Abstract
Rice bran (RB), a byproduct of rice milling industry, is a rich source of nutraceuticals and nutrients. However its utility is limited due to the presence of lipase and lipoxygenase which initiates rancidity on milling. The aim of this investigation is to prevent oxidation of free fatty acids by enzymatic approach for its effective utilization. The enzymatic treatment comprised of alcalase treatment for complete inactivation of lipase along with reduction in lipoxygenase (LOX) activity and endoglucanase for improving the soluble fiber content. The enzyme treated rice bran was drum dried for further use. The nutraceutical molecules like γ-oryzanol, α-tocopherol and polyphenols were retained in the range of 68 to 110 % and the total antioxidant activity was improved. By the action of endoglucanase the complex carbohydrate was converted into glucose (72.28 %), cellobiose (18.36 %) and cellotriose (9.36 %). The prebiotic effect of enzyme treated rice bran was evaluated by the action of lactobacillus which was measured through the release of the short chain free fatty acids (SCFAs) analyzed by HPLC. The SCFAs; acetic acid and propionic acid increased by 1.72 folds and 2.12 folds respectively. B-complex vitamins showed maximum retention with vitamins like B1 (66.3 %), B2 (68.3 %) and B3 (55.0 %) after enzyme treatment. At different humidity levels, storage studies showed no change in LOX activity and also retained ubiquinol-10 in reduced state in enzyme treated RB for a period of 3 months. A stabilized RB has been developed enriched with short chain prebiotics and antioxidant molecules.
Keywords: Nutraceuticals, Lipase, Endoglucanase, Ubiquinone 10, Lipoxygenase, Prebiotics
Introduction
Rice bran (RB) is an underutilized milling byproduct of rice, which has a hard outer layer consisting of combined aleurone and pericarp layer. Along with germ, it forms an integral part of whole grain and is produced regularly as a byproduct of milling. This byproduct is utilized in oil processing industry to produce rice bran oil. Rice bran is sweet in taste, with a slightly toasted nutty flavor, it is light in color and moderately oily (Hu et al. 1996). Texture varies from fine powder to flakes, depending on the method of stabilization. Rice bran is a rich source of oil, protein, fiber and micronutrients. Rice bran proteins have a high nutritional value and are hypoallergenic in nature. These proteins are rich in essential amino acids, especially lysine, hence can be used as an ingredient in food formulations (Fabian and Ju 2011).
Rice bran also contains a significant amount of nutraceutical compounds. Ubiquinone-10 is present in rice bran and may provide protective benefits of antioxidants. Epidemiological and biochemical evidence supports the idea that ubiquinol-10 (CoQ10H2) an important cellular antioxidant in vivo, inhibits lipid peroxidation as well as regenerates other antioxidants such as α-tocopherol (Sunesen et al. 2001). The presence of γ-oryzanol and plant sterols attribute to the hypocholesterolemic activity of rice bran (Sheetharamaiah and Chandrasekhara 1988). Tocotrienols present in rice bran inhibit HMG CoA reductase activity, which is the rate-limiting enzyme for cholesterol synthesis (Qureshi et al. 2000).
Complex carbohydrates are substrates for fermentation, where lactobacillus produce SCFAs, primarily acetate, propionate and butyrate, as end products. SCFAs produced are readily absorbed in the colon. Propionate is mainly utilized by the liver. Butyrate is the major energy source for colonocytes. Acetate passes through the peripheral circulation, which will be absorbed by peripheral tissues (Wong et al. 2006). Some of SCFAs may decrease the risk of developing gastrointestinal diseases and cardiovascular disease. In colon, acetate is a major SCFA, which helps in increasing the cholesterol synthesis, but propionate is shown to inhibit cholesterol synthesis. Thus, sources that can reduce the acetate: propionate ratio may reduce serum lipids and possibly cardiovascular disease risks (Wong et al. 2006).
During the milling process, the bran layers are removed from the endosperm, the individual cells of rice bran are ruptured and the lipids come into contact with highly reactive lipases. These enzymes are endogenously present in the bran and initiate hydrolytic deterioration of kernel oil (Ramezanzadeh et al. 1999). Spoilage caused by oxidative rancidity involves a reaction between the lipid and molecular oxygen which is hastened by the presence of singlet oxygen, free radicals and enzymes with metal prosthetic group such as LOX. LOX specifically oxygenates polyunsaturated fatty acids and their double bond systems in esters and acylglycerols (Schewe et al. 1981). The FFAs are substrates for LOX which oxidizes them to produce peroxides; thus peroxidation exhibits rancidity (Zhang et al. 2007). The schematic representation shows the generation of peroxides in Fig. 1. These reactions make rice bran unfit for human consumption.
