Abstract
Impacts of ethanolic extract from coconut husk (EECH) at 0–0.4 % (w/w, on protein basis) on properties of films from tilapia skin gelatin and gelatin/Cloisite Na+ nanocomposite films were investigated. Young’s Modulus, tensile strength and elongation at break of both films decreased with addition of EECH (P < 0.05). The lowest water vapour permeability (WVP) was obtained for gelatin film containing 0.05 % EECH (w/w) (P < 0.05). Nevertheless, the nanocomposite film showed the lowest WVP when incorporated with 0.4 % EECH (w/w) (P < 0.05). Generally, L*- value (lightness) decreased and a*- value (redness) of films increased (P < 0.05) with increasing levels of EECH, regardless of nanoclay incorporation. Transparency of both films generally decreased as the level of EECH increased (P < 0.05). Intercalated or exfoliated structure of nanocomposite films was revealed by wide angle X-ray diffraction (WAXD) analysis. Based on scanning electron microscopic (SEM) analysis, the rougher surface was found when EECH was added. EECH had varying impact on thermal stability of films as revealed by thermogravimetric (TGA) and differential scanning calorimetric (DSC) analyses. Thus, the incorporation of EECH determined the properties of both gelatin film and nanocomposite film in which the improved water vapour barrier property could be obtained.
Keywords: Fish gelatin film, Bio-nanocomposite, Coconut husk, Phenolic compounds, Water vapour permeability, WAXD
Introduction
Packaging from biopolymers known as ‘bio- or eco- packaging’ can be used to replace foods’ plastic packaging materials, thereby lowering waste disposal (Tharanathan 2003). Biodegradable packaging is universally gaining the great interest for the protection and shelf-life extension of foods (Bao et al. 2009). In recent years, there has been growing public concern about sustainability practices, green chemistry and inherent safe design. Consequently, an urgent need is emerging for the efficient use of natural resources (Gomez-Guillen et al. 2009). Biodegradable films are normally made from renewable biopolymers such as proteins, lipids, polysaccharides (Tharanathan 2003). Proteins are thermoplastic heteropolymers containing both polar and non-polar amino acids, which are able to form numerous intermolecular linkages (Chinabhark et al. 2007). However, resistance of protein films to water vapor transmission is limited due to the inherent hydrophilicity of proteins (Gennadios et al. 1993). Amongst all proteins, gelatin has been attracted the attention for the development of edible films due to its abundance, biodegradability and its broad range of functional properties and applications (Karim and Bhat 2009). Gelatin films have poor water vapour barrier property; however, they have been found to be very effective UV light and oxygen barriers (Gennadios et al. 1993; Jongjareonrak et al. 2008). The hydrophilic nature of proteins induces interaction with water, causing swelling and apparent thickness alteration (Avena-Bustillos and Krochta 1993).
Nowadays, one of the most effective alternatives to amelioration of the barrier and mechanical properties of packaging materials, either synthetic or natural, is the formation of nanocomposites (Bae et al. 2009; Farahnaky et al. 2014). Montmorillonite nanoclays, such as sodium bentonite, a hydrophilic aluminum phyllosilicate with high water sorption and swelling capacity can be homogeneously dispersed in a polymeric matrix to form the new nanocomposite (Ray and Okamoto 2003). Its crystal lattice consists of 1 nm thin layers formed by an octahedral alumina sheet sandwiched between two tetrahedral silica sheets. It has a high surface area (aspect ratio of about 100), and is negatively charged (Ray and Okamoto 2003). The stacking of these layers leads to a Van der Waals gap or gallery, in which alkaline cations, such as Na+, Li+ or Ca2+, can neutralise the charge. The major problem in preparing these composites is to separate the initially agglomerated clay layers. Therefore, a necessary step, in which the clay layers are well dispersed in the polymer matrix, is implemented (Nagarajan et al. 2014a). The improved barrier properties could be obtained from well and organised dispersion of nanoclays in the gelatin matrix (Martucci and Ruseckaite 2010a). In general, the ‘tortuous path’ in nanocomposite films has been reported (Rhim 2007). Gelatin bio-nanocomposite films have been prepared (Bae et al. 2009; Farahnaky et al. 2014; Martucci and Ruseckaite 2010a; Nagarajan et al. 2014b).
Coconut husk, the fibrous external portion of the fruit of coconut palms, is a by-product of the copra extraction process and is generally considered as a waste (Vazquez-Torres et al. 1992). Vazquez-Torres et al. (1992) reported that polymer/antioxidant such as lignin can be successfully extracted from coconut husk. The use of coconut husk extract containing phenolic compounds in gelatin films or nanocomposite films might induce the formation of complex matrix, thereby improving water barrier property. Phenolic compounds from plant origin were reported to improve mechanical property of gelatin-based films (Bitencourt et al. 2014; Hoque et al. 2011; Kavoosi et al. 2013). The present study aimed to investigate the effect of ethanolic extract from coconut husk on the barrier and mechanical properties as well as thermal stability of tilapia skin gelatin films and nanocomposite films.
Materials and methods
Chemicals
Fish skin gelatin from tilapia (~240 bloom) was obtained from Lapi Gelatine (Empoli, Italy). MMT-nanoclay, Cloisite® Na+ was purchased from Southern clay products Inc. (Gonzlaes, TX, USA). Glycerol was procured from Merck (Darmstadt, Germany). Ethanol was purchased from RCI Labscan (Pathumwan, Thailand). All chemicals were of analytical grade.
Extraction of ethanolic extract from coconut husk
Collection and preparation of coconut husk
Coconut husk was obtained from a local market in Hat Yai, Songkhla, Thailand and transported to the Department of Food Technology, Prince of Songkla University, Hat Yai, Thailand. Husk sample was prepared as per the method of Vazquez-Torres et al. (1992) with slight modifications. Husk sample was dried at 60 °C in the cabinet rotary dryer for 16 h and then defibered. Husk sample was then subjected to grinding using a mill (IKA Labortechnik colloid mill, Selangor, Malaysia). The prepared sample was then sieved with the aid of sieve shaker (Model EVJ1, Endecotts Ltd., London, UK) with a sieve size of 6 mm (Woven wire sieves, Endecotts Ltd., London, UK). This coarse form was further blended using a blender (Panasonic, Model MX-898 N, Berkshire, UK) and finally sieved using a stainless steel sieve of 80 mesh. The coconut husk powder obtained was further dried in a hot air oven (Memmert, Schwabach, Germany) at 105 °C overnight. The obtained powder was placed in a polyethylene bag, sealed and kept at room temperature until use.
Preparation of the ethanolic extract
Coconut husk powder was subjected to extraction according to the method of Santoso et al. (2004) with a slight modification. Ten grams of husk powder were mixed with 250 ml of 80 % ethanol (w/v). The mixture was stirred at room temperature (28–30 °C) using a magnetic stirrer (IKA-Werke, Staufen, Germany) for 3 h. The mixture was then centrifuged at 5000g for 30 min at room temperature using a RC-5B plus centrifuge (Beckman, JE-AVANTI, Fullerton, CA, USA). The supernatant was filtered using a Whatman No. 1 filter paper (Whatman International, Ltd., Maidstone, England). The filtrate was then evaporated at 40 °C using an Eyela rotary evaporator (Tokyo Rikakikai, Co. Ltd., Tokyo, Japan). To remove the residual ethanol, the extract was purged with nitrogen gas. The extract was then dried using a Scanvac Model Coolsafe 55 freeze dryer (Coolsafe, Lynge, Denmark) to obtain the dry extract. Dried extract was powdered using a mortar and pestle and was kept in an amber bottle and stored in a desiccator until use. The obtained powder was referred to as ‘ethanolic extract from coconut husk, EECH’. EECH had phenolic content of 436.82 mg tannic acid equivalent/g as determined by Folin–Ciocalteu reagent (Slinkard and Singleton 1977).
Preparation of gelatin films and nanocomposite films
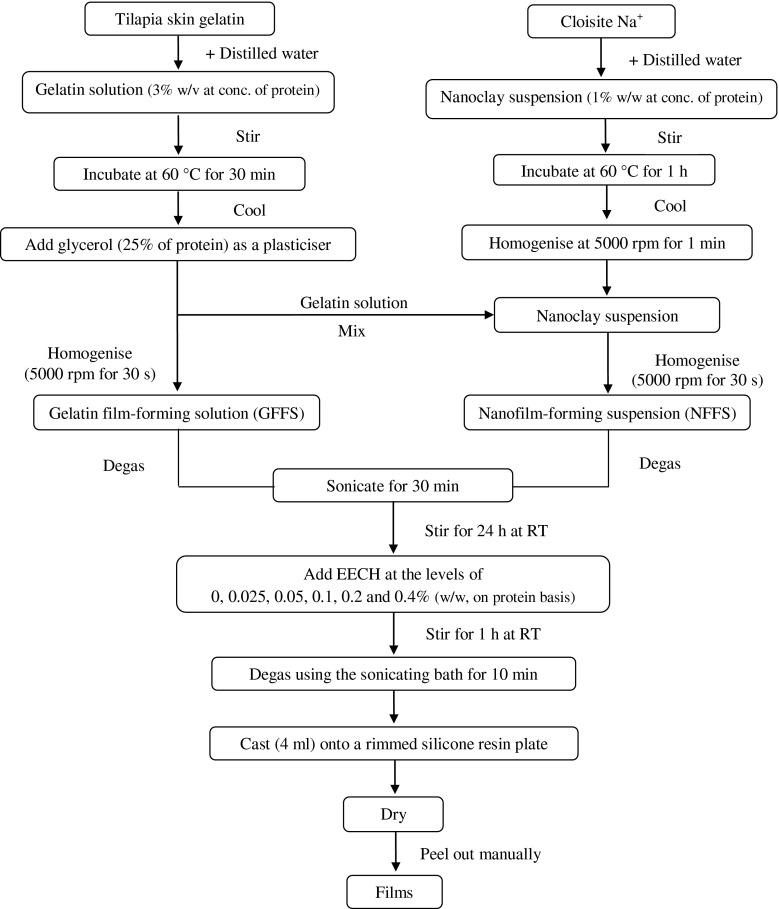
Gelatin films and nanocomposite films were prepared as per the methods of Bae et al. (2009) and Hoque et al. (2011) as illustrated in Fig. 1. Firstly, gelatin solution was prepared by mixing the gelatin powder with distilled water to obtain protein concentration of 3 % (w/v) as determined by the Kjeldhal method (AOAC 2000). Thereafter, glycerol (25 % of protein, w/w) was added into the gelatin solution as a plasticiser. The final volume was made up to 100 ml using the distilled water and referred to as ‘film-forming solution’.
