Abstract
Pursuant to the tendencies of producing functional foods, attractive to a wide range of consumers, in this study chocolates enriched with freeze dried (FD) and concentrated (CE) nettle extracts were formulated, and their polyphenolic and antioxidant capacity stability evaluated during 12 months of storage. A simple aqueous extraction procedure of nettle was developed, and the defined extract evaluated for its cytotoxic and antioxidant/prooxidant activity on human colon cancer cell line (SW 480). An increase in total polyphenolic content, chlorogenic acid and flavonoid derivatives (originating from nettle extract) contents was achieved in enriched chocolates. Implementation of FD extract enabled higher increase of polyphenolic content in comparison to CE extract. During storage, fluctuations of polyphenolic content were observed, but the final bioactive parameters did not differ (or increased) from the initial ones. Nettle enriched chocolates exhibited more intense bitterness and astringency, while dark chocolates were preferred over milk and semisweet ones.
Keywords: Chocolate, Cytotoxicity, Nettle, Polyphenols, Stability
Introduction
A growing body of evidence documents positive health benefits from food components not considered nutrients in the traditional definition. Medicinal plants are a very rich source of such compounds, as well as the richest bio-resource of drugs of traditional medicine systems, food supplements, pharmaceutical intermediates and chemical entities for synthetic drugs (Ncube et al. 2008), since they contain a broad range of bioactive compounds such as lipids, phytochemicals, pharmaceutics, flavors, fragrances and pigments (Wang and Weller 2006). With an emphasis on quality and standardization, food manufacturers may find medicinal plants to be the new source of functional ingredients.
Functional foods enriched with herbal ingredients are not new in the marketplace, since herbal additives have begun to appear in conventional foods ranging from teas and juices to snack chips and energy bars. Different medicinal plants and their extracts have been used to fortify and enhance the antioxidant capacity of cheese (El-Aziz et al. 2012), jelly (Skouroliakou et al. 2009), candies (Gramza-Michalowska and Regula 2007), bread (Glei et al. 2006) and meat products (Ryan et al. 2009). However, little scientific data exists regarding the development and efficiacy of functional confectionery products, one of the most commonly consumed foods today, among consumers of all ages. Chocolate is a unique food product, presenting both a confectionery treat often avoided due to the high calorific value (imparted to high sugar and fat content), but is also promoted as a functional food product with potential health benefits, attributed to its rich bioactive profile. Regardless of its nutritional and phytochemical aspects, the popularity of chocolate has a continous tendency, which makes this product ideal for modifications and development of new products. So far, chocolates enriched with phytosterols (Borges Botelho et al. 2014), probiotics, maltodextrin and lemon fibers (Erdem et al. 2014), dried fruits (Komes et al. 2013) and stevia or other natural or bulk sweeteners (Belščak-Cvitanović et al. 2015) have been produced, while only one study has assessed the potential of using medicinal plant extracts aimed to improve the functional properties of chocolate (Belščak-Cvitanović et al. 2012).
In order to obtain dosage forms of plant materials for their implementation in the production of food products, an extraction step is reqired. Extraction techniques have been extensively investigated in order to obtain valuable natural compounds from plants for commercialization (Wang and Weller 2006), but emphasis has been given to polyphenolic compounds extracted with the use of innovative extraction techniques and organic solvents, while the conventional water-based extraction techniques and the potential effect of other bioactive compounds that are extracted in that way was not clarified. The aim of this study was to develop and characterize water-based polyphenolic extracts of nettle (Urtica dioica L.), a traditional, widely used medicinal plant and to incorporate the obtained extract in the production of enriched, functional chocolates. Phytochemical constituents of Urtica dioica are of medical interest, as extracts of the roots and leaves are widely used in traditional medicine in many areas of the world. High levels of iron and vitamin C in nettle preparations confirm the rationale for its traditionally proclaimed value in the treatment of numerous diseases, such as anaemia and eczema (Pinelli et al. 2008), rheumatism (Jaric et al. 2007), treatment of urinary, bladder and kidney problems (Guarrera and Savo 2013), but could also support the use of this plant for improvement of functional properties of food products.
In this study, in order to avoid the application of organic solvents for the extraction of polyphenols and to ensure a simple extraction technique with low economical costs, infusion, maceration and decoction of nettle were prepared and the extracts examined for their cytotoxic and antioxidative/prooxidative effects on human colon cancer cell line (SW 480), polyphenolic content and profile. Extract which provided maximum polyphenolic content and was validated for its biological activity in vitro was implemented in the production of three types of chocolates and the stability of bioactive compounds of the enriched chocolates was evaluated during 1 year of storage.
Materials and methods
Chemicals
All chemicals used for the experimental procedures were of analytical grade.
Extraction efficiency of polyphenolic compounds from nettle
Dried nettle (Urticae folium) was purchased at a local herbal market specialized for organic food. Moisture (6.89 %), extractable fat (1.32 %), crude fibre (8.87 %), protein (25.43 %) and ash (15.36 %) contents were determined on a dry weight basis as described in AOAC methods (1995). The total carbohydrates content amounted to (25.63 %). Mineral analysis, the content of soluble polysaccharides (15.01 %) and tannins (0.04 %) were determined as presented in the paper by Belščak-Cvitanović et al. (2011). For the extractions, dried nettle was ground to powder using a mortar and pestle and sieved to produce plant material of unified particle size. In order to obtain an extract with the best extraction efficiency of polyphenols, aimed for food purposes, infusion (2.0 g of plant sample was extracted in 200 mL of distilled water (80 °C) and stirred with a glass rod for 10 or 30 min), maceration (2.0 g of sample was extracted in 200 mL of distilled water (room temperature) during 24 h with occasional stirring) or decoction (2.0 g of plant was subjected to boiling in 200 mL of distilled water during 20 min) of dried plant sample were prepared. The final extract used for chocolate production was prepared by pouring 200 mL of boiling water over 10 g of plant sample and extracted for 30 min, without maintaining the water temperature. After extraction, all extracts were filtered through a tea strainer and analyzed.
Determination of biological activity of nettle extract in vitro
Human colon-cancer cells (SW480) were provided as a gift by the Rudjer Boskovic Institute, Zagreb, Croatia. Cells were grown as monolayer cultures in DMEM (GIBCO, USA), supplemented with 10 % of the foetal bovine serum (GIBCO, USA), 4500 mg/L of glucose and 1 % of penicillin/streptomycin. The final concentration of nettle extract obtained for the experiments on SW480 cells (10 g of plant material in 200 mL of water), expressed as the extraction yield, ranged from 17.5 to 176 g/L (concentrations provided for the range of 0.5x – 5x).
Cytotoxicity of nettle extract was determined spectrophotometrically by the neutral red (NR) assay, as described by Belscak-Cvitanovic et al. (2014). SW480 cells were seeded in wells (2 · 104 per well) of 96 well plates. Cells were treated with the extract in different concentration ranges (0.5-5x of concentration present in originally prepared extract) for 1 and 2 h, with and without subsequent recovery period. After the treatment, the NR assay was carried out, as described by Babich and Borenfreund (1990). Cell viability was calculated using the following equation:
where ABSsample is the spectrophotometrically determined absorbance of cells treated with extracts and ABSCtrl is the absorbance of corresponding vehicle control (growth medium).