Fig. 1.
Schematic representation of ubiquinone-10 (CoQ10) in relation with lipase and lipoxygenase in rice bran during storage
The objective of the paper is to develop a stabilized RB which can be utilized as a functional food ingredient, since it is a good source of antioxidant molecules, nutrients and dietary fiber. RB being a source of dietary fiber, attempts would be made towards enriching it with short chained prebiotics. Due to presence of deteriorating enzymes which makes it rancid, it is necessary to stabilize the RB. Shelf life studies at different humidity conditions would be carried out to ensure the lipase and LOX enzyme remain inactive in stabilized rice bran.
Materials and methods
Materials
Rice bran was procured from local rice mill. The bran was sieved to remove the extraneous matter and passed through a mill to obtain a homogenous sample. All the solvents ethanol, methanol, petroleum ether and hexane used were of analytical grade (AR), procured from Rankem fine chemicals Pvt. Ltd. (Bangalore, India). HPLC grade for ubiquinone-10 quantification was procured from Rankem fine chemicals Pvt. Ltd. (Bangalore, India). Ubiquinone-10 standard, pyrogallol and sodium borohydride, were obtained from Sigma Aldrich (Bangalore, India). Food grade protease, Alcalase (Bacillus lechniformis) was purchased from Novozyme (Bangalore, India). Crude cellulase (Aspergillus aculeatus) was obtained from Novozyme (Bangalore, India) which was used for isolation of endoglucanase. A stock solution of Ubiquinone-10 was prepared in hexane and protected from the light by aluminum foil and stored at 4 °C. Before use, the accurate concentration of the standard was determined by spectrophotometer at 275 nm.
Methodology
Enzymatic processing of rice bran
Rice bran was treated with alcalase (0.013 μM/mg/min) at 1 % of protein content of rice bran at optimal conditions of pH (7.5) and temperature (60 °C) for a period of 30 min. The enzyme was inactivated by raising the temperature to 80 °C for 10 min. Later, alcalase treated RB was treated with the endoglucanase (1.4 unit/mg/min) for 1 h for hydrolyzing cellulose present in rice bran at 1 % level at 55 °C and pH 5. Endoglucanase was isolated from crude cellulase from Aspergillus aculeatus (Novozyme) according to the method given by Gajendra et al. (2007). Enzymes were inactivated by raising the temperature to 80 °C for 10 min after the optimum time of incubation. The enzyme treated RB was drum dried and stored in a dessicator until further use. A schematic representation of the enzyme treated rice bran is shown in Fig. 2.
Fig. 2.
Schematic representation of enzyme treatment of rice bran
Lipase assay
The inactivation of lipase in rice bran was monitored by following the modified procedure as described by Sudhindra et al. (1991) using triacetin as substrate in Mettler Toledo DL12 titrator.
Lipoxygenase assay (LOX)
The inactivation of LOX was done using linoleic acid as a substrate, according to the procedure of Schewe et al. (1981).
RP-HPLC profile of reduced and oxidized forms of ubiquinone-10
Ubiquinone-10 was extracted from rice bran (RB) in a mixture of petroleum ether and 95 % methanol (1:1 ratio) by agitating the solvents. The solvents were partitioned; ubiquinone-10 was extracted in pet. Ether which was concentrated in a flash evaporator (Crane and Barr 1971). After extraction, the known volume of the concentrated sample ubiquinone-10 was diluted to 1:10 with hexane. The two states; reduced (ubiquinol) and oxidized (ubiquinone) forms were monitored through RP-HPLC. The concentration of ubiquinone-10 in RB was analyzed using standard from ubiquinone-10 (sigma) as external standard An aliquot of the sample was chromatographed on a C-18 column in RP-HPLC (15 cm × 4.6 mm, 0.3 μm; Waters) protected by 1 cm guard column, using a mobile phase ethanol: methanol (70:30, v/v ratio) at a flow rate of 1.0 mL/min. The eluate was monitored with a photo diode array (PDA) detector (Waters) (Molyneux 2006).
Rate of reduction of ubiquinone-10
The ubiquinone-10 extracts were reconstituted in ethanol and reduced using sodium borohydride (NaBH2), the rate of reduction of ubiquinone-10 was monitored at different time intervals. The assay was based on the difference in the oxidized and reduced absorbance of ubiquinone at 275 nm in a spectrophotometer. The decrease in the absorbance shows the reduction of the quinone (Crane and Barr 1971). The difference in the absorbance was used to measure the rate of quinone reduction in both the samples.
