Abstract
This paper presents a comparison of the contents of capsaicin, dihydrocapsaicin and total phenolics as well as of the antioxidant activities of six types of peppers of the genus Capsicum. The varieties were analyzed in terms of their in vitro antioxidant activity using ferric reducing antioxidant powder (FRAP), 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2’-azinobis 3-ethylbenzothiazoline 6-sulfonate (ABTS●+) assays. The contents of phenolics and capsainoids as well as the antioxidant activities were higher in seeds than in pulps. The correlations (ρ < 0.01) between the phenolic composition and the capsaicinoids levels were high (r = 0.98). Similarly high were also the correlations between the antioxidant activities and the contents of total phenolics and capsaicinoids. Data were analyzed using principal component analysis (PCA), hierarchical cluster analysis (HCA) and multiple linear regression (MLR). PCA explained 97.77 % of the total variance of the data, and their separation into three groups in a scatter plot was divised. Using HCA, three clusters were suggested. Cluster one, formed by pulps (bell pepper, orange habanero, cayenne, dedo de moça and red habanero), showed the lowest levels of the compounds quantified. Most seed samples were grouped in cluster two (bell pepper, cayenne, dedo de moça and malagueta) together with malagueta pulp. Cluster three was formed by orange and red habanero seeds, which showed the highest levels of all compounds analyzed. The MRL revealed that the values of capsaicinoids and total phenols are more adequate to predict the antioxidant activity measured by the FRAP assay.
Keywords: Capsicum, Capsaicinoids, Phenolics, Antioxidant activity, Multivariate analysis
Introduction
Numerous spices contain chemical compounds exhibiting antioxidant properties. These properties are attributed to a variety of active phytochemicals including vitamins, carotenoids, alkaloids, flavonoids, simple phenols, phenolic acids, etc. (Brewer 2011). Peppers (Capsicum spp.), are grown worldwide, used extensively as a natural food colorant and seasoning agent due to their attractive color, flavor, and taste (Reyes-Escogido et al. 2011). Pepper has a high nutritive value and has long been recognized as an excellent source of vitamin C, carotenoids, phenolic compounds and other phytochemicals that are powerful antioxidants that destroy free radicals (Asnin and Park 2015; Kothari et al. 2010). The levels of these compounds in pepper depend on many factors, including cultivar, maturity, growing conditions, and climate (Menichini et al. 2009; Zhuang et al. 2012). Numerous studies have examined peppers mainly to evaluate the chemical composition and/or antioxidant activities of the various cultivars (Deepa et al. 2006; Zhuang et al. 2012) and the effects of drying and cooking methods on their physicochemical properties (Hwang et al. 2012; Scala and Crapiste 2008; Vega-Gálvez et al. 2009; Yaldiz et al. 2010).
One of the important commercial attribute of peppers is its pungency. The pungency is due to the presence of six chemically related compounds knowed as capsaicinoids (Deepa et al. 2007). The two most abundant capsaicinoids in peppers are capsaicin (trans-8-metil-N-vanilil-6-nonenamide) and dihydrocapsaicin (8-metil-N-vanillylnonanamide), both constituting around 90 %, with capsaicin accounting for ~71 % of the total capsaicinoids in most of the pungent varieties (Barbero et al. 2014). The capsaicin content of peppers is one of the major parameters that determine their commercial quality. Capsaicin is also the active principle that accounts for the pharmaceutical properties of peppers. It has been used as an analgesic against arthritis pain and inflammation (Srinivasan 2013). A beneficial role of capsaicin has been reported in obesity, cardiovascular and gastrointestinal conditions, various cancers, neurogenic bladder, and dermatologic conditions (Sharma et al. 2013).
The capsainoid molecules can be divided into three regions, aromatic ring containing a OH- group, an amide bond and a hydrophobic side (Reyes-Escogido et al. 2011). This particular structure is due to the fact that the capsaicinoids are synthesized naturally in the placenta of fruits by enzymatic condensation of vanillylamine (the phenolic portion of the molecule) and different-sized fatty acid chains which are elongated by a fatty acid synthase (Reyes-Escogido et al. 2011). The seeds are not the primary source of pungency but they occasionally absorb capsaicin because they are in close proximity to the placenta (Arora et al. 2011; Pandhair and Sharma 2008). The majority of studies, including those where capsaicin was purified, have been conducted using the whole fruits for quantifying the capsainoids (Yaldiz et al. 2010). The separation of seed and pulps can be useful to evaluate the distribution of capsainoids in the fruits. Moreover, it should be stressed that capsaicinoids and phenolics are biosynthetically derived from the phenylpropanoid pathway and that both possess antioxidant activities (Asnin and Park 2015). This particularity makes it important to correlate the contents of capsaicin and dihidrocapsaicin with the phenolic contents and antioxidant capacities of both pulp and seed extracts.