Reactive oxygen species (ROS) formation in the cells after the treatment with nettle extract was determined by dichlorohydrofluorescein assay using microplate reader (Wang and Joseph 1999), as described by Belscak-Cvitanovic et al. (2014). SW480 cells were seeded in wells (2 · 104 per well) of 96 well black plates and subsequently treated with different concentrations of extract (0.5-5 x of the concentration present in originally prepared extract) for 1 and 2 h, with and without subsequent recovery period. Since H2O2 is an important ROS which acts as a pro-oxidant in the cells affected by a certain imbalance, it initiates a cascade of reactions leading to oxidative stress related changes in the cells (Akyol et al. 2014). In order to determine the nettle extract’s antioxidative/prooxidative nature in the cells in which oxidative stress is already induced, cells were treated with 250 μM H2O2 for 30 min, in order to induce oxidative stress. Each extract concentration was tested in quadruplicate, and each experiment was repeated three times. Data are reported as fluorescence intensity in relation to cell survival data obtained according to the equation:
where ABSsample is the absorbance of cells treated with extracts and ABSCtrl is the absorbance of corresponding vehicle control (growth medium).
Formulation of experimental enriched chocolates
The experimental enriched chocolates were produced as described by Belščak-Cvitanović et al. (2012), through a classical technological process in the confectionery factory (Zvečevo, Požega, Croatia). The addition of two different forms of polyphenolic extract from nettle was evaluated. The strained extracts were freeze-dried (FD) or concentrated (C) by evaporation to thickness of syrup (containing 21.13 % of dry matter, determined by dry matter determination in an oven at 105 °C until constant weight (AOAC, 1995)). The addition of 2 % of freeze dried (FD) and 2 % of concentrated extracts (CE) to milk (M), semisweet (S) and dark chocolates (D), containing 30, 38 and 72 % of cocoa parts, respectively, was evaluated (based on the findings previously established by Belščak-Cvitanović et al. 2012).
All three types of chocolates were refined in two phases: preliminary grinding on two-roll press (Carle & Montanari, Italy) and grinding on five-roll press (Carle & Montanari, Italy). The conching phase was undertaken for a total of 24 h (dry conching for 4 h and liquid conching for 20 h) in a Carle & Montanari conch (Italy). After 16 h of liquid conching, the remaining cocoa butter, emulsifier (soy lecithin) and vanilla flavour were added. The prepared chocolate masses were tempered in a SeedMaster tempering machine (Bühller, Switzerland). 2 kg portions of all chocolates were then excluded, and the above mentioned quantities (2 % of FD, 2 % of CE) of nettle polyphenolic extracts were added in laboratory conditions, followed by moulding into plastic moulds. The moulded chocolates were placed in a temperature-controlled room at 15 °C for 30 min before de-moulding and the finished bars were wrapped in aluminium foil and stored in a refrigerated chamber at 10–18 °C until analysed. In order to examine the stability of polyphenols in the enriched chocolates, polyphenolic content and antioxidant capacity were determined each fourth month during 1 year (shelf life of chocolates).
Determination of bioactive parameters of chocolates
Determination of polyphenolic compounds content
Chocolate extracts were prepared as described by Belščak-Cvitanović et al. (2015), using aqueous methanol (70 %, v/v) as the extraction solvent. Total phenol content (TPC) of chocolate extracts was determined spectrophotometrically by the Folin-Ciocalteu reagent, according to a modified method of Lachman et al. (1998). In order to determine flavonoids, the formaldehyde precipitation was used and the flavonoid content calculated as the difference between total phenol and nonflavonoid contents. All determinations were carried out in triplicates and the results were expressed as mg gallic acid equivalents (GAE)/g of sample. The content of flavan-3-ols was determined using the vanillin assay and the reaction with p-dimethylaminocinnamaldehyde as described by Di Stefano et al. (1989), and expressed as mg (+)-catechin/g. Proanthocyanidins (i.e. condensed tannins) were analysed by the procedure described by Porter et al. (1986), with some modifications and determined from a standard curve of cyanidin chloride treated with BuOH-HCl-FeIII mixture. The results were expressed as mg cyanidin chloride equivalents (CyE)/g sample. HPLC analysis of polyphenolic compounds and methylxanthines in the formulated chocolates was conducted according to the method described by Belščak-Cvitanović et al. (2015). All measurements were performed in triplicate.
Determination of antioxidant capacity
The ferric reducing/antioxidant power (FRAP) assay was carried out according to a standard procedure by Benzie and Strain (1996). Aqueous solutions of FeSO4 × 7H2O (100–1000 μmol/L) were used for the calibration and the results are expressed as mmol Fe(II)/L.
The Trolox equivalent antioxidant capacity (TEAC) of chocolate extracts was also estimated by the ABTS radical cation decolorization assay (Re et al. 1999). The results, obtained from triplicate analyses, were expressed as Trolox equivalents, and derived from a calibration curve determined for this standard (100–1000 μmol Trolox /L).
Sensory evaluation
Chocolates were evaluated using quantitative descriptive analysis procedure excerpt from the literature data on sensory evaluation of chocolate (Camu et al. 2008) and according to ISO standards (International Standard ISO 8586-2/2008 2008). The experimental samples were subjected to sensory evaluation using the internal sensory panel of Faculty of Food Technology and Biotechnology and Zvečevo food industry d.d. The sensory panel was comprised of 15 people, 9 female and 6 male members, who had undergone extensive sensory training and had previous experience in the assessment of chocolates.
The panellists were first trained and calibrated during two sessions in which the judges discussed and familiarized themselves with similar samples, as well as with the scale until reaching a consensus of the sensory attributes and their quantification. The sensory properties were presented on a 10-point scale, according to which 10 meant extremely desired quality and 1 was for a defective product. All samples were evaluated in partitioned booths of sensory laboratory under white light illumination at room temperature. Specimens with a size of 20 × 20 mm were separated from the chocolate bars with a hot knife. For sensory analysis, the samples were equilibrated to room temperature (22 °C) and served in Petri dishes encoded with random alphabet letters. For the experimental samples, a total of three sessions in a period of 1 month were held. Sessions were held once a week, and during each session one type of plain and enriched chocolates was evaluated (plain, CE and FD – in this order) in order to reduce perception fatigue. Eight attributes were evaluated for all chocolates (gloss, surface, breakage, melting, odour, mouthfeel, aftertaste and herbal), while additional two attributes were evaluated specifically for each type of chocolate (sweetness and milk taste for milk chocolate, sweetness and bitterness for semisweet and bitterness and astringency for dark chocolate) and the average scores were calculated. Warm water was provided for rinsing between samples.
Statistical analysis
Statistical analysis was performed using the SPSS v. 8.0 (SPSS Inc., Chicago, IL, USA). A three-way (and subsequently two-way) ANOVA analysis was used for investigation of the effects of three different factors (type of chocolate, extract form and storage time) and their interactions on dependent variables (the evaluated bioactive properties). One-way analysis of variance (ANOVA) was employed to determine whether the means obtained with various groups differ significantly from each other. The significance was established using Tukey post-hoc test. The probability level of p < 0.05 was considered significant.