Analysis of soluble fiber in RB hydrolysate
End product quantification by HPLC method
The quantification of the end product of complex carbohydrates of enzyme treated RB were performed by HPLC (Shimadzu LC, Japan) using aminopropyl column (150 × 4.6 mm) with refractive index detector (RID-10A) (Hernandez et al. 1998). The end products were quantified with respective standards. The method employing RID was performed at a constant temperature of 28 °C using isocratic elution using acetonitrile: water (70:30) as a mobile phase with a flow rate of 1 mL/min.
Evaluation of SCFAs released by lactobacillus action on soluble fiber
To evaluate the enriched soluble fiber in enzyme treated RB, the samples were subjected to lactobacillus strain, which is mostly found in the intestine. The Lactobacillus plantanum available at CFTRI culture collection center was sub cultured at regular intervals for experiments in MRS broth. The cell pellet was separated out from the broth by centrifugation and dissolved in saline suspension. By serial dilution, 1.0 mL of the individual cell suspension was mixed with 9.0 mL of the sterilized normal saline with successive dilutions with the final dilution of 10−6. Individual strains with cell concentration of 1.0 × 107 cells/mL were cultured for 24 h at 37 °C in the MRS broth media with the enzyme treated RB, source of soluble fiber and also with control RB, which served as a control. After incubation, the cultures were subjected to centrifugation at 8500 rpm for 15 min at 4 °C. The supernatant separated was passed through a 0.22 μM membrane filter to concentrate the samples (Weber et al. 1994).
These filtered samples were injected to RP-HPLC to analyze the short chain fatty acids (SCFAs) produced by the lactic acid bacteria due to the consumption of the soluble fiber. SCFAs (acetate, propionate) from the lyophilized samples of control RB and enzyme treated RB were analyzed on a C-18 column (150 × 4.6 mm) protected by 1 cm guard column using acetonitrile: water (70:30) as a mobile phase with a flow rate of 1 mL/min. Detection was performed using PDA detector (Shimadzu LC, Japan). The amount of acetic acid and propionic acids liberated by the action of LAB on short chain prebiotics of RB in the culture broth were quantified by respective standard curves.
Analysis of soluble fiber by gravimetric method
The soluble, insoluble and total dietary fiber was determined by gravimetric method (Asp et al. 1983).
Analysis of nutritional parameters
The enzyme treated RB was estimated for protein (Kjeldhal), fat (Soxhlet) according to the standard methods of AACC (2000). In vitro digestibility of protein was determined by simulating the gastro-intestinal digestion using pepsin and pancreatin (Akeson and Stahmann 1964). The γ-oryzanol content was determined by spectrophotometer method (De and Bhattacharya 2000). Vitamin E was estimated by HPLC method with few modifications (Aguilar-Garcia et al. 2007). Vitamin B1 (AOAC 1990), Vitamin B2 (Raghuramulu et al. 2003), and Vitamin B3 (Tyler and Genzale 1990) were estimated in RB by standard methods. Polyphenol content was measured in the control and enzyme treated rice bran by Folin-Ciocalteau method (Aguilar-Garcia et al. 2007). Values reported were averages of three determinations. Analysis of Zn and Fe after wet digestion was determined by Atomic Absorption Spectrophotometer (Shimadzu Scientific Instrument Inc., USA) by procedures of AOAC (2006).
Storage studies of processed rice bran
The enzyme treated RB samples were packed in polypropylene pouches and were maintained at different relative humidity (RH), ranging from 11, 22, 44, 56 and 64 % at 27 °C. Samples were withdrawn at a period of 30 days interval for the duration of three months for the determination of LOX activity.
Statistical analysis
The data presented represent the mean of triplicate measurements, mean and standard deviation was computed in MS Excel. Paired t-test was applied to check the effect of enzymatic treatment on the content of nutrients and antioxidants in the rice bran, probability level was set to p < 0.05.
Results and discussion
Rice bran is a good source of antioxidants such as γ-oryzanol, ubiquinol-10, tocopherols, vitamins, minerals and dietary fiber. Here, rice bran was enzymatically processed using protease and endoglucanase which resulted in enhanced nutraceutical properties with maximum retention of micronutrients. During dehulling, lipase gets activated and produces free fatty acids (FFAs) by cleaving triglycerides and other fats (Ramezanzadeh et al. 1999). In our study, during enzymatic treatment lipase was completely inactivated by alcalase treatment, along with 50 % reduction in LOX activity in rice bran. For effective stabilization of rice bran thermal treatments like dry heating, microwave, and autoclaving as well as ethanol vaporizing are being used. During thermal processing, tocols bound to proteins/phospholipids are released in significant amounts by breaking the linkages (Sung-Min et al. 2014).
