Abstract
Bacterial cellulose (BC) has been given an ample attention due to its high potential for many industrial applications. However, the high cost of production medium has hindered the commercialization of BC. Several efforts have been made to explore cheep, raw and waste sources for BC production. The current study aims at investigating the BC production from a waste source; the scum obtained during preparation of sugarcane jaggery or gur (JS). JS was five-fold diluted with distilled water and used as culturing medium without any additional nutrients. The production of BC was monitored till 10th days of cultivation both at static and shaking culturing conditions. A maximum of 2.51 g/L and 2.13 g/L BC was produced in shaking and static cultures, respectively, after 10 days. The structure features of BC were confirmed through FTIR, XRD and SEM analysis. The chemical structure and physical appearance strongly resembled the BC produced form synthetic media. It was noteworthy that the BC produced from JS showed higher mechanical and thermal properties. The cell adhesion and proliferation capabilities of produced BC were observed that depicted definite animal cell adhesion without any considerable cytotoxicity. Besides providing an economically feasible way for BC production, the high level of physico-mechanical and biological properties insured the importance in medical fields.
Keywords: Bacterial cellulose, Jaggery Scum, Structural analysis, Physiological characterization, Biocompatibility
Introduction
Cellulose, the most abundant organic polymer on earth surface, has received tremendous economic importance globally. Various products such as paper, textiles, construction materials, cardboards, sports materials are mainly produced from cellulose obtained from cotton and wood (Keshk 2014). Glucose, the basic structural unit of cellulose, is produced in living plants through photosynthesis and assembles to produce cellulose. Although plants are the chief producers of cellulose, some fungi and bacterial species have been recognized for cellulose production (Ul-Islam et al. 2014). The chemical structure of bacterial cellulose (BC) is similar to that of plant cellulose but has shown much superior physical properties, mechanical strength, and crystallinity than the plant cellulose (Ul-Islam et al. 2013a, b).
BC represent a dense reticulated web shaped fibril network structure. The fibril size of BC is much narrower than the plant cellulose that blesses it with improved physiological features. The porous geometry of BC not only absorbs high amount of water (about 100 times its dry weight) but also ensures its slow release (Ul-Islam et al. 2012). These aspects along with high mechanical strength and crystallinity make BC a versatile material for use in medical fields. BC has been successfully used as wound dressing and healing material as it can absorb wound exudates effectively, resulting an easy wound dressing change (Czaja et al. 2006; Shi et al. 2012). Besides, it has received applications in drug delivery systems, novel vascular grafts, and scaffolds for tissue engineering, medical pads, high performance speaker diaphragms, and electronic paper display (Shah et al. 2013).
Since its discovery more than a century ago, BC has remained the subject of high interest to develop some methods for low cost BC production. The expensive nature of the commonly used culture media and the slow production processes are the major concerns so far offering challenges for industrial scale up of BC. Serious attentions were paid to resolve both the issues, and make this essential biomaterial an industrial product for multiple applications. In this regard, efforts were made to develop novel synthetic methods like static, shaking agitation, and use of various bioreactors to enhance the production and productivity (Shah et al. 2013). In spite of achieving a level of success, the goal of economically feasible BC production has not yet been attained successfully. The most important aspect to achieve the goal was the utilization of cheap and waste sources for low cost BC production. In this regard numerous alternative sources including waste of beer fermentation broth, fruit juices, molasses, and industrial wastes have been successfully used so far (Ha et al. 2011; Khattak et al. 2015). These cheap media could provide low cost BC production but still the purification cost, environmental issues and relatively inferior physiological features restricted the process efficacy. It is still an issue of immense importance to explore cheap materials with easy processing possibilities for high quality BC production.
The current study was designed to evaluate the BC production from a cheap waste source, the scum obtained during preparation of JS. JS is disposed in high quantities from brown sugar industry and contains high quantities of glucose and other fermentable sources. The diluted JS media was subjected to BC production through the bacterial strain, Glucanoacetobacter xylinum (ATCC 23768). The results showed that the diluted JS media produced significant amount of BC. BC obtained from JS not only showed homology to the control BC (obtained from synthetic media) but displayed somehow better physical and biological properties. The present investigation can lead towards commercialization and industrial scale up of BC.