Results and discussion
Comparison of different extraction techniques of nettle
Due to safety concerns associated with the use of organic solvents which can cause toxic effects and solvent residues in the extract, and to assure a cost-effective and feasible extraction procedure, several extraction procedures of nettle were compared and a water-based extract developed, which enables its application in food and pharmaceutical sector. As can be seen in Table 1, prolonged extraction (to 30 min) with water heated to 80 °C using stirring to enhance the extraction process, increases the polyphenolic content and antioxidant capacity of nettle. Maceration at room temperature results with the lowest polyphenolic compounds yield, while decoction at 100 °C during 20 min yields lower polyphenolic content in comparison to infusions. Based on the obtained results it can be observed that the content of specific subclasses of polyphenols differentiates depending on the extraction procedure. A low and insignificant (p > 0.05) content of proanthocyanidins was observed among the 10-min extracted infusion and macerate, while longer extraction time allowed the extraction of a higher content of these compounds (infusion30′ and final extract). Similarly, a high content of tannins was obtained by maceration of nettle, while employing higher temperature resulted with a decrease in their content, which is favourable since these compounds provide bitterness and astringency to the extract. Katsube et al. (2009) determined that polyphenolic compounds and their antioxidant capacity in plant substrates are stable up to temperatures of 70–80 °C, whereas above temperatures of 80 °C they start to deteriorate and consequently loose their activity. The most probable cause for a higher polyphenolic content at high temperature is the breakage of bonds between various phenolics (analytes) and the plant matrix (Palma et al. 2001). However, in this study higher solvent (100 °C) temperature and prolonged extraction time (30 min) were selected to obtain the nettle extract for further use. Despite the higher starting extraction temperature (100 °C), it was not maintained during extraction, since due to stirring it decreased and reached 46 °C at the end of extraction. The final extract was characterized with an insignificant content of total phenols when compared to infusion30′, but exhibited higher antioxidant capacity, which indicates that the applied extraction procedure yielded specific polyphenolic compounds with strong radical scavenging properties.
Table 1.
The effect of different aqueous extraction techniques on polyphenolic profile and antioxidant capacity of nettle
| Infusion10′ | Infusion30′ | Maceration | Decoction | Final extract | |
|---|---|---|---|---|---|
| Polyphenolic composition | |||||
| TPC (mg GAE/g dw) | 27.23 ± 1.22 | 31.25 ± 0.25a | 9.09 ± 0.77 | 18.68 ± 2.38 | 32.09 ± 0.54a |
| TFC (mg GAE/g dw) | 21.32 ± 0.71b | 21.47 ± 0.38b | 7.27 ± 0.39 | 13.12 ± 0.94 | 19.13 ± 0.82 |
| Flavan-3-ols (VI) (mg CE/g dw) | 2.25 ± 0.04 | 2.75 ± 0.15c | 1.04 ± 0.00 | 3.02 ± 0.28c | 3.48 ± 0.03 |
| Flavan-3-ols (p-DAC) (mg CE/g dw) | 1.72 ± 0.01d | 1.99 ± 0.10d | 0.97 ± 0.02 | 2.66 ± 0.02e | 2.42 ± 0.19e |
| Proanthocyanidins (mg CyE/g dw) | 0.03 ± 0.01f | 0.13 ± 0.02g | 0.04 ± 0.00f | 0.09 ± 0.01 | 0.14 ± 0.01g |
| Tannins (%) | 0.74 ± 0.08 | 0.84 ± 0.06 | 1.25 ± 0.02 | 0.30 ± 0.01 | 0.44 ± 0.00 |
| Antioxidant capacity | |||||
| ABTS (mM Trolox) | 2.25 ± 0.08 | 4.01 ± 0.28 | 0.28 ± 0.02 | 1.83 ± 0.09 | 5.75 ± 0.29 |
| FRAP (mM Fe(II)) | 5.47 ± 0.15 | 9.27 ± 0.32 | 2.38 ± 0.03 | 4.88 ± 0.03 | 13.47 ± 0.69 |
Values in the same row superscripted with the same alphabet are not significantly (p > 0.05) different
Cytotoxic and antioxidant/proroxidant activity of nettle extract on SW480 cell line
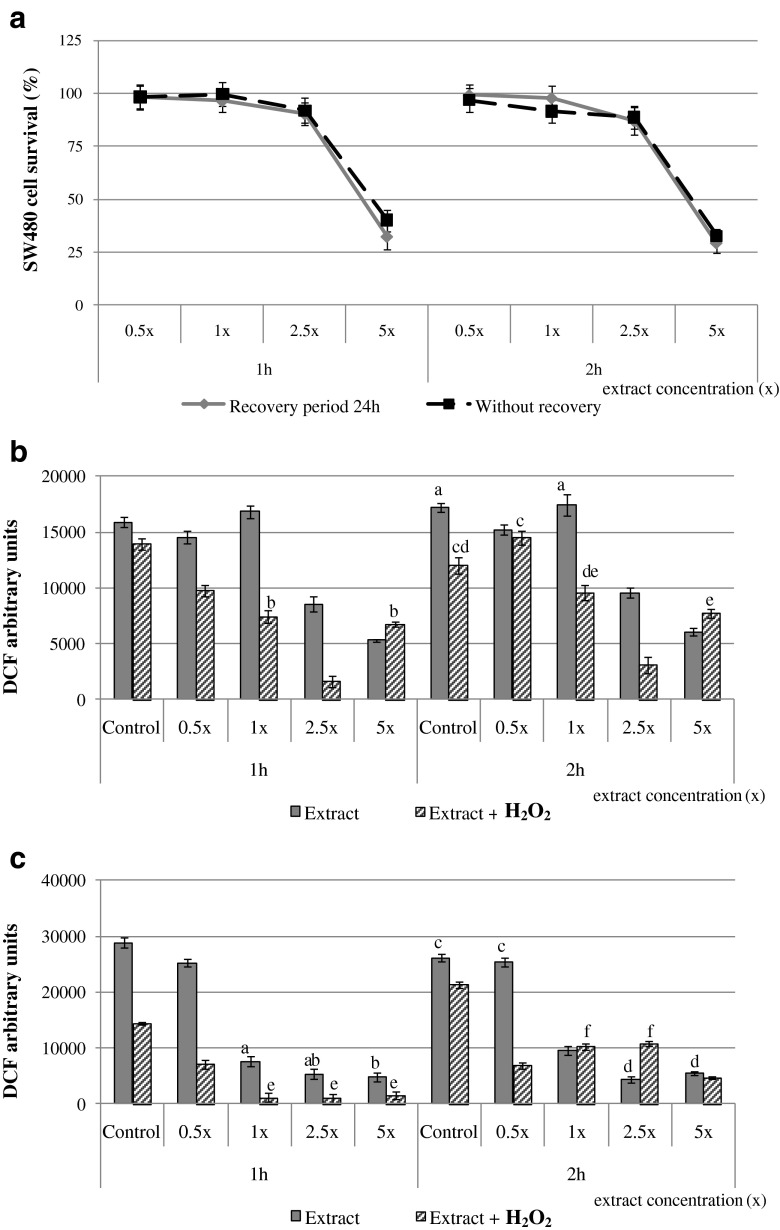
Cytotoxicity of the final nettle extract (Fig. 1a) obtained after the extraction comparisons, was in dependence of the extract concentration and treatment duration (1 or 2 h). Nettle extract treatment of SW 480 cells caused a dose–response decrease in cell survival during both 1 and 2 h of incubation. The cytotoxic effect of the extract was exerted by higher nettle extract concentrations (2.5x and 5x), while prolonged incubation (2 h) with the highest extract concentration (5x) caused a lower but insignificant decrease in cell survival (in comparison to 1 h treatment). After 2 h of incubation with nettle extract (5x), the cell survival exhibited only 32 %, indicating the cytotoxic effect of nettle extract on SW480 colon cancer cell line. The recovery period of 24 h (denoting one cell cycle period) had no significant effect (p < 0.05) on the cell survival of SW 480 cells.
