Abstract
Previously, we have reported the chemical composition, molecular mass distribution and antioxidant activity of rohu roe protein hydrolysates. In the current study, antiproliferative, angiotensin-converting enzyme (ACE)-inhibitory activities and functional properties of protein hydrolysates from rohu (Labeo rohita) roe proteins, prepared by gastrointestinal proteases (pepsin and trypsin), were investigated. Antiproliferative activity was evaluated against human colon cancer cell line Caco-2. The results showed that the pepsin hydrolysate possessed dose dependent inhibitory effect on Caco-2 cell line. Pepsin and trypsin hydrolysates displayed ACE-inhibitory activity in vitro. The ACE-inhibitory activity of the hydrolysate generated by pepsin (47 ± 1.7 %, at 1 mg/ml) is higher than that obtained by trypsin (36 ± 3.2 %). Additionally, the undigested rohu roe proteins and its hydrolysates exhibited functional properties. Solubilities of the hydrolysates were above 81 ± 9.2 % at all pH values tested. Pepsin and trypsin hydrolysates showed good foaming capacity (45–211 %) and emulsification activity (4–29 m2/g). The foaming abilities and emulsifying activity index (EAI) were affected by pH. The results suggest that protein hydrolysates from rohu roe could be useful in food industry for various applications.
Keywords: Rohu egg, Pepsin, Trypsin, Enzymatic hydrolysis, Bioactive peptides, Protein hydrolysates
Introduction
The search for novel bioactive peptides/protein hydrolysates from food proteins is growing rapidly due to their health promoting effects as well as better functional characteristics (He et al. 2013; Chalamaiah et al. 2014). Food protein sources such as milk, meat, fish, egg, soybean, rice, wheat have been extensively investigated for production of bioactive protein hydrolysates (Shahidi and Zhong 2008; Agyei and Danquah 2012). Generation of biologically active protein hydrolysates from fish proteins is very important and economical since large quantities of protein rich by-products from fish processing industries are available for the process (Agyei and Danquah 2012; Chalamaiah et al. 2012).
Food proteins contain a wide variety of bioactive peptides, which are inactive within the sequence of native proteins (Saavedra et al. 2013). Enzymatic hydrolysis of fish proteins under controlled conditions releases the bioactive peptides (Gimenez et al. 2009; Intarasirisawat et al. 2012). In addition, enzymatic hydrolysis affects the molecular size, polar groups, and hydrophobicity of fish proteins, and results in enhanced functional properties such as solubility, emulsifying and foaming properties of protein hydrolysates (Klompong et al. 2007; Kristinsson and Rasco 2000).
Protein hydrolysates/peptides are a group of nutraceuticals that have potential in preventing hypertension and cancer (Shahidi and Zhong 2008). Several studies have reported antihypertensive protein hydrolysates derived from various fish protein sources such as tilapia, tuna frame, sardinelle by-products and tuna liver (Nasri et al. 2013a; Aleman et al. 2013; Lee et al. 2010; Je et al. 2009; Raghavan and Kristinsson 2009; Bougatef et al. 2008). In addition, protein hydrolysates produced from milk, rice, soybean, canola, and chicken egg have been shown to possess ACE inhibitory activity (Sanchez et al. 2011; Li et al. 2007; Yang et al. 2004; Jianping et al. 2009; Chen et al. 2012). Picot et al. (2006) reported antiproliferative activity for fish protein hydrolysates. Protein hydrolysates from tuna dark muscle, anchovy sauce, soybean, egg, milk, rice have been extensively investigated for anticancer activity (Hsu et al. 2011; Lee et al. 2003; Shahidi and Zhong 2008; Revilla et al. 2013).
Fish processing industry generates huge amounts of by-products such as head, skin, bones, liver, trimmings, fins, frames, viscera, meat of underutilized fish species and roes, from which bioactive peptides can be produced (Agyei and Danquah 2012; Chalamaiah et al. 2012). Fish egg is present in ovarian membrane of female fish and is a valuable source of high quality protein (Chalamaiah et al. 2013; Intarasirisawat et al. 2011). In India and other Asian countries, large quantities of fish roes are considered by-products of fish processing industry and are normally processed into low market-value products, such as animal feed, fish meal and fertilizer (Chalamaiah et al. 2012). Hence, production of protein hydrolysates with anticancer, antihypertensive and improved functional properties would improve economic benefits as well as solve the disposal problems (He et al. 2013). In our previous study, the preparation, chemical composition, molecular mass distribution and antioxidant activities of protein hydrolysates from rohu (Labeo rohita) egg were reported (Chalamaiah et al. 2013). The objectives of this study were to evaluate the anticancer, antihypertensive and functional (solubility, foaming properties and emulsifyings activities) properties of protein hydrolysates derived from rohu roe by enzymatic hydrolysis using pepsin and trypsin as gastrointestinal proteases.
Materials and methods
Materials
MTT (3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl tetrazolium bromide), DMEM medium, foetal bovine serum (FBS), phosphate buffered saline (PBS), dimethyl sulfoxide (DMSO), sodium dodecylsulphate (SDS), Hip-His-Leu, Angiotensin I-converting enzyme (ACE) were obtained from Sigma (St. Louis, MO, USA). Enzymes, pepsin (from hog stomach, 1:3000) and trypsin (from bovine pancreas, 1:250) were obtained from Loba Chemie Pvt. Ltd. (Mumbai, India).
Fresh rohu (Labeo rohita) roes were procured from a local fish market (AP Fisheries Department, Hyderabad, India). Roes were separated from blood vessels, and homogenized using a high speed mixer to obtain fish roe homogenate. The roe mass was dried at 48 ± 2 °C for 8 h in a cabinet tray dryer (Chemida, Mumbai), ground to powder using a high speed mixer and sieved through a 180 μ mesh to produce fish roe powder. It was stored in Schott Duran bottles (Germany) at −20 °C until used for preparation of protein hydrolysates.
