Abstract
Little information is available regarding the incorporation of dietary fiber into edible films and coatings. In this work, apple fiber and inulin were incorporated into polysaccharide-based (alginate, pectine and gellan gum) edible coating formulations and their effects on the quality attributes of fresh-cut apples were evaluated. Antioxidant properties, color, firmness, sensory quality and microbial growth of fresh-cut apple were studied during 16 days of storage at 4 °C. Results show that dietary fiber extracts incorporated to gellan gum, pectin and alginate-based coatings together with calcium chloride and ascorbic acid successfully maintained the firmness and color of coated fresh-cut apples in comparison with uncoated control samples, which presented severe texture softening and browning. The firmness of apple pieces coated with polysaccharide-based coating formulations incorporating apple fiber doubled, and sometimes tripled, that of uncoated samples. Any of the assayed coatings exhibited a positive effect on the sensory properties of fresh-cut apples. The incorporation of apple fiber, together with the use of ascorbic acid, contributed to keep the antioxidant potential of the fruit at least during the first week of storage. Furthermore, gellan gum coatings had a marked effect in reducing mesophilic and psychrophilic counts on fresh-cut apples throughout storage regardless the addition of dietary fibers. The results achieved demonstrate the feasibility of the addition of dietary fiber to edible coating formulations for increasing the nutritional value of fresh-cut apples without compromising their fresh-like quality attributes.
Keywords: Edible coatings, Shelf-life, Fresh-cut fruit, Apple fiber, Inulin, Minimal processing
Introduction
Over the last years the market of lightly processed food products has been expanded especially due to an increase in the consumers’ demand for fresh-like products (Gorny 2003; Olaimat and Holley 2012). Fresh-cut fruit is an important and rapidly developing segment of this market, because of its convenience and fresh-like quality. However, it is well known that minimally processed fruits and vegetables are generally more perishable than the original raw materials (Lee et al. 2003; Moreira et al. 2011; Oms-Oliu et al. 2010). Mechanical stress during processing results in biochemical deteriorations such as enzymatic browning, off-flavor, and texture breakdown. Aside, the presence of microorganisms on the surface of fruit may compromise the safety of fresh-cut produce (Alvarez et al. 2013; Rojas-Graü et al. 2007). In consequence, processors are continuously looking for methods that contribute to minimize the deleterious reactions triggered by mechanical bruises, while keeping the fresh-like properties of the raw produce. Among these, edible coatings have a great potential for the development of high-quality fresh-cut commodities with an extended shelf-life. Alginate, extracted from marine brown algae (Phaeophyceae), gellan gum, secreted by the bacterium Sphingomonas elodea (formerly referred to as Pseudomonas elodea) and pectin, extracted from apple or from the peel of citrus fruits, are common polysaccharides used as gelling agents in the food industry. Alginate, gellan gum or low methoxyl pectin gelling properties are mainly due to their capacity to form strong gels or insoluble polymers in the presence of multivalent metal cations such as calcium (Oms-Oliu et al. 2008). In addition, the development of edible films and coatings as carriers of active ingredients is considered as a promising packaging alternative to maintain freshness of fresh-cut fruits and vegetables.
Apple is a very popular fruit, consumed all over the world. Thus, its susceptibility to enzymatic and microbial spoilage during postharvest, handling, and processing operations stands yet as an important topic from the standpoint of food science and technology (Ramos et al. 2013). Dietary fiber is an essential nutrient in our diet which has been related to risk reduction for a number of chronic diseases including diabetes, heart disease, and certain cancers (Anderson et al. 2009). Despite not being an excellent source of dietary fiber, apples provide health benefits associated with the synergies of the fiber fractions they contain with other nutrients. Dietary fibers obtained from apple fruits are of higher quality than those extracted from cereal sources because of their higher solubility and content in other health-promoting bioactive compounds with antioxidant properties (Grigelmo-Miguel and Martin-Belloso 1999a). Inulin is an indigestible polysaccharide that belongs to a class of dietary fibers known as fructans. It is present in many vegetables, fruit and cereals, used as an ingredient in a wide range of food formulations due to both technological and nutritional benefits associated (Rinaldoni et al. 2012). Fibers form fruit and greens are especially used as functional food additives due to their prebiotic properties, promoting the growth of healthy bacteria in the gut. Despite the interest in incorporating nutraceutical compounds into food products, their integration into edible coatings has been scarcely studied. In the present work, the objective was to evaluate the effects of the addition of two different fiber extracts to three different polysaccharide-based coatings for maintaining quality and extending shelf-life of fresh-cut ‘Golden delicious’ apples. Effects of these coatings on color, texture, antioxidant properties, sensory and microbial quality were evaluated through refrigerated storage.
Materials and methods
Materials
‘Golden’ delicious apples were purchased in a local supermarket (Lleida, Spain) at commercial maturity and stored at 4 ± 1 °C until processing. Food grade gellan gum (Kelcogel®, CPKelco, Chicago, IL, USA), sodium alginate (Keltone LV, ISP, San Diego, CA USA) and potassium salt of low methoxyl pectin from citrus fruit (Sigma-Aldrich Chemic, Steinhein, Germany) were used as carbohydrate biopolymers for coating formulations. Glycerol (Merck, Whitehouse Station, NJ, USA) was added as plasticizer. Apple fiber was kindly supplied by the factory Indulleida S. A. (Alguaire, Lleida, Spain). Inulin from artichoke was purchased from Sigma-Aldrich (St. Louis, MO, USA). Calcium chloride (Sigma-Aldrich Chemic, Steinhein, Germany) was used to induce cross-linkage between the polymers chains. Ascorbic acid (Sigma-Aldrich Chemic, Steinhein, Germany) was added as antibrowning agent.
Preparation of the film forming solutions and dipping solutions
Sodium alginate (2 g/100 mL), gellan gum (0.5 g/100 mL) or pectin (2 g/100 mL) powders were dissolved into distilled water by gently stirring at 70 °C until the solution became clear (Rojas-Graü et al. 2008). Film-forming solutions were prepared with and without the addition of two different dietary fibers. Apple fiber obtained from apple pomace was used in concentrations of 0.2 g 100/mL for gellan gum and 0.7 g/100 mL for pectin and alginate solutions, while pure inulin was used at 4 g/100 mL regardless the kind of coating. These concentrations were selected according to the results obtained in preliminary assays. Glycerol was added as plasticizer in a concentration of 1.5 g/100 mL for alginate and pectin solutions and 0.6 g/100 mL for gellan gum solutions. Ascorbic acid (1 g/100 mL) was dissolved in the calcium chloride solution (2 g/100 mL) used to cross-link the carbohydrate polymers. As a consequence of the coatings application to apple pieces the weight gain averaged 12 g/100 g, thus resulting in a fiber addition of approximately 45 mg/100 g (fw) for pectin and alginate-coated fresh-cut apples and 24 mg/100 g (fw) for gellan gum-coated samples.
