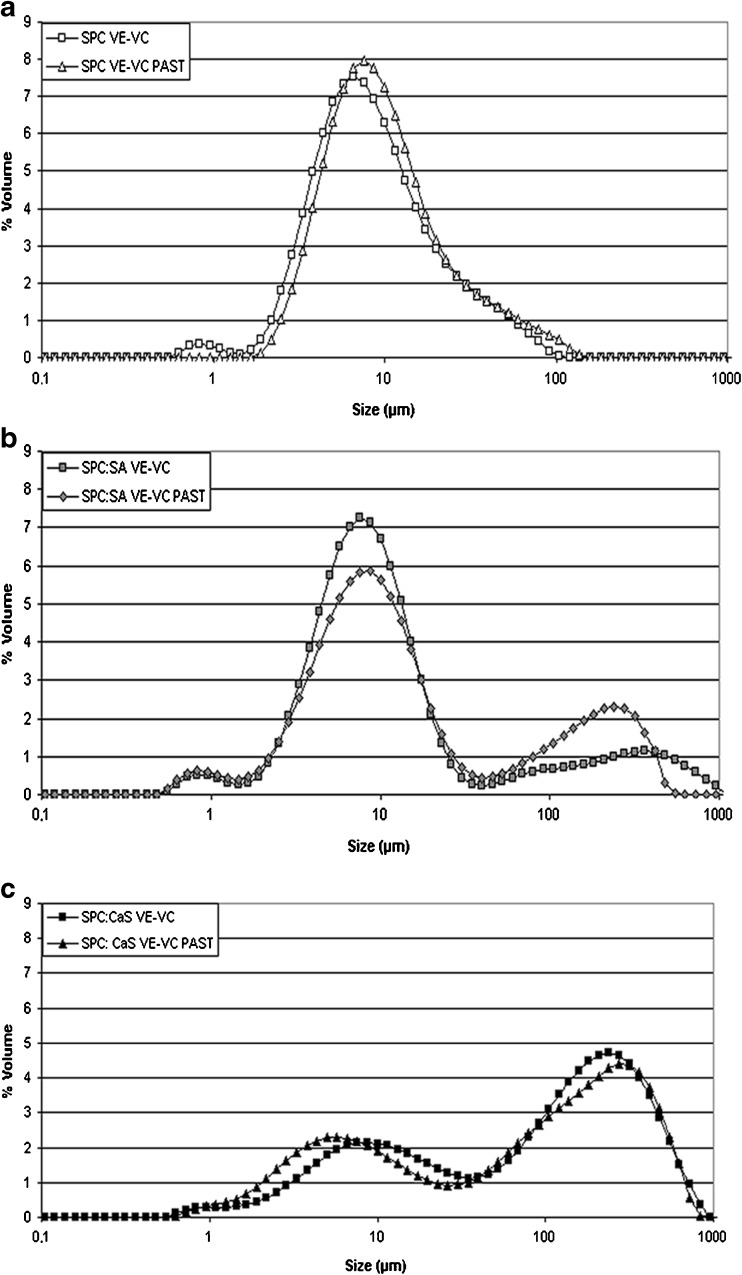
SPC system with vitamins presented a bimodal and a monomodal particle size distribution before and after pasteurization, respectively (Fig. 2a). The particle size distributions were consistent with the size that the system presented in microscopy (Fig. 1a).
Fig. 2.