Analysis of soluble fiber in RB hydrolysate
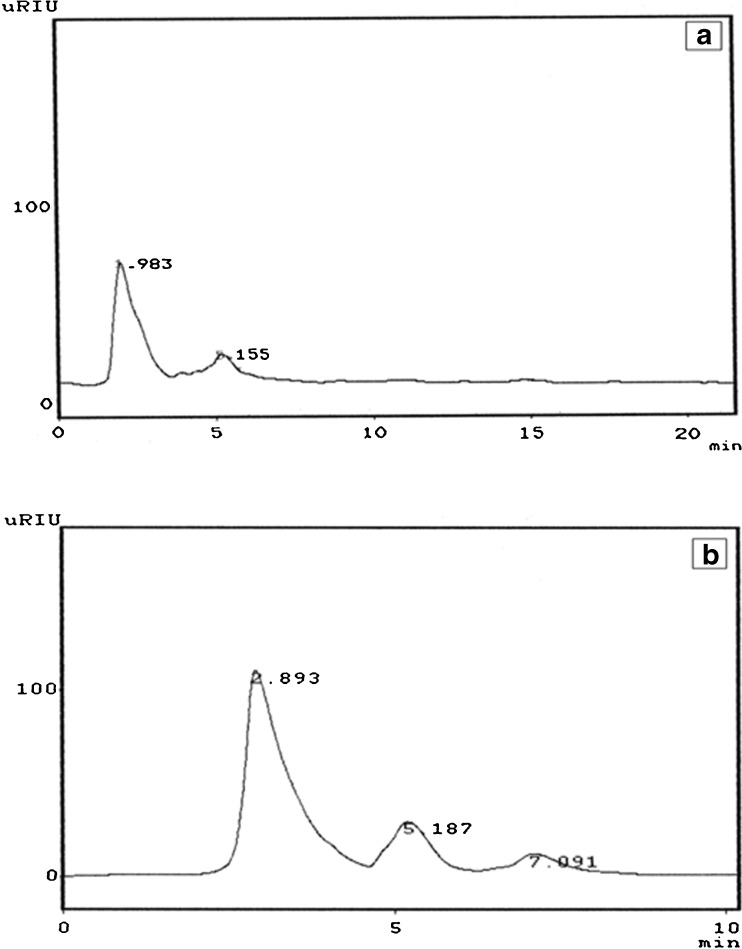
Enzyme treatment increased the soluble fiber content (determined by gravimetric method) from 4.0 ± 0.2 g/100 g in control rice bran to 6.25 ± 0.21 g/100 g in enzyme treated RB which accounts to 1.5 folds. By the action of endoglucanase the complex carbohydrate of RB was converted into simple soluble sugars like glucose (72.28 %), cellobiose (18.36 %) and cellotriose (9.36 %) in enzyme treated RB. Raw RB showed a major peak of complex carbohydrate (86.66 %) and a minor peak of cellobiose (12.50 %) as shown in HPLC profiles (Fig. 3a, b). The retention times of the individual peaks of the sample were matching with their respective standards. The increased content of soluble fiber in enzyme treated rice bran was confirmed by prebiotic activity. This is shown through the release of short chain fatty acids by Lactobacillus plantanum cultured with enzyme treated rice bran enriched with short chain prebiotics, which resulted in an increase in propionic acid (2.12 folds) and acetic acid (1.72 folds) compared to control (Table 1).
Fig. 3.
HPLC profiles showing the degradation products of complex carbohydrate present in rice bran. The endogluconase additional enzyme treated RB was loaded on aminopropyl column (150 × 4.6 mm) Shimadzu LC model, using acetonitrile: water (70:30) as a mobile phase with a flow rate of 1 mL/min. Detection was performed using RID-10A detector. The two RB samples were labeled as a Control RB and b enzyme treated RB with additional treatment with endogluconase
Table 1.