Fig. 1.
Survival of human colon cancer (SW480) cells following 1 and 2 h treatment with nettle extract without and with subsequent recovery period of 24 h (a), reactive oxygen species formation in SW480 cells treated with nettle extract (and pre-treated with H2O2) for 1 and 2 h without recovery period (b), reactive oxygen species formation in SW480 cells treated with blackberry leaf extract (and pre-treated with H2O2) with recovery period of 24 h (c)
Conversely to the cytotoxic effects, the antioxidant/prooxidant activity (ROS formation) was more affected by the recovery period than the incubation (treatment) time. Noncytotoxic concentration of nettle extract (1x) slightly increased ROS formation after 1 h of incubation (Fig. 1b), which was also observed in the case of 2 h of incubation. Increasing the concentrations of nettle extract (2.5x and 5x) exhibited antioxidant activity, which is in correspondence to the exerted cytotoxic effect by these extract concentrations, but can not be attributed to the ROS scavenging activity. After the recovery period of 24 h (Fig. 1c), ROS formation was decreased upon the treatment with all nettle extract concentrations, but a proportional relation between the extract concentration and ROS level can be observed since higher extract concentrations caused lower ROS levels. In case of previous treatment of SW 480 cells with hydrogen peroxide, antioxidant activity of nettle extract was pronounced, since a decrease of ROS levels was observed after the extracts treatment (regardless of the extract dose) in comparison to control. A slight increase of ROS formation was observed only after the tretament with 0.5x concentration of nettle extract, indicating the prooxidant activity, which was already established for non-H2O2 pretreated cells. The prooxidant effect of lower concentrations of ground ivy and hawthorn extracts was also assessed by Belscak-Cvitanovic et al. (2014). Since a correlation between the cytotoxicity and ROS formation was observed, meaning that higher nettle extract concentrations exert a high cytotoxicity and a decrease of ROS levels, the results indicate that different cellular responses are attributed for the observed effects. Due to the complex nettle extract composition, comprising polyphenols, minerals, vitamin C, soluble polysaccharides and other bioactive compounds that were not determined in this study, it seems that the combination of all these bioactive compounds may exerted the observed effects on the examined model SW 480 cell line.
Bioactive compounds and antioxidant capacity of chocolates enriched with nettle extract during storage
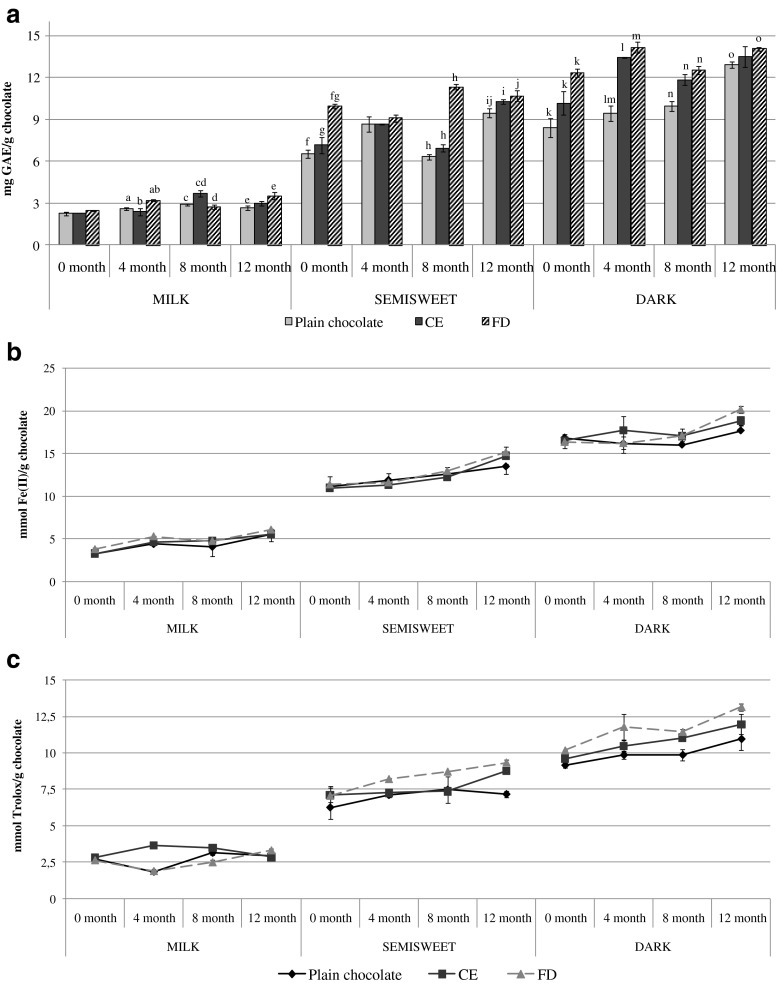
Since the results of toxicological evaluations on SW480 cell line revealed that the concentration of 5x is the most appropriate to induce potent cytotoxic and antioxidative effects, the obtained final nettle extract in that concentration was freeze dried, or even further concentrated to examine its potential for enrichement of chocolate polyphenolic content. The analysis of TPC and antioxidant capacity of enriched chocolates (Fig. 2a–c), revealed that an increase of these parameters was achieved by the addition of nettle extracts to chocolates, regardless of the extract form (CE or FD extracts) or the type of chocolate (cocoa parts content). Three way ANOVA (performed by taking into account the extract form, type of chocolate and storage time as independent variables (factors) and the measured parameters as dependent variables) confirmed significant (p < 0.05) interactions between these three factors regarding the TPC and antioxidant capacity of formulated chocolates. Consequently, two-way and one way ANOVA analysis also revealed significant (p < 0.05) effects of all three individual factors (extract form, type of chocolate and storage time) on the TPC and antioxidant capacity of chocolates. The results displayed on Fig. 2a show a higher TPC of chocolates enriched with freeze dried nettle extract (FD) in comparison to chocolates enriched with concentrated extract (CE), however more pronounced differences and a higher increase were observed in enriched semisweet and dark chocolates. This implies that the presence of water in the extracts aimed for fortifying chocolate based products adversely affects the bioactive compounds and indicates that dehydrated extracts should be employed for that purpose.
Fig. 2.