Preparation of rohu roe (egg) protein hydrolysates
Rohu (Labeo rohita) roe protein hydrolysates were prepared by gastrointestinal proteases (pepsin and trypsin) according to earlier reported method (Chalamaiah et al. 2013). Briefly, rohu roe powder (5 g, protein content basis) was suspended in 150 ml of distilled water and the mixture was adjusted (0.5 M HCl or 0.5 M NaOH) to the optimum pH 2.0 for pepsin and pH 8.0 for trypsin for proteolytic enzyme hydrolysis. The mixtures were pre-incubated at optimum temperature (37 °C) for 10 min prior to enzymatic hydrolysis. The enzymatic reaction was initiated by the addition of 1.5 % of pepsin or 1 % of trypsin (w/w) on the basis of the protein content of the substrate. The enzymatic reaction was performed (120 min for trypsin or 150 min for pepsin) with continuous stirring at 37 °C. The enzymes were inactivated by keeping the mixture in boiling water bath at 85–95 °C for 20 min. After enzyme inactivation, pepsin reaction mixture was neutralized to pH 7.0 using 1 M NaOH. The slurry was then centrifuged at 13,000 g using Eppendorf centrifuge (Model 5810 R, Germany) for 30 min (4 °C) and the soluble aqueous fraction was decanted, vacuum dried, and stored in Schott Duran bottles at −20 °C until used for experimental work. Protein percent of the hydrolysates was estimated by micro-Kjeldahl method using automatic nitrogen analyzer (Foss Kjeltec Nitrogen Analyzer, Model 8400, Sweden).
Antiproliferative activity on caco-2 cell line
Antiproliferative activity of rohu roe protein hydrolysates was determined by the MTT (3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl tetrazolium bromide) assay (Hsu et al. 2011). The Caco-2 cells (human epithelial colorectal adenocarcinoma cells) were obtained from National Centre for Cell Science (NCCS), Pune, India and seeded in a 96-well flat bottom plate at a density of 1 × 104 cells/well in 100 μl of DMEM medium supplemented with 10 % foetal bovine serum (FBS), L-glutamine and streptomycin 125 mg/L. Cells were allowed to adhere for 24 h at 37 °C humidified atmosphere with 5 % CO2. Hydrolysates were dissolved in PBS (pH 7.4) and sterilized by filtration using 0.2 μm filter. After 24 h of adherence, the medium was removed, and the hydrolysates at different concentrations ranging from 4 to 10 (mg/ml) were added to the cells in 100 μl culture medium. After 24 h exposure, the hydrolysate containing media were removed. Hundred microliter MTT (0.5 mg/ml) was added to each well and incubated for 4 h. After 4 h, MTT was removed, 100 μl of DMSO was added to each well and plate was shaken for 10 min to solubilize formazen crystals and absorbance was read at 570 nm. The following formula was used to calculate percent cell viability.
where At is absorbance of treated cells and Ac is absorbance of control cells
Measurement of ACE inhibitory activity
The ACE inhibitory activity was measured by the method of Cushman and Cheung (1971) with slight modification. 50 μl of rohu roe protein hydrolysates (mg, on protein content basis) with 50 μl of ACE solution (25 mU/ml) was pre-incubated at 37 °C for 10 min, and the mixture was incubated with 100 μl of substrate (8.3 mM Hip-His-Leu in 50 mM sodium borate buffer containing 0.3 M NaCl, pH 8.3) for 60 min at 37 °C. Control sample was carried out using distilled water instead of sample. The reaction was terminated with the addition of 250 μl of 1 M HCl. The resulting hippuric acid was extracted with 1.5 ml of ethyl acetate. After centrifugation (3000 g, 10 min), 1 ml upper layer of the extract was evaporated at 80 °C to dryness. The hippuric acid was dissolved in 3.0 ml of distilled water, and the absorbance was read at 228 nm using UV-visible spectrophotometer (Perkin-Elmer Lambda 1, USA). The ACE inhibitory activity was calculated using following formula:
Determination of functional properties
Protein solubility
Solubility of the hydrolysates was measured according to the method of Klompong et al. (2007). Briefly, 200 mg of vacuum-dried hydrolysates of rohu egg was suspended in 20 ml distilled water and the pH of the mixture was adjusted to different values using either 0.5 N HCl or 0.5 N NaOH solutions. The mixture was stirred at room temperature (25 ± 2 °C) for 30 min, and then centrifuged at 4500 g for 30 min. Protein content of the supernatant was determined using the Biuret method (Weichselbaum 1946). Protein solubility was calculated using the following formula.
Foaming properties
Foam capacity and foam stability were measured by following the method of Klompong et al. (2007), with slight modification. Undigested rohu roe protein and its hydrolysates (0.5 %) were dissolved in 20 ml of distilled water, pH was adjusted to 2–10 using 0.5 N HCl or 0.5 N NaOH, and the contents were transferred into 100 ml measuring cylinder and whipped for 30 s. Total volume was noted immediately at 0, 30 and 60 min. Foam capacity and foam stability were calculated according to the following equations.
A = the volume after whipping at ‘0’ min (ml)
B = the volume before whipping (ml)
A = the volume at 30 and 60 min (ml)
B = the volume before whipping (ml)
Emulsifying properties
Emulsifying activity index (EAI) and emulsion stability index (ESI) were measured according to the method of Pearce and Kinsella (1978). Undigested rohu roe proteins and its hydrolysates (1 %, w/v) were dissolved in distilled water for 5 min at room temperature (25 ± 2 °C). Then fifteen milliliters of each solutions was mixed with 5 ml of sunflower oil and pH was adjusted (2–10) using 0.5 N HCl or 0.5 N NaOH. The mixture was homogenized for 1 min at a speed of 18,000 rpm using IKA T25 digital Ultr-Turrax homogenizer. An aliquot of the emulsion (50 μl) was taken from bottom of the tube at 0 and 10 min after homogenization and mixed with 5 ml of 0.1 % sodium dodecylsulphate (SDS) solution (1:100 dilution). The absorbance of the diluted solution was monitored at 500 nm using UV-Visible spectrophotometer (Perkin-Elmer Lambda 1, USA). The absorbances measured immediately (A0) and 10 min (A10) after emulsion formation were used to calculate emulsifying activity index (EAI) and emulsion stability index (ESI). EAI and ESI were calculated using the following formula.
Where dil is the dilution factor (100);
A0 is the absorbance at 500 nm;
c is the protein concentration (g/ml), 0.01;
θ is the disperse phase volume fraction (0.25).