Fruit coating
Apples were thoroughly rinsed with tap water and dried prior to cutting operations. Subsequently, apples were hand-peeled, cored and cut into 1-cm-thick cubes with a sharp stainless steel blade. At most four fruits were processed simultaneously in order to avoid unnecessary exposure to adverse conditions. The apple pieces were dipped into the chilled (5 °C) polysaccharide solutions (sodium alginate, gellam gum or low methoxyl pectin) for 2 min. The excess of coating material was allowed to drip off for 1 min before submerging the fruits again for 2 min in the calcium chloride cross-linking solution. Uncoated samples dipped into distilled water and coated samples without addition of the fiber extracts were used as a reference. Ten apple cubes were placed into 500-cm3 polypropylene trays (Mcp Performance Plastic LTD, Kibbutz Hamaapil, Israel). The packages were thermally sealed with a 64 μm-thick polypropylene film (ILPRA Food Pack Basic V/G, ILPRA Systems, CP. Vigevono, Italy) and stored in darkness at 4 ± 1 °C. Five trays were prepared for each coating condition. Analyses were carried out periodically during 16 days in two independent experimental runs.
Antioxidant capacity determination
The antioxidant capacity was studied by evaluation of the free radical- scavenging effect on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, according to the method described by Oms-Oliu et al. (2008). This assay is not specific to any particular antioxidant compound, thus providing an estimate of the overall antioxidant capacity of a sample. Apple was centrifuged at 10.000 × g for 15 min at 4 °C (Centrifuge Medigifer; Select, Barcelona, Spain) and 100 μL of the supernatant were added to 3.9 mL of methanolic DPPH solution (0.025 g/L). The homogenate was shaken vigorously and kept in darkness for 30 min. Absorption of the samples at 515 nm against a blank of methanol without the DPPH reagent was spectrophotometrically measured (CECIL CE 2021; Cecil Instruments Ltd., Cambridge, UK). Antioxidant capacity was related to the scavenging activity of the sample extracts towards the DPPH radical, which can be monitored through the decrease in absorbance once the sample extract has been incorporated to the DPPH solution. DPPH assays were performed in quadruplicate for each independent experimental run.
Color measurement
Cut apple surface color was determined with a Minolta chroma meter (Model CR-400, Minolta, Tokyo, Japan). An illuminant D75 was used and measurements were carried out with an observer angle of 10°. A white reflector plate (Y = 94.00, x = 0.3158, y = 0.3322) was used as a reference standard. Ten replicate samples were evaluated for each tray. Three measures were read in each replicate by changing the position of the apple cubes. The color was measured through changes in L* (lightness) and h* (hue angle) values. Numerical values of a*(green-red chromaticity) and b* (blue-yellow chromaticity) were used to calculate hue angle (h* = arctan b*/a*).
Firmness measurements
Apple firmness was evaluated with a TA-XT2 Texture Analyzer (Stable Micro Systems Ltd., England, UK). Apple pieces were randomly withdrawn from each tray and placed perpendicularly to the probe. The maximum force required for a 4-mm-diameter probe to penetrate into a 1-cm-high apple cube to a depth of 5 mm at a rate of 5 mm/s was measured.
Microbiological analysis
Naturally-occurring microbial counts on fresh-cut apples were evaluated throughout storage. Mesophilic and psychrophilic aerobic microorganisms and yeasts and molds were plate cultured and counted. A sample of 10 g, obtained from eight different pieces of a same package, was aseptically transferred to a sterile plastic pouch and homogenized for 1 min with 90 mL of saline peptone water (0.1 g peptone/100 mL water, Biokar Diagnostics, Beauvais, France) in a stomacher blender (IUL Instruments, Barcelona, Spain). Serial dilutions were made and pour-plated onto Plate Count Agar (PCA) and Chloramphenicol Glucose Agar (GCA) (Biokar Diagnostics, Beauvais, France). Plates were incubated for 48 h at 30 °C to determine mesophilic, 7 days at 5 °C for psychrophilic counts and 5 days at 25 °C for yeast and mold counts (Alvarez et al. 2013). The colony counts were expressed as CFU/g of apple. Analyses were carried out periodically during 16 days in randomly sampled pairs of trays. Three replicate counts were performed per tray.
Sensory acceptability evaluation
Sensory acceptability of coated and uncoated apple cubes was determined through refrigerated storage by regular apple consumers. For the hedonic tests, the panelists evaluated four pieces of apple uncoated and coated with gellan gum, pectin and alginate, with and without the addition of fiber extracts. Ten individuals who regularly consumed apples were recruited among the personnel of the Department of Food Technology, University of Lleida, Spain. The panelists were trained to evaluate color and firmness of apples. Evaluations were performed immediately after removal from storage conditions. The order of the samples was randomized for each consumer. They were asked to evaluate the samples on a non-structured linear scale with anchor points at each end. Color by visual observation under white illumination, firmness by crushing apple cubes between finger tips, taste by mastication, and overall preference (OVQ) were evaluated in a five point scale, where 5 indicates extreme like and 0 extreme dislike. The judges’ average response was calculated for each attribute. The limit of acceptance was three, indicating that scores below three for any of the attributes evaluated were deemed to indicate end of shelf-life from a sensorial point of view (Alvarez et al. 2013).
Statistical analysis
Data were analyzed using SAS software version 9.0 (SAS Inst. Inc., Cary, N. C., U.S.A.). Specific differences were determined by least significant difference (LSD). PROC GLM (general linear model procedure) was used for the variance analysis (ANOVA) and PROC REG (general linear regression analysis) was used to perform slopes analysis. Differences were determined by the Tukey–Kramer multiple comparison test (p < 0.05). PROC UNIVARIATE was used to validate the ANOVA assumptions.