Fig. 1.
Scheme for preparation of gelatin film-forming solutions and nanocomposite film-forming suspensions incorporated with EECH
To prepare nanocomposite film, nanoclay, Cloisite Na+ was mixed with distilled water to obtain a final concentration of 1 % (w/w, on protein basis). The mixture was stirred at 1000 rpm (IKA Labortechnik stirrer, Selangor, Malaysia) for 5 min at room temperature. Nanoclay suspension was then incubated at 60 °C for 1 h to delaminate the nanoclay in a temperature controlled water bath (W350; Memmert, Schwabach, Germany) with occasional stirring. Nanoclay suspension was cooled down to room temperature and homogenised for 1 min at 5000 rpm (IKA Labortechnik homogeniser, Selangor, Malaysia). Gradually, nanoclay suspension was dropped into the gelatin solution, prepared as mentioned earlier and the mixture was homogenised for 30 s at 5000 rpm. The mixture was degassed using a desiccator equipped with JEIO Model VE-11 electric aspirator (JEIO TECH, Seoul, Korea). The final volume was made up to 100 ml using the distilled water and referred to as ‘film-forming suspension’.
Both film-forming solution and suspension were sonicated for 30 min using the sonicating bath (Elmasonic S 30 H, Singen, Germany), followed by gentle stirring for 24 h at room temperature to obtain a homogenous solution and suspension. Prior to casting, EECH was added to both film-forming solution and suspension at the levels of 0, 0.025, 0.05, 0.1, 0.2 and 0.4 % (w/w, on protein basis) and the mixtures were gently stirred for 1 h at room temperature. The mixtures were degassed for 10 min using the sonicating bath and then cast (4 ± 0.01 ml) onto a rimmed silicone resin plate (5 × 5 cm2), air-blown for 12 h at 25 °C, followed by drying in an environmental chamber (Binder GmbH, Tuttlingen, Germany) at 25 ± 0.5 °C and 50 ± 5 % relative humidity (RH) for 24 h. Films obtained were manually peeled off. Gelatin films (GF) and nanocomposite films (NF) containing different levels of EECH were subjected to analyses.
Analyses
Prior to testing, samples were conditioned in an environmental chamber for 48 h at 50 ± 5 % relative humidity (RH) and 25 ± 0.5 °C. For WAXD, SEM, TGA and DSC studies, films were conditioned in a desiccator containing dried silica gel for 3 weeks at room temperature (28–30 °C) to obtain the most dehydrated films.
Thickness
The thickness of ten film samples of each condition was measured using a digital micrometer (Mitutoyo, Model ID-C112PM, Serial No. 00320, Mituyoto Corp., Kawasaki-shi, Japan). Ten random locations around each film sample were used for determination of thickness.
Mechanical properties
Young’s Modulus (YM), tensile strength (TS) and elongation at break (EAB) of film samples were determined as described by Iwata et al. (2000) using the Universal Testing Machine (Lloyd Instruments, Hampshire, UK). The test was performed in the controlled room at 25 °C and 50 ± 5 % RH. Ten film samples (2 × 5 cm2) with the initial grip length of 3 cm were used for testing. The film samples were clamped and deformed under tensile loading using a 100 N load cell with the cross head speed of 30 mm/min until the samples were broken. The initial slope of the stress–strain curve, the maximum load and final extension at break of the film samples were used to calculate YM, TS and EAB, respectively.
Water vapour permeability
Water vapour permeability (WVP) was measured using a modified ASTM (American Society for Testing and Materials 1989) method as described by Shiku et al. (2004). Film samples were sealed on an aluminium permeation cup containing dried silica gel (0 % RH) with silicone vacuum grease and rubber gasket. The cups were placed at 30 °C in a desiccator containing distilled water, followed by weighing at every 1 h intervals for up to 8 h. Five film samples were used for WVP testing. WVP of the film was calculated as follows:
where, w is the weight gain of the cup (g); l is the film thickness (m); A is the exposed area of film (m2); t is the time of gain (s); (P2-P1) is the vapour pressure difference across the film (Pa).
Colour
Colour of five film samples was determined using a CIE colourimeter (Hunter associates laboratory, Inc., Reston, VA, USA). Colour of the film was expressed as L*- (lightness or brightness), a*- (redness or greenness) and b*- (yellowness or blueness) values. Total difference in colour (∆E*) was calculated according to Gennadios et al. (1996).
where ∆L*, ∆a* and ∆b* are the difference between the colour parameter of corresponding film samples and that of white standard (L* = 92.81, a* = −1.24 and b* = 0.49).
Light transmission and transparency
Light transmission of the films in ultraviolet (UV) and visible range were measured at selected wavelengths between 200 and 800 nm, using a UV-Visible spectrophotometer (model UV-1800, Shimadzu, Kyoto, Japan) according to the method of Jongjareonrak et al. (2008). The transparency value of five film samples was calculated by following the equation of Shiku et al. (2004).
where T600 is the fractional transmittance at 600 nm and x is the film thickness (mm). The higher transparency value represents the lower transparency of films.
Characterisation of selected films
Gelatin films and nanocomposite films incorporated with EECH at the selected levels (GF-0.05 % and NF-0.4 %) were further characterised, in comparison with the corresponding films.
Wide angle x-ray diffraction (WAXD) analysis
WAXD analysis of the film samples was conducted in reflection mode with an incident wavelength (λ) at 0.154 nm of CuKα radiation (Martucci and Ruseckaite 2010b). Measurements were performed for 2θ from 5° to 10° at a scan rate of 1.0°/min. The layer spacing of the clay was calculated from Bragg’s law:
where λ is the wavelength of the radiation; d is the c-dimension distance or the interlayer spacing; and θ is the diffraction angle (Bae et al. 2009).
Scanning electron microscopic (SEM) analysis
Microstructure of the upper surface and cryo-fractured cross-section of the film samples was visualised using a scanning electron microscope (Quanta 400; FEI, Praha, Czech Republic) at an accelerating voltage of 15 kV, as described by Farahnaky et al. (2014). The gelatin film samples were cryo-fractured by immersion in liquid nitrogen. Prior to visualisation, the film samples were mounted on a brass stub and sputtered with gold in order to make the sample conductive, and photographs were taken at 5000× magnification for surface analysis. For cross-sectional analysis, cryo-fractured films were mounted around stubs perpendicularly using double sided adhesive tape, coated with gold and observed at the 3000× magnification.
Thermo-gravimetric analysis (TGA)
Dried film samples were scanned using a thermogravimetric analyser (TGA-7, Perkin Elmer, Norwalk, CT, USA) from 30 to 600 °C using a heating rate of 10 °C/min (Nuthong et al. 2009). Nitrogen was used as the purge gas at a flow rate of 20 ml/min.
Differential scanning calorimetry (DSC)
Thermal properties of film samples were determined using a differential scanning calorimeter (DSC-7, Perkin Elmer, Norwalk, CT, USA) as per the method of Hoque et al. (2011). Temperature calibration was performed using the Indium thermogram. Film samples (2–5 mg) were accurately weighed into aluminium pans, hermetically sealed, and scanned over the temperature range of −30 to 150 °C with a heating rate of 10 °C/min. Dry ice was used as the cooling medium and the system was equilibrated at −30 °C for 5 min prior to scanning. The empty aluminium pan was used as a reference.
Statistical analyses
All experiments were performed in triplicates (n = 3) and a completely randomised design (CRD) was used. Analysis of variance (ANOVA) was performed and the mean comparisons were done by Duncan’s multiple range tests (Steel and Torrie 1980). Data are presented as mean ± standard deviation and the probability value of P < 0.05 was considered as significant. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS 17.0 for windows, SPSS Inc., Chicago, IL, USA).
Results and discussion
Properties of gelatin films and nanocomposite films as affected by EECH addition
Thickness
Thickness of gelatin films and nanocomposite films incorporated with EECH at different levels is shown in Table 1. In general, thickness of the films was not significantly affected by the incorporation of EECH at all levels used (P > 0.05). Nanocomposite films generally showed similar thickness to gelatin films (P > 0.05), regardless of EECH incorporation. Generally, film thickness is influenced by the solid content of the film forming solution (Han and Krochta 1999). The results suggested that the thickness of gelatin films and nanocomposite films was not markedly affected by the incorporation of nanoclay and EECH.