Where ΔA = A0 – A10 and Δt = 10 min.
Statistical analysis
Results are presented as mean ± SD of the triplicates (n = 3). Significant differences (P < 0.05) between means were identified by using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL).
Results and discussion
Protein content of the hydrolysates was 69 ± 0.57 and 73 ± 1.59 %, respectively for pepsin and trypsin hydrolysates (Chalamaiah et al. 2013).
Antiproliferative activity
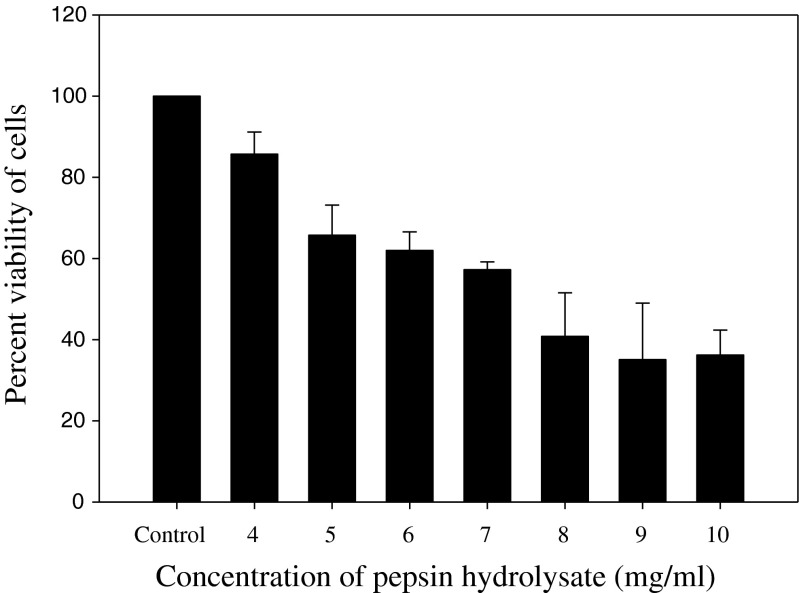
Figure 1 shows the antiproliferative effects of the protein hydrolysates derived from rohu roe at different concentrations on colon cancer cells (Caco-2 cells). Pepsin hydrolysate showed significant antiproliferative activity in a dose dependent manner. The maximum inhibitory effect observed was 65 ± 13.9 % at 9 mg/ml concentration. Trypsin hydrolysate did not show any significant (P < 0.05) antiproliferative activity (data not shown). Hsu et al. (2011) produced protein hydrolysates from tuna dark muscle byproduct using two commercial enzymes, papain and Protease XXIII, and reported antiproliferative activity (~40 % inhibition at 60 min degree of hydrolysis) on human breast cancer cell line MCF-7. Antiproliferative activity (65 %) shown by pepsin hydrolysate from rohu egg is higher than antiproliferative activity of tuna dark muscle byproduct protein hydrolysates (Hsu et al. 2011) and squid gelatin hydrolysates (Aleman et al. 2011). The antiproliferative activity shown in this study by pepsin hydrolysate could be related to different peptide sizes, sequences and compositions from specific cleavage by pepsin (Klompong et al. 2007; Wiriyaphan et al. 2012). The result suggests that the antiproliferative activity could be related to the presence of specific peptides exerting a direct cytotoxicity on cancer cells, as previously reported for an anchovy peptide, able to induce apoptosis in human U937 lymphoma cells through the increase of caspase-3 and caspase-8 activity (Lee et al. 2003; Lee et al. 2004). Previously, whiting, plaice and salmon hydrolysates had been identified as significant growth inhibitors on two human breast carcinoma cell lines, MCF-7/6 and MDA-MB-231 (Picot et al. 2006).
Fig. 1.
Dose dependent anti-proliferative activity of rohu roe protein hydrolysate, produced by pepsin, after 24 h of incubation with Caco-2 cells (n = 4)
ACE-inhibitory activity
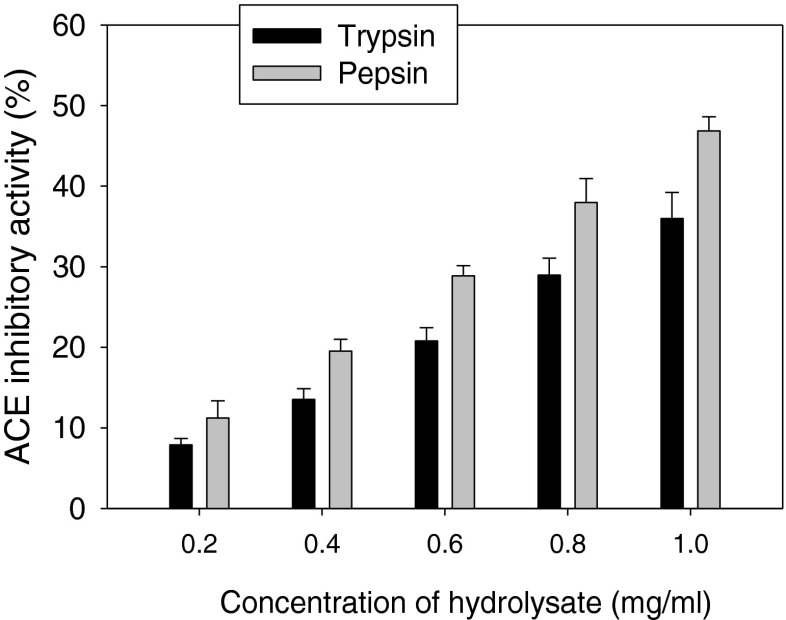
Pepsin and trypsin hydrolysates exhibited an interesting angiotensin I-converting enzyme (ACE) inhibition in a dose dependent manner (Fig. 2). The maximum ACE inhibitory activity was found to be 47 ± 1.7 % and 36 ± 3.2 % for pepsin and trypsin hydrolysates, respectively. ACE plays an important role in the regulation of blood pressure and cardiovascular function (Vercruysse et al. 2005). It catalyzes the conversion of the inactive angiotensin I to angiotensin II, a potent vasoconstrictor and also inactivates the antihypertensive vasodilator bradykinin (Vercruysse et al. 2005). As shown in Fig. 2, the pepsin hydrolysate exhibited higher ACE inhibitory activity than trypsin hydrolysate. The type of proteolytic enzyme significantly (P < 0.05) affected ACE inhibitory activity of protein hydrolysates. Gastrointestinal digestive proteases have often been used for production of bioactive peptides with ACE inhibitory activity (Lin et al. 2012). Many studies have reported that low molecular weight peptides are better ACE inhibitors than high MW peptides (Raghavan and Kristinsson 2009; Barbana and Boye 2010; Nasri et al. 2014). In the current study, the low molecular mass peptides present in the pepsin hydrolysate might have contributed to higher ACE inhibitory activity than trypsin hydrolysate (Chalamaiah et al. 2013). In addition, the aromatic and hydrophobic amino acids present in the pepsin and trypsin hydrolysates play an important role in ACE inhibitory activity (Chalamaiah et al. 2013; Lee et al. 2011; Nasri et al. 2014). The ACE inhibitory activity of rohu roe hydrolysates was lower than those of goby fish muscle protein hydrolysates and squid skin gelatin hydrolysates generated by gastrointestinal proteases (Nasri et al. 2013a, b; Lin et al. 2012), and but higher than that of tilapia protein hydrolysate fractions produced by cryotin and Flavourzyme (Raghavan and Kristinsson 2009).