Multivariate analyses are more accurate than univariate comparisons (Zielinski et al. 2014b), a reason why they are increasingly applied in the characterization, determination of origin, authentication and adulteration, and quality control of food products. In line with this new methodological tendency the objective of the present study was to compare the capsaicin, dihidrocapsaicin and phenolic contents as well as the antioxidant activities of pulp and seed extracts of six different peppers of the genus Capsicum using multivariate statistical techniques.
Materials and methods
Standards and reagents
Capsaicin, dyhydrocapsaicin, gallic acid, Folin-Ciocalteu reagent, sodium carbonate, ethyl alcohol, acetic acid, methanol, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azinobis 3-ethylbenzothiazoline 6-sulfonate (ABTS) and tripyridyltriazine were purchased from Sigma-Aldrich Co (St Louis, USA). All other reagents used in the experiments were of analytical grade.
Samples
Six varieties of the genus Capsicum were used in this work: C. annuum (cayenne pepper and bell pepper), C. baccatum var. pendulum (known in Brazil as dedo de moça pepper), C. chinense (red habañero pepper and orange habañero pepper) and C. frutescens (malagueta pepper). Three samples of each pepper were purchased in three different supermarkets between April and June 2013. They were all produced in the Northwest Paraná region, Southern Brazil (23°21’ South latitude, 52°04’ West longitude and 510 m altitude), between March and May 2013.
Ethanolic extracts
Pulps and seeds were manually separated and grounded in a blender. For extracting the phenolics from the pulps, a volume of 20 mL of 40 % ethanol in water was added to 10 g of pulp (Haminiuk et al. 2011). For extracting the phenolics from the seeds, a volume of 40 mL of 80 % ethanol in water was added to 5 g of each seed (Bae et al. 2012). The mixtures were maintained in a rotary shaker at 22 rpm for 24 h at room temperature in the dark and thereafter centrifuged at 3370 g for 10 min at 5 ° C. The supernatants were then filtered using Whatman paper n° 41 and maintained at −20 °C until analysis.
HPLC analysis
Thermo Scientific Dionex, model UltiMate™ 3000 equipped with LPG- 3400SD Dionex pump, Bannockburn, IL, EUA), column of the sample compartment liquid chromatograph Ultimate 3000, photodiode detector 3000 was employed for the HPLC analyses. Data analysis and instrument control were controlled by Chromeleon BN software An Acclaim® 120, C18 column (4,6 × 250 mm, 5 μm, 120 Å) was used in the analysis. The column was maintained at 40 ° C and the detection was carried out at 278.7 nm. This wavelength represents the mean of maximal absorbance for the various standards used in the present study. A volume of 5 μL of each sample was injected. The binary gradient elution system consisted of water containing 1 % acetic acid (A) and methanol (B). Separation was achieved using a linear gradient of the two mobile phases for 45 min. After this time elution was continued for 5 min with B alone. Finally, solvent B was gradually reduced until the initial conditions for column packing (95 % A and 5 % B), working with flow rate of 1.0 mL.min−1. Identification of the peaks of capsaicin and dihydrocapsaicin was carried out by comparison of their retention times with those obtained by injecting standards in the same conditions, as well as by spiking the samples with stock standard solutions. The concentrations of the identified compounds in the extract samples were calculated by means of the regression parameters obtained from calibration curves. The calibration curves were constructed by separating chromatographically standard solutions of the compounds. Linear relationships were obtained between the concentrations and areas under the elution curves.
Total phenolic contents
The total phenolic contents were determined using the Folin-Ciocalteu method (Singleton and Rossi 1965). A standard curve was constructed using gallic acid (R2 = 0.99). The results were expressed as gallic acid equivalents (GAE)/100 g (fresh weight).
