Abstract
Cold water steeping is reported to maximise tea health benefits, but requires long infusion time. In this work, the employment of a brief hot infusion step followed by ice addition was evaluated. The comparison of this innovative method with hot and cold steeping was investigated on green, black and oolong teas. Catechins, xanthines and gallic acid content, antioxidant power, total phenolics and colour analysis were evaluated. Hot infusion shown rapid extractive power, but relevant compound degradation. On the contrary, cold infusion extracted higher level of healthy molecules with slow kinetic. The innovative method achieved in short time similar properties of cold infusion in terms of antioxidant power. As for bioactive compounds, such as gallic acid and epigallocatechin gallate, highest values, about double than in hot infusion, were recorded for green and black teas. This steeping method may represent an alternative approach for industrial beverage preparation.
Keywords: Green tea, Black tea, Oolong tea, Colour, FRAP, Caffeine
Introduction
Tea is a popular beverage representing the most consumed drink in the world after water. According to processing, such as extent of oxidation (so-called “fermentation”), three types of tea are known: green (non-fermented), oolong (semi-fermented) and black tea (fully fermented). Black tea is the most consumed in Western countries, although great attention has been paid in recent years to green tea, for its higher antioxidant activity and related health effects (Steinmann et al. 2013; Park et al. 2014; Pang et al. 2015).
Traditional way of preparing teas is brewing leaves in hot water at a temperature depending on the type (70–100 °C, Yang et al. 2007). Many studies reported differences in quality and antioxidant property depending on polyphenols and bioactive compounds content of different teas (Chen et al. 2012; El-Shahawi 2012; Rahim et al. 2014; Zhang et al. 2013) according to tea type (Bae et al. 2015; Lin et al. 2014a, b; Yang and Liu 2013), geographic origin (Dias et al. 2014) and seasonal variation (Laddi et al. 2014). Effect of infusion conditions on bioactive compounds extraction has been studied (Araújo Ramalho et al. 2013; Bae et al. 2015; Rusak et al. 2008) showing that temperature and time are the most crucial parameters affecting polyphenol content and antioxidant capacity (Araújo Ramalho et al. 2013; Damiani et al. 2014). For best extraction of antioxidant compounds from green tea it is recommended to employ low temperatures (Banerjee and Chatterjee 2014), since over 90 °C polyphenols are destroyed and sensory properties have been found to decrease (Saklar et al. 2015); agitation and dosage form have no important influence (Samaniego-Sánchez et al. 2011).
Recently, cold water (4 °C or room temperature) steeping become a new popular way for infusion. It provides lower caffeine, lower bitter taste and higher aroma (Lin et al. 2014a, b). Infusion rates of caffeine, catechins and gallic acid were lower than in hot water, and increased with infusion time (Liao et al. 2012). Venditti et al. (2010) studied the influence of temperature to maximise potential health benefits. However, the promising cold steeping technology requires long infusion. To overcome it, a potential modification involves an infusion step with hot water followed by ice addition, avoiding the slow cooling process, responsible for changes in the contents of functional compounds (Ananingsih et al. 2013).
Effects of cold water on functional compounds and organoleptic quality were not yet investigated and deserve more attention considering the advertised beneficial effects of tea consumption on health. In this paper, the effects of three different steeping methods on three tea types (black, green and oolong) are described. The methodologies followed for infuse preparation involved hot, cold, and hot water followed by ice addition. Antioxidant activity, colour, caffeine, theobromine, theophylline, catechins and gallic acid content were evaluated to compare the characteristics of the obtained drinks.
Material and methods
Chemicals
Gallic acid (GA), ascorbic acid, theobromine, theophylline, caffeine, (-)-epicatechin (EC), (-)-epigallocatechin gallate (EGCG), 2,4,6-Tripyridyl-s-triazine (TPTZ), glacial acetic acid, methanol (MeOH), acetonitrile (ACN), Folin Ciocalteu’s phenol reagent and 2,2-difenil-1-picrilhydrazyl (DPPH) were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA). Sodium acetate and ferric chloride were obtained from Carlo Erba Reagents (Milano, Italy). Phosphoric acid was supplied by J.T. Baker (Milano, Italy) and sodium carbonate by Sigma–Aldrich (Milano, Italy). Deionized water (‹ 18 MΩ cm resistivity) was obtained from a Milli-Q (Millipore, Bedford, MA, USA); bottled mineral water (80.5 mg/l dry residue at 180 °C), 124 μS/cm conductivity at 25 °C, and 5.9 °F) was purchased from market.
Teas and infuses preparation
Sencha Needle unfermented green, Tung Ting semi-fermented oolong and Orange Pekoe Flowery fully-fermented black teas donated by “Ferri dal 1905” (Mantova, Italy) were analysed. Green and black leaf size was about 1.5 mm; oolong tea leaves were rolled with 3 mm diameter. Estimated total surface available for mass diffusion was 104.4, 44.8, and 16.8 mm2 for oolong, green and black tea, respectively. Steeping methods tuned by a panel of experienced tea tasters are reported in Table 1. Extraction with hot water (“hot”): tea (12 g) was placed into 1 L of water brought to specific temperature. Cold tea (“cold”) was prepared by infusion at refrigeration temperature (4 °C ± 1) along 12 h. Ice tea (“hot + ice”) was prepared by placing 30 g of tea in 600 mL of water at 80 °C and adding 400 g of ice after the required time of infusion and leaves removing. All samples were prepared in triplicate and filtered (0.45 mm).