Fig. 2.
In vitro ACE-inhibitory activity of rohu roe protein hydrolysates produced by pepsin and trypsin enzymes. Values are mean of triplicates ± SD
Functional properties
Protein solubility
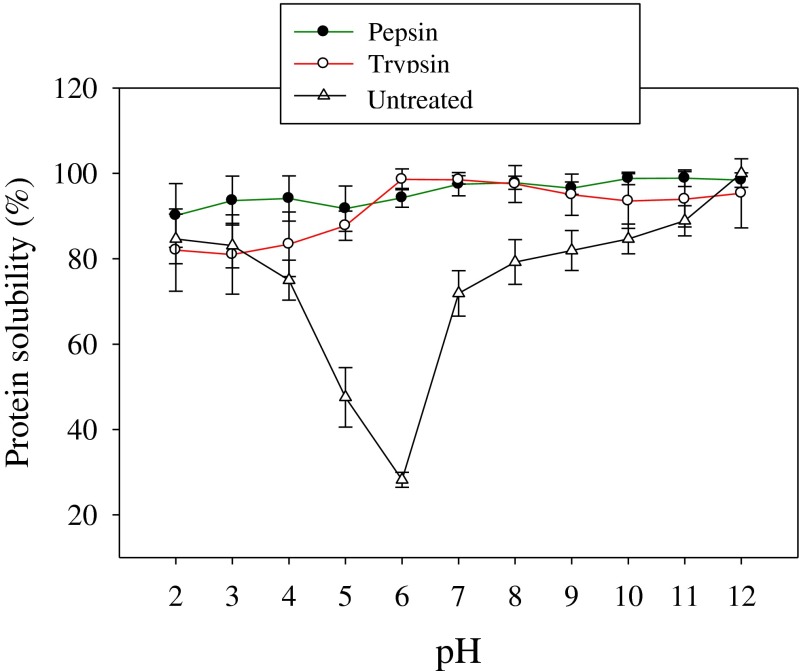
Solubility is one of the most important functional properties of protein hydrolysates and it also affects other functional properties such as emulsification and foaming properties (Kristinsson and Rasco 2000). Production of high-solubility protein hydrolysates is essential for many food applications (Thiansilakul et al. 2007; Ben Khaled et al. 2014). Figure 3 shows protein solubility profiles for rohu roe proteins and hydrolysates. Enzymatic hydrolysis by pepsin and trypsin significantly improved the solubility of rohu roe proteins. Both hydrolysates share solubility profiles, exhibiting higher solubility values above 81 % over wide pH range of 2–12. This profile is quite similar to those of protein hydrolysates from zebra blenny fish (Ktari et al. 2012). Furthermore, the maximum solubility >98 % was observed under alkaline conditions for both hydrolysates. The solubility profile of undigested roe protein decreased at pH 5 and 6 which may be the isoelectric point of protein. Hydrophobic and ionic interactions are the major factors that influence the solubility characteristics of proteins/protein hydrolysates (Kristinsson and Rasco 2000). From pH values 2 to 4, pepsin and trypsin hydrolysates exhibited a slight difference in solubility. The differences in solubility of roe hydrolysates may be related to the hydrophobic–hydrophilic balance and as well as the charge group of the peptides produced during the hydrolysis process (Ktari et al. 2012). Hydrophobic interactions promote protein-protein interactions and result in decreased solubility, whereas ionic interactions promote protein-water interactions and result in increased solubility (Kristinsson and Rasco 2000). As shown in the Fig. 3, the solubility of pepsin and trypsin hydrolysates from rohu roe proteins was higher than that obtained by the protein hydrolysates from pink perch muscle (Naqash and Nazeer 2013) and the protein hydrolysates prepared from Cirrhinus mrigala roe (Chalamaiah et al. 2010) exhibited comparable solubility profiles. The present results clearly indicated that the enzymatic hydrolysis by pepsin and trypsin was an effective way to increase solubility of rohu roe proteins.
Fig. 3.