Particle size distribution expressed as volume percentage for liposomal formulations with both vitamins before and after pasteurization (PAST). Data correspond to a SPC, b SPC:SA and c SPC:CaS
The SPC:SA formulation with vitamins presented a multimodal particle size distributions (Fig. 2b), with a population of greater values corresponding to aggregates with different sizes of liposomes, as also observed with the optical microscopy (Fig. 1b). Significant differences were found in D4,3 values with respect to the SPC, before and after pasteurization. It should be mentioned that the volume particle size distribution is more sensitive to larger particles, which is related to the aggregation of the liposomes (Marsanasco et al. 2011), and corroborated by the differences between D4,3 and D3,2 values obtained (Table 1). Besides, the SPC:SA system did not show significant differences in D3,2 value with respect to SPC. The addition of SA favors the aggregation of liposomes but did not change the size. However, after pasteurization the aggregation decreases with respect to the same system without pasteurization. The aggregation is an example of physical instability according to McClements (1999), for that it can be inferred that a decrease in aggregation favors the stability of the system. This result was corroborated with the D4,3 value (Table 1) and particle size distribution reflected in a decrement of percentage volume values in the population size ranging between 40 and 1000 μm (Fig. 2b).
Table 1.
Size distribution of the liposomal formulations with both vitamins before and after pasteurization (PAST)
| Liposomal formulation | D4,3 (μm) | D3,2 (μm) |
|---|---|---|
| SPC with VE and VC | 12.37 ± 0.97 | 5.91 ± 0.19 |
| SPC:SA with VE and VC | 166.0 ± 1.95*** | 8.44 ± 0.01 |
| SPC:CaS with VE and VC | 171.10 ± 22.75*** | 15.82 ± 2.34*** |
| SPC with VE and VC PAST | 13.67 ± 0.07 | 7.20 ± 0.16 |
| SPC:SA with VE and VC PAST | 55.23 ± 0.89**▲▲▲ | 6.04 ± 0.08 |
| SPC:CaS with VE and VC PAST | 147.4 ± 4.51*** | 10.31 ± 0.07*▲▲▲ |
The results are shown as the mean ± SD of three independent assays. Statistical comparison was made:
-Respect to SPC with the Dunnett test. Significant differences respect to the control are shown as *p < 0.05, **p < 0.01, ***p < 0.001
-In each system before and after pasteurization with the Tukey test. Significant differences respect to the control are shown as ▲ p < 0.05, ▲▲ p < 0.01, ▲▲▲ p < 0.001
The SPC:CaS system with vitamins showed a multimodal particle size distributions (Fig. 2c). In the D3,2 value, the addition of CaS presented a significant increase with respect to SPC, before and after pasteurization, related with the increment of size (Table 1). Also, the addition of CaS presented a shift in the particle size distribution to higher values with a significant increase in the D4,3 value with respect to SPC (before and after pasteurization). These results demonstrate that the aggregation is favored in SPC:CaS. A possible explanation could be related to compact areas of the membrane formed by the binding in the phosphatidylcholines and Ca2+ (Yeap et al. 2008). Sukhija and Palmquist (1990) demonstrated by the acid pH that favors the dissociation of the CaS (pKa of SA is 4.5). Calcium ion is free to bind with two adjacent phosphatidylcholines, inducing more compact areas in the bilayer packing, accompanied by a structural reorganization of phosphatidylcholines molecules. Negative charges of phosphate groups attract Ca2+, while the positive charges of the trimethylammonium group repel it. This additional repulsion supports the position of calcium along the area of the phosphate group (Yeap et al. 2008). Also, Ca2+ ions favor the dehydration at the interfacial region facilitating vesicle aggregation by lowering the repulsive hydration forces (Hincha 2003). These results are in agreement with the particle size distributions (Fig. 2c) and morphology (Fig. 1c), where SPC:CaS showed a higher aggregation respect to the other two systems.
Comparing the systems with vitamins before and after pasteurization, SPC:CaS system showed a significant decrease in D3,2 value. And SPC:SA system presented a significant decrease in D4,3 value. For the other results in D4,3 and D3,2 values (Table 1), the formulations did not show significant differences. The results demonstrated the thermal stability of the liposomes with a low aggregation tendency and a smaller liposome size after pasteurization.
Zeta potential
The results of zeta potential (ZP) showed positive values (Table 2). The phosphatidylcholine at neutral pH had a ZP value in the range of −40 mV/−50 mV (Mosca et al. 2011). In our results, ZP had positive values due to the H+ concentration of the acetic acid 3 % w/v (pH = 3.0).