Table 1.
Schematic description of the infusion preparations
| Tea | Extraction | Leaves (g/L) | Temperature (°C) | Time (min) | Water (L) | Ice (kg)a |
|---|---|---|---|---|---|---|
| Green | Hot | 12 | 75 | 4 | 1 | - |
| Cold | 8 | 4 | 720 | 1 | - | |
| Hot + Ice | 30 | 80 | 5 | 0.6 | 0.4 | |
| Oolong | Hot | 12 | 85 | 4 | 1 | - |
| Cold | 7 | 4 | 720 | 1 | - | |
| Hot + Ice | 30 | 80 | 4 | 0.6 | 0.4 | |
| Black | Hot | 12 | 90 | 3 | 1 | - |
| Cold | 7 | 4 | 720 | 1 | - | |
| Hot + Ice | 30 | 80 | 3 | 0.6 | 0.4 |
aadded after infusion
High-performance liquid chromatography
Separations were performed on Agilent Technologies 1200 liquid chromatograph with Kinetex C18 column (100 mm × 2.1 mm ID, 2.6 μ) modifying a previous method (Wang et al. 2000). Mobile phase (0.2 ml/min) consisted of 85 % solvent A (water containing 0.1 % v/v orthophosphoric acid) and 15 % of solvent B (MeOH). Solvent B increased linearly to 27 % at 16 min. Gradient returned to 15 % B in 1 min, standing for 13 min. Injected volume 20 μl. Monitoring wavelengths: 210 and 280 nm.
Studies on linearity, precision, selectivity, and recovery were performed, according to Eurachem guidelines (www.eurachem.org).
Detection limit (yD) and quantitation limit (yQ) were calculated as signals based on the mean blank () and the standard deviation (sb) of the blank signals:
t = constant of t-Student distribution (one-sided) depending on confidence level and degrees of freedom (ν = n-1; n = number of measurements). Ten blanks were performed to calculate and sb. Values of yD and yQ were converted from signal domain to concentration domain to estimate LOD and LOQ respectively, using an appropriate calibration function.
Linearity was established in the range of between 0.05 and 100 mgL−1 for gallic acid and caffeine, 0.05–50 mgL−1 for theobromine, 0.05–25 mgL−1 for theophylline, 0.5–25 mgL−1 for epicathechin and 0.5–100 mgL−1 for epigallocathechin gallate. Five equispaced concentration levels were chosen and three replicated injections were performed at each level. The homoscedasticity test was run and the goodness of fit of the calibration curve was assessed applying Mandel’s fitting test. A t-test was carried out to verify the significance of the intercept (confidence level 95 %). Precision was calculated in terms of inter-day and intra-day repeatability both of area and retention times as RSD% at two concentration levels for analysis.
Intraday repeatability was calculated on peak areas and on retention times using five determinations at two concentration levels in triplicate. Inter-day repeatability was calculated performing the same analyses in two different days.
Percentage of recovery, on samples fortified with all analytes at three levels of concentration was calculated by:
(C1 concentration determined in fortified sample, C2 concentration determined in unfortified sample, C3 concentration of fortification).
Total phenolic content
Total phenol content (TPC) was determined according to Folin–Ciocalteau procedure (Jayasekera et al. 2011), with some modification. Briefly, to an aliquot of 1 mL, 4 mL of 7.5 % sodium carbonate solution and 5 mL Folin–Ciocalteau reagent (10 %) were added. Mixture was allowed to react for 60 min at room temperature in the dark. Absorbance was read at 750 nm. Calibration was achieved with an aqueous gallic acid solution (25–300 μg/mL). Total phenol values were expressed as gallic acid equivalents (GAE). Duplicate extractions of each sample were performed; each assay was carried out in triplicate (n = 6).
Determination of ferric reducing antioxidant power (FRAP)
Ferric reducing/antioxidant power (FRAP) assay was carried out according to Benzie and Strain (1996) by measuring the change in absorbance at 593 nm owing to the formation of a blue coloured FeII-tripyridyltriazine compound from the colourless oxidized FeIII form by the action of electron donating antioxidants. FRAP was prepared by mixing acetate buffer 300 mM, pH 3.6, TPTZ 10 mM and FeCl3 6H2O (20 mM) at a ratio of 10:1:1. To 200 mL of tea infuse, 380 μL of working FRAP warmed at 37 °C was added. Immediately, and again after 4 min, the absorbance at 593 nm was measured against a blank sample.
Determination of liquor total colour and CIElab parameters
Liquid total colour (LTC) was determined according to Obanda et al. (2004). Three mL of infusion were diluted with 45 ml distilled water. Absorbance was read at 460 nm against distilled water. Results were corrected for dry matter content: values of 93.5, 92.6 and 93.7 % for black, green and oolong respectively were obtained with thermo-balance method (BEL Engineering, Monza, Italy).