Total phenol content (TPC) in mg GAE/g of chocolate (a) and antioxidant capacity of experimental chocolates determined by the FRAP (mmol Fe(II)/g chocolate) (b) and ABTS assays (mmol Trolox/ g chocolate) (c). Results are expressed as means of mg/g or mmol/g ± SD
Regarding the stability of polyphenolic compounds and antioxidant capacity during the storage period of 12 months (the shelf life of chocolates), an increasing tendency in the TPC and antioxidant capacity (Figs. 2a–c) of enriched chocolates during the storage time was established, although fluctuations in the periodically determined parameters can be observed. Available literature data on the stability of polyphenolic compounds during storage of various plant substrates and food products reveal very contradictory results, however in dependence of the plant substrate (polyphenols source) and type of polyphenolic compound. Hurst et al. (2009) evaluated the stability of polyphenolic antioxidants and flavan-3-ols from milk and dark chocolates over time and determined their stability, i.e. no marked differences in their levels during storage. Conversely to these results, our study indicated a slight increase of these parameters, which is supported by the study of Mazur et al. (2014), who determined an increase in total phenols during 6 months of storage of raspberry jams. According to the authors explanation, the release of certain polyphenolic compounds after hydrolysis during storage may be the cause of the observed increase, since in their free forms polyphenols can exhibit higher reducing capacity, thus contributing more to TPC. However, since in the present study marked fluctuations of the polyphenolic content was observed, it seems that degradation of these compounds may have also occured. The reduction of total phenol content during storage may be the result of degradation processes that occur in the more complex substrates and oxidation activities that modify their structure. Namely, previous studies (Galmarini et al. 2013) have reported about the significant decrease in the content of catechins during storage. The observed fluctuations can also be attributed to the presence of sugars, polysaccharides and proteins in the chocolates, since polyphenols can bind with proteins and peptides forming soluble and insoluble complexes through hydrophobic interaction and hydrogen bonding (Jobstl et al. 2006), which may interfere during analytical determination. According to Jobstl et al. (2006) polyphenols also have the ability to bind to iron and reduce its availability. Nettle leaves were noted for their particularly high content of some metals (Se, Zn and Fe, Mg) (Rzemykowska and Ostrowska 1994). Due to the presence of these compounds in enriched chocolates, deriving from both chocolate (sugars, proteins) and nettle (polysaccharides, metals) the observed fluctuations of polyphenolic content could have occured.
Despite the observed fluctuations the final TPC and antioxidant capacity of enriched chocolates, obtained after 12 months of storage are higher than the initial values (after the production). The same was observed for specific sub-classes of polyphenolic compounds, flavonoids, flavan-3-ols and proanthocyanidins (Table 2). The percentage increase after the implementation of nettle extracts, calculated as the ratio between the polyphenolic content of enriched chocolate (in a certain storage time) and plain milk chocolate after production (0 month) was the highest in case of total flavonoids, even up to 34.5 % (enriched milk chocolate after 12 months of storage). Although cocoa parts are the major source of flavan-3-ols and proanthocyanidins, marked fluctuations during the storage period of 12 months were established. This is not surprising if taking into account that catechins can undergo degradation, oxidation, epimerization and polymerization (Ananingsih et al. 2011) and many factors could contribute to these chemical changes.
Table 2.
The content of total flavonoids (mg GAE/g chocolate), flavan-3-ols (mg CE/g chocolate) and proanthocyanidins (mg CyE/g chocolate) of experimental chocolates affected by storage time
| Flavonoids | Flavan-3-ols (mg CE/g chocolate) | Proanthocyanidins | |||
|---|---|---|---|---|---|
| mg GAE/g chocolate | Vanillin index | p-DAC | mg CyE/g chocolate | ||
| Milk chocolate | |||||
| 0 month | M | 1.19 ± 0.15 | 0.83 ± 0.02 | 0.44 ± 0.09 | 0.26 ± 0.06 |
| M CE | 1.25 ± 0.07 | 1.05 ± 0.04 | 0.48 ± 0.01 | 0.35 ± 0.09 | |
| M FD | 1.58 ± 0.10 | 1.05 ± 0.01 | 0.47 ± 0.01 | 0.39 ± 0.00 | |
| 4 month | M | 1.35 ± 0.12 | 0.86 ± 0.08 | 0.43 ± 0.00 | 0.32 ± 0.05 |
| M CE | 1.56 ± 0.21 | 1.11 ± 0.13 | 0.48 ± 0.03 | 0.38 ± 0.03 | |
| M FD | 1.82 ± 0.13 | 1.27 ± 0.08 | 0.55 ± 0.00 | 0.38 ± 0.07 | |
| 8 month | M | 1.44 ± 0.11 | 1.45 ± 0.17 | 0.53 ± 0.02 | 0.30 ± 0.02 |
| M CE | 1.67 ± 0.10 | 1.31 ± 0.15 | 0.51 ± 0.01 | 0.43 ± 0.01 | |
| M FD | 1.90 ± 0.17 | 1.66 ± 0.19 | 0.54 ± 0.02 | 0.44 ± 0.01 | |
| 12 month | M | 1.42 ± 0.15 | 1.55 ± 0.04 | 0.64 ± 0.04 | 0.40 ± 0.07 |
| M CE | 1.58 ± 0.14 | 1.32 ± 0.03 | 0.69 ± 0.05 | 0.58 ± 0.08 | |
| M FD | 2.17 ± 0.09 | 1.82 ± 0.06 | 0.65 ± 0.01 | 0.55 ± 0.03 | |
| Semisweet chocolate | |||||
| 0 month | S | 5.48 ± 0.85 | 2.77 ± 0.11 | 1.61 ± 0.01 | 1.14 ± 0.01 |
| S CE | 5.59 ± 1.77 | 2.35 ± 0.19 | 1.63 ± 0.00 | 1.28 ± 0.06 | |
| S FD | 6.20 ± 1.11 | 2.64 ± 0.02 | 1.58 ± 0.00 | 1.66 ± 0.15 | |
| 4 month | S | 6.25 ± 0.19 | 2.41 ± 0.10 | 1.52 ± 0.06 | 1.32 ± 0.26 |
| S CE | 6.30 ± 0.13 | 2.94 ± 0.04 | 1.65 ± 0.00 | 1.30 ± 0.23 | |
| S FD | 6.35 ± 0.31 | 2.62 ± 0.47 | 1.68 ± 0.04 | 1.65 ± 0.05 | |
| 8 month | S | 7.05 ± 0.42 | 2.90 ± 0.15 | 1.55 ± 0.04 | 1.32 ± 0.02 |
| S CE | 6.02 ± 0.19 | 2.86 ± 0.48 | 1.71 ± 0.07 | 1.83 ± 0.07 | |
| S FD | 7.11 ± 0.34 | 2.69 ± 0.04 | 1.63 ± 0.01 | 1.63 ± 0.04 | |