Materials and methods
Microorganism and culture maintenance
The freeze-dried culture of Glucanoacetobacterxylinum (ATCC 23768) was suspended in 5 ml of sterilized Hestrin-Schramm (HS) medium containing 2.0 % D-glucose, 0.5 % peptone, 0.5 % yeast extract, 0.27 % Na2HPO4 and 0.115 % citric acid in distilled water (pH 6.0) and spread on HS-agar plates (1.5 % Agar). Agar plates were incubated at 30 °C until the colonies appeared on plates. Colonies of G. xylinum were inoculated into a 50 ml HS medium in a 250 ml Erlenmeyer flask shaken at 200 rpm and 30 °C for 24 h.
JS media preparation and fermentation
JS media was a thick liquid obtained from traditional JS factory located in the rural area of Khyber Pakhtunkhwa, Pakistan. The JS was five-fold diluted with distilled water and centrifuge at 6000 rpm for 15 min to remove solid particles. The pH of supernatant was adjusted to 6.0 by using 1 M NaOH solution and then sterilized at 121 °C for 15 min at 1.5 bar pressure before use.
Fermentation
Fermentation was carried out by inoculating 5 % culture broth into JS medium in static and shaking (200 rpm) conditions at 30 °C for 10 days. The BC production was checked at each alternate day of cultivation.
Analysis of BC and cell
The produced BC was harvested by centrifuging the culture broth twice at 5000 rpm for 15 min each time washing it with dilute NaOH and then with distilled water to remove the attached bacterial cells and medium constituent. The dissolved materials were removed by filtration using an aspirator. The filter cake was repeatedly washed with distilled water until the pH of the filtrate became neutral. It was then freeze-dried at −50 °C and the dry weight of BC was measured (Ha et al. 2008).
Characterization of BC
Scanning electron microscopy (SEM) of the BC samples was performed using a Hitachi S-4800 & EDX-350 (Horiba) FE-SEM (Tokyo Japan). Samples were fixed onto a brass holder and coated with osmium tetra oxide (OsO4) by a VD HPC-ISW osmium coater (Tokyo Japan) prior to FE-SEM observation. FT-IR spectra were recorded by using a Perkin Elmer FTIR spectrophotometer (Spectrum GX &Autoimage, USA, Spectral range: 4000 ~ 400 cm−1; Beam splitter: Ge coated on KBr; Detector: DTGS; Resolution: 0.25 cm−1 (step selectable)). XRD patterns of the samples were determined through an X-Ray Diffractometer (X’Pert-APD PHILIPS, Netherland) with an X-ray generator (3 KW) and anode (LFF Cu). The radiation was Cu Kα of wavelength 1.54 Å. The X-ray generator tension and current were 40 kv and 30 mA, respectively. Thermogravimetry was carried out using a thermogravimetric/ differential thermal analyzer (Seiko Instruments Inc.). A thermogram was obtained in the range of 30–600 °C, under nitrogen atmosphere with a gradual temperature increase of 10 °C min−1. The tensile properties were measured using an Instron Universal Testing Machine (Model 4465, USA) according to the procedure of the American Society for Testing and Materials (ASTM D 882) (Shezad et al. 2010) Two metal clamps were placed at either end of each 100 mm × 10 mm rectangular strip of dried sample. The clamps were then mounted on an Instron 4465 that measured both elongation and maximum tensile load before fracture. All the analyses were repeated several times and the average values were calculated.
Biocompatibility and cell toxicity of the BC
Round pieces of BC (15 mm diameter × 2 mm thickness) were used for the cell attachment studies. Briefly, samples were sterilized and pre-treated by immersion in DMEM (Dilbecco’s Modified Eagle Medium) for 24 h. After pre-treatment, the samples were carefully placed in 6 well plates and the osteoblast cells (MC3T3-E1 ATCC) were seeded at a density of 2.5 × 105 cells/well. The samples were then incubated at 37 °C for 24 h after which the cell attachment was investigated by FE-SEM analysis.
Result and discussion
Chemical analysis of JS medium
The composition and physical properties of the culture medium has a great impact on the production and physico-chemical properties of the microbial polysaccharides (Khan et al. 2007; Khan and Park 2008; Kim et al. 2012). Thus, suggesting a particular media for production of any biomaterial is primarily based on its chemical constituents. The presence of sufficient amount of important constituents and the physical properties of the medium can ensure the BC production from JS. Therefore, the chemical composition of the JS was investigated and the results have been displayed in Table 1. The most important factor among all the chemical constituents is the high levels of glucose (68.8 g/L). Suitable quantities of glucose in the fermentation media is of prime importance because it is the major precursor of cellulose. It has been observed that the presence of higher quantities of glucose in media can inhibit the process of BC production (Masaoka et al. 1993). As the concentration of glucose in JS was quite high thus the media was five times diluted with distilled water to ensure avoiding any inhibitory effects. The second reason of media dilution was the viscosity. The viscosity of media was too high that can seriously restrict the BC production process because highly viscid media decelerate the bacterial movements and oxygen-transfer rate and thus result in reducing the overall yield and productivity. The tested media was five times diluted in order to ensure the free moment of bacterial cells, better oxygen transfer and thus better BC production. Besides it contained small quantities of ethanol, proteins and other metabolites that might also have significant influence in the BC production process.