Table 1.
Young’s Modulus (YM), tensile strength (TS), elongation at break (EAB), water vapour permeability (WVP) and thickness of gelatin films and nanocomposite films incorporated with EECH at different levels
| Film Samples | YM (MPa) | TS (MPa) | EAB (%) | WVP (X10−11 g.m.m−2.s−1.Pa−1) | Thickness (mm) |
|---|---|---|---|---|---|
| GF-0 % | 1048.03 ± 31.40bB | 41.93 ± 0.49bB | 7.90 ± 0.03bA | 2.76 ± 0.05aA | 0.048 ± 0.0053bA |
| GF-0.025 % | 1046.27 ± 5.89bB | 40.00 ± 0.66cC | 7.24 ± 0.13cdC | 2.09 ± 0.07gC | 0.049 ± 0.0004abA |
| GF-0.05 % | 1129.63 ± 25.58aA | 43.65 ± 0.68aA | 7.63 ± 0.01bcB | 1.79 ± 0.02iD | 0.050 ± 0.0009abA |
| GF-0.1 % | 1030.87 ± 34.09bB | 39.33 ± 0.80cC | 7.08 ± 0.11dCD | 2.10 ± 0.05gC | 0.049 ± 0.0003abA |
| GF-0.2 % | 977.69 ± 15.81cC | 35.61 ± 1.14ghD | 6.94 ± 0.30dD | 2.17 ± 0.09fBC | 0.050 ± 0.0001abA |
| GF-0.4 % | 923.98 ± 19.96dD | 34.97 ± 0.59hD | 6.16 ± 0.01eE | 2.25 ± 0.01eB | 0.049 ± 0.0002abA |
| NF-0 % | 1144.85 ± 1.75aA | 43.99 ± 0.48aA | 9.20 ± 0.12aA | 2.19 ± 0.02efD | 0.049 ± 0.0023abC |
| NF-0.025 % | 989.67 ± 6.19cC | 36.06 ± 0.02fghD | 7.57 ± 0.43bcBC | 2.50 ± 0.03cdB | 0.052 ± 0.0019aA |
| NF-0.05 % | 972.16 ± 2.64cC | 36.45 ± 0.38fgD | 7.61 ± 0.30bcBC | 2.56 ± 0.02bcA | 0.051 ± 0.0010aABC |
| NF-0.1 % | 1037.73 ± 12.73bB | 37.90 ± 0.99deBC | 7.95 ± 0.19bB | 2.59 ± 0.03bA | 0.050 ± 0.0013abBC |
| NF-0.2 % | 981.10 ± 17.68cC | 37.18 ± 0.82efCD | 7.89 ± 0.07bB | 2.44 ± 0.05dC | 0.051 ± 0.0014aABC |
| NF-0.4 % | 1044.60 ± 15.70bB | 38.82 ± 0.61cdB | 7.19 ± 0.50cdC | 1.94 ± 0.04hE | 0.052 ± 0.0001aAB |
Mean ± SD (n = 3).
Different lowercase letters in the same column indicate significant differences between the different groups (P < 0.05)
Different uppercase letters in the same column indicate significant differences in the same group (P < 0.05)
Mechanical properties
Mechanical properties, including YM, TS and EAB of gelatin films and nanocomposite films incorporated with EECH at different levels are shown in Table 1. Mechanical properties of films varied with the levels of EECH incorporated (P < 0.05). YM and TS of gelatin films increased when EECH at a level of 0.05 % (w/w, on protein basis) was added (P < 0.05). Further increasing levels of EECH decreased both YM and TS of resulting gelatin films. Nevertheless, protein precipitation obviously occurred in gelatin film-forming solutions and nanocomposite film-forming suspensions when the incorporation level of EECH was higher than 0.4 % (w/w). Hydroxyl group of phenolic compounds in EECH possibly acted as hydrogen donor and hydrogen bonds could be formed between phenolic functional groups and gelatin molecules at an appropriate level of EECH. Film formation generally takes place by the development of a three dimensional network of protein molecules by ionic, hydrophobic, hydrogen and covalent (non-disulfide) bonds (Hoque et al. 2011). Hoque et al. (2011) reported that integrity and chain length of gelatin molecules directly contributed to the formation of film network. The decrease in YM and TS of gelatin film incorporated with EECH at high amount was probably caused by aggregation of phenolics and gelatin molecules, in which the ordered network of film was not formed. This resulted in the lessened integrity of film structure (Jongjareonrak et al. 2008). Haslam (1998) stated that polyphenols have the ability to form complexes with proteins. Kavoosi et al. (2013) reported that addition of carvacrol resulted in the lowered interaction between gelatin monomers, and might hinder polymer chain-to-chain interactions. As a consequence, the decrease in mechanical property could be obtained (Gomez-Estaca et al. 2014).
When comparing YM and TS between the control gelatin film and nanocomposite film (without EECH addition), it was found that the latter showed the higher values than the former (P < 0.05). The increase in YM and TS of gelatin nanocomposite film might be owing to the uniform dispersion of MMT nanoclay in gelatin matrix and a strong interaction between carbonyl group of gelatin and hydroxyl group of MMT (Martucci and Ruseckaite 2010a). The enhancement of YM and TS of nanocomposite film was directly attributed to the reinforcement provided by the high aspect ratio and the high surface area of silicate layers, to the good dispersion of clay layers in the gelatin matrix (Gutierrez et al. 2012). It was noted that YM and TS of gelatin nanocomposite films decreased with the addition of EECH. The incorporation of foreign components at an excessive amount is more likely associated with the development of heterogeneous film structure with the presence of discontinuous areas (Hoque et al. 2011; Li et al. 2014). Thus, EECH more likely lowered the mechanical properties of gelatin film containing nanoclay.
EAB decreased with increasing levels of EECH (P < 0.05). Control films (GF-0 % and NF-0 %) showed higher EAB (P < 0.05) than those added with EECH. In general, higher EAB was obtained for nanocomposite films (P < 0.05) than gelatin films. Similar result was observed by Li et al. (2014), who reported that the control gelatin film had the higher EAB than the films incorporated with natural antioxidants. Gelatin–phenolics, gelatin–clay or gelatin-phenolics–clay interactions at various degrees more likely led to the formation of different polymer matrices. These complex systems with reduced molecular mobility might have lower elasticity as indicated by the decreased EAB. Nunez-Flores et al. (2012) also reported that the number and size of the lignosulphonate domains led to the greater steric hindrance in gelatin film matrix, which considerably restricted the biopolymer molecular motion. Lignosulphonate was shown to form supra-molecular complexes by inter- and intra-molecular hydrogen bonding of its polar groups. Moreover, the triple-helix content in films might be decreased due to the strong interaction between phenolics and gelatin (Bao et al. 2009). Polyphenolic compounds could form hydrogen and covalent (non-disulphide) bonds with amino and hydroxyl groups of polypeptide in gelatin, which would weaken the protein-protein interactions in film network (Li et al. 2014). Thus, the incorporation of EECH and nanoclay directly affected the mechanical properties of gelatin films.
Water vapour permeability (WVP)
WVP of gelatin films and nanocomposite films incorporated with EECH at different levels is shown in Table 1. Control gelatin film (GF-0 %) showed the highest WVP, compared with others (P < 0.05). Gelatin is hydrophilic in nature, due to its polar amino acids and large number of hydroxyl groups (−OH). As a consequence, gelatin film has the lower moisture barrier property. Gelatin film incorporated with EECH at the level of 0.05 % (w/w) had the lowest WVP (P < 0.05). The interaction between gelatin and phenolic compounds in EECH could lower the available or free charged or polar residues of gelatins and this might result in the decreased water adsorptivity and thus decreasing the WVP of films (Bitencourt et al. 2014). Additionally, incorporation of EECH more likely increased the compactness of films, thereby lowering the adsorptivity as well as diffusivity of water vapour through the film as indicated by lower WVP. Interactions of polymers also reduced the free volume in the film matrix, limiting the diffusion of small molecules through the polymer film. The phenolic compounds were able to form hydrogen and covalent bonds (non-disulphide) with reactive groups of polypeptide in gelatin (Li et al. 2014). These bonds limited the availability of hydrogen groups to bind with water, thereby decreasing the affinity of gelatin films with water. Wu et al. (2013) reported that polyphenolic compounds could fit into the gelatin matrix and establish crosslinks through hydrogen bonds or through hydrophobic interactions with the reactive groups of the gelatin. Consequently, the network structure of films became denser and less permeable (Jongjareonrak et al. 2008). The degree of crosslinking also affects the barrier characteristics of gelatin films (Hoque et al. 2011). However, WVP of gelatin films seemed to increase when EECH was incorporated at the levels higher than 0.05 % (w/w). The highest WVP was observed when the highest level of EECH (0.4 %, w/w) was incorporated into the gelatin films. The excessive interaction between phenolic compounds and gelatins could bring about the coagulation. As a result, non-uniform film matrix was developed. This might be associated with the increasing number of micro-pores or voids in film networks. Thus, water migration through the films was increased.