Determination of antioxidant activity
The antioxidant capacities of the pulp and seed extracts were evaluated using three methods and expressed as μmol trolox equivalents per g of pulp or seed (μmol TE⋅g−1). The radical scavenging capacity was evaluated using the DPPH● (2,2-diphenyl-1-picrylhydrazil) method, as described previously (Mensor et al. 2001) with slight modifications. To 1000 μL of a DPPH● (0.3 mmol⋅L−1) methanolic solution, a volume of 2500 μL of each extract at different concentrations was added and mixed in a vortex for 10 s. After 30 min at room temperature in the dark, the absorbance was read at 518 nm. The results were compared with the trolox calibration curve (10–60 μmol⋅L−1, Y = −0.197× + 1.0639, r2 = 0.9948, p < 0.001).
The antioxidant capacities of the pulp and seed extracts were also evaluated using the radical cation ABTS●+ (2.2’-azinobis 3-ethylbenzothiazoline 6-sulfonate), as described previously (Thaipong et al. 2006). The radical ABTS●+ was prepared 12 h prior to the assay by mixing equal volumes of 7.4 mmol⋅L−1 ABTS and potassium persulfate (2.6 mmol⋅L−1). The radical was diluted in methanol until the absorbance at 734 nm reached a value of 1.10 ± 0.01. A volume of 150 μl of each extract at different concentrations was added to 2850 μL of ABTS●+. After 2 h at room temperature in the dark, the absorbance was read at 734 nm. The results were compared with the trolox calibration curve (50–500 μmol at 734 nm⋅L−1, Y = − 0.0012× + 0.8507, R2 = 0.9908, p < 0.001).
The third indicator of the antioxidant capacity was the capacity of reducing the ferric ion (FRAP assay) (Benzie and Strain 1996). The FRAP reagent was prepared with 6-tripyridyl-s-triazine (TPTZ, 10 mmol⋅L−1) in 40 mmol⋅L−1 HCl, FeCl3 (20 mmol⋅L−1) and acetate buffer (300 mmol⋅L−1 pH 3.6) in the proportions of 1:1:10 (v/v/v). The reagent was maintained at 37 ° C. A volume of 100 μl of each extract at different concentrations was added to 3000 μL of the FRAP reagent. After 30 min at 37 °C, the absorbance was read at 593 nm. The difference between the initial and final absorbances was correlated with the standard calibration curve with trolox (50–1000 μmol⋅L−1, Y = 0.0012×–0.0017, R2 = 0.9928, p < 0.001).
Statistical analysis
The results are presented as the mean ± standard deviation of three replicates of each experiment A p-value ≤ 0.05 was used to indicate significant differences between the mean values determined by analysis of variance (ANOVA). Pearson linear correlation (ρ) was used to evaluate the association between two variables. A chemometric approach, composed of principal component analysis (PCA), hierarchical cluster analysis (HCA) and multiple linear regression (MLR), implemented in Statistica 7.0 software (Stat-Soft Inc., Tulsa, Okla., U.S.A.), was used to analyze the data. A composite sample (n = 12) and responses (n = 6) matrix was constructed, totaling 72 points. The results obtained for each parameter were used as variables (columns) and the pulp and seeds from the peppers were used as individual samples (lines). Before the chemometrics application, all variables were autoscaled to standardize the statistical importance of all responses (Zielinski et al. 2014a).
PCA was applied to separate the samples according to the contents in capsaicin, dihydrocapsain, total phenolics and antioxidant activities. Analysis was based on linear correlations and variances were computed as sums of squares/(n-1). Eigen-values higher than 1.0 were adopted to explain the projection of the samples on the factor plane, in which a bidimensional graph was built to project both responses and samples.
HCA was performed to assess similarities among the peppers according to the analyzed variables. In this sense, sample similarities were calculated on the basis of the Euclidean metric distance, and Ward’s method was used to form and suggest groups of similar samples. Finally, a dendrogram was constructed for visualizing the similarity between samples in the 2-dimensional plane.