Table 1.
Initial composition of the scum obtained during preparation of JS that was used for the production of bacterial cellulose
| Parameter | Concentration |
|---|---|
| Moisture (%) | 58.38 ± 2.31 |
| Viscosity (CP) | 882 ± 85 |
| Glucose (g/L) | 68.8 ± 4.75 |
| Ethanol (g/L) | 2.8 ± 0.47 |
| Acetaldehyde (g/L) | 0.085 ± 0.013 |
| Protein (g/L) | 1.75 ± 0.152 |
BC production in JS media
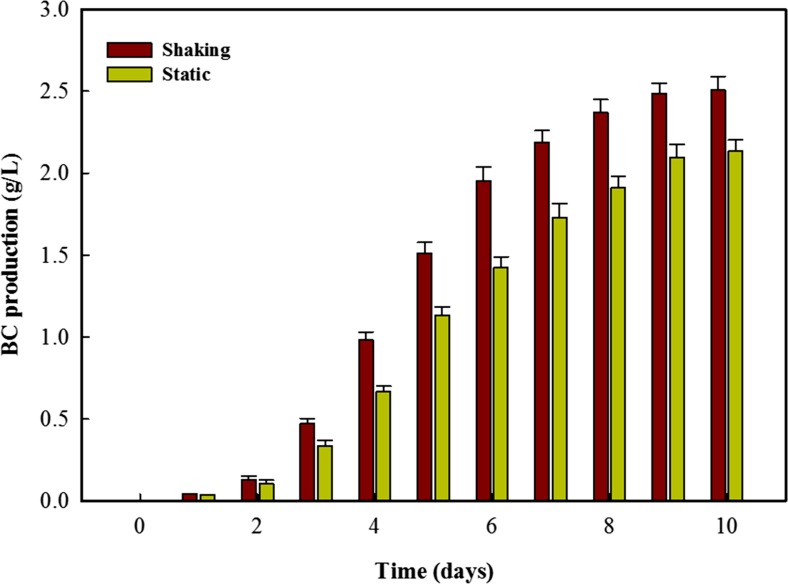
BC has been produced through a number of synthetic strategies including static, shaking and through use of differently designed applicators (Shah et al. 2013). The static cultures usually produced BC in the form of pellicle on the surface while in shaking cultures small pellets or granules are produced. The production conditions are usually defined by the desired application (Khattak et al. 2015). We observed the BC production from JS media through both static and shaking cultures. The results depicted in Fig. 1 indicate that BC production continuously increased till 10th day of cultivation in both culture. It is also clear that the production rate was higher in shaking culture compared to static culture that is in agreement with our previous study (Khattak et al. 2015). It is obvious that both the cultures showed very slow production till first 2 days of cultivation that could be attributed to the lag growth phase of bacterial cells. Besides the higher glucose concentration might also initially retard the activity of bacterial cells. However, visible increase in the production rate was observed after second day of cultivation in both cultures. A slight increase in the production rate of shaking culture is mainly due to faster moment of cells and better availability of nutrients (Masaoka et al. 1993; Khattak et al. 2015). After second day an exponential increase was observed in BC production rate in both the cultures and last till 8th day of cultivation. This increased production rate is basically caused by the increasing number of cells and better utilization of glucose content of media. The production rate decreased beyond that and negligible increase in total production was observed in next 2 days. The total BC production in shaking and static culture was 2.51 and 2.13 g/L, respectively. The higher BC production under shaking conditions has been already reported previously (Son et al. 2003; Mohite et al. 2013; Khattak et al. 2015). The fast movement of cells, easy availability of nutrients and better air diffusion in the media collectively affect the production rate in shaking cultures.
Fig. 1.
Bacterial cellulose production from the scum obtained during preparation of JS for 10 days under shaking and static cultivations strategy
Characterization of produced BC
The chemical structure of BC is similar to that of plant cellulose; however, its physical appearance is much different. The superior properties of BC are basically attributed to its physico-mechamical characteristics. After getting success in preparing BC from a new cheap source, it was imperative to investigate its structural features because the physico-chemical properties of the microbial polysaccharides are greatly influenced by the composition and physical properties of the culture medium (Khan et al. 2007; Khan and Park 2008). The chemical structure and purity of BC was investigated through FT-IR, XRD analysis.