Solubility profiles of rohu roe protein hydrolysates as affected by pH. Values are mean of triplicates ± SD
Foaming properties
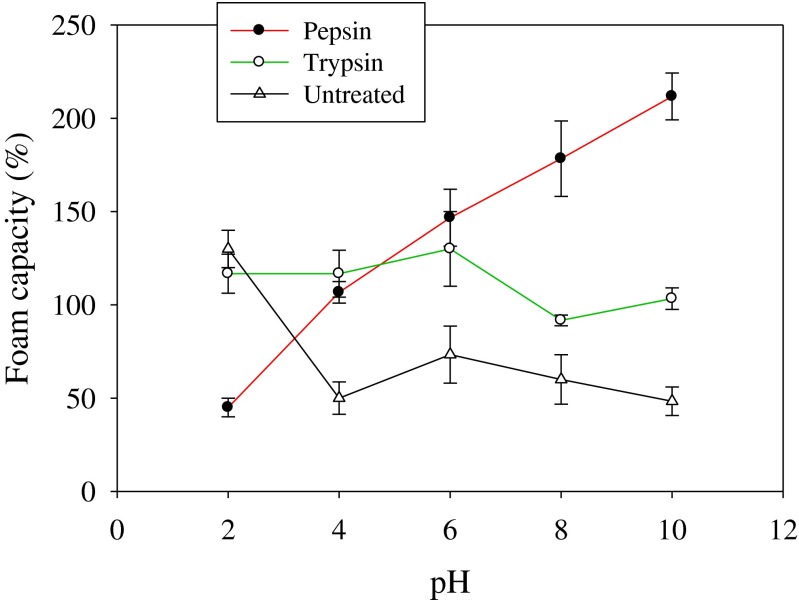
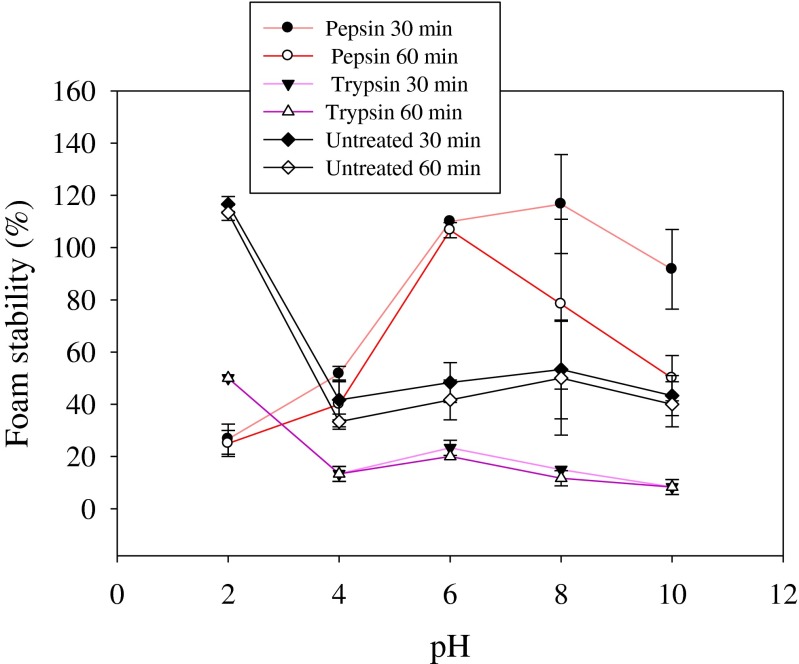
Foam capacity (FC) and foam stability (FS) of the hydrolysates and the control at different pH (2–10) are shown in Figs. 4 and 5, respectively. Foam formation is important in food applications such as beverages, mousses, meringue cakes and whipped toppings (Boye et al. 2010). As shown in Fig. 4, the hydrolysis of rohu roe proteins using pepsin and trypsin improves FC, except at pH 2. Furthermore, FC of pepsin hydrolysate increases with the increase of pH, despite its decreases for trypsin hydrolysate. Highest foaming capacity (211 %) was observed for pepsin hydrolysate at pH 10.0. The pH of the dispersing medium significantly influences foaming properties, especially foam stability (Hmidet et al. 2011). The results are in line with previous findings of foaming properties of protein hydrolysates prepared from cuttlefish (Sepia officinalis) muscle (Hmidet et al. 2011). Pepsin hydrolysate showed superior foam stability than undigested roe proteins, while trypsin hydrolysate exhibited lower foam stability than roe proteins. Hydrolysates with longer peptide chain lengths produce foam with higher stability (Intarasirisawat et al. 2012). Longer chain peptides could form thicker and stronger films surrounding air bubbles, thereby increasing the foam stability. Recently, Intarasirisawat et al. (2012) studied the foaming properties of skipjack roe protein hydrolysates at 0 and 30 min after whipping and reported that the hydrolysates exhibited good foam expansion and foam stability, which were in line with the present study results.
Fig. 4.
Foam capacity of rohu roe protein hydrolysates produced by pepsin and trypsin enzymes. Values are mean of triplicates ± SD
Fig. 5.
Foam stability of rohu roe protein hydrolysates, produced by pepsin and trypsin enzymes, as affected by pH. Values are mean of triplicates ± SD
Emulsifying properties
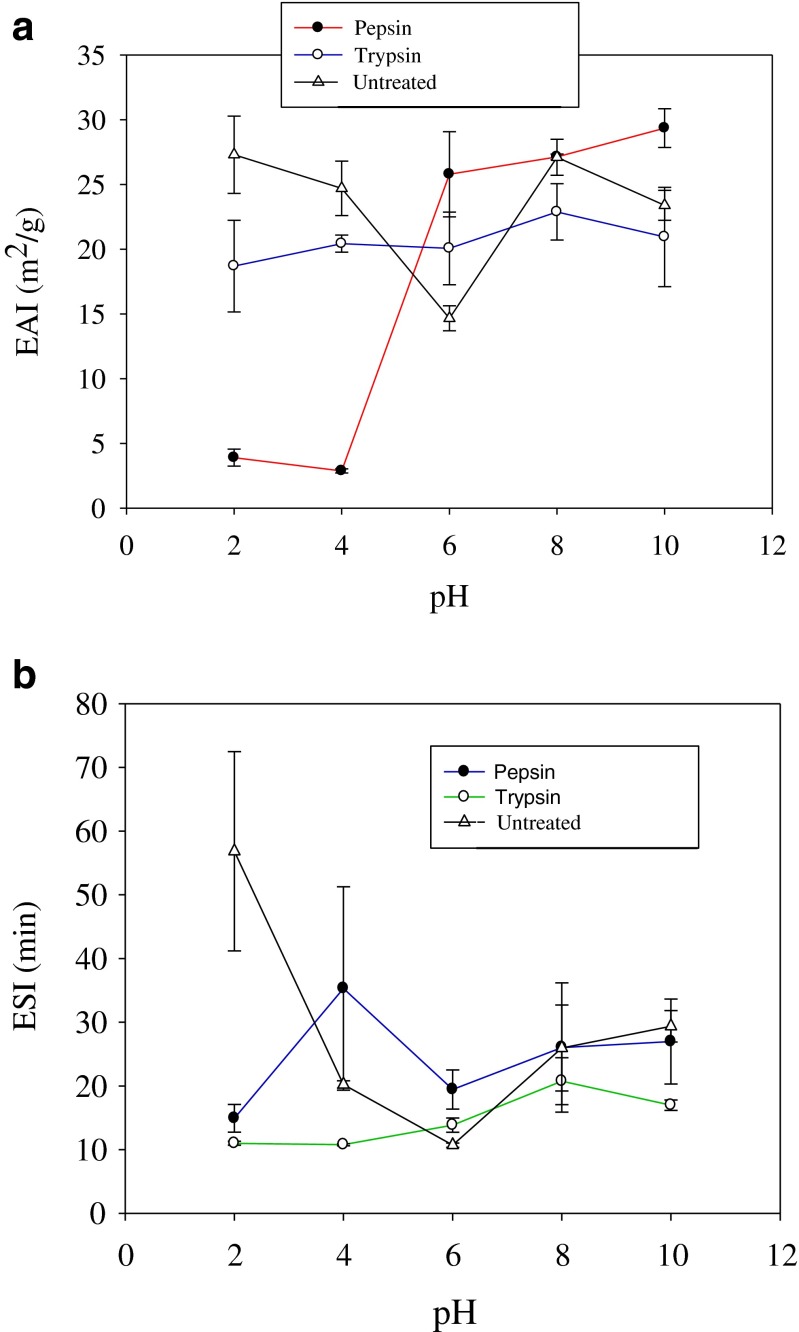
The capacity of protein hydrolysates/proteins to form stable emulsions is important since interactions between proteins and lipids are essential for application in many food systems. Emulsifying activity index (EAI) and emulsion stability index (ESI) are two methods commonly used to measure the ability of protein hydrolysates to form and stabilize emulsions (Klompong et al. 2007; Ben Khaled et al. 2014). Figure 6a, b shows the EAI and ESI of rohu roe hydrolysates at different pH values of 2–10. Both hydrolysates exhibited good EAI and ESI at pH 6. Protein hydrolysates are surface active substances that promote oil in water emulsion because of their hydrophobic and hydrophilic