Table 2.
Partition coefficient (PC) and zeta potential (ZP) values of the liposomal formulations with and without vitamins or before and after pasteurization (PAST)
| Liposomal formulation | PC | ZP (mV) |
|---|---|---|
| SPC | 1.93 ± 0.03 | 21.38 ± 0.34 |
| SPC:SA | 2.21 ± 0.02 | 16.64 ± 0.38 |
| SPC:CaS | 2.37 ± 0.01 | 13.92 ± 0.44 |
| SPC with VE and VC | 2.37 ± 0.01*** | 17.82 ± 0.58*** |
| SPC:SA with VE and VC | 2.57 ± 0.03*** | 14.52 ± 0.53*** |
| SPC:CaS with VE and VC | 2.54 ± 0.05*** | 12.40 ± 0.47*** |
| SPC PAST | 1.94 ± 0.04 | |
| SPC:SA PAST | 2.32 ± 0.05 | |
| SPC:CaS PAST | 2.38 ± 0.01 | |
| SPC with VE and VC PAST | 2.39 ± 0.01*** | |
| SPC:SA with VE and VC PAST | 2.62 ± 0.01*** | |
| SPC:CaS with VE and VC PAST | 2.87 ± 0.04***▲▲▲ |
The results are shown as the mean ± SD of three independent assays. Statistical comparison was made:
-Between each system with vitamins respect to the same system without vitamins (control) through the Tukey test before and after pasteurization. Significant differences respect to the control are shown as *p < 0.05, **p < 0.01, ***p < 0.001
-In each system with vitamins before and after pasteurization with the Tukey test. Significant differences respect to the control are shown as ▲ p < 0.05, ▲▲ p < 0.01, ▲▲▲ p < 0.001
For systems without vitamins, SPC:SA and SPC:CaS showed a significant decrease in ZP values compared to SPC (***p < 0.01 in both cases with the Dunnett test, statistic not shown in Table 2). With the addition of SA (pKa = 4.5), there was a contribution of 25 % less negative charge (1:0.25 M ratio in SPC:SA). A smaller negative surface charge generated a significant reduction in the ZP value with respect to the SPC system. In the SPC:CaS system, the acid pH and the binding of phosphatidylcholines with the Ca2+ generated a decrease in negative charges. The effect of Ca2+ ion reduced the ZP value with respect to the SPC system.
The three systems with vitamins presented values of ZP significantly lower with respect to their controls, related with the possible of VE effect on the decrease of the surface charge (Yoshioka 1991) as the VC (pKa1 = 4.04) did not contribute with charges to the systems at the acid pH.
According to Henriksen et al. (1997), the neutralization of the charges favors aggregation, so a lower surface charge induces the presence of aggregates. In our results, the formulation that has the lower ZP (SPC:CaS) was that with the higher amount of aggregates. This trend was maintained in the three formulations: the lower ZP, the higher the aggregation.
Membrane packing
In the membrane packing study with the probe MC540 (Table 2), SPC:SA and SPC:CaS systems showed a PC value significantly higher relative to SPC, before and after pasteurization (***p < 0.01 in both cases with the Dunnett test, statistic not shown in Table 2). According to Mateašik et al. (2002), lower surface charge increase incorporation of the anionic MC540. SPC:SA and SPC:CaS systems presented a ZP value lower with respect to SPC, favoring higher incorporation of probe. Especially in SPC:CaS system which showed the lower ZP value.
In all three systems, the addition of the vitamins showed a significant increase in PC values with respect to their controls (before and after pasteurization), indicating that a greater amount of probe entered the membrane (Table 2). A possible explanation is that VE produced a general increase in the mobility of the polar head group, previously reported by Hincha (2008).
Finally, by comparing each system before and after pasteurization, the systems did not show significant differences. Only SPC:CaS system with vitamins presented a significant increase in PC value. This finding is noteworthy because it corroborates the thermal stability of the membrane with regard to membrane packing.