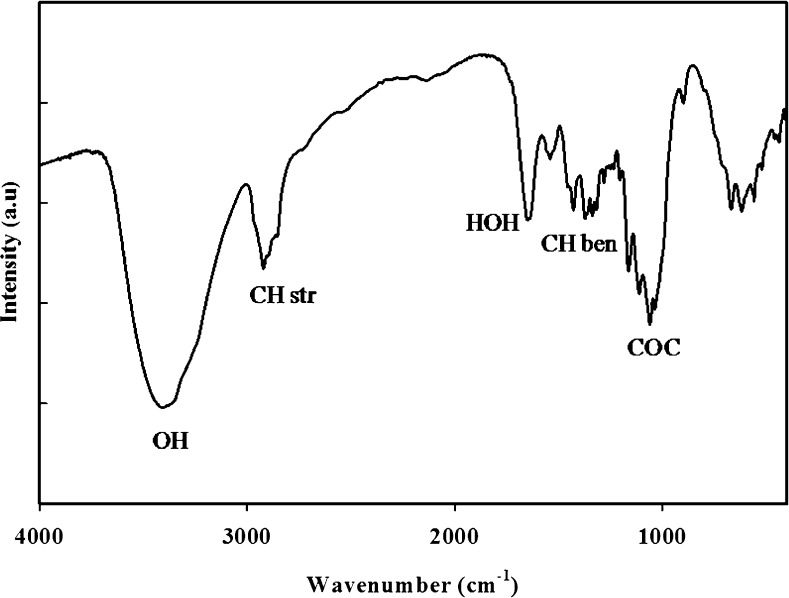
The FT-IR results presented in Fig. 2 indicates the existence of all the common functional groups of cellulose. Broad peaks centred at 3316 cm−1 represents the O-H group. A sharp peak showing the C-H stretching appeared at ~2907 cm−1 that is further strengthened by a couple of peaks at 1408 cm−1 and 1363 cm−1governed by the symmetric deformation and bending vibration of CH, respectively (Ul-Islam et al. 2011). The band at around 1640 cm−1 is due to stretching of H–O–H adsorbed on the surface (Liu et al. 2012). The peak at ~1030 cm−1 is produced by the C-O-C stretching (Ul-Islam et al. 2013a, b). The observed results are in accordance with the FT-IR spectra of cellulose and absence of any additional peaks provides evidence about the purity of BC.
Fig. 2.
FTIR spectrum of bacterial cellulose produced from the scum obtained during preparation of JS
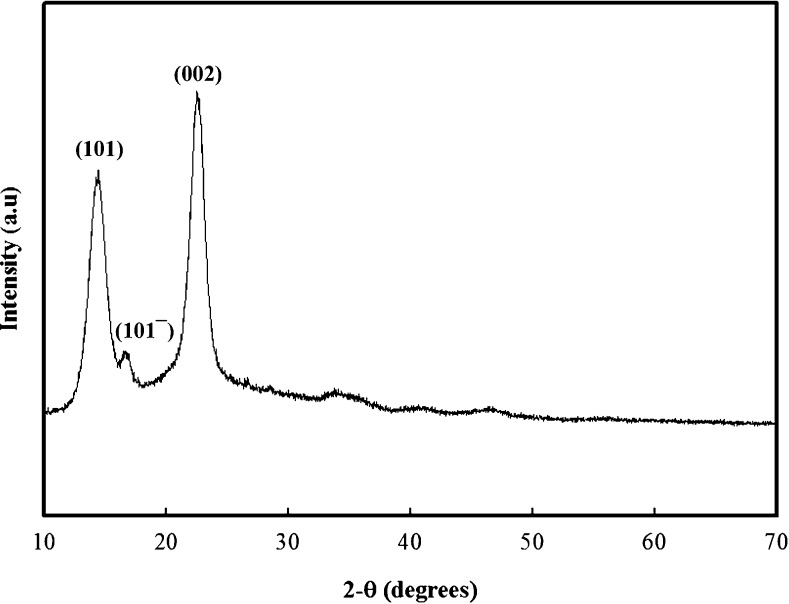
The XRD of BC has well-defined patterns because of the crystalline nature of its molecules. The XRD spectra of BC produced from JS have been shown in Fig. 3, which represent three distinct crystalline peaks at 2θ = 14.62, 16.55 and 22.72 rising from the (101), (101¯) and (002) crystalline plane, respectively. This represents the cellulose-1 crystalline form. The XRD results obtained for BC are exactly in accordance with the previous studies and show the complete purity of BC (Ul-Islam et al. 2014). Besides these three crystalline peaks BC exhibits an amorphous halo at 2 θ 19.5. BC is a semi crystallinic material and the ultimate crystallinity is calculated from total crystalline and amorphous regions of the BC as shown by Ul-Islam et al. (2012). The relative crystallinity of the BC was 63 %. The crystallinity of pure BC produced in various media usually varies in the range of 40 to 75. The current % crystallinity of BC is quite high that urge better crystalline properties of BC.