For gelatin nanocomposite films, those without and with EECH addition exhibited lower WVP than did the control gelatin film (without nanoclay and EECH addition) (P < 0.05). The lowest WVP was observed when EECH at 0.4 % (w/w) was incorporated in the gelatin nanocomposite film (P < 0.05). The improved barrier properties of the gelatin/nanocomposite films (NF-0 %) could be attributed to the hindrance caused by nanoclay (Ray and Okamoto 2003; Rhim and Ng 2007). The formation of network induced by the hydrogen bonds between the gelatin chains and phenolics and the exfoliation/intercalation of gelatin molecules into the silicate galleries of nanoclays might lead to the improved water vapour barrier property (Abdollahi et al. 2012). OH groups may form hydrogen linkages between phenolic compounds and gelatin chains or Cloisite Na+ (Gutierrez et al. 2012). Furthermore, the incorporation of Cloisite Na+ to the gelatin matrix provides a ‘tortuous pathway’ for water molecules to pass through (Martucci and Ruseckaite 2010a). When EECH was added into nanocomposite films, higher WVP was found in comparison with gelatin films. Phenolic compounds might enhance the formation of coagulated gelatins in the matrix or interfere with the interaction between gelatin and nanoclays. This resulted in the discontinuous network with the poorer water vapour barrier property. Thus, gelatin films had varying WVP, depending on the concentration of phenolic compounds as well as the incorporation of nanoclay.
Colour
Colour of gelatin films and nanocomposite films incorporated with EECH at different levels is shown in Table 2. The colour of the packaging is an important factor in terms of general appearance and consumer acceptance (Rawdkuen et al. 2012). Lightness (L*- value) of both films generally decreased with increasing levels of EECH (P < 0.05). However, redness (a*- value) and yellowness (b*- value) of films increased (P < 0.05). This was in accordance with the increases in ∆E* value. The lowest L* and the highest a* and b*- values were obtained for both films incorporated with the highest level (0.4 %, w/w) of EECH (P < 0.05). Generally, ∆E* was higher in nanocomposite films than gelatin films, especially at high level of EECH. The phenolic compounds in EECH might interact with matrix or nanoclays, in the ways which yielded the higher redness or yellowness. Similar results were observed for gelatin films incorporated with natural spices (Hoque et al. 2011) and ethanolic extract of curcuma (Bitencourt et al. 2014). Films from cuttlefish (Sepia pharaonis) skin gelatin incorporated with cinnamon, clove and star anise extracts and gelatin-based films added with curcuma ethanol extract showed lower L* and higher b* values than the control gelatin film (without added herbal/curcuma extracts). Thus, colour of films was affected by the incorporation of EECH as well as nanoclay.
Table 2.
Colour of gelatin films and nanocomposite films incorporated with EECH at different levels
| Film samples | L* | a* | b* | ∆E* |
|---|---|---|---|---|
| GF-0 % | 90.11 ± 0.01eB | −1.25 ± 0.02ghD | 1.92 ± 0.01eB | 3.05 ± 0.06gC |
| GF-0.025 % | 90.14 ± 0.01dA | −1.24 ± 0.01fgD | 1.94 ± 0.02eB | 3.04 ± 0.01ghCD |
| GF-0.05 % | 90.05 ± 0.01gC | −1.22 ± 0.01efgD | 1.75 ± 0.01fC | 3.03 ± 0.01hDE |
| GF-0.1 % | 90.03 ± 0.01hD | −1.11 ± 0.01cB | 1.93 ± 0.02eB | 3.14 ± 0.02 dB |
| GF-0.2 % | 90.15 ± 0.01cA | −1.19 ± 0.02dC | 1.92 ± 0.02eB | 3.02 ± 0.01hiE |
| GF-0.4 % | 89.92 ± 0.01jE | −1.02 ± 0.02bA | 2.23 ± 0.01bA | 3.38 ± 0.01bA |
| NF-0 % | 90.15 ± 0.01cC | −1.25 ± 0.06ghDE | 2.04 ± 0.02cC | 3.08 ± 0.01fD |
| NF-0.025 % | 90.18 ± 0.01bB | −1.27 ± 0.01hE | 1.93 ± 0.03eE | 3.00 ± 0.01iE |
| NF-0.05 % | 90.09 ± 0.00fD | −1.21 ± 0.01defCD | 1.99 ± 0.03dD | 3.11 ± 0.01eC |
| NF-0.1 % | 90.25 ± 0.01aA | −1.20 ± 0.02deC | 1.76 ± 0.02fF | 2.86 ± 0.01jF |
| NF-0.2 % | 89.97 ± 0.01iE | −1.12 ± 0.01cB | 2.22 ± 0.01bB | 3.33 ± 0.01cB |
| NF-0.4 % | 89.25 ± 0.01kF | −0.66 ± 0.02aA | 3.08 ± 0.01aA | 4.44 ± 0.01aA |
Mean ± SD (n = 3)
Different lowercase letters in the same column indicate significant differences between the different groups (P < 0.05)
Different uppercase letters in the same column indicate significant differences in the same group (P < 0.05)
Light transmission and transparency
Transmission of UV and visible light at selected wavelengths in the range of 200–800 nm of gelatin films and nanocomposite films incorporated with EECH at various levels is presented in Table 3. Decreases in light transmission of both films at all wavelengths were observed as the levels of EECH increased. However, the degree of decrease varied with the levels of EECH for nanocomposite films. The transmission of UV light was low at 200 and 280 nm for both gelatin film and nanocomposite film and NF-0.4 % had the lowest transmission. It was found that film added with 0.4 % EECH (w/w) also showed the lowest transmission in visible range. This might be due to the light scattering effect of gelatin-phenolic complexes (Papadopoulou and Frazier 2004). The result suggested that film effectively prevented the UV light. High UV light barrier ability was reported for gelatin films (Gomez-Guillen et al. 2009; Hoque et al. 2011; Jongjareonrak et al. 2008). Hamaguchi et al. (2007) reported that protein-based films exhibited the good UV barrier properties, owing to their high content of aromatic amino acids that absorb UV light. In general, light transmission in visible range (350–800 nm) for all films was in the range of 73.12–89.78 %. In visible range, gelatin film incorporated with EECH showed higher light transmission, as compared with nanocomposite film containing EECH. The result suggested that phenolic compounds in EECH might form the complex with gelatin or nanoclays in the film network. As a result, light could not pass through the film with ease. Furthermore, nanoclays might result in the light reflection of films, leading to the lower transmission.
Table 3.
Light transmittance and transparency values of gelatin films and nanocomposite films incorporated with EECH at different levels
| Film samples | Transmittance (%) | Transparency values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 | 280 | 350 | 400 | 500 | 600 | 700 | 800 | ||
| GF-0 % | 0.02 | 41.10 | 83.27 | 86.57 | 88.13 | 88.79 | 89.27 | 89.78 | 1.02 ± 0.0005kF |
| GF-0.025 % | 0.02 | 41.64 | 81.18 | 84.79 | 86.74 | 87.61 | 88.29 | 88.95 | 1.15 ± 0.0034jE |
| GF-0.05 % | 0.02 | 43.91 | 81.84 | 85.15 | 86.98 | 87.88 | 88.58 | 89.25 | 1.21 ± 0.0016iD |
| GF-0.1 % | 0.03 | 43.15 | 80.56 | 84.21 | 86.15 | 87.06 | 87.73 | 88.39 | 1.27 ± 0.0012hC |
| GF-0.2 % | 0.02 | 40.16 | 79.36 | 83.00 | 85.14 | 86.26 | 87.09 | 87.84 | 1.28 ± 0.0015gB |
| GF-0.4 % | 0.01 | 32.91 | 77.13 | 81.47 | 83.89 | 85.13 | 86.11 | 86.98 | 1.42 ± 0.0005eA |
| NF-0 % | 0.02 | 37.52 | 77.53 | 81.78 | 84.68 | 86.31 | 87.47 | 88.44 | 1.28 ± 0.0026gF |
| NF-0.025 % | 0.02 | 34.45 | 76.64 | 80.86 | 83.84 | 85.48 | 86.72 | 87.76 | 1.31 ± 0.0024fE |
| NF-0.05 % | 0.02 | 35.99 | 73.65 | 77.99 | 81.15 | 83.02 | 84.43 | 85.65 | 1.53 ± 0.0017dD |
| NF-0.1 % | 0.01 | 34.91 | 73.93 | 78.59 | 81.95 | 83.88 | 85.35 | 86.55 | 1.56 ± 0.0010cC |
| NF-0.2 % | 0.01 | 37.61 | 75.57 | 79.61 | 82.46 | 84.12 | 85.36 | 86.43 | 1.56 ± 0.0016bB |
| NF-0.4 % | 0.01 | 24.96 | 73.12 | 78.44 | 81.98 | 83.99 | 85.45 | 86.61 | 1.57 ± 0.0006aA |
Mean ± SD (n = 3)
Different lowercase letters in the same column indicate significant differences between the different groups (P < 0.05)
Different uppercase letters in the same column indicate significant differences in the same group (P < 0.05)
Transparency value of both gelatin films and nanocomposite films increased as the level of EECH increased (P < 0.05). Both films added with EECH at a level of 0.4 % (w/w) showed the highest transparency value (P < 0.05), regardless of nanoclay incorporation. Higher transparency value indicated that the films had lower transparency. Transparency of protein-based films is generally affected by additives, processing conditions, thickness as well as compatibility between polymer and nanoclay (Farahnaky et al. 2014; Hoque et al. 2011; Martucci and Ruseckaite 2010b; Nagarajan et al. 2014a, 2014b; Rhim 2007). The aggregation of gelatin molecules due to the incorporation of EECH, especially at higher level might result in large agglomeration (Papadopoulou and Frazier 2004). Consequently, the films possessed more internal light scattering property and became turbid (Martucci and Ruseckaite 2008). In general, gelatin films containing EECH were more transparent (lower transparency value) than those incorporated with both EECH and nanoclay (P < 0.05). Additionally, phenolic compounds themselves also contributed to the opaqueness of films. Therefore, the incorporation of EECH and nanoclay had an impact on the appearance and light barrier properties of gelatin films.