charges (Kristinsson and Rasco 2000). EAI and ESI of protein hydrolysate produced by pepsin showed significantly (P < 0.05) higher values than trypsin hydrolysate. Protease specificity plays a key role in the emulsification properties of protein hydrolysates (Kristinsson and Rasco 2000). The hydrolysates showed lowest EAI at pH 2 and 4 than un-hydrolyzed roe proteins. This may be due to the presence of smaller peptides, which are less effective in stabilizing emulsions (Kristinsson and Rasco 2000). Klompong et al. (2007) have also reported lowest EAI at pH 4 for yellow stripe trevally (Selaroides leptolepis) meat protein hydrolysates. In a recent study, Chalamaiah et al. (2015) reported significant effect of pH on EAI and ESI of common carp (Cyprinus carpio) roe protein hydrolysates. In Fig. 6b the results indicate that enzymatic hydrolysis strongly influenced the emulsion stability index (ESI) of rohu egg protein hydrolysates. Unhydrolysed (untreated) egg proteins showed good emulsion stability index (ESI) than the hydrolysates. The large molecular weight proteins are known to form stable emulsions than the hydrolysates. Enzymatic hydrolysis brings about the loss of emulsifying properties (Klompong et al. 2007; Kristinsson and Rasco 2000). The low molecular mass peptides in the hydrolysates are generally less efficient in stabilizing the emulsions. Hence, untreated group showed greater ESI than the hydrolysates. Intarasirisawat et al. (2012) reported EAI and ESI in the range of 5.1–25.16 m2/g and 14.2–24.3 min respectively, for skipjack roe protein hydrolysate which is in agreement with our results.
Fig. 6.
a Emulsifying activity index (EAI) of rohu roe protein hydrolysates, produced by pepsin and trypsin enzymes, as affected by pH. Values are mean of triplicates ± SD. b Emulsion stability index (ESI) of rohu roe protein hydrolysates, produced by pepsin and trypsin enzymes, as affected by pH. Values are mean of triplicates ± SD
Conclusion
Pepsin hydrolysate derived from rohu roe showed potent antiproliferative activity against Caco-2 cells. Pepsin and trypsin hydrolysates exhibited ACE inhibitory activity in a dose dependent manner. The roe hydrolysates showed high solubility (>81 %) at wide pH range of 2–12, good foaming and emulsification properties. Furthermore functional properties were affected by the pH. Bioactive hydrolysates obtained from rohu roe could be used as functional ingredient in food/nutraceutical/pharmaceutical industry.
Acknowledgments
We are grateful to the Indian Council of Medical Research (ICMR), New Delhi for Senior Research Fellowship (SRF) (No. 3/1/2/19/2010-RHN) for M. Chalamaiah. Authors wish to thank Director, NIN (ICMR) and Director, CSIR-CFTRI for their keen interest and permission for publication.