Fig. 3.
FTIR spectrum of bacterial cellulose produced from the scum obtained during preparation of JS
Morphological features
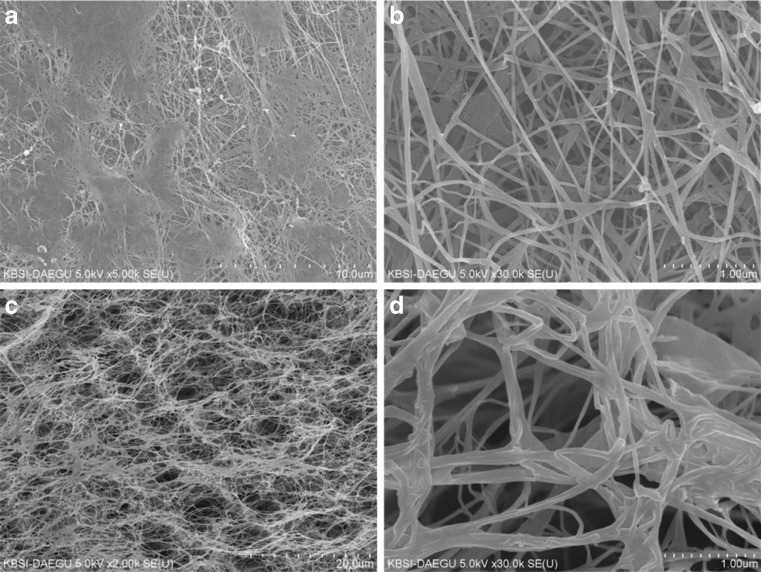
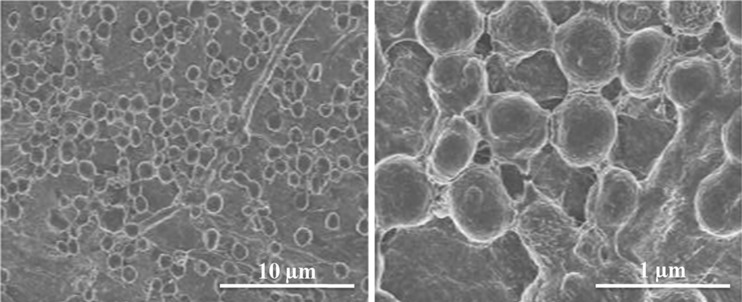
The physical properties and mechanical strength of BC is mainly dependent on its structural features. The fibril arrangement in BC production process mainly defines its porosity, surface area and ultimately liquid absorbing capabilities. The surface and internal fibril arrangement of BC were investigated through FE-SEM analysis. Figure 4a–d represents the surface and cross sectional morphology of the produced BC. A well-arranged fibril network could be observed from surface morphology. The fibril thickness is around 100 nm that is much smaller than the typical plant cellulose fibrils. The porous nature, fibril arrangement and fibril layers can be observed easily in the cross section view of sample. The existing free spaces between the fibrils could grant BC with high porosity and water accumulating properties. Moreover, these are responsible for water retention time that is important in using BC for medical purposes. The fibril density, size and arrangement are mainly dependent on the medial components, viscosity and activities of the BC producing cells (Ul-Islam et al. 2012; Khattak et al. 2015). The currently observed morphology of BC produced in JS media is equivalent to those produced in expensive synthetic media.
Fig. 4.