MRL was used to predict the antioxidant activity of peppers. The antioxidant assays were defined as the dependent variable (Yi) and the capsaicin, dihydrocapsaicin, and total phenols as independent variables (Xn). Linear models were constructed as:
| 1 |
where Yi is the predicted response, b0, b1, b2, and b3 are the regression coefficients, and X1, X2, and X3 are the independent variables. As well as PCA, initially all variables were autoscaled and the models were built. The statistical significance of the equations was examined by ANOVA and goodness-of-fit was evaluated based on the regression coefficients (R2), adjusted R2, accuracy factor (AF), and bias factor (BF) (Alberti et al. 2014; Patras et al. 2009).
Results and discussion
Capsaicin and dihydrocapsaicin contents
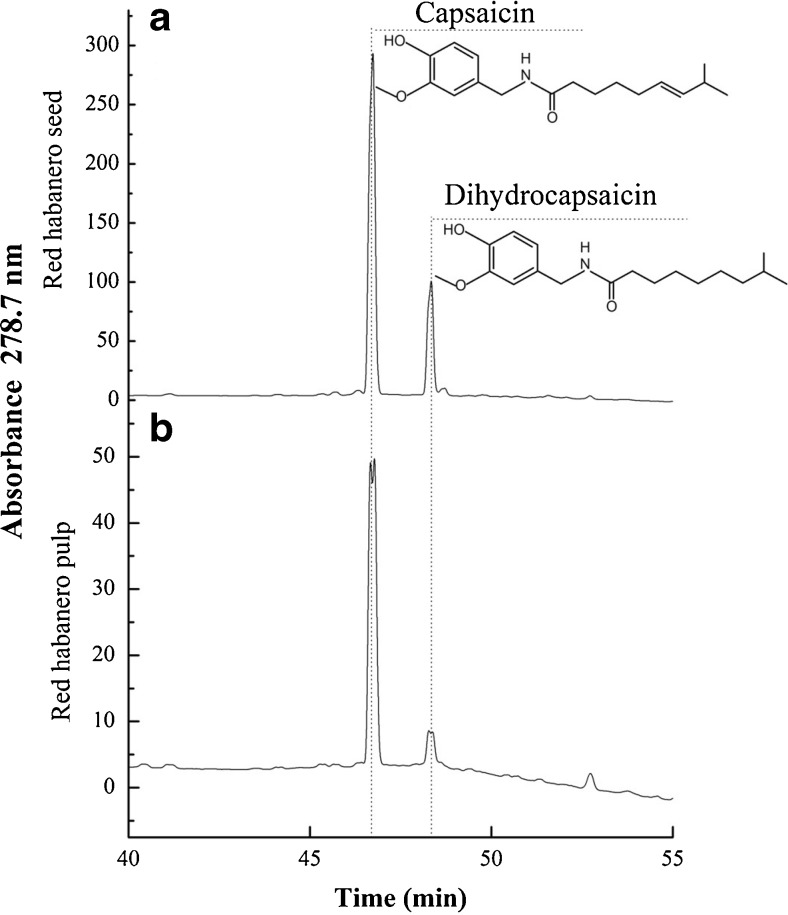
A typical HPLC chromatogram obtained with a red habanero pepper extract is shown in Fig. 1. The spectrophotometric detection was done at 278.7 nm. By using the calibration curves that were done with the capsainoid standards, the amounts of capsaicin and dihydrocapsaicin in all extracts were determined (Table 1). For all peppers, the seed extracts were richer in capsaicin and dihydrocapsaicin than the pulp extracts. These data confirm the high differences found between seeds and pulps previously reported by several studies (Andrew 1994; Arora et al. 2011; Cheema and Pant 2011; Pandhair and Sharma 2008; Reyes-Escogido et al. 2011),
Fig. 1.
HPLC chromatogram of red habanero pepper extracts. a seed extract; b pulp extract
Table 1.