Colour of infuses was measured by image analysis: samples put into transparent containers were scanned by desktop flatbed scanner (Hewlett Packard Scanjet 8200, Palo Alto, CA, USA) at 236 pixels per cm (600 dpi of resolution; true colour – 24 bit), equipped with a cold cathode lamp for reflective scanning. During acquisition, scanner was held in a black box to exclude surrounding light and external reflections. Flatbed scanner colour was characterized and corrected as previously reported by N’Dri et al. (2010).
Consumer test
Tea were subjected to a preference ranking test (ISO 2006) conducted with 24 subjects, young adults (10 men and 14 women) between 18 and 40 years. Samples were ranked on the basis of overall acceptability and organoleptic quality. Data were analyzed using Friedman’s chi-square non-parametric test providing an overall view of sample discrimination (p-value). Least significant rank differences (LSRD) were calculated to identify pair-wise sample differences among rank sums resulting from preference ranking.
Statistical analysis
Means and standard deviations (SD) of data were calculated with SPSS (Version 20.0) software used also to perform one-way analysis of variance (ANOVA) and the least significant difference (LSD) test at a 95 % confidence level (p < 0.05), to identify differences among samples. The same software was used to perform Pearson correlation between analytical data (0.01 level, 2-tailed).
Results and discussion
High-performance liquid chromatography (HPLC)
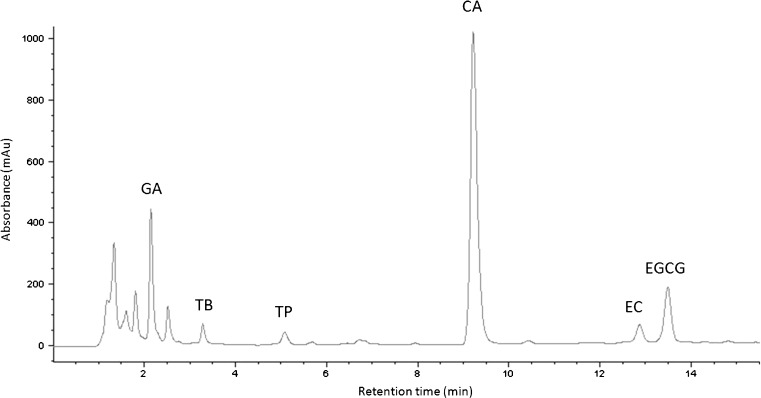
The analytes of interest were xanthines (caffeine, theophylline and theobromine), catechins (epicatechin, and epigallocatechin gallate) and gallic acid, well separated in about 14 min (Fig. 1). Quantitative data reported in Table 2 show the effectiveness of extraction notwithstanding the differences in the leaves:water ratio employed. Significant differences between the steeping methods were observed.
Fig. 1.
HPLC chromatogram of green tea infuse. GA gallic acid, TB theobromine, TP theophylline, CA caffeine, EC epicatechin, EGCG epigallocatechine gallate
Table 2.
Analytical results of tea samples.Data (mean ± standard deviation) expressed as mg/g leaves
| Tea | Extraction | FRAP | TPC | GA | EC | EGCG | TP | TB |
|---|---|---|---|---|---|---|---|---|
| Green | Hot | 1463.1 ± 129.7a | 13.5 ± 0.1c | 3.46 ± 0.03c | 1.36 ± 0.06b | 4.09 ± 0.12b | 0.20 ± 0.00a | 0.63 ± 0.01b |
| Cold | 1653.5 ± 147.1a | 19.7 ± 0.1a | 3.93 ± 0.03b | n.d. | 4.13 ± 0.10b | 0.19 ± 0.01a | 0.65 ± 0.03b | |
| Hot + Ice | 1432.6 ± 80.8a | 14.2 ± 0.0b | 5.13 ± 0.06a | 1.61 ± 0.05a | 7.36 ± 0.02a | 0.19 ± 0.00a | 1.22 ± 0.03a | |
| Oolong | Hot | 325.4 ± 64.9b | 12.8 ± 0.2c | 1.05 ± 0.01c | 0.23 ± 0.05a | 0.62 ± 0.01c | 0.06 ± 0.01b | 0.25 ± 0.01b |
| Cold | 1278.5 ± 81.1a | 21.0 ± 0.1a | 2.94 ± 0.02a | n.d. | 2.45 ± 0.01a | 0.24 ± 0.01a | 0.43 ± 0.01a | |
| Hot + Ice | 1183.5 ± 124.3a | 14.1 ± 0.2b | 2.12 ± 0.02b | 0.11 ± 0.02b | 1.17 ± 0.07b | 0.06 ± 0.00b | 0.25 ± 0.00b | |
| Black | Hot | 1111.8 ± 139.2b | 14.2 ± 0.1b | 3.62 ± 0.15b | n.d. | 0.43 ± 0.01c | 0.09 ± 0.00a | 1.29 ± 0.01b |
| Cold | 1218.8 ± 38.0b | 20.4 ± 0.1a | 4.11 ± 0.14a | n.d. | 0.60 ± 0.01b | 0.10 ± 0.01a | 1.15 ± 0.00c | |
| Hot + Ice | 1629.0 ± 189.6a | 14.8 ± 0.2b | 4.07 ± 0.15a | 0.03 ± 0.00 | 0.76 ± 0.03a | 0.11 ± 0.01a | 1.62 ± 0.06a |
Abbreviations: GA gallic acid, EGC (−)-epigallocatechin, EC (−)-epicatechin, EGCG (−)-epigallocatechin gallate, TP Theophylline, TB Theobromine
a,b,cSame letters within each column do not significantly differ (n = 5; p < 0.05)
EGCG was the most predominant catechin (El-Shahawi et al. 2012). Among xanthines, caffeine showed the highest content, followed by theobromine and theophylline, in accord to ranges reported in literature (Friedman et al. 2006). Gallic acid was detected in significant amounts, ranging from 1.05 to 5.13 mg/g, according to recent studies (Bae et al. 2015).