FE-SEM analysis of the surface (a, b) and cross section (c, d) of bacterial cellulose produced from the scum obtained during preparation of JS
Thermal degradation of BC
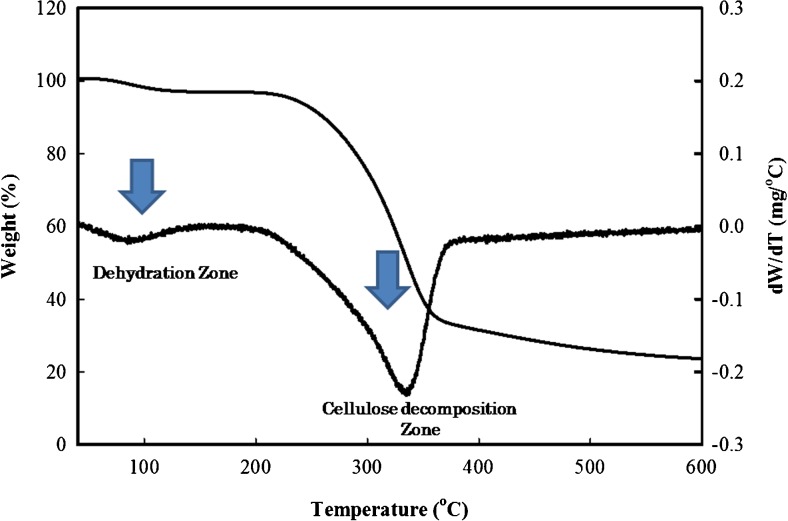
All the compounds have specific thermal degradation temperature. The thermogravimetric analysis (TGA) not only provides the heat tolerance capabilities of specific materials but also insures the purity. Normally the TGA results for BC shows two definite degradation zones. These involve the weight loss caused by dehydration and decomposition of glycosyl units of cellulose (Ul-Islam et al. 2013a, b, 2014). TGA results of BC are shown in Fig. 5. The weight loss for dehydration observed around 100 °C was about 5 %. Although the dried BC samples are used for TGA analysis, due to hydrophilic nature it absorb some moisture. Besides, the interlayer coordinated water molecules also contribute in this observed initial weight loss (Darder et al. 2003; Li et al. 2010). The second degradation phase starts above 220 °C and remains till complete degradation of cellulose chains above 350 °C. Around 70 % weight loss was observed in the second phase of degradation. The thermogravimetric curves better represent the weight loss zones. Literature study reveals that main cellulose skeleton of BC degrades above 200 °C and the overall degradation is completed above 300 °C (Ul-Islam et al. 2012). A slight variation in the degradation temperature could be attributed to the variation in BC fibrils size, their arrangement, and compactness. The thermal degradation results not only justify the well-arranged fibril nature of BC but also support its purity due to absence of additional degradation zones (Ul-Islam et al. 2012).
Fig. 5.
Thermal degradation analysis of bacterial cellulose produced from the scum obtained during preparation of JS
Mechanical properties of BC
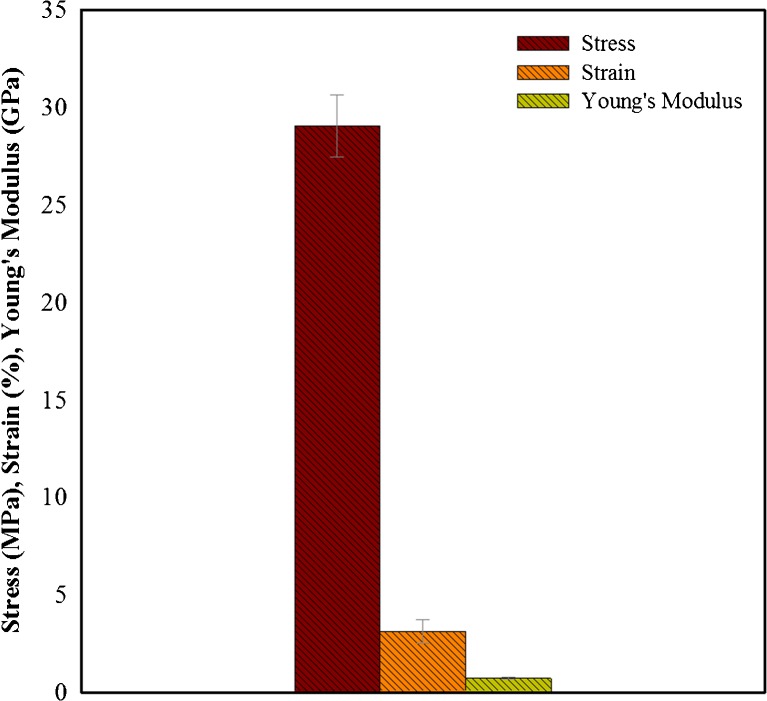
The mechanical properties of BC produced from JS media were investigated in order to assess any impact of the change of culture medium. The results have been compiled in Fig. 6. The mechanical properties are completely dependent on the physical nature of fibril together with the strength of intermolecular hydrogen bonding between cellulose chains (Ha et al. 2011). Figure 6 shows that tensile stress of the samples was about 29.05 MPa while the elongation at break was 3.12 %. The average values of Young’s modulus were 0.711 GPa. The observed mechanical properties depict the high strength of BC samples. As mentioned, the mechanical features are dependent on fibril size and arrangements, FE-SEM analysis results can explain these observations. The uniform sized well-arranged fibrils provide higher strength to the BC as observed in the present study. Slightly lower values of strain indicate that samples were completely dried and do not have much space to get high elasticity. The closely packed fibrils resist against the applied force hence results in higher tensile strength values; however, these directly break after certain pressure without showing much elastic behaviour.