Results
Microbiological evaluation
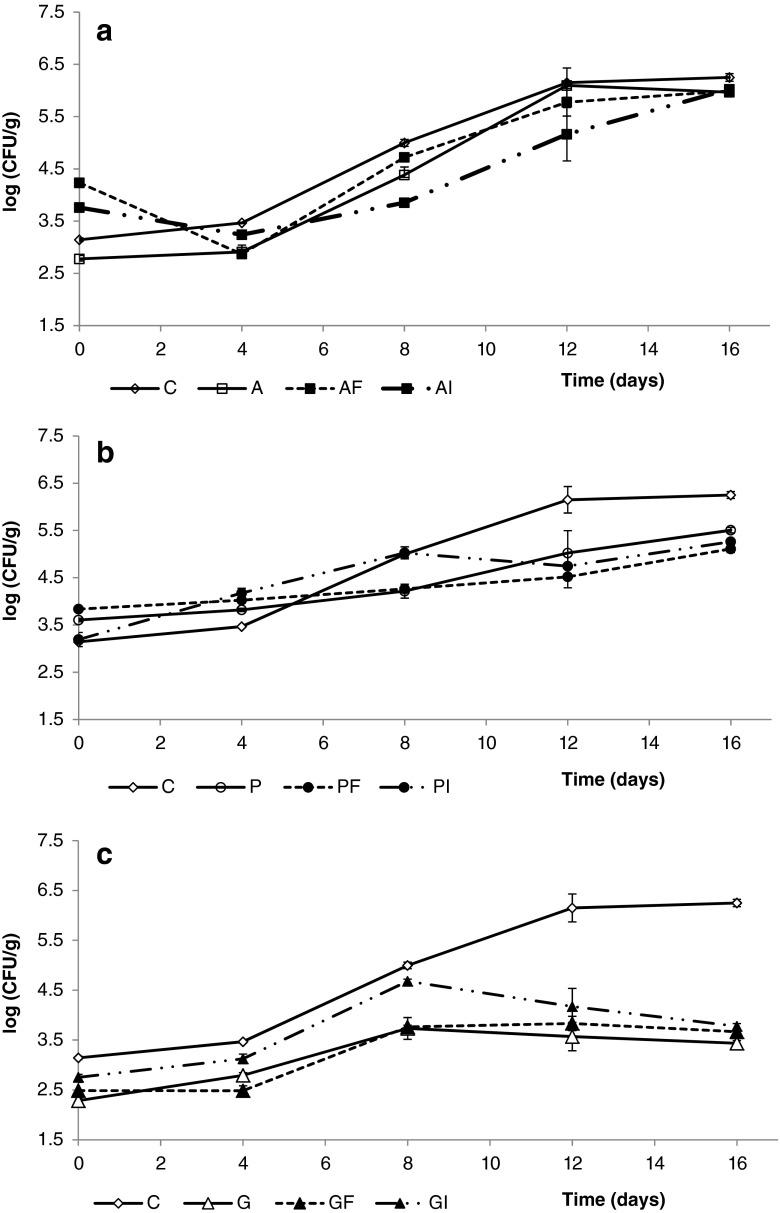
Figure 1 shows the changes in the counts of mesophilic aerobic microorganisms on fresh-cut apples coated with alginate, pectin and gellan gum with and without added dietary fibers during refrigerated storage. Alginate- and pectin-coated fresh-cut apples exhibited counts ranging from 2.8 to 4.2 CFU/g (Fig. 1a–b), similar to the counts initially found on uncoated apple pieces (3.1 CFU/g). The initial counts on gellan gum-coated samples were significantly the lowest and values ranged from 2.2 to 2.5 CFU/g (Fig. 1c). An influence of the addition of apple fiber and inulin on the initial microbial loads was only observed for alginate-coated apples, whose counts were slightly but significantly higher when dietary fibers were present in the formulations. Significant differences (p < 0.05) between the counts on alginate-coated, pectin-coated and uncoated fresh-cut apples were not observed through storage (Fig. 1a–b). Nevertheless, gellan gum-coated apple cubes exhibited the lowest counts throughout storage regardless the addition of fiber extracts (Fig. 1c). Hence, counts of aerobic mesophiles on gellan gum-coated fresh-cut apples stored for 16 days were at least 2.0 log CFU/g lower than those observed for other coatings.
Fig. 1.
Effect of alginate (a), pectin (b) and gellan-based coatings (c) on mesophilic counts (log CFU/g of fruit) of apple wedges, during 16 days of storage at 4 °C. Fresh control (C), Alginate (A), A plus Fiber (AF), A plus Inulin (AI); Pectin (P), P plus Fiber (PF), P plus Inulin (PI); Gellan (G), G plus F (GF), G plus I (GI). Data shown are the means ± standard deviation
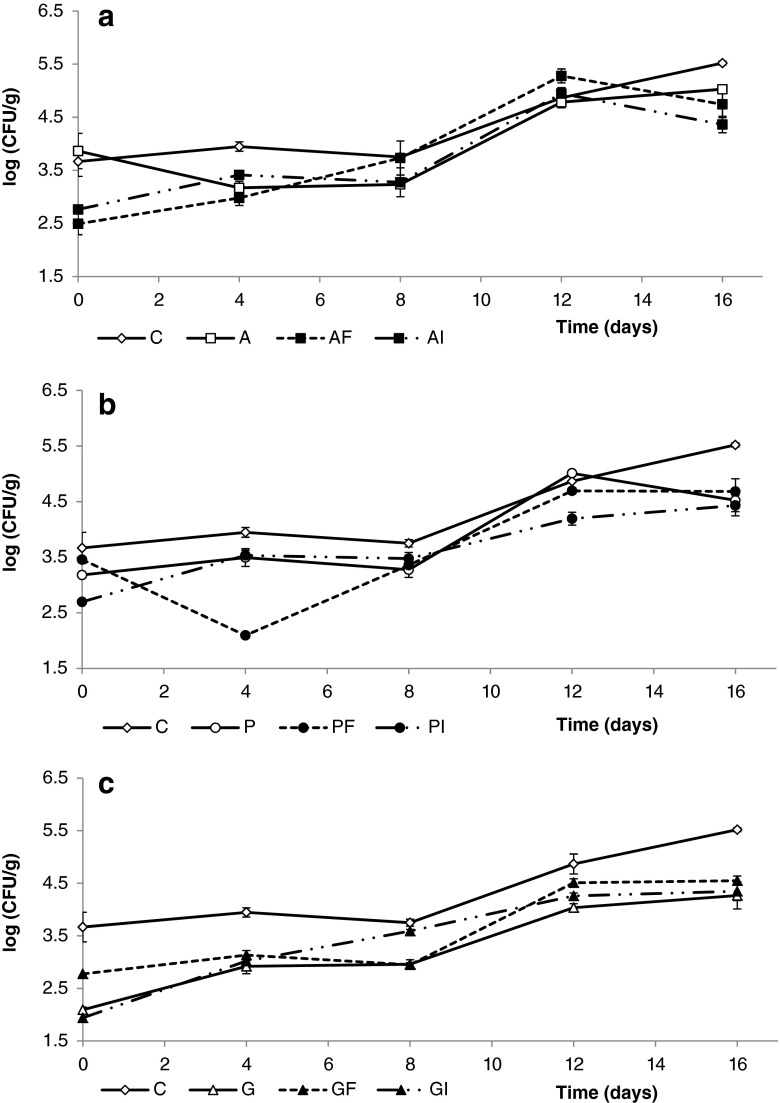
The growth of psychrophilic aerobic microorganisms on fresh-cut apples is displayed in Fig. 2. In line with the results reported for aerobic mesophiles, the initial counts of aerobic psycrophiles on alginate- and pectin-coated apple pieces ranged from 2.5 to 3.8 log CFU/g and were above those found for gellan gum-coated samples, which fell in the range of 2.0–2.7 log CFU/g. Regardless the kind of polysaccharide base, the addition of fiber was generally not found to be relevant in terms of microbial counts. However, the addition of inulin or apple fiber to alginate-based coatings initially led to slightly reduced psychrophilic aerobic counts compared to their corresponding reference treatments. Statistically significant differences (p < 0.05) between coated and uncoated apples were scarcely noticeable throughout storage. However, gellan gum coatings were apparently more effective in preventing microbial growth throughout the whole evaluated storage period (Fig. 2c). In that case, psychrophilic microbial loads were consistently lower than those counted on uncoated apples with no significant effect (p < 0.05) attributable to the addition of fiber extracts.