For green and black tea, hot + ice infusion allowed extracting the highest amount of all analytes, except TP whose amount was constant (Table 2). Extraction of compounds clearly depends on both temperature and time of infusion. Temperature seems the most influent parameter in agree with Yang et al. (2007) reporting lower rates of gallic acid from green tea with cold than with hot water.
For both green and black tea, cold method allowed the extraction of slightly higher amounts than hot method, showing that longer time of contact ensures migration of a relevant amount of some compounds even at low temperature. It can also be hypothesized that higher amounts of analytes are extracted during long time, but then are destroyed by oxidation or side reactions such as polymerization phenomena occurring along 12 h (Samaniego-Sánchez et al. 2011; Ananingsih et al. 2013). Such phenomena could occur after infusion, during cooling process, while hot + ice infuses reach quickly a low temperature keeping higher amount of active compounds. Oxidative degradation of EGCG has been previously reported to occur during storage (Yang et al. 2007); accordingly, its amount was definitely higher in both “hot + ice” and cold infuses (Table 2). Similarly, Kim et al. (2007) observed a decrease in catechins at high temperature, suggesting that epimerization or oxidation could take place. Moreover it can be noticed that EC was not found in cold infuse (Wang and Helliwell 2000).
For oolong tea the highest values of analytes were found after cold extraction. This apparently unexpected behaviour can be explained considering the shape and dimension of the rolled leaves (diameter definitely higher than others). Their shape leads to a difficult extraction kinetics, slowing the migration process. Longer time of contact with water, although at low temperature, causes the leaves unrolling, exposing a bigger total surface available for mass diffusion (Astill et al. 2001). As for the comparison between hot and hot + ice methods, similarly to green tea, a double amount of gallic acid and ECGC was recorded in the latter extract respect to the former, while the opposite happened for EC.
In black tea, smaller differences between the three steeping procedures were observed, with slight higher values for hot + ice method, and lower values for hot extraction. Since hot extraction was performed at highest temperature employed, our results confirm previous findings reporting that high temperature leads to compounds degradation (Samaniego-Sánchez et al. 2011; Kim et al. 2007).
In conclusion, data about catechins content in different teas are in agreement with Zhang et al. (2013) and Kim et al. (2007) who observed that the amounts of total catechins were ordered according to the fermentation processes as follows: non-fermented (green tea) > partially fermented (oolong tea) > fully fermented (black tea).
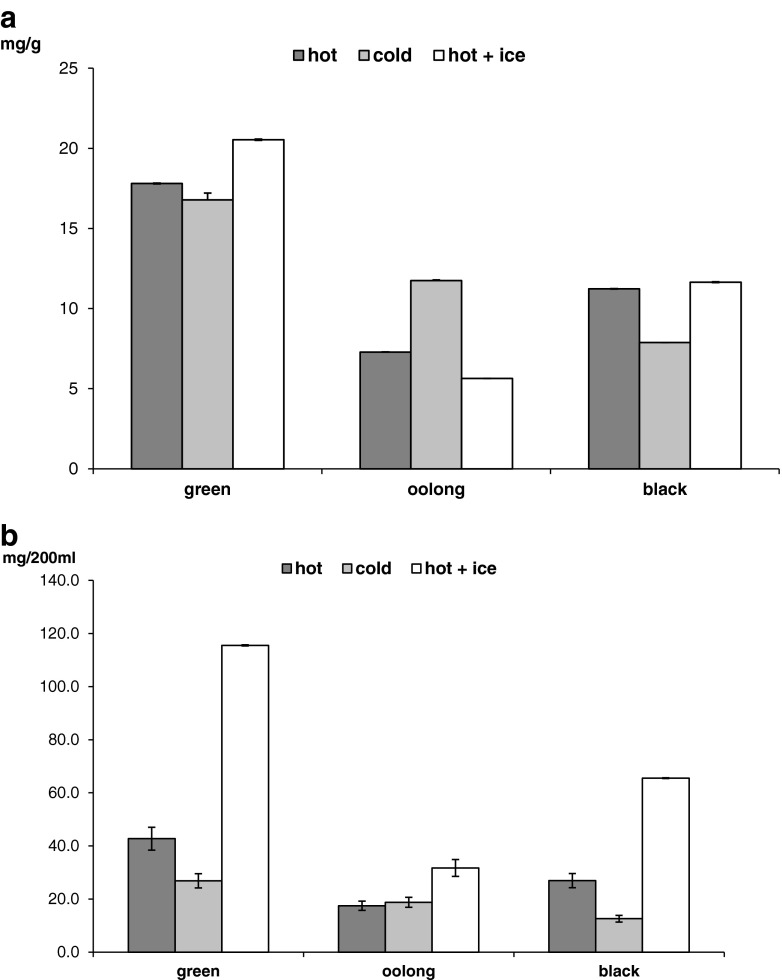
Caffeine contents reported in Fig. 2 (panel a) were in the ranges previously reported (Bae et al. 2015; Rahim et al. 2014) with green tea showing higher values (Yang et al. 2007). Caffeine extraction directly depends on temperature with a higher mass transfer rate with increasing water temperature (Perva-Uzunalić et al. 2006). For green tea, the highest value was recorded for hot + ice extraction, similarly to black tea. On the contrary, for oolong tea, cold method gave the highest value. Thus, for green and black tea the leaves dimension represents the limiting factor for mass transfer, and water temperature played an important role. Besides, in oolong tea the great surface/volume ratio allowed efficient extraction also at low temperature due to longer infusion time.