Capsainoids, total phenolics and antioxidant activities of seed and pulp pepper extracts
| Sample | Bioactive concentration | Antioxidant activity | ||||
|---|---|---|---|---|---|---|
| Capsaicin mg/100 g | Dihydrocapsaicin (mg/100 g−1) | Total phenolics (mg GAE 100 g−1) | DPPH•(μmol TE.g−1) | ABTS• + (μmol TE.g−1) | FRAP (μmol TE.g−1) | |
| Bell pepper pulp | NDi | 3,00 ± 0.08g | 119.97 ± 3.44h | 2.28 ± 0.02h | 17.17 ± 0.07i | 3.99 ± 0.15h |
| Bell pepper seed | NDi | 12.27 ± 0.22g | 409.45 ± 7.27e | 11.32 ± 0,23f | 89.25 ± 2.12e | 9.94 ± 0.32f g |
| Cayenne pepper pulp | 7.72 ± 0.24g h | 10.02 ± 0.40g | 205.12 ± 4.57f g | 2.57 ± 0.02i | 32.08 ± 0.42g | 4.28 ± 0.07h |
| Cayenne pepper seed | 61.95 ± 0.14d | 102.70 ± 0.36d | 547.64 ± 19.24d | 13.89 ± 0,10d | 106.66 ± 1.92f | 14.74 ± 0.78e |
| Dedo de moça pepper pulp | 2.99 ± 0.14h i | 7.58 ± 0.04g | 164.51 ± 3.67g h | 2.24 ± 0.01h | 28.66 ± 0.15g h | 3.21 ± 0.015h |
| Dedo de moça pepper seed | 10.01 ± 0.50g | 39.47 ± 0.54f | 508.85 ± 18.30d | 12.90 ± 0.02e | 105.09 ± 2.00f | 13.37 ± 0.33e f |
| Red Habanero pepper pulp | 50.62 ± 0.23f | 16.27 ± 0.54f g | 232.70 ± 5.01f | 2.60 ± 0.02i | 26.94 ± 0.18h | 8.98 ± 0.29g |
| Red Habanero pepper seed | 1024.32 ± 0,41a | 1207.84 ± 0.38a | 2666.18 ± 28.48a | 16.26 ± 0.03a | 132.93 ± 0.61b | 82.67 ± 4.24a |
| Orange Habanero pepper pulp | 21.70 ± 0.03e | 14.15 ± 0.23g | 169.97 ± 4.58f g h | 2.60 ± 0.02i | 20.27 ± 0.30i | 4.81 ± 0.06h |
| Orange Habanero pepper seed | 907.44 ± 4.73b | 781.44 ± 3.38b | 2060.12 ± 20.56b | 15.64 ± 0.04b | 122.74 ± 2.12c | 64.73 ± 1.55b |
| Malagueta pepper pulp | 48.27 ± 0.06f | 68.32 ± 0.69e | 438.76 ± 9.72e | 3.09 ± 0.03g | 97.40 ± 0.36d | 21.17 ± 0.79d |
| Malagueta pepper seed | 203.84 ± 1.60c | 410.30 ± 20.59c | 843.39 ± 11.11c | 15.06 ± 0.04c | 141.25 ± 2.12a | 26.32 ± 0.98c |
Different letters within the same column indicate that the values are significantly different (p ≤ 0.05)
Red habanero and orange habanero seed extracts showed the highest capsaicin and dihydrocapsaicin contents and the bell pepper extracts the lowest. Actually, capsaicin was not detected in the bell pepper extracts (pulp and seed). Bell pepper is generally known as sweet pepper (Carvalho and Bianchetti, 2008). The absence of capsaicin in bell pepper can be related to the high moisture content of the fruits, considering that some authors propose hydric stress as a necessary condition for increasing the capsaicinoid levels (Estrada et al. 1999; Sung et al. 2005). As capsaicin and dihydricapsaicin are responsible for the pungency of the peppers, it is possible to conclude that seeds are more pungent than the pulp and to set up de following order of decreasing seed pungency: red habanero > orange habanero > malagueta > cayenne > dedo de moça pepper > bell pepper. Regarding to the pulps, the order of decreasing pungency is malagueta > red habanero > orange habanero > cayenne > red pepper > bell pepper.
Phenolic contents and antioxidant acitivities
The phenolic contents of the peppers (pulp and seed) ranged from 119.97 ± 3.44 to 2060.12 ± 20.56 mg GAE/100 g (Table 1). For all peppers, the values of phenolics were higher in seeds than in pulps. In relation to the phenolic contents, the order for the seed extracts was red habanero > orange habanero > malagueta > cayenne > red pepper > bell pepper. For pulps, malagueta pepper presented the highest levels of phenolics, while the lowest values were found in bell pepper.
Concerning the antioxidant capacities, for all peppers, they were higher in seed than in pulp extracts. Red habanero seed, orange habanero seed and malagueta seed extracts presented the highest values (Table 1).