Determination of short chain fatty acids released by Lactobacillus plantanum grown on RB dietary fiber with short chain prebiotics
| Samples | Short chain fatty acids | Concentration (ug/ul) |
|---|---|---|
| Control RB | Acetic acid | 12.67 ± 0.05 |
| Propionic acid | 0.62 ± 0.01 | |
| Enzyme treated RB | Acetic acid | 21.88 ± 0.1 |
| Propionic acid | 1.30 ± 0.06 |
All the above values (mean ± SEM) are of triplicate values
Endoglucanase attacks the carboxymethyl cellulose in a random fashion resulting in a rapid decrease in the chain length (Wood and McCrae 1979). Degradation of oligosaccharides by enzyme treatment would make the rice bran available for human consumption. According to Slavin et al. (2009), dietary fiber in the soluble form helps to prevent the inhibition of micronutrient absorption caused by total dietary fiber and stabilizes blood glucose and lipid levels. Stabilization by microwave heating and conventional roasting have shown reduction in soluble fiber content by 50 and 93 % respectively (Faria et al. 2012). Acetate acts as a fuel for skeletal and cardiac muscle, kidney and the brain. Propionate is the only SCFA that can be a major source of glucose after metabolism, used for energy production and may play a role in cholesterol lowering (Slavin et al. 2009).
Antioxidant activity in rice bran
Antioxidant molecules like α-tocopherol, polyphenol, γ-oryzanol, ubiquinone-10 were retained in the range of 68 to 110 % with total antioxidant activity improved to 102 % (Table 2). These contributed to the total antioxidant activity. The enzyme treated RB extracts showed higher antioxidant activity compared to control. α-tocopherol was retained upto 67.7 % where as polyphenol and γ-oryzanol increased from 382 to 408 mgGAE/100 g and 200-220 mg/100 g, respectively. Antioxidant molecules such as tocols are active in-vivo combating lipid peroxidation in the biological membranes. The Polyphenols (604–684 μmol gallic acid eq/100 g dry wt) in different rice varieties (Carlos et al. 2007). The increase in the polyphenol content and total antioxidant activity was found to be highly significant. In different rice varieties of rice bran γ-Oryzanol content was reported to be in the range of 155–272 mg/100 g dry wt (Carlos et al. 2007). Also the reduced state of ubiquinone was maintained which otherwise would be oxidized (CoQ10H2 to CoQ10) due to the peroxidation of free fatty acids (FFAs) resulting in the formation of free radicals (James et al. 2005). Nutraceuticals present in rice bran have the potential to prevent a range of chronic diseases. The complete exploitation of these may lead to health promoting value added products which have cholesterol lowering properties, cardiovascular health benefits and anti-tumor activity (Nagendra Prasad et al. 2011).
Table 2.
Determination of nutritional and nutraceutical molecules in enzyme treated rice bran
| Nutritional parameters | Control (Per 100 g) | Enzyme treated (Per 100 g) | Retention (%) | p value | |
|---|---|---|---|---|---|
| Macronutrients | Fat (g) | 21.2 ± 1.0 | 21.2 ± 2.0 | 100.0 | p = 0.500 |
| Protein (g) | 15.0 ± 1.0 | 15.0 ± 1.0 | 100.0 | p = 0.500 | |
| Dietary fiber (g) | 27.0 ± 1.0 | 24.5 ± 1.0 | 91.0 | p = 0.054 | |
| Nutraceuticals | Vitamin E (mg) | 15.5 ± 1.5 | 10.0 ± 1.0 | 67.7 | p = 0.003 |
| γ- Oryzanol (mg) | 200 ± 2.0 | 220 ± 2.0 | 110.0 | p = 0.0001 | |
| Ubiquinone-10 (mg) | 42.0 ± 4.0 | 44.0 ± 2.0 | 100.0 | p = 0.240 | |
| Polyphenol content (mg GAE) | 382 ± 1.0 | 408 ± 1.6 | 107.0 | p = 0.0009 | |