Fig. 2.
Panel a Caffeine content, mg/g leaves. Panel b Caffeine content, mg per cup - 200 mL
Finally, since caffeine is an important psychoactive drug, whose consumption should be taken under control, we calculated the amount of caffeine occurring in a cup to evaluate the intake for a tea habitual consumer notwithstanding the different procedures (Fig. 2, panel b). For all teas, the hot + ice method gave highest values of caffeine, because of the greater leaves:water ratio used (Table 1). Great differences between hot + ice and other methods were observed for green and black teas due to their low surface/volume value. As expected, hot method gave higher amount of caffeine than cold one because of higher leaves:water ratio employed, but also since high temperature favoured the extraction process.
Total phenolic content
Folin-Ciocolteau method gave an estimation of the total reducing power of the extract, since is sensible to all reducing compounds. The amount of phenolics is generally connected to beneficial health effects of food, considered a major source of antioxidants.
No great differences were observed between the different infusions, as data follow a similar trend (Table 2): cold infusion gave the highest phenolic content, followed by hot + ice and then hot method, in accordance to Damiani et al. (2014). Two possible reasons could explain this behaviour: the longer time of extraction determined the migration of higher amount of active compounds and/or low temperature protected molecules from degradation.
To understand the predominant variable between time and temperature, we took into account data from other extracts: time seems having a predominant effect, while high temperature lead to destruction of the molecules causing oxidation, epimerization and polymerization phenomena. Therefore, we can assume that the rapid cooling in the hot + ice method limited those effects, exerting a protective effect on the molecules not subjected to long exposition to high temperature.
FRAP value
High values were recorded for green tea with no differences between extraction methods (Table 2). This confirms that it is richer of antioxidant compounds and suggests that compounds responsible for this activity are also stable at high temperature.
On the contrary, lowest values were recorded for oolong. Besides, since cold and hot + ice extractions gave higher values than hot infusion, we may assume that the compounds reacting in FRAP test for oolong tea are quite sensible to high temperature (Damiani et al. 2014). In addition, we can hypothesize that the high exchanging surface at the longer time of extraction in cold method determined the extraction of higher amount of active compounds. At the same time, low temperature protected the antioxidant molecules from degradation.
Similar behaviour was noticed for black tea: hot + ice infusion gave the highest value, while no significant differences between the other extracts were observed.
The high variability between the behaviour of teas suggests that the pattern of the compounds responsible for the antioxidant activity is different and/or a different kinetic of migration of the active molecules from the leaves occurred.
Nevertheless, it is important to bear in mind that tea leaves composition and infusion concentration depends on many parameters such as cultivar type, growing environment, plucking practices and manufacturing conditions (Astill et al. 2001; Dias et al. 2014).
Liquor total colour (LTC) and colorimetric parameters
Samples of hot infusion presented a significantly higher total liquor colour compared to cold ones (Table 3), which presented a significantly higher value than hot + ice ones. Kim et al. (2007) reported for green tea that L* decreased while a* and b* increased with heating temperature, suggesting that the tea liquor becomes less green and deeper yellow due to oxidation of catechins and degradation of chlorophyll. Ice infuses presented lowest value of colour, even though solid/liquid ratio was the highest one, probably due to the rapid cooling process preventing these phenomena and reducing extraction of coloured compounds.
Table 3.