Fig. 2.
Effect of alginate (a), pectin (b) and gellan-based coatings (c) on psychrotrophic counts (log CFU/g of fruit) of apple wedges, during 16 days of storage at 4 °C. Fresh control (C), Alginate (A), A plus Fiber (AF), A plus Inulin (AI); Pectin (P), P plus Fiber (PF), P plus Inulin (PI); Gellan (G), G plus F (GF), G plus I (GI). Data shown are the means ± standard deviation
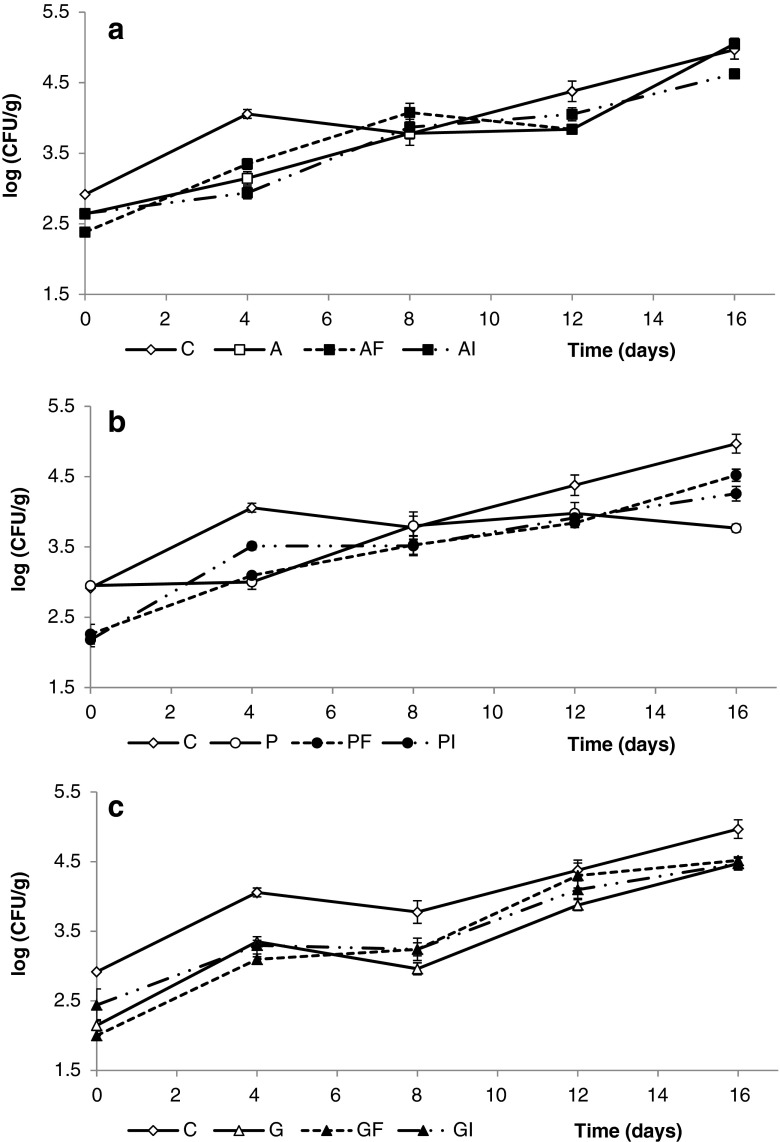
The changes in yeast and mold loads growing on fresh-cut apples through refrigerated storage are shown in Fig. 3. Initial yeast and mold counts were in the range of 2.0–3.0 log CFU/g. These counts presented a sustained increase that ranged between 2.0 and 2.5 log CFU/g throughout 16 days of storage. Yeast and molds were generally found to be significantly (p < 0.05) inhibited by the coatings application especially during the first days of storage. Pectin and gellan gum coatings provided the lowest counts for moulds and yeasts after prolonged storage (Fig. 3b and c). Neither positive nor negative effects could be attributed to the incorporation of dietary fibers disregarding their source.
Fig. 3.
Effect of alginate (a), pectin (b) and gellan-based coatings (c) on yeast and molds counts (log CFU/g of fruit) of apple wedges, during 16 days of storage at 4 °C. Fresh control (C), Alginate (A), A plus Fiber (AF), A plus Inulin (AI); Pectin (P), P plus Fiber (PF), P plus Inulin (PI); Gellan (G), G plus F (GF), G plus I (GI). Data shown are the means ± standard deviation
Antioxidant activity
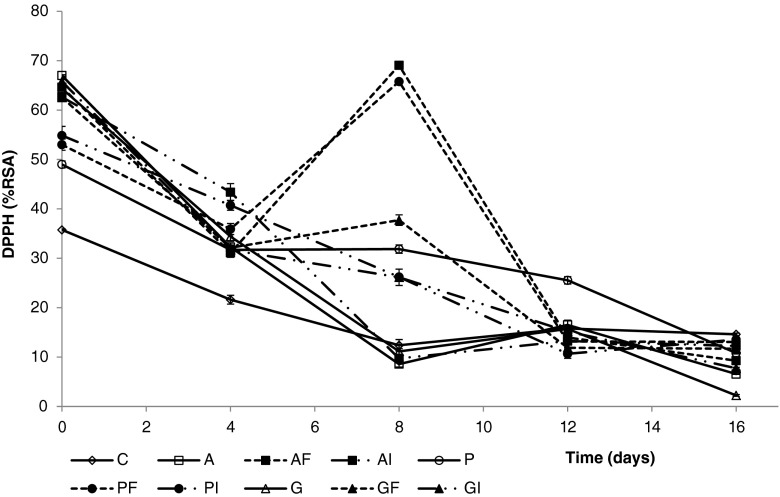
Figure 4 displays the DPPH · radical scavenging antioxidant activity of fresh-cut apples coated with gellan gum, pectin and alginate, with and without added dietary fiber extracts. The application of coatings resulted into a significant (p < 0.05) increase of the antioxidant activity of fresh-cut apples just after processing. Alginate- and gellan gum-coated apple pieces exhibited the highest initial antioxidant activity values, which almost doubled those observed for uncoated apples. The incorporation of apple fiber or inulin was generally not evidenced by the radical scavenging activity values of the just processed samples. However, fresh-cut apples coated with alginate, pectin and gellan gum enriched with apple fiber better maintained their antioxidant capacity during the first storage week. A sharp increase was noticed at day 8 of refrigerated storage, at which point radical scavenging capacity values of coated apples with addition of apple fiber ranged from 40 to 70 %. These values contrast with those observed for apples uncoated or coated without added dietary fiber (10–30 %). Similar results have been previously reported by Oms-Oliu et al. (2008), who related the increase in the antioxidant potential of fresh-cut melon to the accumulation of phenolic compounds caused by the induction of the phenylpropanoid metabolism. The presence of antioxidants bond to the apple fiber extract could be behind this observation, having a protective effect against oxidation and, at the same time, contributing to the activation of the production of the phenolic compounds by the fruit tissues. During the subsequent storage period, the antioxidant activity of coated apple cubes with fiber addition decreased sharply and reached residual values similar to those found for their corresponding reference treatments.