Characteristics of selected gelatin film and nanocomposite film added with EECH
Gelatin film and nanocomposite film incorporated with EECH (GF-0.05 % and NF-0.4 %) were further subjected to characterisation, in comparison with the control gelatin film (GF-0 %) and nanocomposite film (NF-0 %) (without EECH incorporation).
Wide angle x-ray diffraction (WAXD) analysis
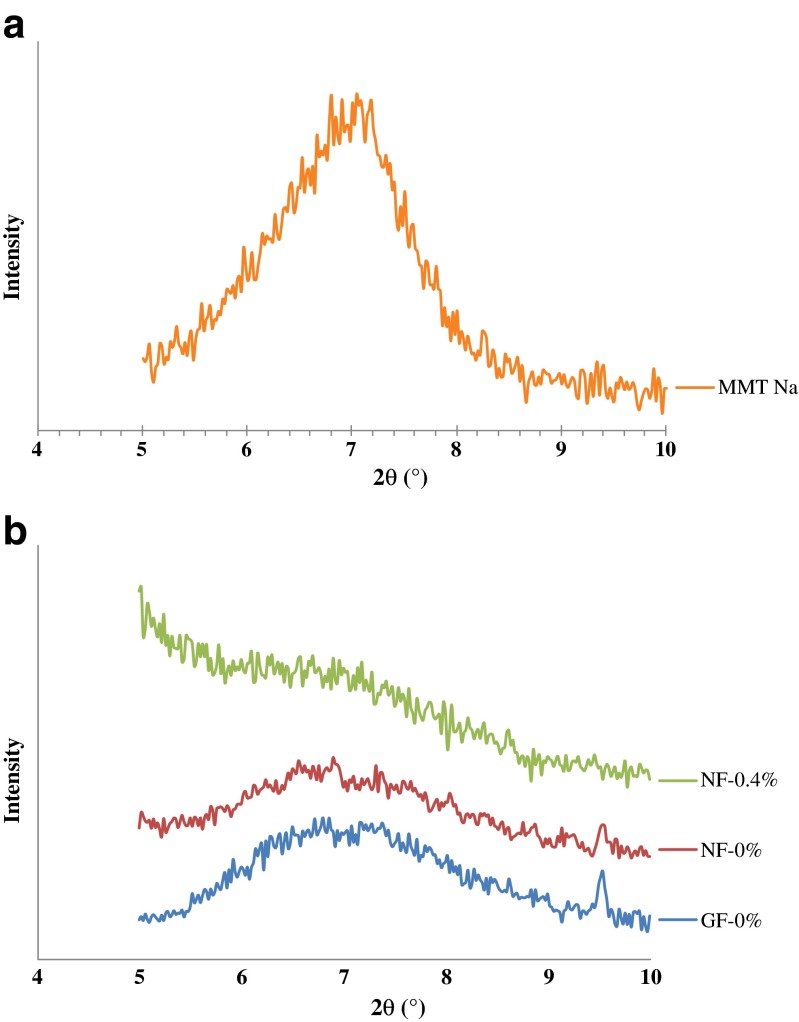
WAXD analysis was performed in order to determine the dispersion of Cloisite Na+ in the films from tilapia skin gelatin incorporated with and without EECH. WAXD patterns of Cloisite Na+ and the selected films are illustrated in Fig. 2a and b, respectively. Intercalated or exfoliated structures of different nanocomposite films were normally revealed by the d-spacing due to the interlayer spacing of the nanoclay gallery in gelatin matrix (Martucci et al. 2007). Characteristic diffraction peak of Cloisite Na+ (Fig. 2a) was found at 2θ of 7.04° (d-spacing = 1.25 nm, based on Bragg’s equation; nλ = 2d sinθ). This result was consistent with previous report and was also quite similar to the suggested value given by manufacturer (Gutierrez et al. 2012; Koh et al. 2010; Pradhan et al. 2012). WAXD patterns of films were different (Fig. 2b), compared to that of hydrophilic nanoclay, Cloisite Na+. The characteristic halo peak of amorphous proteins was obtained for control gelatin film (GF-0 %) in the 2θ range of 6.2 to 9.5°. Similar WAXD pattern of gelatin-based film has been reported (Grevellec et al. 2001; Martucci and Ruseckaite 2010a). For nanocomposite films incorporated with or without EECH, the absence of characteristic diffraction peak (2θ = 7.04°) of Cloisite Na+ nanoclay was noticed in their WAXD patterns. This suggested the intercalated/exfoliated structure of obtained nanocomposite films (Abdollahi et al. 2012; Gutierrez et al. 2012). However, WAXD pattern of NF-0.4 % was different from that of NF-0 % (without EECH addition). This suggested the varying degrees of intercalation or exfoliation of nanoclays in gelatin matrix, as influenced by EECH addition (Gutierrez et al. 2012). In particular, the WAXD pattern of NF-0.4 % exhibited broader halo peak of amorphous gelatin. This might be due to the non-homogeneous aggregation of gelatin molecules at highest incorporation level (0.4 %, w/w) of EECH. WAXD analysis is a classical method for determining the gallery height (d-spacing distance) in clay particles. During intercalation or exfoliaton, the insertion of polymer into the organoclay galleries forces the platelets apart and increases the d-spacing, resulting in a shift of the diffraction peak to lower angles or even disappeared (Abdollahi et al. 2012; Xu et al. 2006). Therefore, phenolic compounds in EECH more likely affected intercalated or exfoliated structure as well as the organisation of amorphous gelatin in the film matrix. In general, the intercalated or exfoliated structures of gelatin nanocomposite films were dependent on the addition of nanoclay and EECH.
Fig. 2.
WAXD patterns of Cloisite Na+ (a) and gelatin films and nanocomposite films incorporated with EECH at different levels (b). MMT Na - Cloisite Na+; GF-0 % and NF-0 % - control gelatin film and nanocomposite film, respectively; NF-0.4 % - nanocomposite film incorporated with 0.4 % EECH (w/w)
Microstructure
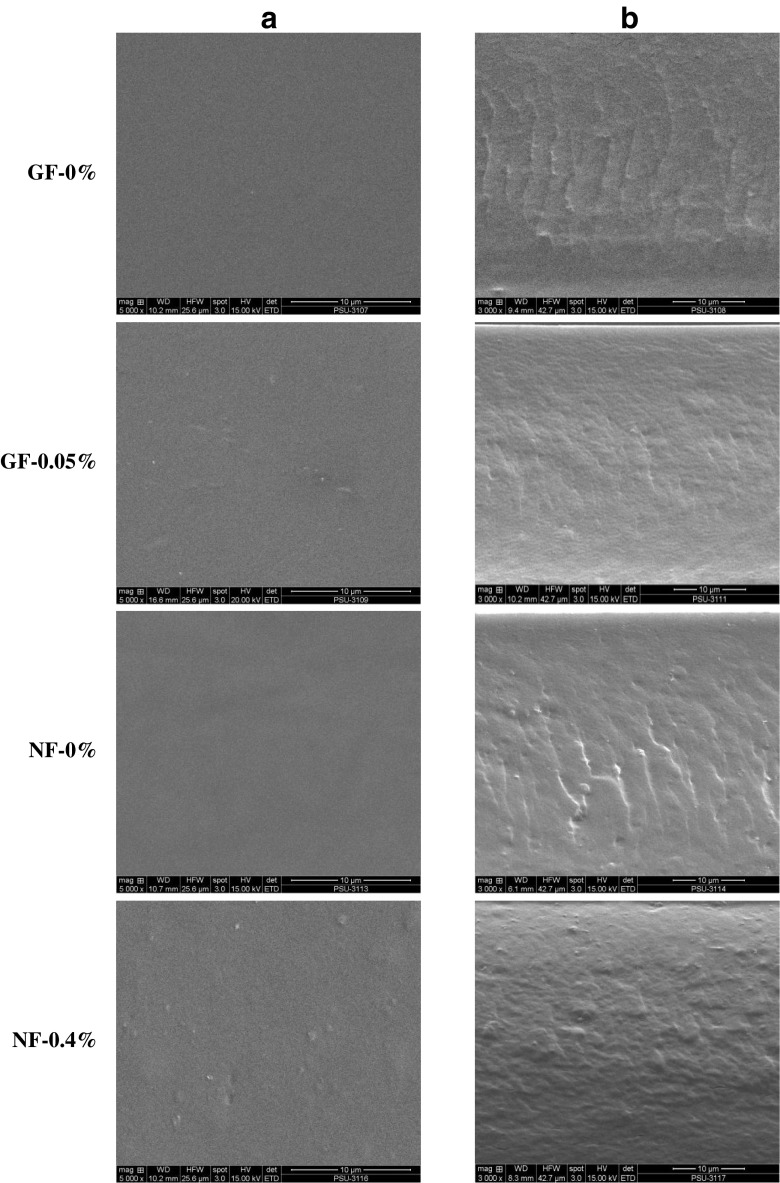
SEM micrographs of the surface (5000×) and cryo-fractured cross-section (3000×) of selected gelatin films and nanocomposite films incorporated with or without EECH are shown in Fig. 3. All films showed the smooth surface and free of crack or void. Homogeneity and smoothness of film surface were varied upon the inclusion of EECH and nanoclays. The control gelatin film (GF-0 %) and Cloisite Na+ incorporated film (NF-0%) had no differences in film surface morphology. Smooth, homogenous and compact film surface of both films indicated an ordered film matrix. The continuous and strong film network with a great number of junction zones was developed and the thorough dispersion of hydrophilic nanoclay into the hydrophilic polymer was obtained (Bao et al. 2009; Theng 1979). However, the incorporation of EECH resulted in slightly coarser/non-homogenous film surface. Gelatin film incorporated with both EECH and nanoclay showed larger aggregates or agglomerates on surface (NF-0.4 % films). The highest EECH level (0.4 %, w/w on protein basis) plausibly induced the formation of coagulation, as evidenced by the increased rougher surface.