Liquor total colour and colour determination values (L*, a*, b*) of tea infusions
| Green | Oolong | Black | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hot | Cold | Hot + Ice | Hot | Cold | Hot + Ice | Hot | Cold | Hot + Ice | |
| LTC | 10.1 ± 0.0a | 10.1 ± 0.0a | 9.5 ± 0.0a | 9.9 ± 0.0a | 9.8 ± 0.0a | 10.0 ± 0.0a | 11.1 ± 0.0a | 10.2 ± 0.0a | 10.4 ± 0.0a |
| L* | 56.3 ± 0.5a | 57.5 ± 0.6a | 56.4 ± 0.5a | 56.1 ± 0.3a | 56.9 ± 0.5a | 57.2 ± 0.8a | 47.3 ± 0.8b | 54.4 ± 0.9a | 46.7 ± 0.8b |
| a* | −4.4 ± 0.1a | −4.8 ± 0.1b | −4.9 ± 0.1b | −4.7 ± 0.1a | −4.6 ± 0.1a | −5.1 ± 0.4a | 1.1 ± 0.1b | −3.8 ± 0.0c | 2.6 ± 0.1a |
| b* | 7.9 ± 0.2b | 4.7 ± 0.5c | 11.3 ± 0.2a | 10.1 ± 0.2a | 7.9 ± 0.1b | 9.5 ± 1.7a | 35.6 ± 1.2b | 18.3 ± 0.2c | 44.7 ± 2.0a |
Abbreviations: LTC liquid total colour
a,b,cSame letters within each row do not significantly differ (n = 10; p < 0.05)
Regarding indices measured by image analysis, no significant differences were observed for L*, a* and b* for oolong, giving a very light infuse; probably this method was not feasible to distinguish colour differences due to its low sensibility. On the contrary, for green teas significant differences were observed for a* with hot sample significantly less green compared to others, confirming catechins oxidation and chlorophyll degradation due to high temperature in hot infusion, and for b* with the order hot + ice < hot < cold: cold infusions presented the highest b* value meaning a more yellow colour.
Finally, for black tea, L* value in cold extraction was significantly higher compared to both hot + ice and hot method; on the contrary, cold sample presented a negative a* value corresponding to a green colour, while both hot and hot + ice showed a positive value, corresponding to a red colour with a significantly higher value for the latter. Probably, in cold samples chlorophyll was not degraded into pheophytin by the effect of high temperature, and colour of leaves was maintained after the infusion process.
In addition, b* values presented significant differences among all samples with hot + ice being the more yellow colour and cold the less one.
Consumer test
Ranking test was performed by 24 not-expert panellists, therefore the significant value for assuming a preference was equal to 13.6 (ISO 2006). Results shown a significant preference only between cold vs. hot + ice for black tea, between cold vs. hot + ice and hot vs. hot + ice for green one with a preference for the former samples. For oolong tea no significant differences were observed. Thus, it appears that consumers, not expert tasters, did not appreciate hot + ice method probably due to the higher astringency and higher concentration of compounds with extensive aromatic notes.
Correlations
Data were subjected to analysis by evaluating Pearson’s correlation indices. For black tea, FRAP data resulted significantly correlated with catechins (R2 > 0.86, p < 0.01) but not with total phenolic content (TPC), as also previously observed (Agbor et al. 2005). This behaviour can be explained taking into account that FRAP and Folin reagents reacts differently and at different rates with the several antioxidant compounds contained in the infuses.
TPC was significantly directly correlated with L* (R2 > 0.96, p < 0.01) and inversely correlated with other indices (a* and b*, R2 < −0.90, p < 0.01) and with ranking test results (R2 = −0.92, p < 0.01) confirming contribution of phenolic compounds to the colour and the astringency.
In oolong tea, caffeine was inversely correlated with ranking test (R2 = −0.94, p < 0.01) according to a previous report that identified it as key taste for contribution to bitter taste (Scharbert and Hofmann 2005). Besides, in oolong tea, catechins and gallic acid were recognised to be strongly responsible for antioxidant value since TPC and FRAP showed a high correlation with them (R2 > 0.89, p < 0.01). In the case of TPC a high correlation was also found with all xanthynes (R2 > 0.92, p < 0.01).
Differently from others, in green tea correlation between TPC and FRAP was found (R2 = 0.71, p < 0.01), confirming a linkage between phenolic compounds and antioxidant activity (Samaniego-Sánchez et al. 2011).
For green and oolong samples, EC and EGCG were correlated with b* value (R2 > 0.86, p < 0.01) in accord with Kim et al. (2007) suggesting that catechin oxidation might be responsible for the changes in colour. A remarkable point was that no correlation with total liquor colour (TLC) was recorded, showing that this analysis was not suitable for comparing different methods.
Conclusions
Comparing classical hot extraction with alternative methods, differences on compounds extraction, antioxidant activity, total phenolic content, colour and taste were recorded. Cold extraction allowed highest antioxidant activity, total phenolics and gallic acid content, supporting the raising popularity of this technique. The innovative infusion method prevents degradation of bioactive molecules caused by exposure to high temperature, permitting to enhance antioxidant properties. Besides, it allows obtaining, in a definitely shorter time, similar properties to those achieved by cold infusion.
Pearson’s correlations can be interpreted as indicating that consumers acceptance was significantly correlated with parameters such as caffeine, catechins, total phenolic content and liquor colour contributing to the overall taste.