| 12 month | S | 7.91 ± 0.27 | 2.93 ± 0.01 | 1.69 ± 0.02 | 1.33 ± 0.05 |
| S CE | 8.39 ± 0.22 | 3.19 ± 0.07 | 1.67 ± 0.06 | 1.84 ± 0.17 | |
| S FD | 8.68 ± 0.19 | 3.34 ± 0.17 | 1.61 ± 0.03 | 1.81 ± 0.21 | |
| Dark chocolate | |||||
| 0 month | D | 6.45 ± 0.26 | 4.32 ± 0.06 | 1.79 ± 0.33 | 1.62 ± 0.23 |
| D CE | 6.98 ± 0.66 | 4.55 ± 0.29 | 1.70 ± 0.01 | 1.75 ± 0.01 | |
| D FD | 7.61 ± 0.76 | 4.72 ± 0.11 | 2.01 ± 0.01 | 1.77 ± 0.13 | |
| 4 month | D | 7.41 ± 0.13 | 4.21 ± 0.34 | 1.81 ± 0.01 | 1.85 ± 0.14 |
| D CE | 8.09 ± 0.35 | 4.83 ± 0.02 | 1.87 ± 0.08 | 2.09 ± 0.84 | |
| D FD | 8.84 ± 0.05 | 4.61 ± 0.65 | 1.93 ± 0.09 | 2.17 ± 0.25 | |
| 8 month | D | 7.66 ± 0.66 | 4.13 ± 0.13 | 1.91 ± 0.02 | 1.91 ± 0.04 |
| D CE | 7.89 ± 0.21 | 4.58 ± 0.05 | 2.01 ± 0.10 | 1.97 ± 0.01 | |
| D FD | 8.80 ± 0.34 | 4.67 ± 0.03 | 2.10 ± 0.03 | 2.32 ± 0.04 | |
| 12 month | D | 8.89 ± 0.18 | 4.22 ± 0.16 | 2.06 ± 0.05 | 1.94 ± 0.05 |
| D CE | 8.07 ± 0.21 | 4.64 ± 0.20 | 2.09 ± 0.05 | 2.03 ± 0.08 | |
| D FD | 9.80 ± 0.43 | 4.95 ± 0.07 | 2.26 ± 0.06 | 2.27 ± 0.09 | |
M milk chocolate, S semisweet chocolate, D dark chocolate
The analysis of specific polyphenolic compounds performed using HPLC-PDA (Table 3), revealed extensive fluctuations in the identified specific polyphenols, affected by both the form of nettle extract and storage duration. The content of all identified compounds increases with the increase of cocoa parts content of chocolates, i.e. in the following ranking: milk > semisweet > dark chocolate, indicating the prevalence of cocoa-derived polyphenolic compounds in the TPC (rather than the ones from nettle extract). Generally, epicatechin (−)-EC, catechin (+)-C and procyanidin B2 (Proc B2) were identified among flavan-3-ols, with EC being the most abundant compound (followed by proc B2). The content of flavonoid derivatives (ΣFlavonoidD) was expressed as the sum of identified quercetin derivatives, since this was the dominant flavonoid compound (other than flavan-3-ols) identified in both chocolate and nettle. The HPLC analysis performed in this study revealed an increase of quercetin derivatives in nettle-enriched chocolates (regardless of the extract dosage form) in comparison to plain chocolates, which confirms that the addition of nettle to chocolates enhances its polyphenolic profile. This is also applicable to the content of flavonoid derivatives since an increase in the overall flavonoid derivatives content of both enriched chocolates (CE and FD) was observed when compared to plain chocolates (Table 3).
Table 3.
Content (mg/g chocolate) of flavan-3-ols [epicatechin (EC), catechin (C), procyanidin B2], sum of phenolic acids and the sum of flavonoid derivatives [Ʃ Flavonoid D] in the formulated enriched chocolates during 12 months of storage*The standard deviation of all measurements amounts to < 10 % of the mean value
| Flavan-3-ols | ∑ Phenolic acids | Ʃ Flavonoid D | ||||
|---|---|---|---|---|---|---|
| (−) – EC | (+)- C | Proc B2 | ||||
| 0 month | Mplain | 0212 | n.d. | 0223 | 0.288 | 0027 |
| M CE | 0183 | 0023 | 0081 | 0.06 | 0036 | |
| M FD | 0231 | 0021 | 0110 | 0.083 | 0050 | |
| 4 month | Mplain | 0209 | 0024 | 0095 | 0.121 | 0020 |
| M CE | 0220 | 0035 | 0097 | 0.088 | 0035 | |
| M FD | 0296 | 0028 | 0179 | 0.13 | n.d. | |
| 8 month | Mplain | 0245 | 0060 | 0156 | 0.285 | 0058 |
| M CE | 0163 | 0038 | 0106 | 0.132 | 0033 | |
| M FD | 0235 | 0036 | 0053 | 0.123 | 0050 | |
| 12 month | Mplain | 0136 | 0045 | 0076 | 0.099 | traces |
| M CE | 0254 | 0039 | 0061 | 0.154 | 0533 | |
| M FD | 0294 | 0041 | 0087 | 0.126 | 0125 | |
| 0 month | Splain | 0227 | 0137 | 0358 | 0.053 | 0245 |
| S CE | 0296 | 0113 | 0299 | 0.068 | 0191 | |
| S FD | 0241 | 0110 | 0315 | 0.066 | 0089 | |
| 4 month | Splain | 0336 | 0094 | 0252 | 0.152 | 0211 |
| S CE | 0274 | 0134 | 0199 | 0.201 | 0270 | |
| S FD | 0225 | n.d. | 0179 | 0.052 | n.d. | |
| 8 month | Splain | 0342 | 0139 | 0237 | 0.083 | 0141 |
| S CE | 0247 | 0158 | 0119 | 0.164 | 0331 | |
| S FD | 0140 | 0121 | 0234 | 0.04 | n.d. | |
| 12 month | Splain | 0385 | 0094 | 0271 | 0.089 | 0166 |
| S CE | 0169 | 0158 | 0223 | 0.046 | 0164 | |
| S FD | 0260 | 0118 | 0094 | 0.079 | 0090 | |
| 0 month | Dplain | 0923 | 0211 | 0718 | 0.184 | 0185 |
| D CE | 1386 | 0217 | 0569 | 0.088 | 0422 | |
| D FD | 1887 | 0209 | 0317 | 0.476 | 1028 | |
| 4 month | Dplain | 1186 | 0219 | 0640 | 0.28 | 0163 |
| D CE | 0875 | n.d. | 0520 | 0.294 | n.d. | |
| D FD | 1189 | n.d. | 0459 | 0.28 | n.d. | |
| 8 month | Dplain | 1923 | 0386 | 0959 | 0.261 | 0192 |
| D CE | 1064 | 0312 | 0825 | 0.34 | 0432 | |
| D FD | 2258 | 0289 | 0587 | 0.383 | 0448 | |
| 12 month | Dplain | 0732 | 0257 | 0486 | 0.317 | n.d. |
| D CE | 1546 | 0369 | 0714 | 0.565 | 0498 | |
| D FD | 2076 | 0444 | 0779 | 1.099 | 0543 | |
M milk chocolate, S semisweet chocolate, D dark chocolate
*The values on figures b and c superscripted with the same alphabet (a–f) are not significant (p > 0.05)
*The TPC values on figure a) superscripted with the same alphabet (a–o) are significant (p < 0.05)
Among the phenolic acids displayed as a sum in Table 3, chlorogenic acid (ChlA) was the most abundant, followed by caffeic acid (CA), while gallic (GA) and protocatehuic acids (PA) were quantitatively least represented in formulated chocolates. The addition of nettle extracts to chocolates regardless of the type of chocolate (cocoa parts content) generally increased the content of specific phenolic acids, although discrepancies can be observed. With regard to extract form, the addition of freeze dried (FD) extract resulted with slightly higher content of specific phenolic acids in enriched chocolates in comparison to concentrated extract (CE), which is in agreement with the results obtained for TPC and antioxidant capacity.