Fig. 3.
SEM micrographs of surface (a) and cryo-fractured cross-section (b) of gelatin films and nanocomposite films incorporated with EECH at different levels. Magnification: 5000× for surface and 3000× for cross-section. GF-0 % and NF-0 % - control gelatin film and nanocomposite film, respectively; GF-0.05 % and NF-0.4 % - gelatin film and nanocomposite film incorporated with EECH at the levels of 0.05 and 0.4 % (w/w), respectively
For cross-section, gelatin film incorporated with EECH at 0.05 % (w/w, on protein basis) level showed a more compact and smoother structure, compared to the control gelatin film (without EECH and nanoclay). Similar result was reported for pig skin gelatin films incorporated with curcuma ethanol extract (Bitencourt et al. 2014). The compact structure regulated by interaction between phenolic compounds of EECH and gelatin was also responsible for the improved mechanical and water vapour barrier properties (Table 1). NF-0 % film showed some roughness in the film network. This was plausibly due to the presence of Cloisite Na+ in the film matrix. Films incorporated with EECH at the level of 0.4 % (w/w, on protein basis) along with the nanoclay had the compactness with some protrusions. This result was concomitant with lower mechanical but improved water vapour barrier properties (Table 1). Phenolic compounds in EECH more likely interacted with protein chains and caused protein aggregation. Therefore, the microstructures of gelatin films were governed by the incorporation of EECH and nanoclay.
TGA thermograms
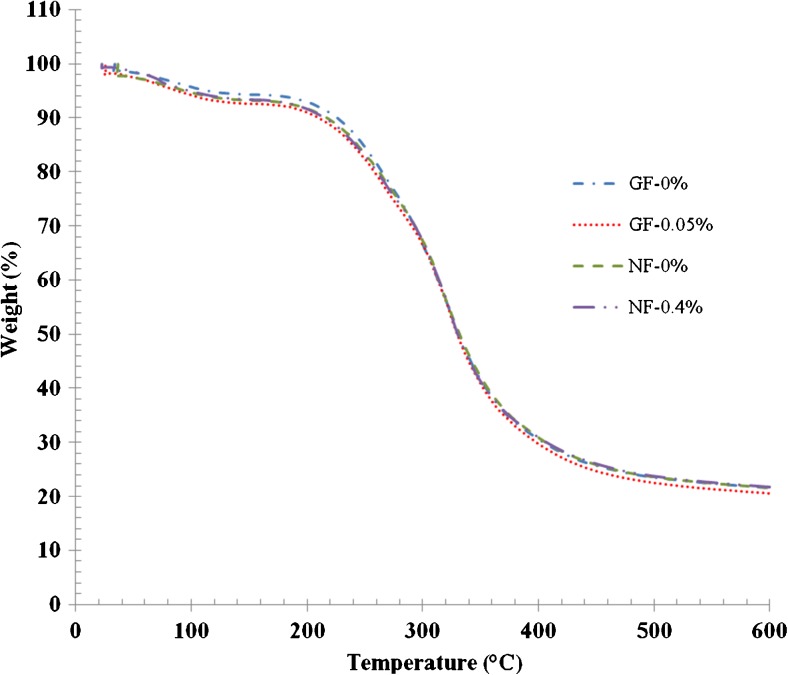
TGA curves of selected gelatin films and nanocomposite films incorporated with or without EECH are illustrated in Fig. 4. Degradation temperatures (Td) and weight loss (∆w) of corresponding films are presented in Table 4. In general, two stages of weight loss were observed for all films, irrespective of nanoclay or EECH incorporation. First stage of weight loss (∆w1 = 5.64 - 6.98 %) was observed for all films approximately at onset temperatures (Td1) of 52.38–71.43 °C, mostly associated with the continuous loss of free moisture absorbed in the films. At the first stage of weight loss, GF-0.05 % films showed higher weight loss than NF-0.4 % films at the same onset temperatures. Enhanced interaction between gelatin chains and Cloisite Na+ sheets plausibly consumed some hydrophilic groups and depressed the water uptake through capillary action at the interface (Li et al. 2003). Td1 of GF-0.05 % was higher than that of GF-0 %. For all films, the second stage of weight loss (∆w2 = 71.49–72.70 %) was observed approximately at a temperature (Td2) of 258.33–267.86 °C. Here, Td2 referred to as thermal degradation temperature of the films. This change was most likely due to the degradation or decomposition of protein components and the plasticiser, glycerol. GF-0.05 % had the higher Td2 than GF-0 %. GF-0 % also had higher weight loss (72.70 %) with the lower Td2 (258.33 °C). This result revealed that control gelatin film showed higher heat susceptibility than that added with EECH. In general, increasing thermal degradation temperatures (Td2) was related with decreasing weight loss (∆w2). Nanocomposite films added with EECH showed the slightly higher Td2 than the control nanocomposite film (NF-0 %). NF-0 % also showed higher Td1 and Td2 than GF-0 %. This was possibly due to the decrease in free volume of polymer matrix by stronger interaction between the gelatin molecules and nanoclay (Bae et al. 2009). Effective crosslinking of gelatin by phenolics in EECH could lead to the enhancement in thermal stability of films. Higher amount of bondings between phenolic compounds and gelatin molecules yielded stronger film network. As a result, films became stiffer and more compact, thereby improving thermal stability (Hoque et al. 2011; Wu et al. 2014). The increased thermal stability of nanocomposite films might be due to the thermal resistance of Cloisite Na+ and the nano-dispersion of montmorillonite sheets in the polymer matrix (Zheng et al. 2002). Martucci and Ruseckaite (2010a) reported the increased thermal stabilisation of gelatin nanocomposite films. Additionally, all films had slight difference in residual mass (representing char content) at 600 °C in the range of 21.08–21.78 %. The results suggested that the incorporation of nanoclay and EECH into the films based on tilapia skin gelatin contributed to differences in thermal stability.
Fig. 4.
Thermogravimetric curves of gelatin films and nanocomposite films incorporated with EECH at different levels. Key: see Fig. 3 caption
Table 4.
Thermal degradation temperature (Td, °C), weight loss (∆w, %), residue (%) and glass transition temperature (Tg, °C) of gelatin films and nanocomposite films incorporated with EECH at different levels
| Film samples | ∆1 | ∆2 | Residue | Tg | ||
|---|---|---|---|---|---|---|
| Td1 | ∆w1 | Td2 | ∆w2 | |||
| GF-0 % | 52.38 | 5.64 | 258.33 | 72.70 | 21.66 | 47.14 |
| GF-0.05 % | 69.05 | 6.98 | 264.29 | 71.94 | 21.08 | 53.57 |
| NF-0 % | 71.43 | 6.76 | 266.66 | 71.49 | 21.75 | 54.99 |
| NF-0.4 % | 69.05 | 6.60 | 267.86 | 71.62 | 21.78 | 58.57 |
∆1 and ∆2 denote the first and second stage weight loss, respectively, of films during TGA heating scan
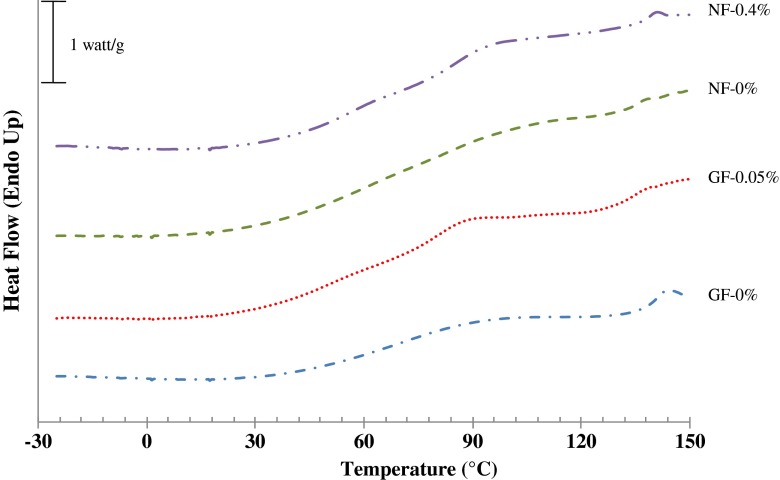
DSC thermograms
DSC thermograms of gelatin films and nanocomposite films incorporated with or without EECH are depicted in Fig. 5. Glass transition temperatures (Tg) of films are presented in Table 4. Thermograms of the first heating scan of all film samples exhibited step-like or glass transition at temperatures (Tg) ranging from 47.14 to 58.57 °C (Fig. 5). Tg is normally correlated to the segmental motion of polymer molecules in the amorphous phase (Slade and Levine 1991). The control gelatin film (GF-0 %) had the lowest Tg (47.14 °C). In contrast, the incorporation of Cloisite Na+ (NF-0 %) or EECH (GF-0.05 %) into the control gelatin film resulted in the higher Tg (54.99 and 53.57 °C, respectively). However, gelatin film incorporated with both nanoclay and EECH showed the highest Tg (58.57 °C). Incorporation of EECH along with the nanoclay decreased the molecular mobility of gelatin. For nanocomposite films, nano-dispersion of hydrophilic nanoclay in hydrophilic gelatin matrix might enhance the stronger interaction via hydrogen bonds, thus increasing the rigidity of gelatin molecules in the matrix of film (Martucci et al. 2007). Li et al. (2003) reported that Cloisite Na+ serves as physical crosslinking sites, which could enhance the stability of film network. Moreover, hydrogen bond or hydroxyl group interaction between the gelatin molecules and phenolic compounds in EECH was also responsible for increasing Tg of NF-0.4 % films. Oxford et al. (1989) stated that Tg is one of the important parameters which determines both the mechanical and barrier properties of corresponding films and controls the crystallisation kinetics of the gelatins. In general, the DSC results were correlated well with the mechanical and water vapour barrier properties of films (Table 1). EECH incorporation at higher level might induce cross-linking of gelatin matrix. This resulted in the stronger and more heat stable gelatin film network/matrix.