This study adds new information on the effect of non-conventional preparation methods and on the extraction of active compounds from leaves. Obtained results may contribute to investigate new ways for maximising potential health benefits by means of different methods of tea preparation.
Footnotes
Highlights
• Alternative steeping methods for green, black and oolong tea infusion were studied
• Catechins, xanthines, antioxidant value and colour analysis were performed
• The innovative ice-based method tested prevented degradation of active molecules
• Similar properties to those achieved by cold infusion were obtained in shorter time
References
- Agbor GA, Oben JE, Ngogang JY, Xinxing C, Vinson JA. Antioxidant capacity of some herbs/spices from Cameroon: a comparative study of two methods. J Agric Food Chem. 2005;53:6819–6824. doi: 10.1021/jf050445c. [DOI] [PubMed] [Google Scholar]
- Ananingsih VK, Sharma A, Zhou W. Green tea catechins during food processing and storage: a review on stability and detection. Food Res Int. 2013;50:469–479. doi: 10.1016/j.foodres.2011.03.004. [DOI] [Google Scholar]
- Araújo Ramalho S, Nigam N, Barbosa Oliveira G, Alves de Oliveira P, Matos Silva TO, Geovânia Passos dos Santos A, Narendra N. Effect of infusion time on phenolic compounds and caffeine content in black tea. Food Res Int. 2013;51:155–161. doi: 10.1016/j.foodres.2012.11.031. [DOI] [Google Scholar]
- Astill C, Birch MR, Dacombe C, Humphrey PG, Martin PT. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J Agric Food Chem. 2001;49:5340–5347. doi: 10.1021/jf010759+. [DOI] [PubMed] [Google Scholar]
- Bae IK, Ham HI, Jeong MH, Dong HK, Kim HJ. Simultaneous determination of 15 phenolic compounds and caffeine in teas and mate using RP-HPLC/UV detection: method development and optimization of extraction process. Food Chem. 2015;172:469–475. doi: 10.1016/j.foodchem.2014.09.050. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Chatterjee J. Efficient extraction strategies of tea (Camellia sinensis) biomolecules. J Food Sci Technol. 2014 doi: 10.1007/s13197-014-1487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang Y, Lu X, Qu Z. Comparative studies on the physicochemical and antioxidant properties of different tea extracts. J Food Sci Technol. 2012;49:356–361. doi: 10.1007/s13197-011-0291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani E, Bacchetti T, Padella L, Tiano L, Carloni P. Antioxidant activity of different white teas: comparison of hot and cold tea infusions. J Food Compos Anal. 2014;33:59–66. doi: 10.1016/j.jfca.2013.09.010. [DOI] [Google Scholar]
- Dias PM, Changarath J, Damodaran A, Joshi MK. Compositional variation among black tea across geographies and their potential influence on endothelial nitric oxide and antioxidant activity. J Agric Food Chem. 2014;62:6655–6668. doi: 10.1021/jf501611w. [DOI] [PubMed] [Google Scholar]
- El-Shahawi MS, Hamza A, Bahaffi Al-Sibaai AA, Abduljabbar TN. Analysis of some selected catechins and caffeine in green tea by high performance liquid chromatography. Food Chem. 2012;134:2268–2275. doi: 10.1016/j.foodchem.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Friedman M, Levin CE, Choi S-H, Kozukue E, Kozukue N. HPLC analysis of catechins, theaflavins, and alkaloids in commercial teas and green tea dietary supplements: comparison of water and 80 % ethanol/water extracts. J Food Sci. 2006;71:328–337. doi: 10.1111/j.1750-3841.2006.00090.x. [DOI] [Google Scholar]
- ISO 8587 (2006). Sensory analysis. Methodology—ranking
- Jayasekera S, Molan AL, Garg M, Moughan PJ. Variation in antioxidant potential and total polyphenol content of fresh and fully-fermented Sri Lankan tea. Food Chem. 2011;125:536–541. doi: 10.1016/j.foodchem.2010.09.045. [DOI] [Google Scholar]
- Kim ES, Liang YR, Jin J, Sun QF, Lu JL, Du YY, Lin C. Impact of heating on chemical compositions of green tea liquor. Food Chem. 2007;103:1263–1267. doi: 10.1016/j.foodchem.2006.10.031. [DOI] [Google Scholar]
- Laddi A, Prakash NR, Kumar A. Quality evaluation of black CTC teas based upon seasonal variations. Int J Food Sci Technol. 2014;49:493–500. doi: 10.1111/ijfs.12327. [DOI] [Google Scholar]
- Liao WC, Wu WH, Lai ST, Lin WJ, Liou H-C, Chan C-F. Kinetics investigation of antioxidant capacity and total phenols of low-temperature steeping Bi Luo Chun green tea. Int J Food Sci Technol. 2012;47:2009–2014. doi: 10.1111/j.1365-2621.2012.03064.x. [DOI] [Google Scholar]