Oxidative stability of liposomes with BC and VC
The three liposomal formulations without vitamins, presented the same oxidative stability by the ORAC method without significant differences regarding SPC (Dunnett Test, Fig. 3). SPC based liposomes revealed a low peroxidative trend. Monroig et al. (2007) also arrived to the same conclusion. The addition of SA or CaS to SPC favors such oxidative stability. According to Soto-Arriaza et al. (2008), the increased rigidity of 1,2-dipalmitoyl-sn-glycerol-3-phosphatidylcholine in the hydrophilic-hydrophobic region affects propagation of the radical initiation and produces a reduction in the water flow rate, decreasing lipid oxidation. Even though a lower oxidative value would be expected in SPC:SA and SPC:CaS compared to SPC, this difference was not detected probably because of the low peroxidation in SPC.
Fig. 3.
Peroxidation assay in liposomal formulations after pasteurization. Each column represents the mean ± SD of four independent assays. Statistical comparison was made: -Between each system with vitamin/s with respect to the same system without vitamins (control) through the Dunnett test. Significant differences respect to the control are shown as ***p < 0.001. -Respect to SPC in systems without vitamins with the Dunnett test. No significant differences were observed
None of the three liposomal systems containing VE showed significant differences with respect to their controls. However, in a previous work (Marsanasco et al. 2011), VE had an antioxidant activity when entrapped in SPC, SPC:SA and SPC:CaS systems, with a percentage encapsulation efficiency around 99 % and determined by a different methodology (Thiobarbituric acid).
The three systems with VC (alone or with VE) showed a significant higher value compared to the controls, related to the antioxidant activity after pasteurization process. This result was corroborated statistically when systems with both vitamins did not show significant differences with respect to the same formulations with VC (Fig. 3 with the Tukey Test). It is important to mention that different types of pasteurization, including the LTLT, dramatically reduced this vitamin stability (Abioye et al. 2013). In previous results, the percentage encapsulation efficiency of VC for these liposomes was determined and was c.a. 86 % (Marsanasco et al. 2011). So, it is possible to infer that the encapsulation efficiency of these liposomes protected efficiently most of the VC and hence, maintained its antioxidant activity.
According to studies by Gramlich et al. (2002) the pH 2.7 favors the interaction of the VC (pKa = 4.04) with the hydrophobic surface of the phospholipids. Our experimental conditions generated the same effect considering that the pH medium was 3.0. This interaction of VC with phospholipids, favored a higher incorporation of MC540. This was corroborated with the results obtained as PC values (Table 2). According to Fukuzawa (2008), the peroxyl radical phospholipids become more polar and are located near the polar area of the membrane. Thus the polar region of the membrane is more fluid favoring the interaction of the vitamin with peroxyl radicals, which would enhance its antioxidant activity.
The results obtained with ORAC confirmed that VC maintains an antioxidant activity after pasteurization with the possible protection of the BC, and liposomes protected VC against damage induced by the LTLT process. Even though the ORAC method did not corroborate the antioxidant activity of VE, possibly due to its lipophilic nature (Prior et al. 2003), it did help to demonstrate VC antioxidant activity.
Rheology
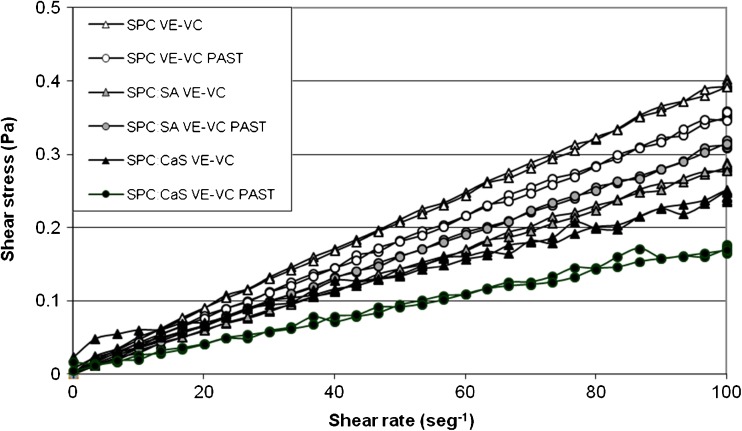
SPC and SPC:SA systems exhibited similar behavior to a Newtonian fluid either before and after pasteurization (Fig. 4). This result was corroborated by the values of n (with or without pasteurization) which were lower than one but close to this value (Table 3). In contrast, SPC:CaS showed a pseudoplastic behavior (especially before pasteurization). SPC:CaS formulation showed the highest aggregation with a tendency to pseudoplastic behavior. SPC:SA system had a higher aggregation in relation to SPC but lower than SPC:CaS favoring a Newtonian behavior. For that, a higher aggregation favors the trend towards a pseudoplastic fluid behavior.