Fig. 4.
DPPH radical scavenging activity of fresh-cut apple coated with alginate, pectin and gellan (with and without apple fiber and inulin) during 16 days of storage at 4 °C. Fresh control (C), Alginate (A), A plus Fiber (AF), A plus Inulin (AI); Pectin (P), P plus Fiber (PF), P plus Inulin (PI); Gellan (G), G plus F (GF), G plus I (GI). Data shown are mean ± standard deviation
Color
Table 1 shows the effect of alginate-, pectin- and gellan gum-based edible coatings with or without the addition of fiber extracts on lightness (L*) and hue (h*) values of fresh-cut apples during storage at 4 °C. L* values tended to decrease in all cases with the exception of pectin-coated apple samples, either in coated or uncoated ones and especially beyond the 8th day of storage. The analysis of variance indicated that the use of edible coatings generally had a significant (p < 0.05) effect on the color parameters L* and h* of fresh-cut apples. Pectin-coated apple pieces, either with or without incorporated apple fiber, better maintained their lightness values with minor changes throughout 2 week of storage. As well, coated apple cubes with addition of apple fiber extracts generally exhibited the highest hue values at prolonged storage (16 days).
Table 1.
Changes in color parameters of fresh-cut apple coated with alginate, pectin and gellan plus apple fiber and inulin during 16 days of storage at 4 °C
| Storage time (days) | 0 | 4 | 8 | 12 | 16 |
|---|---|---|---|---|---|
| L* | |||||
| C | 78.09 ± 0.50ab | 76.11 ± 0.62abc | 72.66 ± 0.86e | 67.57 ± 1.15cd | 67.93 ± 1.00d |
| A | 78.11 ± 0.73ab | 68.87 ± 1.12d | 77.30 ± 0.91abc | 68.82 ± 1.02cd | 62.37 ± 1.23e |
| AF | 78.24 ± 0.48ab | 75.67 ± 0.82abc | 74.68 ± 1.35cde | 61.59 ± 0.98e | 72.38 ± 0.86bc |
| AI | 76.99 ± 0.60b | 74.33 ± 0.87bc | 76.25 ± 0.39bcd | 70.17 ± 1.25bc | 74.20 ± 0.68bc |
| P | 78.93 ± 0.54ab | 76.12 ± 1.17abc | 76.67 ± 0.50bcd | 76.23 ± 0.71a | 76.26 ± 0.74ab |
| PF | 78.88 ± 0.68ab | 79.21 ± 0.54a | 80.01 ± 0.35a | 70.19 ± 0.59bc | 79.36 ± 0.66a |
| PI | 79.71 ± 0.49a | 76.83 ± 0.70abc | 77.94 ± 0.37abc | 69.89 ± 1.09bcd | 70.71 ± 0.45cd |
| G | 78.34 ± 0.58ab | 73.37 ± 01.26c | 75.62 ± 0.80cde | 65.24 ± 1.47de | 71.33 ± 0.73cd |
| GF | 80.19 ± 0.53a | 78.04 ± 0.64ab | 79.05 ± 0.55ab | 74.40 ± 0.90ab | 72.24 ± 0.30c |
| GI | 79.54 ± 0.43ab | 77.01 ± 0.93abc | 73.84 ± 0.42de | 65.80 ± 0.83cde | 71.61 ± 0.83cd |
| °hue | |||||
| C | 99.34 ± 0.43b | 98.47 ± 0.48c | 100.31 ± 0.59f | 94.18 ± 0.48c | 97.86 ± 0.70c |
| A | 102.94 ± 0.39a | 105.05 ± 0.71a | 103.07 ± 0.57bcd | 102.85 ± 0.50a | 98.60 ± 1.07bc |
| AF | 102.82 ± 0.41a | 102.46 ± 0.35ab | 101.93 ± 0.40def | 101.22 ± 0.98ab | 101.92 ± 0.83abc |
| AI | 103.22 ± 0.75a | 102.54 ± 0.40ab | 100.80 ± 0.65ef | 102.42 ± 0.56a | 101.71 ± 0.88abc |
| P | 103.93 ± 0.61a | 103.45 ± 0.47ab | 102.50 ± 0.46cde | 99.83 ± 1.46ab | 101.06 ± 0.48abc |
| PF | 103.94 ± 0.64a | 103.72 ± 0.54ab | 105.18 ± 0.43ab | 100.97 ± 0.79ab | 102.55 ± 0.68ab |
| PI | 103.19 ± 0.87a | 103.50 ± 0.87ab | 106.02 ± 0.42a | 97.25 ± 1.12bc | 104.05 ± 0.47a |
| G | 103.21 ± 0.71a | 101.44 ± 0.72b | 103.94 ± 0.33abcd | 97.31 ± 1.20bc | 99.67 ± 0.78abc |
| GF | 103.72 ± 0.34a | 104.99 ± 0.41a | 104.25 ± 0.27abc | 99.26 ± 0.84ab | 98.55 ± 2.15bc |
| GI | 103.26 ± 0.48a | 103.02 ± 0.60ab | 102.43 ± 0.25cdef | 98.95 ± 1.12ab | 100.26 ± 0.51abc |
Mean values ± SD with different letters in the same column indicate significant differences (p < 0.05) among treatments
Firmness
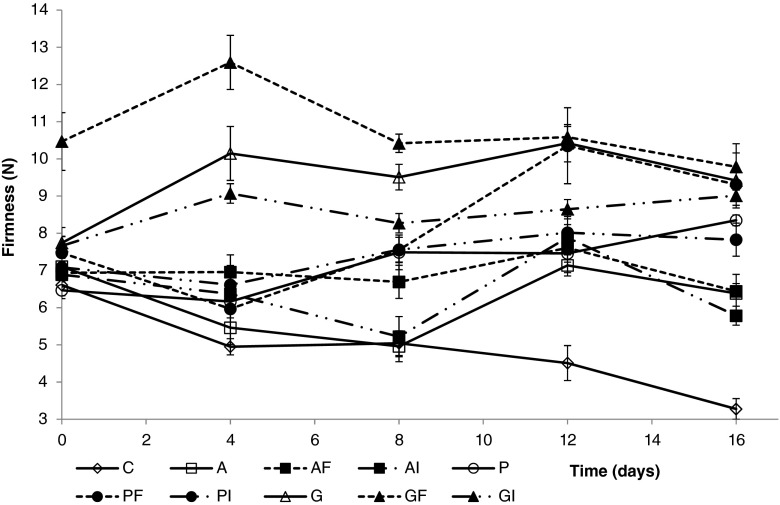
Firmness values of fresh-cut apple pieces through 16 days of storage are shown in Fig. 5. The incorporation of dietary fiber into the coating formulations had a significant effect (p < 0.05) on the fruit firmness. Hence, coated samples containing apple fiber or inulin extracts maintained or even improved their firmness through storage. In contrast, the initial texture values of uncoated pieces (6.60 N) gradually declined from the beginning of storage reaching values of almost half of the initial (3.20 N) after 2 weeks. Regarding the influence of polysaccharide type, apple pieces coated with gellan gum kept the highest firmness values throughout refrigerated storage.