Sensory properties of chocolates enriched with nettle extract
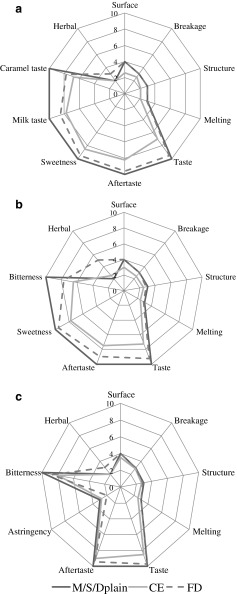
Based on the results displayed on Fig. 3a–c, as a general trend it can be observed that plain chocolates (regarding of the type of chocolate) were preferred by the panel in terms of taste and aftertaste, over the nettle-enriched chocolates. The textural properties (surface, breakage, structure and melting) of plain chocolates did not differ significantly (p < 0.05) from the chocolates enriched with freeze dried extract (FD), regardless of the chocolate type, while the addition of concentrated extract (CE) decreased the scores of all sensory properties of chocolates. The obtained results indicate that the use of concentrated extracts adversely affected taste and flavour of chocolates, suggesting the use of freeze dried extract for that purpose. This was also confirmed by the more pronounced bitterness and astringency of semisweet (Fig. 3b) and dark (Fig. 3c) chocolates enriched with concentrated extract (CE), in comparison to freeze dried extract. Since the water infusions of nettle are well known for their salty and astringent sensory profile (Kavalali 2003) which can be attributed to the nutritional and phytochemical composition of this plant, the bitterness and astringency of enriched chocolates may be derived (and synergistically enhanced) from both nettle and cocoa. While the bitterness is a univocal criterion, the recent study by Childs and Drake (2010) reported that the degree of astringency considered by the subjects as desirable or undesirable attribute of a food product is a person-specific feature. The results obtained in the present study indicate that the addition of nettle extract to chocolates decreased their sensation of sweetness and augmented their astringency and bitterness, which may be regarded as unfavourable by consumers.
Fig. 3.
Spider charts representing mean scores of the evaluated sensory attributes for experimental a milk chocolates, b semisweet chocolates and c dark chocolates enriched with freeze dried and concentrated nettle extracts
Dark chocolate (Fig. 3c) enriched with both forms of nettle extract (CE and FD) exhibited the least changes in sensory properties when compared to plain chocolate, which may be attributed to the higher cocoa parts content of that type of chocolate and consequently more intensive cocoa flavour which supressed the flavour of nettle. However, the addition of freeze dried extract to all three types of chocolates augmented the herbal aroma intensity, in comparison to plain chocolates. This outcome is in agreement with the previous results of Belščak-Cvitanović et al. (2012), where freeze dried extract of raspberry leaf also provided the most pronounced herbal aroma in enriched chocolates.
Conclusions
In this study, chocolates enriched with nettle extract were produced and their bioactive and sensory profile were evaluated upon production, and during storage period of 12 months. By comparing conventional extraction techniques (maceration, infusion and decoction), the best extraction procedure of nettle polyphenolic compounds was established, consisting of infusing nettle leaves during 30 min. The obtained nettle extract exhibited cytotoxic and antioxidative properties on human colon cancer cell lines (SW480) at higher extract concentrations. Implementation of freeze dried nettle extract to chocolate enables a higher increase of polyphenolic content and their better sensory properties, than the addition of concentrated extract. Marked fluctuations of polyphenolic compounds in enriched chocolates during 12 months of storage were observed, but all enriched chocolates exhibited higher polyphenolic content and antioxidant capacity than plain, control chocolates after the 12 months of storage. Dark chocolates enriched with nettle extracts was preferred over milk and semisweet chocolates. In general, using freeze dried extract of nettle, produced by a simple, infusion based extraction procedure enables to produce chocolates with an enhanced and stable polyphenolic profile during 12 months of storage, but further optimization of their sensory properties must be conducted in future.
Acknowledgments
This work was supported by the Ministry of Science, Education and Sports, Republic of Croatia project 058 3470. The authors are grateful to Croatian food industry Zvečevo d.d. for supplying the materials used in the study.
References
- Akyol S, Yükselten Y, Çakmak Ö, Uğurcu V, Altuntaş A, Gürler M, Akyol Ö, Demircan K. Hydrogen peroxide-induced oxidative damage in human chondrocytes: the prophylactic effects of hypericum perforatum linn extract on deoxyribonucleic acid damage, apoptosis and matrix remodeling by a disintegrin-like and metalloproteinase with thrombospondin motifs proteinases. Arch Rheumatol. 2014;29(3):203–214. doi: 10.5606/ArchRheumatol.2014.5203. [DOI] [Google Scholar]
- Ananingsih VK, Sharma A, Zhou W. Green tea catechins during food processing and storage: a review on stability and detection. Food Res Int. 2011;50:469–479. doi: 10.1016/j.foodres.2011.03.004. [DOI] [Google Scholar]
- A.O.A.C . Official methods of analysis. 15. Washington: The Association of Official Analytical Chemists; 1995. [Google Scholar]
- Babich H, Borenfreund E. Applications of the neutral red cytotoxicity assay to in vitro toxicology (review) ATLA. 1990;18:129–144. [Google Scholar]
- Belščak-Cvitanović A, Stojanović R, Manojlović V, Komes D, Juranović Cindrić I, Nedović V, Bugarski B. Encapsulation of polyphenolic antioxidants from medicinal plant extracts in alginate-chitosan system enhanced with ascorbic acid by electrostatic extrusion. Food Res Int. 2011;44:1094–1101. doi: 10.1016/j.foodres.2011.03.030. [DOI] [Google Scholar]
- Belščak-Cvitanović A, Komes D, Benković M, Karlović S, Hečimović I, Ježek D, Bauman I. Innovative formulations of chocolates enriched with plant polyphenols from Rubus idaeus L. leaves and characterization of their physical, bioactive and sensory properties. Food Res Int. 2012;48:820–830. doi: 10.1016/j.foodres.2012.06.023. [DOI] [Google Scholar]
- Belscak-Cvitanovic A, Durgo K, Busic A, Franekić J, Komes D. Phytochemical attributes of four conventionally extracted medicinal plants and cytotoxic evaluation of their extracts on human laryngeal carcinoma (HEp2) cells. J Med Food. 2014;17:206–217. doi: 10.1089/jmf.2013.0071. [DOI] [PubMed] [Google Scholar]