Fig. 4.
Rheological behavior of liposomal formulations with both vitamins before and after pasteurization (PAST) in acetic acid 3 % w/v
Table 3.
Flow behavior index (n) values of the liposomal formulations with both vitamins, before and after pasteurization (PAST). Values are show as mean ± SD of three independent samples
| Liposomal formulation | n |
|---|---|
| SPC VE-VC | 0.92 ± 0.01 |
| SPC VE-VE PAST | 0.95 ± 0.01 |
| SPC:SA VE-VC | 0.89 ± 0.11 |
| SPC:SA VE-VC PAST | 0.95 ± 0.00 |
| SPC:CaS VE-VC | 0.58 ± 0.03 |
| SPC:CaS VE-VC PAST | 0.67 ± 0.26 |
Ordinary food such as water, milk, apple juice and corn syrup has a Newtonian behavior. And the pseudoplastic behavior is present also in ordinary food like sauces and concentrated orange juice (Sharma et al. 2003). For that, the behavior of this type of liposomes, which resembles a Newtonian or pseudoplastic fluid, is a great advantage to implement these systems, considering a production at larger scales.
Sensory evaluation of liposomes in orange juice
The numbers of the correct answers in the triangular test for liposomes with VC and BC were 43/78 for SPC, 35/78 for SPC:SA and 38/78 for SPC:CaS. The correct answer means that the evaluator found the difference between samples.
Applying the statistical table for the triangular test with a significance level of 0.10 for 78 evaluators, the minimum number of correct answers from which the samples show significant differences is 32 (Meilgaard et al. 1999). From the above, it is concluded that there are significant differences between commercial juice with or without liposomes with VC and BC.
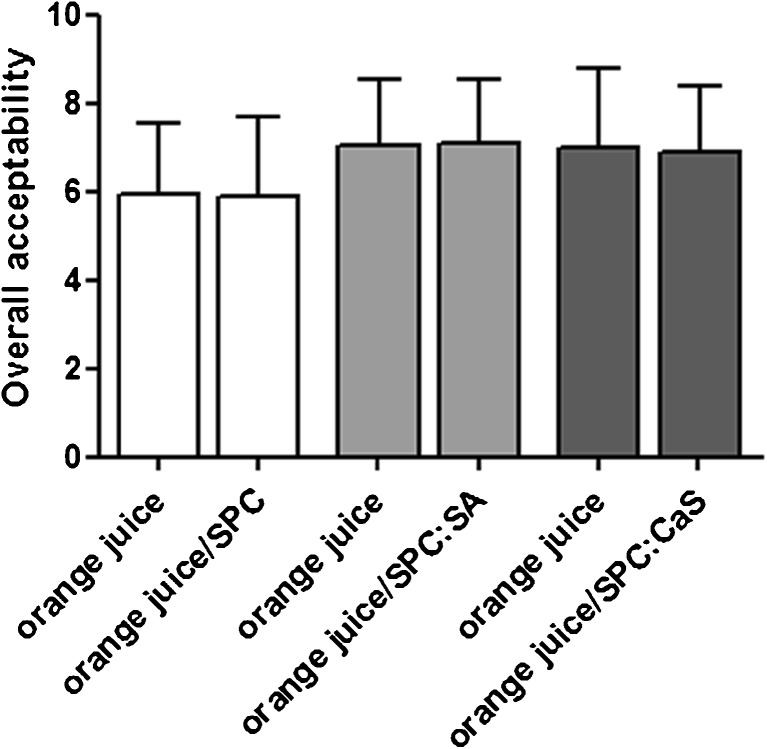
Even though the significant differences were obtained in the triangular test, the addition of liposomes with BC and VC did not change the acceptability of the product. These results are reflected in Fig. 5 where the juice with the three added formulations showed no significant differences with respect to commercial juice. Also, it is noteworthy, that although there was aggregation after pasteurization in SPC:SA and SPC:CaS formulations with BC and VC, this physical behavior did not affect the acceptability of the final product. The results obtained showed that all three liposomal formulations were potentially applicable in the product.