Fig. 5.
Changes in firmness of fresh-cut apple coated with alginate, pectin and gellan (with and without apple fiber and inulin) during 16 days of storage at 4 °C. Fresh control (C), Alginate (A), A plus Fiber (AF), A plus Inulin (AI); Pectin (P), P plus Fiber (PF), P plus Inulin (PI); Gellan (G), G plus F (GF), G plus I (GI). Data shown are mean ± standard deviation
Sensorial evaluation
Figure 6 displays the color, texture, odor, taste and overall visual quality scores for fresh-cut apple coated with alginate, pectin and gellan gum. Fresh-cut apples incorporating apple fiber or inulin could not be discriminated from their corresponding references without fiber addition. Therefore, results are expressed as mean values of samples coated with the same polysaccharide base. It can be observed that cut fruit coated with alginate, pectin and gellan gum initially obtained higher scores than uncoated fruit, while no differences were observed between the odor scores received by coated and uncoated pieces. Alginate- and pectin-coated samples received lower scores for taste compared to uncoated and gellan gum-coated pieces. Similar results were observed at days 2 and 4 of storage. Between days 12 to 16 of the storage, panelists expressed a preference for coated apple cubes over the uncoated ones. In addition, overall visual scores for the treated samples were similar, indicating that coated samples were well accepted by the panelists (p > 0.05). Preference over uncoated control samples was attributed to softer texture and evident signs of browning. Hence, the application of pectin, gellan gum and alginate coating allowed apple samples to reach prolonged storage periods with sensory scores above the sensory acceptability threshold (3) for any attributes evaluated.
Fig. 6.
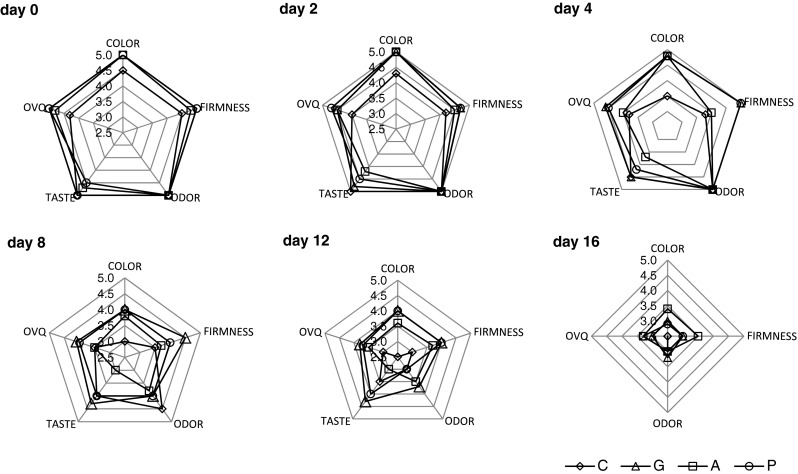
Sensory characteristics of fresh-cut apple coated with alginate, pectin and gellan during 16 days of storage at 4 °C. Fresh control (C), Alginate (A), Pectin (P), and Gellan (G). Data shown are mean ± standard deviation
Discussion
Edible coatings could be an excellent vehicle to enhance the nutritional value of fruits and vegetables by carrying several nutrients, such as dietary fiber. However, only a few studies have suggested their integration into edible coatings. Chien et al. (2007) maintained the vitamin C content of sliced dragon fruit coated with low molecular weight chitosan. Tapia et al. (2008) reported that the addition of ascorbic acid to the alginate edible coating helped to preserve the natural vitamin C content in fresh-cut papaya. Hernández-Muñoz et al. (2006) indicated that chitosan-coated strawberries retained more calcium gluconate (3079 g/kg dry matter) than strawberries dipped into calcium solutions (2340 g/kg). In the present study three edible coatings enriched with dietary fiber were assayed and their ability to enhance the nutritional value of apple cubes without unacceptable modifications of their quality attributes was demonstrated.
The obtained results indicate that gellan gum coatings applied on fresh-cut apples have a remarkable effect in reducing mesophilic and psychrophilic counts as compared to uncoated, alginate-coated and pectin-coated apple pieces. This is in line with the finding of other authors, who reported similar results for minimally processed apples with various types of carbohydrate polymers (Lee et al. 2003; Oms-Oliu et al. 2008; Rojas-Graü et al. 2009). On the other hand, pectin and gellan gum-coated samples exhibited the highest inhibition of yeast and molds growth during the second week of storage, compared to uncoated samples. Lee et al. (2003) reported consistent results for fresh-cut apples coated with various types of carbohydrate polymers and whey protein concentrate, using ascorbic acid as antibrowning agent. Also, Oms-Oliu et al. (2008) working with fresh-cut pears observed that pectin and gellan gum coatings, containing N-acetylcisteine as an antibrowning agent, had a marked effect on reducing yeast and molds counts.