Contributor Information
M. Chalamaiah, Email: chalamaiah_230@rediffmail.com
B. Dinesh Kumar, Email: pctgeneral@gmail.com
References
- Agyei D, Danquah MK. Rethinking food-derived bioactive peptides for antimicrobial and immunomodulatory activities. Trends Food Sci Technol. 2012;23:62–69. doi: 10.1016/j.tifs.2011.08.010. [DOI] [Google Scholar]
- Aleman A, Perez-Santin E, Bordenave-Juchereau S, Arnaudin I, Gomez-Guillen MC, Montero P. Squid gelatin hydrolysates with antihypertensive, anticancer and antioxidant activity. Food Res Int. 2011;44:1044–1051. doi: 10.1016/j.foodres.2011.03.010. [DOI] [Google Scholar]
- Aleman A, Gomez-Guillen MC, Montero P. Identification of ace-inhibitory peptides from squid skin collagen after in vitro gastrointestinal digestion. Food Res Int. 2013;54:790–795. doi: 10.1016/j.foodres.2013.08.027. [DOI] [Google Scholar]
- Barbana C, Boye JI. Angiotensin I-converting enzyme inhibitory activity of chickpea and pea protein hydrolysates. Food Res Int. 2010;43:1642–1649. doi: 10.1016/j.foodres.2010.05.003. [DOI] [Google Scholar]
- Ben Khaled H, Ktari N, Ghorbel-Bellaaj O, Jridi M, Lassoued I, Nasri M. Composition, functional properties and in vitro antioxidant activity of protein hydrolysates prepared from sardinelle (Sardinella aurita) muscle. J Food Sci Technol. 2014;51:622–633. doi: 10.1007/s13197-011-0544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougatef A, Nedjar-Arroume N, Ravallec-Ple R, Leroy Y, Guillochon D, Barkia A, Nasri M. Angiotensin I-converting enzyme (ACE) inhibitory activities of sardinelle (Sardinella aurita) by-products protein hydrolysates obtained by treatment with microbial and visceral fish serine proteases. Food Chem. 2008;111:350–356. doi: 10.1016/j.foodchem.2008.03.074. [DOI] [PubMed] [Google Scholar]
- Boye J, Zare F, Pletch A. Pulse proteins: processing, characterization, functional properties and applications in food and feed. Food Res Int. 2010;43:414–431. doi: 10.1016/j.foodres.2009.09.003. [DOI] [Google Scholar]
- Chalamaiah M, Narsing Rao G, Rao DG, Jyothirmayi T. Protein hydrolysates from meriga (Cirrhinus mrigala) roe and evaluation of their functional properties. Food Chem. 2010;120:652–657. doi: 10.1016/j.foodchem.2009.10.057. [DOI] [Google Scholar]
- Chalamaiah M, Dinesh Kumar B, Hemalatha R, Jyothirmayi T. Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem. 2012;135:3020–3038. doi: 10.1016/j.foodchem.2012.06.100. [DOI] [PubMed] [Google Scholar]
- Chalamaiah M, Jyothirmayi T, Bhaskarachary K, Vajreswari A, Hemalatha R, Dinesh Kumar B. Chemical composition, molecular mass distribution and antioxidant capacity of rohu (Labeo rohita) roe (egg) protein hydrolysates prepared by gastrointestinal proteases. Food Res Int. 2013;52:221–229. doi: 10.1016/j.foodres.2013.03.020. [DOI] [Google Scholar]
- Chalamaiah M, Hemalatha R, Jyothirmayi T, Diwan P.V., Uday Kumar P, Chetan N, Dinesh Kumar B. Immunomodulatory effects of protein hydrolysates from rohu (Labeo rohita) egg in BALB/c mice. Food Res Int. 2014;62:1054–1061. doi: 10.1016/j.foodres.2014.05.050. [DOI] [Google Scholar]
- Chalamaiah M, Jyothirmayi T, Diwan P.V., Dinesh Kumar B. Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg) J Food Sci Technol. 2015 doi: 10.1007/s13197-015-1714-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chi Y, Zhao M, Xu W. Influence of degree of hydrolysis on functional properties, antioxidant and ACE inhibitory activities of egg white protein hydrolysate. Food Sci Biotechnol. 2012;21:27–34. doi: 10.1007/s10068-012-0004-6. [DOI] [Google Scholar]
- Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- Gimenez B, Aleman A, Montero P, Gomez-Guillen MC. Antioxidant and functional properties of gelatin hydrolysates obtained from skin of sole and squid. Food Chem. 2009;114:976–983. doi: 10.1016/j.foodchem.2008.10.050. [DOI] [Google Scholar]
- He S, Franco C, Zhang W. Functions, applications and production of protein hydrolysates from fish processing co-products (FPCP) Food Res Int. 2013;50:289–297. doi: 10.1016/j.foodres.2012.10.031. [DOI] [Google Scholar]
- Hmidet N, Balti R, Nasri R, Sila A, Bougatef A, Nasri M. Improvement of functional properties and antioxidant activities of cuttlefish (Sepia officinalis) muscle proteins hydrolyzed by Bacillus mojavensis A21 proteases. Food Res Int. 2011;44:2703–2711. doi: 10.1016/j.foodres.2011.05.023. [DOI] [Google Scholar]
- Hsu K, Li-Chan ECY, Jao C. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011;126:617–622. doi: 10.1016/j.foodchem.2010.11.066. [DOI] [Google Scholar]
- Intarasirisawat R, Benjakul S, Visessanguan W. Chemical compositions of the roes from skipjack, tongol and bonita. Food Chem. 2011;124:1328–1334. doi: 10.1016/j.foodchem.2010.07.076. [DOI] [Google Scholar]
- Intarasirisawat R, Benjakul S, Visessanguan W. Antioxidative and functional properties of protein hydrolysate from defatted skipjack (Katsuwonous pelamis) roe. Food Chem. 2012;135:3039–3048. doi: 10.1016/j.foodchem.2012.06.076. [DOI] [PubMed] [Google Scholar]
- Je JY, Lee KH, Lee MH, Ahn CB. Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Res Int. 2009;42:1266–1272. doi: 10.1016/j.foodres.2009.06.013. [DOI] [Google Scholar]
- Jianping W, Rotimi EA, Alister DM. Production of angiotensin I converting enzyme inhibitory peptides from defatted canola meal. Bioresour Technol. 2009;100:5283–5287. doi: 10.1016/j.biortech.2009.03.090. [DOI] [PubMed] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Kristinsson HG, Rasco BA. Fish protein hydrolysates: production, biochemical and functional properties. Crit Rev Food Sci Nutr. 2000;40:43–81. doi: 10.1080/10408690091189266. [DOI] [PubMed] [Google Scholar]