Fig. 6.
Tensile strength, Young’s modulus and % strain of bacterial cellulose produced from the scum obtained during preparation of JS
Biocompatibility of BC
Biocompatible and non-toxic nature of any materials is of primary importance in defining its rule as biomedical material. BC has been widely used in medical fields specifically in wound healing and skin regeneration (Torres et al. 2012; Ul-Islam et al. 2014). In the present study, the biocompatibility of the BC produced from JS medium with skin fibroblast cells. The results of biocompatibility have been shown in Fig. 7. It could be seen that after 2 days of incubation the cells are attached throughout the BC surface. The growth of fibroblast cells on BC surface indicates the biocompatible and non-toxic nature of the BC. Higher resolution image further indicated the strength of cells attachment and depicts their spreading. The successful attachment of skin cells on BC surface not only clarify its biocompatible nature but also advocated its importance in medical applications.
Fig. 7.
Biocompatible behaviour of bacterial cellulose produced from the scum obtained during preparation of JS against animal osteoblast cells
Conclusion
A new cheap and raw medium (JS) was successfully evaluated for BC production through static and shaking cultivation strategies. The BC production and productivity rate was comparable to the one obtained from expensive synthetic media. Structural features insured the purity of produced BC while the physical measurements depicted high mechanical and thermal properties. Besides, BC produced from JS showed excellent level of biocompatible properties. The study provides a broad idea about the potential of waste materials for eco-friendly and inexpensive BC production.
Contributor Information
Taous Khan, Phone: (92)992-383591-6, Email: taouskhan@ciit.net.pk.
Joong Kon Park, Phone: (82) 53-950-5621, Email: parkjk@knu.ac.kr.
References
- Czaja W, Krystynowicz A, Bielecki S, Brown RM. Microbial cellulose-the natural power to heal wounds. Biomaterials. 2006;27(2):145–151. doi: 10.1016/j.biomaterials.2005.07.035. [DOI] [PubMed] [Google Scholar]
- Darder M, Colilla M, Ruiz-Hitzky E. Biopolymer-clay nanocomposites based on chitosan intercalated in montmorillonite. Chem Mater. 2003;15(20):3774–3780. doi: 10.1021/cm0343047. [DOI] [Google Scholar]
- Ha JH, Shehzad O, Khan S, Lee SY, Park JW, Khan T, Park JK. Production of bacterial cellulose by a static cultivation using the waste from beer culture broth. Korean J Chem Eng. 2008;25(4):812–815. doi: 10.1007/s11814-008-0134-y. [DOI] [Google Scholar]
- Ha JH, Shah N, Ul-Islam M, Khan T, Park JK. Bacterial cellulose production from a single sugar α-linked glucuronic acid-based oligosaccharide. Process Biochem. 2011;46(9):1717–1723. doi: 10.1016/j.procbio.2011.05.024. [DOI] [Google Scholar]
- Keshk SMA. Bacterial cellulose production and its industrial applications. J Bioprocess Biotechnol. 2014;4(2):150. doi: 10.4172/2155-9821.1000150. [DOI] [Google Scholar]
- Khan T, Park JK. The structure and physical properties of glucuronic acid oligomers produced by a Gluconacetobacter hansenii strain using the waste from beer fermentation broth. Carbohydr Polym. 2008;73(3):438–445. doi: 10.1016/j.carbpol.2007.12.010. [DOI] [Google Scholar]
- Khan T, Hyun SH, Park JK. Production of glucuronan oligosaccharides using the waste of beer fermentation broth as a basal medium. Enzym Microb Technol. 2007;42(1):89–92. doi: 10.1016/j.enzmictec.2007.08.007. [DOI] [Google Scholar]
- Khattak WA, Khan T, Ul-Islam M, Wahid F, Park JK. Production, characterization and physico-mechanical properties of bacterial cellulose from industrial wastes. J Polym Environ. 2015;23(1):45–53. doi: 10.1007/s10924-014-0663-x. [DOI] [Google Scholar]