Regulations affecting fresh-cut produce have sometimes limited the counts of aerobic microorganisms to 6–7 log CFU/g at expiry date. In the present study, counts of overall aerobic bacteria were significantly lower than 6 log CFU/g through the entire evaluated period. Gellan gum and pectin coatings were found to be the most successful in terms of microbial control. Lee et al. (2003) and Rojas-Graü et al. (2009) reported similar results for fresh-cut apples coated with various types of carbohydrate polymers. Besides, Oms-Oliu et al. (2008) reported that aerobic mesophilic and psychrophilic bacteria on fresh-cut pears coated with polysaccharide-based formulation did not exceed 5.0 log CFU/g through 14 days of refrigerated storage.
Antioxidant capacity has been used to evaluate the antioxidant potential status of a sample, which is a function of the type and amount of bioactive compounds present. As expected, the combination of polysaccharide coatings with a dipping treatment containing ascorbic acid resulted into a substantial initial increase of the antioxidant capacity values. Our results are supported by those of Robles-Sánchez et al. (2013), who reported that ascorbic acid applied as a dipping treatment dramatically increased the antioxidant capacity of fresh-cut mangoes. Differences between samples coated with different polysaccharide matrices during the first storage week could be attributed to the uneven accumulation of phenolics synthesized through the phenilpropanoid pathway, whose response has been shown to be modulated by certain processing and storage conditions (Ramos et al. 2013). Regarding the positive effect of the incorporated fiber extracts, it may be hypothesized that these may act as protective agents against oxidative stress sources. Several studies have highlighted the presence of antioxidant compounds, namely polypenols, associated to dietary fibers derived from orange and apple, highlighting their antioxidant properties (Figuerola et al. 2005; Grigelmo-Miguel and Martín-Belloso 1999b; Marín et al. 2007; Moraes Crizel et al. 2013). This fact could explain that apple fiber incorporated to coating formulations resulted into a better retention of the antioxidant properties of the fruit, compared to inulin.
Color is a critical quality property of fresh-cut fruits such as pears, apples and bananas, since cutting operations may lead to enzymatic browning, which could limit the shelf-life of fresh-cut cubes. In this study, ascorbic acid was incorporated into the edible coating formulations with the purpose of preventing browning. L* and h* values have been used as indicators of enzymatic browning reactions. Hue angle (h*) may be used to determine the commercial acceptance or rejection of produce, as it is related to the chromatic perception of a sample (Robles-Sánchez et al. 2013). All coating formulations used in this work contained ascorbic acid, which helped to keep the apple pieces free from browning during the entire period of storage, in line with the results obtained in previous studies (Oms-Oliu et al. 2008; Robles-Sánchez et al. 2013). On the other hand, the slightly better maintenance of color values observed for coated samples with incorporation of apple fiber could be attributed to the presence of polyphenolic compounds from apple pomace, which would retard the initiation of oxidative stress phenomena that would subsequently result into increased browning rates.
Tissue softening, together with oxidative browning, may dramatically curb the shelf life of fresh-cut produce. Different techniques have been developed to extend the shelf life of minimally processed fruits. In particular, refrigeration in combination with the use of antibrowning agents and calcium salts is critical to delay firmness loss and to control browning (Olivas et al. 2007). The beneficial result of the incorporation of dietary fiber on the firmness retention of ‘Golden delicious’ apple cubes during 16 days of storage could be attributed to the antioxidant content of the fiber extracts in combination with the texture protective effects calcium chloride used for cross-linking the polysaccharide polymer chains. These results are in agreement with those reported by Rojas-Graü (2007, 2009), who found significant differences between firmness of coated and uncoated samples, for gellan gum, pectin and alginate edible coatings on fresh-cut apple. Also, Lee et al. (2003) demonstrated that incorporation of calcium chloride (1 %) within the coating formulation helped to maintain firmness of apple pieces coated with a whey protein concentrate. The effect of calcium chloride as firming agent has been extensively documented in the literature. Possible interaction of dietary fiber with calcium and their potentially beneficial effects regarding firmness retention have not been studied in depth.
Edible coatings should not impart undesirable flavors that can be detected once the product is consumed (Ponce et al. 2008; Rojas-Graü et al. 2007). Many nutraceutical compounds have bitter or astringent off-flavors that could lead to rejection of the product by consumers. As a result of this, the incorporation of dietary fiber into alginate, gellan gum and pectin edible coatings could change the original sensory attributes of fresh-cut apples. However, preliminary assays (data not shown) performed for the sensory analysis indicated that the addition of inulin and apple fiber into the edible coatings applied on fresh-cut apple did not produce any difference in color, texture, flavor and OVQ respect to apple pieces coated with gellan gum, pectin and alginate. Therefore, the results pertaining to the sensory acceptability of fresh control samples and apple cubes coated with gellan gum, pectin and alginate with and without fiber addition have been reported jointly. In accordance with our results, Moraes Crizel et al. (2013) analyzed the use of orange fiber as a potential fat replacer in ice cream and reported that sensory attributes such as color, odor and texture do not differ among the ice cream formulations with and without orange fiber addition. Also, Rinaldoni et al. (2012) reported that yogurt enriched with inulin presented similar global acceptability respect to control sample. In this work, sensory evaluation was performed through the storage period to indicate the differences between uncoated samples and apple pieces coated with gellan gum, pectin and alginate. It was observed that the application of pectin, gellan gum and alginate films (with and without fibers addition) allowed apple samples to reach the end of storage with sensory scores above the sensory acceptability threshold (above 3) for any attribute evaluated.
Conclusions
Alginate, pectin and gellan gum edible coatings with dietary fibers addition may help to maintain desirable quality characteristics of fresh-cut apples. The coated apples cubes kept their initial firmness and color throughout refrigerated storage, which confirms their good ability to carry different compounds incorporated to maintain their quality and extend their shelf-life. Regarding microbiological results, gellan gum edible coating applied on fresh-cut apple had a marked effect in reducing mesophilic and psychrophilic counts during all storage period. The incorporation of an apple fiber extract into different polysaccharide coating formulations contributed to the preservation of the antioxidant activity of fresh-cut apples during their storage. At the end of the evaluated period, apple samples coated with polysaccharide-based edible coatings incorporating dietary fiber obtained higher sensory scores and, from an organoleptical point of view, remained marketable. Therefore, addition of dietary fiber extracts stands as a good alternative for enhancing the nutritional value of fresh-cut apples while maintaining their quality attributes.
Acknowledgments
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, Argentina) and by Spanish Ministry of Economy and Competitiveness, through the project AGL2010-21572. An ICREA Academia Award is also acknowledged.