- Lin S-D, Udompornmongkol P, Yang J-H, Chen S-Y, Mau J-L. Quality and antioxidant property of three types of tea infusions. J Food Process Preserv. 2014;38:1401–1408. doi: 10.1111/jfpp.12099. [DOI] [Google Scholar]
- Lin S-D, Yang J-H, Hsih Y-J, Liu EH, Mau J-L. Effect of different brewing methods on quality of green tea. J Food Process Preserv. 2014;38:1234–1243. doi: 10.1111/jfpp.12084. [DOI] [Google Scholar]
- N’Dri D, Calani L, Mazzeo T, Scazzina F, Rinaldi M, Del Rio D, Brighenti F. Effects of different maturity stages on antioxidant content of Ivorian Gnagnan (Solanum indicum L.) berries. Molecules. 2010;15:7125–7138. doi: 10.3390/molecules15107125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obanda M, Owuora PO, Mangòka R, Kavoic MM. Changes in thearubigin fractions and theaflavin levels due to variations in processing conditions and their influence on black tea liquor brightness and total colour. Food Chem. 2004;85:163–173. doi: 10.1016/S0308-8146(02)00183-8. [DOI] [Google Scholar]
- Pang J, Zhang Z, Zheng T, Yang Y-J, Li N, Bai Y, Peng Y, Zhang J, Li Q, Zhang B. Association of green tea consumption with risk of coronary heart disease in Chinese population. Int J Cardiol. 2015;179:275–278. doi: 10.1016/j.ijcard.2014.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-H, Bae J-H, Im S-S, Song D-K. Green tea and type 2 diabetes. Integr Med Res. 2014;3:4–10. doi: 10.1016/j.imr.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perva-Uzunalić A, Škerget M, Knez Ž, Weinreich B, Otto F, Grüner S. Extraction of active ingredients from green tea (Camellia sinensis): extraction efficiency of major catechins and caffeine. Food Chem. 2006;96:597–605. doi: 10.1016/j.foodchem.2005.03.015. [DOI] [Google Scholar]
- Rahim AA, Nofrizal S, Saad B. Rapid tea catechins and caffeine determination by HPLC using microwave-assisted extraction and silica monolithic column. Food Chem. 2014;147:262–268. doi: 10.1016/j.foodchem.2013.09.131. [DOI] [PubMed] [Google Scholar]
- Rusak G, Komes D, Likić S, Horžić D, Kovač M. Phenolic content and antioxidative capacity of green and white tea extracts depending on extraction conditions and the solvent used. Food Chem. 2008;110:852–858. doi: 10.1016/j.foodchem.2008.02.072. [DOI] [PubMed] [Google Scholar]
- Saklar S, Ertas E, Ozdemir IS, Karadeniz B. Effects of different brewing conditions on catechin content and sensory acceptance in Turkish green tea infusions. J Food Sci Technol. 2015 doi: 10.1007/s13197-015-1746-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaniego-Sánchez C, Inurreta-Salinas Y, Quesada-Granados JJ, Blanca-Herrera R, Villalón-Mir M, López-García de la Serrana H, López Martínez MC. The influence of domestic culinary processes on the trolox equivalent antioxidant capacity of green tea infusions. J Food Compos Anal. 2011;24:79–86. doi: 10.1016/j.jfca.2010.06.003. [DOI] [Google Scholar]
- Scharbert S, Hofmann T. Molecular definition of black tea taste by means of quantitative studies, taste reconstitution, and omission experiments. J Agric Food Chem. 2005;53:5377–5384. doi: 10.1021/jf050294d. [DOI] [PubMed] [Google Scholar]
- Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Brit J Pharmacol. 2013;168:1059–1073. doi: 10.1111/bph.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti E, Bacchetti T, Tiano L, Carloni P, Greci L, Damiani E. Hot vs. cold water steeping of different teas: do they affect antioxidant activity? Food Chem. 2010;119:1597–1604. doi: 10.1016/j.foodchem.2009.09.049. [DOI] [Google Scholar]
- Wang H, Helliwell K. Epimerisation of catechins in green tea infusions. Food Chem. 2000;70:337–344. doi: 10.1016/S0308-8146(00)00099-6. [DOI] [Google Scholar]
- Wang H, Helliwell K, You X. Isocratic elution system for the determination of catechins, caffeine and gallic acid in green tea using HPLC. Food Chem. 2000;68:115–121. doi: 10.1016/S0308-8146(99)00179-X. [DOI] [Google Scholar]
- Yang Y, Liu RH. The phenolic profiles and antioxidant activity in different types of tea. Int J Food Sci Technol. 2013;48:163–171. doi: 10.1111/j.1365-2621.2012.03173.x. [DOI] [Google Scholar]
- Yang DJ, Lucy SH, Jau-Tien L. Effects of different steeping methods and storage on caffeine, catechins and gallic acid in bag tea infusions. J Chromatogr A. 2007;1156:312–320. doi: 10.1016/j.chroma.2006.11.088. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li Q, Xing H, Lu X, Zhao L, Qu K, Bi K. Evaluation of antioxidant activity of ten compounds in different tea samples by means of an on-line HPLC–DPPH assay. Food Res Int. 2013;53:847–856. doi: 10.1016/j.foodres.2013.03.026. [DOI] [Google Scholar]