Fig. 5.
Overall acceptability of 40 panelists in commercial orange juice with or without liposomes with both vitamins. Statistics were performed using the test for paired samples between each commercial orange juice sample with and without liposomes. No significant differences were obtained
Conclusions
This study presents a simple and feasible approach to enhance food nutritional value in the human diet by delivering bioactive constituents in liposomes to orange juice, a food product that is consumed worldwide. The three liposomal formulations studied remained stable even after pasteurization, as demonstrated by morphology, size, membrane packing, and high oxidative stability. Besides, all systems showed protection of the thermolabile VC that maintained their antioxidant activity after pasteurization.
SPC and SPC:SA systems had a rheological behavior similar to a Newtonian fluid whereas SPC:CaS had a pseudoplastic one. There was an increment in aggregation that favored the pseudoplastic behavior but did not change acceptability of the product.
From all the aspects discussed above, it can be concluded that these liposomes with BC and VC can be added to orange juice for industrial application with added commercial and nutritional value.
Acknowledgments
This work was funded by the following sources:
Universidad Nacional de Quilmes-Project PUNQ974/11.
Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) – Project PIP-CONICET # 11220110100214.
Ministerio Nacional de Ciencia y Tecnología (MINCyT)- Project: MINCyT-CAPES N°. BR/11/01 (2012–2013).
Footnotes
Highlights
Highlight 1 Liposomes with omega-3, omega-6 and vitamins E and C were obtained.
Highlight 2 Liposomes were stable in structure, morphology, size, oxidation and membrane packing.
Highlight 3 High stability of liposomes was maintained before and after pasteurization.
Highlight 4 Liposomes presented a protective effect over the thermolabile vitamin C.
Highlight 5 Liposomes showed an excellent acceptability of commercial orange juice.
References
- Abioye A. O., Abioye V. F., Ade-Omowaye B. I.O., Adedeji A. A. Kinetic modeling of ascorbic acid loss in baobab drink at pasteurization and storage temperatures. J Environ Sci Toxicol Food Technol. 2013;7(2):17–23. doi: 10.9790/2402-0721723. [DOI] [Google Scholar]
- Anzaldúa-Morales A. La evaluación sensorial de los alimentos en la teoría y la práctica. Zaragoza: Acribia; 1994. [Google Scholar]
- Atala E., Vásquez L., Speisky H., Lissi E., López-Alarcón C. Ascorbic acid contribution to ORAC values in berry extracts: an evaluation by the ORAC-pyrogallol red methodology. Food Chem. 2009;113:331–335. doi: 10.1016/j.foodchem.2008.07.063. [DOI] [Google Scholar]
- Atkinson J., Epand R. F., Epand R. M. Tocopherols and tocotrienols in membranes: a critical review. Free Radic Biol Med. 2008;44(5):739–764. doi: 10.1016/j.freeradbiomed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Bangham A.D. Model membranes. Chem Phys Lipids. 1972;4:386–392. doi: 10.1016/0009-3084(72)90069-2. [DOI] [PubMed] [Google Scholar]
- Bernik D. L., Disalvo E. A. Gel state surface properties of phosphatidylcholine liposomes as measured with merocyanine 540. Biochim Biophys Acta. 1993;1146:169–177. doi: 10.1016/0005-2736(93)90352-Z. [DOI] [PubMed] [Google Scholar]