- Belščak-Cvitanović A, Komes D, Dujmović M, Karlović S, Biškić M, Brnčić M, Ježek D. Physical, bioactive and sensory quality parameters of reduced sugar chocolates formulated with natural sweeteners as sucrose alternatives. Food Chem. 2015;167:61–70. doi: 10.1016/j.foodchem.2014.06.064. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Borges Botelho P, Galasso M, Dias V, Mandrioli M, Pereira Lobato L, Rodriguez-Estrada TM, Alves Castro I. Oxidative stability of functional phytosterol-enriched dark chocolate. LWT - Food Sci Technol. 2014;55:444–451. doi: 10.1016/j.lwt.2013.09.002. [DOI] [Google Scholar]
- Camu N, De Winter T, Addo SK, Takrama JS, Bernaert H, De Vuyst L. Fermentation of cocoa beans: influence of microbial activities and polyphenol concentrations on the flavour of chocolate. J Sci Food Agric. 2008;88:2288–2297. doi: 10.1002/jsfa.3349. [DOI] [Google Scholar]
- Childs JL, Drake M. Consumer perception of astringency in clear acidic whey protein beverages. J Food Sci. 2010;75:S513–S521. doi: 10.1111/j.1750-3841.2010.01834.x. [DOI] [PubMed] [Google Scholar]
- Di Stefano R, Cravero MC, Gentilini N. Metodi per lo studio dei polifenoli dei vini. L’Enotecnico. 1989;25:83–89. [Google Scholar]
- El-Aziz MA, Mohamed SHS, Seleet FL. Production and evaluation of soft cheese fortified with ginger extract as a functional dairy food. Pol J Food Nutr Sci. 2012;62:77–83. [Google Scholar]
- Erdem Ö, Gültekin-Özgüven M, Berktas I, Ersana S, Tuna HE, Karada A, Özçelik B, Günes G, Cutting SM. Development of a novel synbiotic dark chocolate enriched with bacillus indicus HU36, maltodextrin and lemon fiber: optimization by response surface methodology. LWT - Food Sci Technol. 2014;56:187–193. doi: 10.1016/j.lwt.2013.10.020. [DOI] [Google Scholar]
- Galmarini MV, Maury C, Mehinagic E, Sanchez V, Baeza R, Mignot S, Zamora MC, Chirife J. Stability of individual phenolic compounds and antioxidant activity during storage of a red wine powder. Food Bioprocess Technol. 2013;12:3585–3595. doi: 10.1007/s11947-012-1035-y. [DOI] [Google Scholar]
- Glei M, Kirmse A, Habermann N, Persin C, Pool-Zobel BL. Bread enriched with green coffee extract has chemoprotective and antigenotoxic activities in human cells. Nutr Cancer. 2006;56:182–192. doi: 10.1207/s15327914nc5602_9. [DOI] [PubMed] [Google Scholar]
- Gramza-Michalowska A, Regula J. Use of tea extracts (Camelia sinensis) in jelly candies as polyphenols sources in human diet. Asia Pac J Clin Nutr. 2007;16(Suppl 1):43–46. [PubMed] [Google Scholar]
- Guarrera PM, Savo V. Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: a review. J Ethnopharmacol. 2013;146:659–680. doi: 10.1016/j.jep.2013.01.036. [DOI] [PubMed] [Google Scholar]
- Hurst WJ, Payne MJ, Miller KB, Stuart DA. Stability of cocoa antioxidants and flavan-3-ols over time. J Agric Food Chem. 2009;57:9547–9550. doi: 10.1021/jf901457s. [DOI] [PubMed] [Google Scholar]
- International Standard ISO 8586-2/2008 (2008) Sensory analysis – general guidance for selection, training and monitoring of assessors, part 2: expert sensory assessors. ISO, Tour Europe, Cedex 7, 92080 Paris La Defense
- Jaric S, Popovic Z, Macukanovic-Jocic M, Djurdjevic L, Mijatovic M, Karadzic B, Mitrovic M, Pavlovic P. An ethnobotanical study on the usage of wild medicinal herbs from Kopaonik Mountain (Central Serbia) J Ethnopharmacol. 2007;111:160–175. doi: 10.1016/j.jep.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Jobstl E, Howse JR, Fairclough JP, Williamson MP. Noncovalent cross-linking of casein by epigallocatechin gallate characterized by single molecule force microscopy. J Agric Food Chem. 2006;54:4077–4081. doi: 10.1021/jf053259f. [DOI] [PubMed] [Google Scholar]
- Katsube T, Tsurunaga Y, Sugiyama M, Furuno T, Yamasaki Y. Effect of air-drying temperature on antioxidant capacity and stability of polyphenolic compounds in mulberry (Morus alba L.) leaves. Food Chem. 2009;113:964–969. doi: 10.1016/j.foodchem.2008.08.041. [DOI] [Google Scholar]
- Kavalali GM. Urtica: the genus urtica (medicinal and aromatic plants – industrial profiles) London: Taylor & Francis; 2003. [Google Scholar]
- Komes D, Belščak-Cvitanović A, Škrabal S, Vojvodić A, Bušić A. The influence of dried fruits enrichment on sensory properties of bitter and milk chocolates and bioactive content of their extracts affected by different solvents. LWT - Food Sci Technol. 2013;53:360–369. doi: 10.1016/j.lwt.2013.02.016. [DOI] [Google Scholar]
- Lachman J, Hosnedl V, Pivec V, Orsák M (1998) Polyphenols in cereals and their positive and negative role in human and animal nutrition. In: Proceedings of conference cereals for human health and preventive nutrition. Brno, p 118–125
- Mazur SP, Nesa A, Wold A-B, Rembergb SF, Martinsen BK, Aaby K. Effect of genotype and storage time on stability of colour, phenolic compounds and ascorbic acid in red raspberry (Rubus idaeus L.) jams. Acta Agric Scand B. 2014;64:442–453. [Google Scholar]
- Ncube NS, Afolayan AJ, Okoh AI. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr J Biotechnol. 2008;7:1797–1806. [Google Scholar]
- Palma M, Zulema P, Carmelo GB. Stability of phenolic compounds during extraction with superheated solvents. J Chromatogr A. 2001;921:169–174. doi: 10.1016/S0021-9673(01)00882-2. [DOI] [PubMed] [Google Scholar]
- Pinelli P, Ieri F, Vignolini P, Bacci L, Baronti S, Romani A. Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica dioica L. J Agric Food Chem. 2008;56:9127–9132. doi: 10.1021/jf801552d. [DOI] [PubMed] [Google Scholar]
- Porter LJ, Hrstich L, Chan BG. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry. 1986;2:223–230. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Ryan E, Aherne SA, O’Grady MN, McGovern L, Kerry JP, O’Brien NM. Bioactivity of herb-enriched beef patties. J Med Food. 2009;12:893–901. doi: 10.1089/jmf.2008.0069. [DOI] [PubMed] [Google Scholar]
- Rzemykowska Z, Ostrowska B. The method of quantitative determination of magnesium in the juice from the fresh nettle Urtica dioica L. Herba Pol. 1994;40:95–98. [Google Scholar]
- Skouroliakou M, Kastanidou O, Stathopoulou M, Vourli G. Evaluation of the antioxidant effect of a new functional food enriched with Sideritis euboea in healthy subjects. J Med Food. 2009;12:1105–1110. doi: 10.1089/jmf.2008.0172. [DOI] [PubMed] [Google Scholar]
- Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/S0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Wang L, Weller C. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol. 2006;17:300–312. doi: 10.1016/j.tifs.2005.12.004. [DOI] [Google Scholar]