| Total antioxidant activity (μmole/g α - tocopherol) | 163 ± 1.1 | 166.3 ± 0.6 | 102.0 | p = 0.0051 | |
| Vitamins | Vitamin B1 (mg) | 5.16 ± 0.3 | 3.42 ± 0.7 | 66.3 | p = 0.0001 |
| Vitamin B2 (mg) | 1.71 ± 0.3 | 1.17 ± 0.4 | 68.4 | p = 0.023 | |
| Vitamin B3 (mg) | 42.13 ± 1.1 | 23.17 ± 0.6 | 55.0 | p = 0.0005 | |
| Micronutrients | Fe (mg) | 12.6 ± 0.35 | 11.05 ± 0.55 | 87.7 | p = 0.005 |
| Zn (mg) | 4.87 ± 0.31 | 4.14 ± 0.38 | 85.0 | p = 0.032 |
p < 0.05 – significant at 5 % level; p < 0.01 - significant at 1 % level; p < 0.001 - significant at 0.1 % level
Determining the two forms of ubiquinone-10 and its rate of reduction
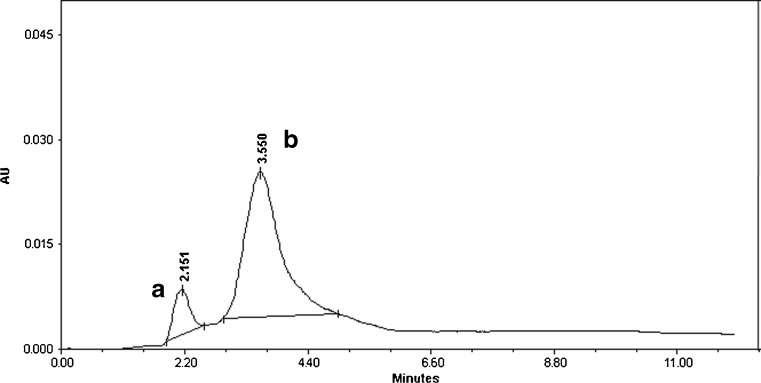
The HPLC profile depicts the two forms of ubiquinone-10, reduced (CoQ10H2) and oxidized (CoQ10) shown in Fig. 4. Kinetic studies show that the rate of reduction of ubiquinone-10 in control RB was 0.079 μM−1 min−1 whereas in case of enzyme treated RB it was 0.0591 μM−1 min−1. This confirms that enzyme treated RB has higher potency to scavenge the free radicals compared to control RB as depicted through the area under the peak A (reduced form) in Fig. 4. Ubiquinol-10, in rice bran is an important nutraceutical molecule regenerates antioxidants and also prevents oxidation of free fatty acids. CoQ10 and CoQ9 were determined in over 70 samples using the HPLC technique (Kamei et al. 1986).
Fig. 4.
HPLC profile showing two different forms; reduced and oxidized forms of ubiquinone-10, a ubiquinol-10 (CoQ10H2) and b ubiquinone-10 (CoQ10)
Nutritional parameters
The enzymatically stabilized rice bran has 15 % protein and 21 % fat. The in-vitro digestibility of protein in control rice bran was 62.6 ± 1.87 % which increased to 86.4 ± 2.6 % on enzyme treatment thereby improving the in-vitro protein digestibility by 1.37 folds. Vitamin B-complex showed maximum retention with 66.3 % vitamin B1 compared to 68.4 % vitamin B2, and 55 % vitamin B3 on enzyme treatment. The micronutrients iron and zinc were retained in the range of 85 to 88 % on enzyme treatment. The total dietary fiber was retained up to 90 %. (Table 2). Similar composition was reported for protein ranging between 11.53–14.58 % and fat 13.3–19.85 % in different varieties of RB (Amissah et al. 2003). The extent of losses is maximum in vitamin B3. Vitamin B1 is of <0.1 mg/100 g, Vitamin B2 is of 24.1 mg/100 g (Parrado et al. 2006). Enzyme treatment made it palatable and also could retain maximum amount of nutrients. Dietary fibers have been reported to produce numerous health benefits, such as decreasing cardiovascular and gastrointestinal disease, decreasing blood cholesterol, and also preventing colon cancer (Burton-Freeman 2000).
Effect of humidity on enzyme treated RB during storage
The enzyme treated RB samples stored at different humidity (11, 22, 44, 56 and 64 %) conditions exhibit no significant change in the LOX activity as shown in Table 3. When these samples were stored for a period of 3 months, there was no significant difference in LOX activity at different humidity levels. LOX activity persists as long as FFA is available for the LOX, which decreases with time during the storage of enzyme treated RB (Ramezanzadeh et al. 1999). Roasting and extrusion cooking have shown significant reduction in FFA and peroxide value, due to inactivation of lipases and lipoxygenases, thus stabilizing the rice bran for 180 days (Ahmad Mujahid et al. 2005). Heating in the presence of moisture is much more effective in permanently denaturing lipases (Ramezanzadeh et al. 2000).
Table 3.