Fig. 5.
DSC thermograms of the first heating scan of gelatin films and nanocomposite films incorporated with EECH at different levels. Key: see Fig. 3 caption
For the second heating scan, no clear transition was generally observed for both gelatin films and nanocomposite films incorporated with or without EECH (data not shown). Absorbed water, acting as plasticiser, might be removed during the first heating scan. As a consequence, the interactions between gelatin chains and also between gelatin molecules and nanoclays along with the phenolic compounds of EECH could be enhanced by the formation of more rigid film network. Therefore, the solid-state molecular transition was limited and could not be noticed during the second heating scan in the temperature range tested.
Gelatin-phenolics-nanoclay interaction occurred by different possible interactions such as hydrogen, hydrophobic, ionic and covalent (non-disulphide) interactions as illustrated in Fig. 6. Polyphenolic compounds contain many hydrophobic groups, which can form hydrophobic interaction with hydrophobic region of gelatin molecule and hydroxyl groups of polyphenolic compounds and nanoclay were able to interact via hydrogen bonds (Bae et al. 2009; Hoque et al. 2011; Martucci and Ruseckaite 2010a; Rattaya et al. 2009). As a result, the compact and stronger network with the improved water vapour barrier property could be achieved.
Fig. 6.
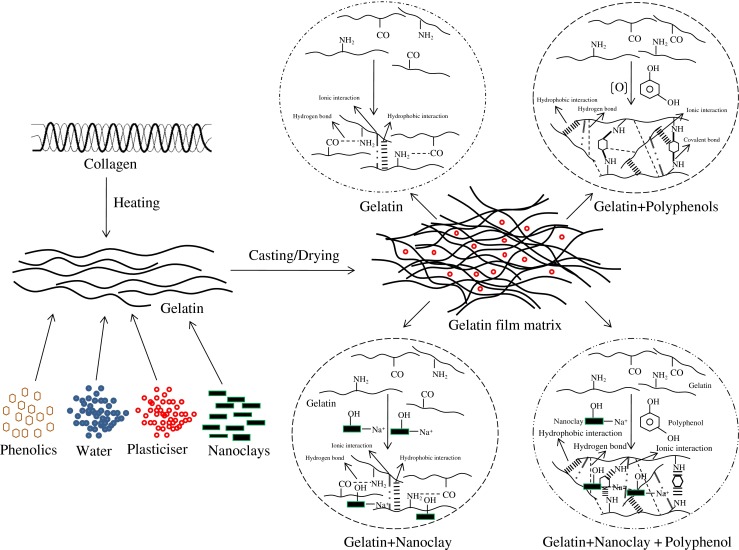
Proposed scheme of possible interaction between gelatin, phenolic compounds, and nanoclay in film matrix
Conclusion
Properties of gelatin films and nanocomposite films from tilapia skin gelatin were governed by EECH. Gelatin film incorporated with EECH at 0.05 % (w/w, on protein basis) showed the improved mechanical and water vapour barrier properties, possibly due to the augmented interactions between functional group of gelatin and phenolics. Thus, the appropriate level of EECH could effectively improve the film properties, particularly in conjunction with nanoclay incorporation.
Acknowledgments
The authors would like to express their sincere thanks to Prince of Songkla University and the TRF Distinguished Research Professor Grant for financial support. The ICAR-International Fellowship from Indian Council of Agricultural Research (ICAR), New Delhi, India, for Muralidharan Nagarajan is gratefully acknowledged.
References
- Abdollahi M, Rezaei M, Farzi G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J Food Eng. 2012;111:343–350. doi: 10.1016/j.jfoodeng.2012.02.012. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 17. Gaithersberg: Association of Official Analytical Chemists; 2000. [Google Scholar]
- ASTM (1989) Standard test methods for water vapor transmission of materials (E96-E80). In: Annual book of ASTM standards. ASTM, Philadelphia. 730–739
- Avena-Bustillos RJ, Krochta JM. Water vapor permeability of caseinate-based edible films as affected by pH, calcium crosslinking and lipid content. J Food Sci. 1993;58:904–907. doi: 10.1111/j.1365-2621.1993.tb09388.x. [DOI] [Google Scholar]
- Bae HJ, Park HJ, Hong SI, Byun YJ, Darby DO, Kimmel RM, Whiteside WS. Effect of clay content, homogenisation RPM, pH, and ultrasonification on mechanical and barrier properties of fish gelatin/montmorillonite nanocomposite films. LWT Food Sci Technol. 2009;42:1179–1186. doi: 10.1016/j.lwt.2008.12.016. [DOI] [Google Scholar]
- Bao S, Xu S, Wang Z. Antioxidant activity and properties of gelatin films incorporated with tea polyphenol-loaded chitosan nanoparticles. J Sci Food Agric. 2009;89:2692–2700. doi: 10.1002/jsfa.3775. [DOI] [Google Scholar]
- Bitencourt CM, Favaro-Trindade CS, Sobral PJA, Carvalho RA. Gelatin-based films additivated with curcuma ethanol extract: antioxidant activity and physical properties of films. Food Hydrocoll. 2014;40:145–152. doi: 10.1016/j.foodhyd.2014.02.014. [DOI] [Google Scholar]
- Chinabhark K, Benjakul S, Prodpran T. Effect of pH on the properties of protein-based film from bigeye snapper (Priacanthus tayenus) surimi. Bioresour Technol. 2007;98:221–225. doi: 10.1016/j.biortech.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Farahnaky A, Mohammad S, Dadfar M, Shahbazi M. Physical and mechanical properties of gelatin–clay nanocomposite. J Food Eng. 2014;122:78–83. doi: 10.1016/j.jfoodeng.2013.06.016. [DOI] [Google Scholar]
- Gennadios A, Brandenburg AH, Weller CL, Testin RF. Effect of pH on properties of wheat gluten and soy protein isolate films. J Agric Food Chem. 1993;41:1835–1839. doi: 10.1021/jf00035a006. [DOI] [Google Scholar]
- Gennadios A, Weller CL, Hanna MA, Froning GW. Mechanical and barrier properties of egg albumen films. J Food Sci. 1996;61:585–589. doi: 10.1111/j.1365-2621.1996.tb13164.x. [DOI] [Google Scholar]
- Gomez-Estaca J, Lopez-de-Dicastillo C, Hernandez-Munoz P, Catala R, Gavara R. Advances in antioxidant active food packaging. Trends Food Sci Technol. 2014;35:42–51. doi: 10.1016/j.tifs.2013.10.008. [DOI] [Google Scholar]
- Gomez-Guillen MC, Porez-Mateos M, Gomez-Estaca J, Lopez-Caballero E, Gimenez B, Montero P. Fish gelatin: a renewable material for developing active biodegradable films. Trends Food Sci Technol. 2009;20:3–16. doi: 10.1016/j.tifs.2008.10.002. [DOI] [Google Scholar]
- Grevellec JI, Marquie C, Ferry L, Crespy A, Vialettes V. Processability of cottonseed proteins into biodegradable materials. Biomacromolecules. 2001;2:1104–1109. doi: 10.1021/bm015525d. [DOI] [PubMed] [Google Scholar]
- Gutierrez MQ, Echeverria I, Ihl M, Bifani V, Mauri AN. Carboxymethylcellulose–montmorillonite nanocomposite films activated with murta (Ugni molinae Turcz) leaves extract. Carbohydr Polym. 2012;87:1495–1502. doi: 10.1016/j.carbpol.2011.09.040. [DOI] [Google Scholar]
- Hamaguchi PY, WuYin W, Tanaka M. Effect of pH on the formation of edible films made from the muscle proteins of blue marlin (Makaira mazara) Food Chem. 2007;100:914–920. doi: 10.1016/j.foodchem.2005.10.045. [DOI] [Google Scholar]
- Han JH, Krochta JM. Water vapor permeability and wetting properties of whey protein coating on paper. AM Soc Agric Eng. 1999;42:1375–1382. doi: 10.13031/2013.13300. [DOI] [Google Scholar]
- Haslam E. Practical polyphenolics. From structure to molecular recognition and physiological action. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Hoque MS, Benjakul S, Prodpran T. Properties of film from cuttlefish (Sepia pharaonis) skin gelatin incorporated with cinnamon, clove and star anise extracts. Food Hydrocoll. 2011;25:1085–1097. doi: 10.1016/j.foodhyd.2010.10.005. [DOI] [Google Scholar]
- Iwata KI, Ishizaki SH, Handa AK, Tanaka MU. Preparation and characterisation of edible films from fish water-soluble proteins. Fish Sci. 2000;66:372–378. doi: 10.1046/j.1444-2906.2000.00057.x. [DOI] [Google Scholar]
- Jongjareonrak A, Benjakul S, Visessanguan W, Tanaka M. Antioxidant activity and properties of skin gelatin films incorporated with BHT and α-tocopherol. Food Hydrocoll. 2008;22:449–458. doi: 10.1016/j.foodhyd.2007.01.002. [DOI] [Google Scholar]