- Biesalski H. K., Dragsted L. O., Elmadfa I., Grossklaus R., Müller M., Schrenk D., Walter P., Weber P. Bioactive compounds: definition and assessment of activity. Nutrition. 2009;25:1202–1205. doi: 10.1016/j.nut.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Chen C., Zhu S., Huang T., Wang S., Yan X. Analytical techniques for single-liposome characterization. Anal Methods. 2013;5(9):2150–2157. doi: 10.1039/c3ay40219c. [DOI] [Google Scholar]
- Disalvo E. A., Arroyo J., Bernik D. L. Surface properties of liposomes as measured with fluorescent methods. In: Duzgunes N., editor. Methods in enzymology: vol-367, part-A: liposomes. 1st. New York: Academic Press; 2003. pp. 213–233. [DOI] [PubMed] [Google Scholar]
- Fukuzawa K. Dynamics of lipid peroxidation and antioxidion of alpha-tocopherol in membranes. J Nutr Sci Vitaminol (Tokyo) 2008;54(4):273–285. doi: 10.3177/jnsv.54.273. [DOI] [PubMed] [Google Scholar]
- Gramlich G., Zhang J., Nau W. N. Increased antioxidant reactivity of vitamin C at low pH in model Membranes. J Am Chem Soc. 2002;124(38):11252–11253. doi: 10.1021/ja026927b. [DOI] [PubMed] [Google Scholar]
- Hincha D. K. Effects of calcium-induced aggregation on the physical stability of liposomes containing plant glycolipids. Biochim Biophys Acta. 2003;1611:180–186. doi: 10.1016/S0005-2736(03)00053-1. [DOI] [PubMed] [Google Scholar]
- Hincha D. K. Effects of alpha-tocopherol (vitamin E) on the stability and lipid dynamics of model membranes mimicking the lipid composition of plant chloroplast membranes. FEBS Lett. 2008;582:3687–3692. doi: 10.1016/j.febslet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Henriksen I., Våagen S. R., Sande S. A., Smistad G., Karlsen J. Interactions between liposomes and chitosan II: effect of selected parameters on aggregation and leakage. Int J Pharm. 1997;146(2):193–203. doi: 10.1016/S0378-5173(96)04801-6. [DOI] [Google Scholar]
- Jenski L. J., Zerouga M., Stillwell W. Omega-3 fatty acid-containing liposomes in cancer therapy. Proc Soc Exp Biol Med. 1995;210(3):227–233. doi: 10.3181/00379727-210-43943. [DOI] [PubMed] [Google Scholar]
- Keller B. C. Liposomes in nutrition. Trends Food Sci Technol. 2001;12:25–31. doi: 10.1016/S0924-2244(01)00044-9. [DOI] [Google Scholar]
- Kris-Etherton P. M., Hecker K. D., Bonanome A., Coval S. M., Binkoski A. E., Hilpert K. F., Griel A. E., Etherton T. D. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113(9B):71S–88S. doi: 10.1016/S0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- Lee K. W., Lip G. Y. H. The role of omega-3 fatty acids in the secondary prevention of cardiovascular disease. QJM. 2003;96:465–480. doi: 10.1093/qjmed/hcg092. [DOI] [PubMed] [Google Scholar]
- Lelkes P.I., Miller I.R. Perturbations of membrane structure by optical probes. I. Location and structural sensitivity of merocyanine 540 bound to phospholipid membranes. J Membr Biol. 1980;52:1–15. doi: 10.1007/BF01869001. [DOI] [PubMed] [Google Scholar]
- López L. B., Suárez M. M. Fundamentos de Nutrición normal. Buenos Aires: El Ateneo; 2003. [Google Scholar]
- Marsanasco M., Márquez A. L., Wagner J. R., SdelV Alonso, Chiaramoni N. S. Liposomes as vehicles for vitamins E and C: an alternative to fortify orange juice and offer vitamin C protection after heat treatment. Food Res Int. 2011;44:3039–3046. doi: 10.1016/j.foodres.2011.07.025. [DOI] [Google Scholar]