Comparison of LOX activity in Enzyme treated rice bran at two different humidity levels
| Samples | Humidity | LOX activity (U/min/g) |
|---|---|---|
| Control rice bran | <10 % | 0.178 ± 0.020 |
| Enzyme treated rice bran | <10 % | 0.110 ± 0.002 |
| 11 % | 0.080 ± 0.002 | |
| 22 % | 0.088 ± 0.002 | |
| 44 % | 0.120 ± 0.002 | |
| 56 % | 0.104 ± 0.002 | |
| 64 % | 0.104 ± 0.002 |
All the above values (mean ± SEM) are of triplicate values
Conclusion
Our results show that the enzymatic process has retained the nutrients and nutraceuticals present in the rice bran which can be used as a functional food ingredient. Also, enzyme treatment has shown significant increase in the prebiotic activity through release of the SCFAs like acetate and propionate. The ubiquinol-10 was retained in the enzyme treated RB by inactivation of lipase and lipoxygenase (LOX) activities, this helped to retain the reduced form of ubiquinol. Rice bran has not been much exploited as a food/health supplement, there is great potential for using processed rice bran in food preparations as a supplement for improving their nutritional value and health benefits.
Acknowledgments
The authors are thankful to the Director, CSIR-CFTRI for providing the facilities to carry out the work. We acknowledge Planning commission, New Delhi, India for the financial support.
Conflict of interest
The authors report no conflict of interest. The authors are responsible for the content and writing of the paper.
References
- AACC . Approved methods of the American Association of Cereal Chemists, US. St. Paul: American Association of Cereal Chemists; 2000. [Google Scholar]
- Akeson WR, Stahmann MA. A pepsin pancreatin digest index of protein quality evaluation. J Nutr. 1964;83:257–261. doi: 10.1093/jn/83.3.257. [DOI] [PubMed] [Google Scholar]
- Amissah JGN, Ellis WO, Oduro I, Manful JT (2003) Nutrient composition of bran from new rice varieties under study in Ghana. Food Control 14:21–24
- AOAC . Official methods of analysis. Arlington: Association of Analytical Chemists; 1990. [Google Scholar]
- AOAC . Official methods of analysis. Arlington: Association of Analytical Chemists; 2006. [Google Scholar]
- Asp NG, Johansson CG, Hallmer H, Siljestrom M. Rapid enzymatic assay of insoluble and soluble dietary fiber. J Agric Food Chem. 1983;31(3):476–482. doi: 10.1021/jf00117a003. [DOI] [PubMed] [Google Scholar]
- Burton-Freeman B (2000) Dietary fiber and energy regulation. J Nutr 130:272S–275S [DOI] [PubMed]
- Aguilar-Garcia C, Gravino G, Baragaño-Mosqueda M, Hevia P, Gavino VC (2007) Correlation of tocopherol, tocotrienol, γ-oryzanol and total polyphenol content in rice bran with different antioxidant capacity assays. Food Chem 102:1228–1232
- Crane FL, Barr R (1971) Determination of ubiquinones. In: Donald BM, Lemuel DW (Eds), Method Enzymol, Part C, Academic Press, 18: 137–165
- De BK, Bhattacharya DK. Detection of rice bran oil in other edible oils by spectrophotometric assay of oryzanol. J Oil Technol Assoc India. 2000;32:10–11. [Google Scholar]
- Fabian C, Ju YH (2011) A review on rice bran protein: its properties and extraction methods. Crit Rev Food Sci Nutr 51:816–827 [DOI] [PubMed]
- Faria SASC, Bassinello PZ, Penteado MVC (2012) Nutritional composition of rice bran submitted to different stabilization procedures. Braz J Pharm Sci 48:651–657
- Gajendra SN, Purnima K, Prakash V. Purification and characterization of a new endoglucanase from Aspergillus aculeatus. J Agric Food Chem. 2007;55:7566–7572. doi: 10.1021/jf070710p. [DOI] [PubMed] [Google Scholar]
- Hernandez JL, González-Castro MJ, Alba IN, de la Cruz García C. High-performace liquid chromatographic determination of mono- and oligosaccharides in vegetables with evaporative light-scattering detection and refractive index detection. J Chromatogr Sci. 1998;36(6):293–298. doi: 10.1093/chromsci/36.6.293. [DOI] [Google Scholar]
- Hu W, Wells JH, Shin T-S, Godber JS (1996) Comparsion of isopropanol and hexane for extraction of vitamin E and oryzanols from stabilized rice bran. J Am Oil Chem Soc 73(12):1653–1656