- Kim SJ, Li H, Oh I, Kee CD, Kim MJ. Effect of viscosity-inducing factors on oxygen transfer in production culture of bacterial cellulose. Korean J Chem Eng. 2012;29(6):792–797. doi: 10.1007/s11814-011-0245-8. [DOI] [Google Scholar]
- Li SM, Jia N, Zhu JF, Ma MG, Sun RC. Synthesis of cellulose-calcium silicate nanocomposites in ethanol/water mixed solvents and their characterization. Carbohydr Polym. 2010;80(1):270–275. doi: 10.1016/j.carbpol.2009.11.024. [DOI] [Google Scholar]
- Liu W, Hou Y, Wu W, Ren S, Wang W. Complete conversion of cellulose to water soluble substances by pretreatment with ionic liquids. Korean J Chem Eng. 2012;29(10):1403–1408. doi: 10.1007/s11814-012-0023-2. [DOI] [Google Scholar]
- Masaoka S, Ohe T, Sakota N. Production of cellulose from glucose by Acetobacter xylinum. J Ferment Bioeng. 1993;75(1):18–22. doi: 10.1016/0922-338X(93)90171-4. [DOI] [Google Scholar]
- Mohite BV, Salunke BK, Patil SV. Enhanced production of bacterial cellulose by using Gluconacetobacter hansenii NCIM 2529 strain under shaking conditions. Appl Biochem Biotechnol. 2013;169(5):1497–1511. doi: 10.1007/s12010-013-0092-7. [DOI] [PubMed] [Google Scholar]
- Shah N, Ul-Islam M, Khattak WA, Park JK. Overview of bacterial cellulose composites: a multipurpose advanced material. Carbohydr Polym. 2013;98(2):1585–1598. doi: 10.1016/j.carbpol.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Shezad O, Khan S, Khan T, Park JK. Physicochemical and mechanical characterization of bacterial cellulose produced with an excellent productivity in static conditions using a simple fed-batch cultivation strategy. Carbohydr Polym. 2010;82(1):173–180. doi: 10.1016/j.carbpol.2010.04.052. [DOI] [Google Scholar]
- Shi Q, Li Y, Sun J, Zhang H, Chen L, Chen B, Yang H, Wang Z. The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein-2. Biomaterials. 2012;33(28):6644–6649. doi: 10.1016/j.biomaterials.2012.05.071. [DOI] [PubMed] [Google Scholar]
- Son HJ, Kim HG, Kim KK, Kim HS, Kim YG, Lee SJ. Increased production of bacterial cellulose by Acetobacter sp. V6 in synthetic media under shaking culture conditions. Bioresour Technol. 2003;86(3):215–219. doi: 10.1016/S0960-8524(02)00176-1. [DOI] [PubMed] [Google Scholar]
- Torres FG, Commeaux S, Troncoso OP. Biocompatibility of bacterial cellulose based biomaterials. J Funct Biomater. 2012;3(12):864–878. doi: 10.3390/jfb3040864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ul-Islam M, Shah N, Ha JH, Park JK. Effect of chitosan penetration on physico-chemical and mechanical properties of bacterial cellulose. Korean J Chem Eng. 2011;28(8):1736–1743. doi: 10.1007/s11814-011-0042-4. [DOI] [Google Scholar]
- Ul-Islam M, Khan T, Park JK. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr Polym. 2012;88(2):596–603. doi: 10.1016/j.carbpol.2012.01.006. [DOI] [Google Scholar]
- Ul-Islam M, Khattak WA, Kang M, Kim SM, Khan T, Park JK. Effect of post-synthetic processing conditions on structural variations and applications of bacterial cellulose. Cellulose. 2013;28(1):253–263. doi: 10.1007/s10570-012-9799-9. [DOI] [Google Scholar]
- Ul-Islam M, Khattak WA, Kang M, Kim SM, Khan T, Park JK. Effect of post-synthetic processing conditions on structural variations and applications of bacterial cellulose. Cellulose. 2013;20(1):253–263. doi: 10.1007/s10570-012-9799-9. [DOI] [Google Scholar]
- Ul-Islam M, Khattak WA, Ullah MW, Khan S, Park JK. Synthesis of regenerated bacterial cellulose-zinc oxide nanocomposite films for biomedical applications. Cellulose. 2014;21(1):433–447. doi: 10.1007/s10570-013-0109-y. [DOI] [Google Scholar]