- Ktari N, Jridi M, Bkhairia I, Sayari N, Salah RB, Nasri M. Functionalities and antioxidant properties of protein hydrolysates from muscle of zebra blenny (Salaria basilisca) obtained with different crude protease extracts. Food Res Int. 2012;49:747–756. doi: 10.1016/j.foodres.2012.09.024. [DOI] [Google Scholar]
- Lee YG, Kim JY, Lee KW, Kim KH, Lee HJ. Peptides from anchovy sauce induce apoptosis in a human lymphoma cell (U937) through the increase of caspase-3 and -8 activity. Ann N Y Acad Sci. 2003;1010:399–404. doi: 10.1196/annals.1299.073. [DOI] [PubMed] [Google Scholar]
- Lee YG, Lee KW, Kim JY, Kim KH, Lee HJ. Induction of apoptosis in a human lymphoma cell line by hydrophobic peptide fraction separated from anchovy sauce. Biofact. 2004;21:63–67. doi: 10.1002/biof.552210112. [DOI] [PubMed] [Google Scholar]
- Lee SH, Qian ZJ, Kim SK. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. J Agric Food Chem. 2010;118:96–102. doi: 10.1016/j.foodchem.2009.04.086. [DOI] [Google Scholar]
- Lee JK, Jeon J, Byun H. Effect of angiotensin I converting enzyme inhibitory peptide purified from skate skin hydrolysate. Food Chem. 2011;125:495–499. doi: 10.1016/j.foodchem.2010.09.039. [DOI] [Google Scholar]
- Li G, Qu M, Wan J, You J. Antihypertensive effect of rice protein hydrolysate with in vitro angiotensin I-converting enzyme inhibitory activity in spontaneously hypertensive rats. Asia Pac J Clin Nutr. 2007;16:275–280. [PubMed] [Google Scholar]
- Lin L, Lv S, Li B. Angiotensin-I-converting enzyme (ACE)-inhibitory and antihypertensive properties of squid skin gelatin hydrolysates. Food Chem. 2012;131:225–230. doi: 10.1016/j.foodchem.2011.08.064. [DOI] [Google Scholar]
- Naqash SY, Nazeer RA. Antioxidant and functional properties of protein hydrolysates from pink perch (Nemipterus japonicus) muscle. J Food Sci Technol. 2013;50:972–978. doi: 10.1007/s13197-011-0416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasri R, Chataigne G, Dhulster P, Nasri M, Karra-Chaabouni M, Nedjar Arroume N. Novel angiotensin I-converting enzyme inhibitory peptides from enzymatic hydrolysates of Goby (Zosterisessor ophioccephalus) muscle proteins. J Proteome. 2013;91:444–452. doi: 10.1016/j.jprot.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Nasri R, Younes I, Jridi M, Trigui M, Bougatef A, Nedjar-Arroume N, Dhulster P, Nasri M, Karra-Chaabouni M. ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophiocephalus) fish protein hydrolysates: effect on meat lipid oxidation. Food Res Int. 2013;54:552–561. doi: 10.1016/j.foodres.2013.07.001. [DOI] [Google Scholar]
- Nasri R, Jridi M, Lassoued I, Jemil I, Ben Slama-Ben Salem R, Nasri M, Karra-Chaabouni M. The influence of the extent of enzymatic hydrolysis on oxidative activities and ACE inhibitory activities of protein hydrolysates from goby (Zosterisessor ophiocephalus) muscle. Appl Biochem Biotechnol. 2014;173:1121–1134. doi: 10.1007/s12010-014-0905-3. [DOI] [PubMed] [Google Scholar]
- Pearce KN, Kinsella JE. Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem. 1978;26:716–723. doi: 10.1021/jf60217a041. [DOI] [Google Scholar]
- Picot L, Bordenave S, Didelot S, Fruitier-Arnaudin I, Sannier F, Thorkelsson G, Berge JP, Chabeaud A, Piot JM. Antiproliferative activity of fish protein hydrolysates on human breast cancer cell lines. Process Biochem. 2006;41:1217–1222. doi: 10.1016/j.procbio.2005.11.024. [DOI] [Google Scholar]
- Raghavan S, Kristinsson HG. ACE-inhibitory activity of tilapia protein hydrolysates. Food Chem. 2009;117:582–588. doi: 10.1016/j.foodchem.2009.04.058. [DOI] [Google Scholar]
- Revilla E, Santa-Maria C, Miramontes E, Candiracci M, Rodriguez-Morgado B, Carballo M, Bautista J, Castano A, Parrado J. Antiproliferative and immunoactivatory ability of an enzymatic extract from rice bran. Food Chem. 2013;136:526–531. doi: 10.1016/j.foodchem.2012.08.044. [DOI] [PubMed] [Google Scholar]
- Saavedra L, Hebert EM, Minahk C, Ferranti P. An overview of “omic” analytical methods applied in bioactive peptide studies. Food Res Int. 2013;54:925–934. doi: 10.1016/j.foodres.2013.02.034. [DOI] [Google Scholar]
- Sanchez D, Kassan M, Contreras MM, Carron R, Recio I, Montero M, Sevilla MA. Long-term intake of a milk casein hydrolysate attenuates the development of hypertension and involves cardiovascular benefits. Pharmacol Res. 2011;63:398–404. doi: 10.1016/j.phrs.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Zhong Y. Bioactive peptides. J AOAC Int. 2008;91:914–931. [PubMed] [Google Scholar]
- Thiansilakul Y, Benjakul S, Shahidi F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi) Food Chem. 2007;103:1385–1394. doi: 10.1016/j.foodchem.2006.10.055. [DOI] [Google Scholar]
- Vercruysse L, Van Camp J, Smagghe G. ACE inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein: a review. J Agric Food Chem. 2005;53:8106–8015. doi: 10.1021/jf0508908. [DOI] [PubMed] [Google Scholar]
- Weichselbaum TE. An accurate and rapid method for the determination of proteins in small amounts of blood serum and plasma. Am J Clin Pathol. 1946;16(Tech. Suppl.):40. [PubMed] [Google Scholar]
- Wiriyaphan C, Chitsomboon B, Yongsawadigul J. Antioxidant activity of protein hydrolysates derived from threadfin bream surimi byproducts. Food Chem. 2012;132:104–111. doi: 10.1016/j.foodchem.2011.10.040. [DOI] [PubMed] [Google Scholar]
- Yang HY, Yang SC, Chen RR, Tzeng YH, Han BC. Soybean protein hydrolysate prevents the development of hypertension in spontaneously hypertensive rats. British J Nutr. 2004;92:507–512. doi: 10.1079/BJN20041218. [DOI] [PubMed] [Google Scholar]