- James AM, Cocheme HM, Smith RA, Murphy MP. Metabolism and bioenergetics: interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species: implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem. 2005;280(22):21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- Kamei M, Fujita T, Kanbe T, Sasaki K, Oshiba K, Otani S, Matsuiyuasa I, Morisawa S. The distribution and content of ubiquinone in foods. Int J Vitam Nutr Res. 1986;56:57–63. [PubMed] [Google Scholar]
- Molyneux SL (2006) Development of assays for coenzyme Q10 and vitamin K and their application in clinical trials. Unpublished thesis, University of Canterbury, New Zealand
- Mujahid A, Ikram UH, Musaddiq A, Abrar HG. Effect of various processing techniques and different levels of antioxidant on stability of rice bran during storage. J Sci Food Agric. 2005;85:847–852. doi: 10.1002/jsfa.2026. [DOI] [Google Scholar]
- Nagendra Prasad MN, Sanjay KR, Shravya Khatokar M, Vismaya MN, Nanjunda Swamy S. Health benefits of rice bran - a review. J Nutr Food Sci. 2011;1:108. doi: 10.4172/2155-9600.1000108. [DOI] [Google Scholar]
- Parrado J, Miramontes E, Jover M, Gutierrez JF, de Teran LC, Bautista J (2006) Preparation of a rice bran enzymatic extract with potential use as functional food. Food Chem 98(4):742–748
- Qureshi AA, Mo H, Packer L, Peterson DM. Isolation and identification of novel tocotrienols from rice bran with hypocholesterolemic, antioxidant, and antitumor properties. J Agric Food Chem. 2000;48:3130–3140. doi: 10.1021/jf000099t. [DOI] [PubMed] [Google Scholar]
- Raghuramulu N, Nair MK, Kalyanasundaram S. A manual of laboratory techniques. Hyderabad: National Institute of Nutrition, ICMR; 2003. [Google Scholar]
- Ramezanzadeh FM, Rao RM, Windhauser M, Prinyawiwatkul W, Marshall WE. Prevention of oxidative rancidity in rice bran during storage. J Agric Food Chem. 1999;47(8):2997–3000. doi: 10.1021/jf981168v. [DOI] [PubMed] [Google Scholar]
- Ramezanzadeh FM, Rao RM, Witoon P, Marshall WE, Windhauser M (2000) Effects of microwave heat, packaging and storage temperature on fatty acid and proximate composition in rice bran. J Agric Food Chem 48:464–467 [DOI] [PubMed]
- Schewe T, Wiesner R, Rapoport SM (1981) Lipoxygenase from rabbit reticulocytes. In: John ML. Methods Enzymol. Academic Press, 71: 430–441 [DOI] [PubMed]
- Sheetharamaiah GS, Chandrasekhara N. Hypocholesterolemic activity of oryzanol in rats. Nutr Rep Int. 1988;38:927–935. [Google Scholar]
- Slavin JL, Savarino V, Aparedes-Diaz Fotopoulos G. A review of the role of soluble fiber in health with specific reference to wheat dextrin. J Int Med Res. 2009;37:1–17. doi: 10.1177/147323000903700101. [DOI] [PubMed] [Google Scholar]
- Sudhindra RK, Rajendran S, Rajeshwara AN, Prakash V. Structural stability of lipase from wheat germ in alkaline pH 1. J Protein Chem. 1991;10(3):291–299. doi: 10.1007/BF01025628. [DOI] [PubMed] [Google Scholar]
- Sunesen VH, Weber C, Holmer G. Lipophilic antioxidants and polyunsaturated fatty acids in lipoprotein classes: distribution and interaction. Eur J Clin Nutr. 2001;55(2):115–123. doi: 10.1038/sj.ejcn.1601127. [DOI] [PubMed] [Google Scholar]
- Sung-Min K, Hyun-Jung C, Seung-Taik L. Effect of various heat treatments on rancidity and some bioactive compounds of rice bran. J Cereal Sci. 2014;60:243–248. doi: 10.1016/j.jcs.2014.04.001. [DOI] [Google Scholar]
- Tyler TA, Genzale A. Liquid chromatographic determination of total niacin in beef, semolina, and cottage cheese. J Assoc Off Anal Chem. 1990;73(3):467–469. [PubMed] [Google Scholar]
- Weber C, Sejersgard JT, Mortensen SA, Paulsen G, Holme G. Antioxidative effect of dietary coenzyme Q10 in human blood plasma. Int J Vitam Nutr Res. 1994;64(4):311–315. [PubMed] [Google Scholar]
- Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- Wood TM, McCrae SI. Synergism between enzymes involved in the solubilization of native cellulose. Adv Chem Ser. 1979;181:181–209. doi: 10.1021/ba-1979-0181.ch010. [DOI] [Google Scholar]
- Zhang Y, Yu Z, Lu Y, Wang Y, She D, Song M, Wu Y. Effect of the absence of lipoxygenase isoenzymes on the storage characteristics of rice grains. J Stored Prod Res. 2007;43(1):87–91. doi: 10.1016/j.jspr.2005.11.004. [DOI] [Google Scholar]