- Mateašik A., Šikurová L., Chorvát D., Jr Interaction of merocyanine 540 with charged membranes. Bioelectrochemistry. 2002;55:173–175. doi: 10.1016/S1567-5394(01)00140-2. [DOI] [PubMed] [Google Scholar]
- McClements D. J. Food emulsions. Principles, practice and techniques. Boca Raton: CRC Press; 1999. [Google Scholar]
- Meilgaard M., Civille G. V., Carr T. B. Sensory evaluation techniques. Florida: CRC Press; 1999. [Google Scholar]
- Monroig O., Navarro J.C., Amat F., González P., Hontoria F. Oxidative stability and changes in the particle size of liposomes used in the Artemia enrichment. Aquaculture. 2007;266:200–210. doi: 10.1016/j.aquaculture.2006.12.016. [DOI] [Google Scholar]
- Mosca M., Ceglie A., Ambrosone L. Effect of membrane composition on lipid oxidation in liposomes. Chem Phys Lipids. 2011;164:158–165. doi: 10.1016/j.chemphyslip.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Nacka F., Cansell M., Gouygou J. P., Gerbeaud C., Méléard P., Entressangles B. Physical and chemical stability of marine lipid-based liposomes under acid conditions. Colloids Surf B Biointerfaces. 2001;20:257–266. doi: 10.1016/S0927-7765(00)00205-8. [DOI] [PubMed] [Google Scholar]
- Niva M. ‘All foods affect health’: understandings of functional foods and healthy eating among health-oriented Finns. Appetite. 2007;48:384–393. doi: 10.1016/j.appet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Primo Yúfera E. Química de los Alimentos. Madrid: Síntesis; 1998. [Google Scholar]
- Prior R. L., Hoang H., Gu L., Wu X., Bacchiocca M., Howard L., Hampsch-Woodill M., Huang D., Ou B., Jacob R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL)) of plasma and other biological and food samples. J Agric Food Chem. 2003;51(11):3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- Santa Cruz M. J., Martínez C., Varela P. Principios básicos del análisis sensorial. In: Hough G., Fiszman S., editors. Estimación de la vida útil sensorial de los alimentos. 1st. Madrid: Martín Impresores S. L.; 2005. pp. 24–26. [Google Scholar]
- Sharma S. K., Mulvaney S. J., Rizvi S. S. H. Ingeniería de los alimentos. Operaciones prácticas y de laboratorio. Distrito Federal: Limusa S. A.; 2003. [Google Scholar]
- Soto-Arriaza M. A., Sotomayor C. P., Lissi E. A. Relationship between lipid peroxidation and rigidity in L-α-phosphatidylcholine-DPPC vesicles. J Colloid Interface Sci. 2008;323:70–74. doi: 10.1016/j.jcis.2008.04.034. [DOI] [PubMed] [Google Scholar]
- Sukhija P. S., Palmquist D. L. Dissociation of calcium soaps of long-chain fatty acids in fumen fluid. J Dairy Sci. 1990;73:1784–1787. doi: 10.3168/jds.S0022-0302(90)78858-3. [DOI] [PubMed] [Google Scholar]
- Yeap P. K., Lim K. O., Chong C. S., Teng T. T. Effect of calcium ions on the density of lecithin and its effective molecular volume in lecithin–water dispersions. Chem Phys Lipids. 2008;151:1–9. doi: 10.1016/j.chemphyslip.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Yoshioka H. Surface modification of haemoglobin-containing liposomes with polyethylene glycol prevents liposome aggregation in blood plasma. Biomaterials. 1991;12:861–864. doi: 10.1016/0142-9612(91)90075-L. [DOI] [PubMed] [Google Scholar]