Footnotes
Research highlights
• Edible coatings with dietary fiber addition maintain quality attributes of fresh-cut apples
• Dietary fiber incorporated into edible coatings maintained firmness and color
• Edible coatings incorporating fiber had a positive effect on apples sensory attributes
• Edible coatings with dietary fiber addition kept the antioxidant activity of the fruit
• Gellan coatings had marked inhibitory effect on microbial growth during fruit storage
References
- Alvarez MV, Ponce A, Moreira MR. Antimicrobial efficiency of chitosan coating enriched with bioactive compounds to improve the safety of fresh cut broccoli. LWT Food Sci Technol. 2013;50:78–87. doi: 10.1016/j.lwt.2012.06.021. [DOI] [Google Scholar]
- Anderson JW, Baird P, Davis RH, et al. Health benefits of dietary fiber. Nutr Rev. 2009;67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- Chien P, Sheu F, Yang F. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J Food Eng. 2007;78(1):225–229. doi: 10.1016/j.jfoodeng.2005.09.022. [DOI] [Google Scholar]
- Figuerola F, Hurtado M, Estévez A, Chiffelle I, Asenjo F. Fiber concentrates from apple pomace and citrus peel as potential fiber sources for food enrichment. Food Chem. 2005;91:395–401. doi: 10.1016/j.foodchem.2004.04.036. [DOI] [Google Scholar]
- Gorny J. New opportunities for fresh-cut apples. Fresh Cut. 2003;11:14–15. [Google Scholar]
- Grigelmo-Miguel N, Martin-Belloso O. Characterization of dietary fiber from orange juice extraction. Food Res Internat. 1999;31:355–361. doi: 10.1016/S0963-9969(98)00087-8. [DOI] [Google Scholar]
- Grigelmo-Miguel N, Martín-Belloso O. Comparison of dietary fiber from by-products of processing fruits and greens and from cereals. LWT-Food Sci Technol. 1999;32:503–508. doi: 10.1006/fstl.1999.0587. [DOI] [Google Scholar]
- Hernández-Muñoz P, Almenar E, Ocio M, Gavara R. Effect of calcium dips and chitosan coatings on postharvest life of strawberries (Fragaria x ananassa) Postharvest Biol Technol. 2006;39(3):247–253. doi: 10.1016/j.postharvbio.2005.11.006. [DOI] [Google Scholar]
- Lee J, Park H, Lee C, Choi W. Extending shelf-life of minimally processed apples with edible coatings and antibrowning agents. LWT Food Sci Technol. 2003;36:323–329. doi: 10.1016/S0023-6438(03)00014-8. [DOI] [Google Scholar]
- Marín F, Soler-Rivas C, Benavente-García O, Castillo J, Pérez-Alvarez J. By-products from different citrus processes as a source of customized functional fibres. Food Chem. 2007;100:736–74. doi: 10.1016/j.foodchem.2005.04.040. [DOI] [Google Scholar]
- Moraes Crizel T, Jablonski A, Oliveira–Rios A, Rech R. Dietary fiber from orange byproducts as a potential fat replacer. LWT-Food Sci Technol. 2013;53:9–14. doi: 10.1016/j.lwt.2013.02.002. [DOI] [Google Scholar]
- Moreira M, Roura S, Ponce A. Effectiveness of Chitosan edible coatings to improve microbiological and sensory quality of fresh cut broccoli. LWT Food Sci Technol. 2011;44(10):2335–2341. doi: 10.1016/j.lwt.2011.04.009. [DOI] [Google Scholar]
- Olaimat A, Holley R. Factors influencing the microbial safety of fresh-produce: a review. Food Microbiol. 2012;32:1–19. doi: 10.1016/j.fm.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Olivas G, Mattinson D, Barbosa-Cánovas G. Alginate coatings for preservation of minimally processed apple. Postharvest Biol Technol. 2007;45(1):89–96. doi: 10.1016/j.postharvbio.2006.11.018. [DOI] [Google Scholar]
- Oms-Oliu G, Soliva-Fortuny R, Martín Belloso O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol Technol. 2008;50:87–94. doi: 10.1016/j.postharvbio.2008.03.005. [DOI] [Google Scholar]
- Oms-Oliu G, Rojas-Graü A, González L, Varela P, Soliva-Fortuny R, Hernando M, Pérez Munuera I, Fiszman S, Martín-Belloso O. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: a review. Postharvest Biol Technol. 2010;57:139–148. doi: 10.1016/j.postharvbio.2010.04.001. [DOI] [Google Scholar]
- Ponce A, Roura S, Moreira MR. Antimicrobial and antioxidant activities of edible coatings enriched with natural plant extracts: in vitro and in vivo studies. Postharvest Biol Technol. 2008;49:294–300. doi: 10.1016/j.postharvbio.2008.02.013. [DOI] [Google Scholar]
- Ramos B, Miller F, Brandao T, Teixeira P, Silva C. Fresh fruits and vegetables- an overview on applied methodologies to improve its quality and safety. Innovative Food Sci Emerg Technol. 2013;20:1–15. doi: 10.1016/j.ifset.2013.07.002. [DOI] [Google Scholar]
- Rinaldoni A, Campderrós M, Padilla A. Physico-chemical and sensory properties of yogurt from ultrafiltered soy milk concentrate added with inulin. LWT-Food Sci Technol. 2012;45:142–147. doi: 10.1016/j.lwt.2011.09.009. [DOI] [Google Scholar]
- Robles-Sánchez R, Rojas-Graü A, Odriozola-Serrano I, González-Aguilar G, Martín-Belloso O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT-Food Sci Technol. 2013;50:240–246. doi: 10.1016/j.lwt.2012.05.021. [DOI] [Google Scholar]
- Rojas-Graü A, Raybaudi-Massilia R, Soliva-Fortuny R, Avena-Bustillos T, Martín-Belloso O. Apple puree-alginate coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol Technol. 2007;45:254–264. doi: 10.1016/j.postharvbio.2007.01.017. [DOI] [Google Scholar]
- Rojas-Graü MA, Tapia MS, Martin-Belloso O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT-Food Sci Technol. 2008;41:139–147. doi: 10.1016/j.lwt.2007.01.009. [DOI] [Google Scholar]
- Rojas-Graü A, Soliva-Fortuny R, Martín Belloso O. Edible coatings to incorporate active ingredients to fresh-cut fruits: a review. Trends in Food Sci Technol. 2009;38:438–447. doi: 10.1016/j.tifs.2009.05.002. [DOI] [Google Scholar]
- Tapia MS, Rojas-Graü MA, Carmona J, Rodríguez S, Soliva-Fortuny R, Martin-Belloso O. Use of Alginate- and gellan-based coatings for improving barrier, texture and nutritional properties of fresh-cut papaya. Food Hydrocoll. 2008;22:1493–1503. doi: 10.1016/j.foodhyd.2007.10.004. [DOI] [Google Scholar]