- Karim AA, Bhat R. Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatin. Food Hydrocoll. 2009;23:563–576. doi: 10.1016/j.foodhyd.2008.07.002. [DOI] [Google Scholar]
- Kavoosi G, Dadfar SMM, Purfard AM, Mehrabi R. Antioxidant and antibacterial properties of gelatin films incorporated with carvacrol. J Food Saf. 2013;33:423–432. doi: 10.1111/jfs.12071. [DOI] [Google Scholar]
- Koh MJ, Hwang HY, Kim DJ, Kim HJ, Hong YT, Nam SY. Preparation and characterization of porous PVdF-HFP/clay nanocomposite membranes. J Mater Sci Technol. 2010;26:633–638. doi: 10.1016/S1005-0302(10)60098-9. [DOI] [Google Scholar]
- Li P, Zheng JP, Ma YL, Yao KD. Gelatin/Montmorillonite hybrid nanocomposite. II Swelling behavior. J Applied Polym Sci. 2003;88:322–326. doi: 10.1002/app.11689. [DOI] [Google Scholar]
- Li J, Miao J, Wu J, Chen S, Zhang Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014;37:166–173. doi: 10.1016/j.foodhyd.2013.10.015. [DOI] [Google Scholar]
- Martucci JF, Ruseckaite RA. Structure and properties of gelatin/montomorillonite nanocomposite films. In: Jimenez A, Zaikov GE, editors. Recent advances in research on biodegradable polymers and sustainable polymers. Huntington: Nova Publishers; 2008. pp. 27–36. [Google Scholar]
- Martucci JF, Ruseckaite RA. Biodegradable bovine gelatin/Na+-montmorillonite nanocomposite films. Structure, barrier and dynamic mechanical properties. Polym -Plast Technol Eng. 2010;49:581–588. doi: 10.1080/03602551003652730. [DOI] [Google Scholar]
- Martucci JF, Ruseckaite RA. Biodegradable three-layer film derived from bovine gelatin. J Food Eng. 2010;99:377–383. doi: 10.1016/j.jfoodeng.2010.02.023. [DOI] [Google Scholar]
- Martucci JF, Vazquez A, Ruseckaite RA. Nanocomposites based on gelatin and montmorillonite. Morphological and thermal studies. J Therm Anal Calorim. 2007;89:117–122. doi: 10.1007/s10973-006-7454-0. [DOI] [Google Scholar]
- Nagarajan M, Benjakul S, Prodpran T, Songtipya P. Characteristics of bio-nanocomposite films from tilapia skin gelatin incorporated with hydrophilic and hydrophobic nanoclays. J Food Eng. 2014;143:195–204. doi: 10.1016/j.jfoodeng.2014.06.038. [DOI] [Google Scholar]
- Nagarajan M, Benjakul S, Prodpran T, Songtipya P. Properties of bio-nanocomposite films from tilapia skin gelatin as affected by different nanoclays and homogenising conditions. Food Bioprocess Technol. 2014;7:3269–3281. doi: 10.1007/s11947-014-1319-5. [DOI] [Google Scholar]
- Nunez-Flores R, Gimenez B, Fernandez-Martin F, Lopez-Caballero ME, Montero MP, Gomez-Guillen MC. Role of lignosulphonate in properties of fish gelatin films. Food Hydrocoll. 2012;27:60–71. doi: 10.1016/j.foodhyd.2011.08.015. [DOI] [Google Scholar]
- Nuthong P, Benjakul S, Prodpran T. Characterization of porcine plasma protein-based films as affected by pretreatment and cross-linking agents. Int J Biol Macromol. 2009;44:143–148. doi: 10.1016/j.ijbiomac.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Oxford P, Parker R, Ring S. Effect of water as a diluent on the glass transition behaviour of malto-oligosaccharides, amylase and amylopectin. Int J Biol Macromol. 1989;11:91–96. doi: 10.1016/0141-8130(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Papadopoulou A, Frazier RA. Characterisation of protein–polyphenol interactions. Trends Food Sci Technol. 2004;15:186–190. doi: 10.1016/j.tifs.2003.09.017. [DOI] [Google Scholar]
- Pradhan NK, Das M, Palve YP, Nayak PL. Synthesis and characterization of soya protein isolate/cloisite 30B (MMT) nanocomposite for controlled release of anticancer drug curcumin. Int J Res Pharm Biomed Sci. 2012;3:1513–1522. [Google Scholar]
- Rattaya S, Benjakul S, Prodpran T. Properties of fish skin gelatin film incorporated with seaweed extract. J Food Eng. 2009;95:151–157. doi: 10.1016/j.jfoodeng.2009.04.022. [DOI] [Google Scholar]
- Rawdkuen S, Suthiluk P, Kamhangwong D, Benjakul S. Mechanical, physico-chemical, and antimicrobial properties of gelatin-based film incorporated with catechin-lysozyme. Chem Cent J. 2012;6:1–10. doi: 10.1186/1752-153X-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SS, Okamoto M. Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog Polym Sci. 2003;28:1539–1641. doi: 10.1016/j.progpolymsci.2003.08.002. [DOI] [Google Scholar]
- Rhim J. Potential use of biopolymer-based nanocomposite films in food packaging applications. Food Sci Biotechnol. 2007;16:691–709. doi: 10.1080/10408390600846366. [DOI] [PubMed] [Google Scholar]
- Rhim J, Ng P. Natural biopolymer-based nanocomposite films for packaging applications. Crit Rev Food Sci Nutr. 2007;47:411–433. doi: 10.1080/10408390600846366. [DOI] [PubMed] [Google Scholar]
- Santoso J, Yoshie-stark Y, Suzuki T. Antioxidant activity of methanol extracts from Indonesian seaweeds in an oil emulsion model. Fish Sci. 2004;70:183–188. doi: 10.1111/j.1444-2906.2003.00787.x. [DOI] [Google Scholar]
- Shiku Y, Hamaguchi PY, Benjakul S, Visessanguan W, Tanaka M. Effect of surimi quality on properties of edible films based on Alaska Pollack. Food Chem. 2004;86:493–499. doi: 10.1016/j.foodchem.2003.09.022. [DOI] [Google Scholar]
- Slade L, Levine H. Beyond water activity: recent advances based on an alternative approach to the assessment of food quality and safety. Crit Rev Food Sci Nutr. 1991;30:115–360. doi: 10.1080/10408399109527543. [DOI] [PubMed] [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. AM J Enol Vitic. 1977;28:49–55. [Google Scholar]
- Steel RGD, Torrie JH. Principles and procedures of statistics: a biometrical approach. 2. New York: McGraw-Hill; 1980. p. 633. [Google Scholar]
- Tharanathan RN. Biodegradable films and composite coatings: past, present and future. Trends Food Sci Technol. 2003;14:71–78. doi: 10.1016/S0924-2244(02)00280-7. [DOI] [Google Scholar]
- Theng BKG. Formation and properties of clay–polymer complexes. Amsterdam: Elsevier Scientific Publishing Company; 1979. [Google Scholar]
- Vazquez-Torres H, Canche-Escamilla G, Cruz-Ramos CA. Coconut husk lignin. I Extraction and characterisation. J Applied Polym Sci. 1992;45:633–644. doi: 10.1002/app.1992.070450410. [DOI] [Google Scholar]
- Wu J, Chen S, Ge S, Miao J, Li J, Zhang Q. Preparation, properties and antioxidant activity of an active film from silver carp (Hypophthalmichthys molitrix) skin gelatin incorporated with green tea extract. Food Hydrocoll. 2013;32:42–51. doi: 10.1016/j.foodhyd.2012.11.029. [DOI] [Google Scholar]
- Wu J, Ge S, Liu H, Wang S, Chen S, Wang J, Li J, Zhang Q. Properties and antimicrobial activity of silver carp (Hypophthalmichthys molitrix) skin gelatin-chitosan films incorporated with oregano essential oil for fish preservation. Food Pack Shelf Life. 2014;2:7–16. doi: 10.1016/j.fpsl.2014.04.004. [DOI] [Google Scholar]
- Xu Y, Ren X, Hanna M. Chitosan/clay nanocomposite film preparation and characterization. J Appl Polym Sci. 2006;99:1684–1691. doi: 10.1002/app.22664. [DOI] [Google Scholar]
- Zheng JP, Li P, Ma YL, Yao KD. Gelatin/montmorillonite hybrid nanocomposite. 1. Preparation and properties. J Appl Polym Sci. 2002;86:1189–1194. doi: 10.1002/app.11062. [DOI] [Google Scholar]