The relatively stable organic radical DPPH has been widely used in the determination of the antioxidant activity of single compounds as well as of different plant extracts. Small hydrophilic molecules may have a better chance to react with the radical with consequent higher antioxidant values (Apak et al. 2007). The method is based on the reduction of DPPH in methanol in the presence of a hydrogen donating antioxidant. DPPH solutions show a strong absorption band at 517 nm with a deep violet colour. The absorption vanishes and the resulting decolorization is stoichiometric with respect to the degree of reduction. The remaining DPPH, measured after a certain time corresponds inversely to the radical scavenging activity of the antioxidant. The method was used to evaluate the antioxidant properties of the pepper extracts. The red habanero seed extract (6.26 μmol TE⋅g−1), orange habanero seed extract (15.64 μmol TE⋅g−1) and malagueta seed extract (15.06 μmol TE⋅g−1) presented the highest values. Among the pulp extracts, the antioxidant activities determined by the DPPH assay ranged from 3.09 (malagueta extract) to 2.24 μmol TE⋅g−1 (red pepper).
The ABTS radical cation (ABTS•+) is reactive toward most antioxidants, and it is soluble in both aqueous and organic solvents. The ABTS •+ method is a useful tool in determining the antioxidant activity of both lipophilic and hydrophilic antioxidants in various matrices (Cano et al. 2000). ABTS•+ reacts rapidly with antioxidants, and it can be applied over a wide pH range. Selected substances, including most phenolic compounds, reduce ABTS•+ if its redox potential is lower than that of ABTS (0.68 V). In this work, the ABTS•+ method yielded higher antioxidant values than the DPPH assay, ranging from 89.25 to 141.25 μmol TE⋅g−1 for seed extracts and from 17.17 to 97.40 μmol TE⋅g−1 for pulp extracts. These higher values are probably due to the fact the ABTS•+ assay is more sensitive and presents fewer limitations than the DPPH assay in the evaluation of both hydrophilic and lipophilic antioxidant molecules (Kuskoski et al. 2005).
Finally, the antioxidant activities of pepper extracts were also evaluated using the FRAP assay. Differently of the DPPH and ABTS•+ assays, the FRAP assay is based on the reduction of a ferroin analog, the Fe3+ complex of tripyridyltriazine Fe(TPTZ)3+, to the intensely blue coloured Fe2+ complex Fe (TPTZ)2+ by antioxidants in acidic medium (Antolovich et al. 2002). For this method, highest antioxidant activities were also found for the red habanero seed extract (82.67 μmol TE⋅g−1) and the orange habanero seed extract (64.73 μmol TE⋅g−1).
Correlation analysis
The correlations between the contents of capsaicin and dihydrocapsaicin, the contents of total phenolics and the antioxidant activities obtained in all assays were high (Table 2). It is well known that the phenolic composition is related to the synthesis of capsaicinoids (Arora et al. 2011). The highest r values were those obtained with the FRAP assay (0.9) (Table 2). Similar correlations were reported previously for several pepper extracts (Materska and Perucka 2005; Alvarez-Parrilla et al. 2011; Medina-Juarez et al. 2012). Also, the high correlation between the DPPH, ABTS and FRAP assays (Table 2) is indicative of redundancy in using all three methods to evaluate the antioxidant activity of pepper extracts.
Table 2.
Correlation matrix between the variables antioxidant assays (DPPH, ABTS and FRAP), total phenolics, capsaicin and dihydrocapsacin contents
| Capsaicin | Dihydro-Capsaicina | Total phenolics | DPPH• | ABTS•+ | FRAP | |
|---|---|---|---|---|---|---|
| Capsaicin | 1 | |||||
| Dihydro-Capsaicin | 0.97 p < 0.001 |
1 | ||||
| Total phenolics | 0.98 p < 0.001 |
0.98 p < 0.001 |
1 | |||
| DPPH | 0.63 p = 0.028 |
0.68 p = 0.014 |
0.69 p = 0.014 |
1 | ||
| ABTS | 0.58 p = 0.049 |
0.65 p = 0.021 |
0.63 p = 0.027 |
0.91 p < 0.001 |
1 | |
| FRAP | 0.98 p < 0.001 |
0.98 p < 0.001 |
0.97 p < 0.001 |
0.69 p = 0.013 |
0.69 p = 0.013 |
1 |
Multivariate analysis
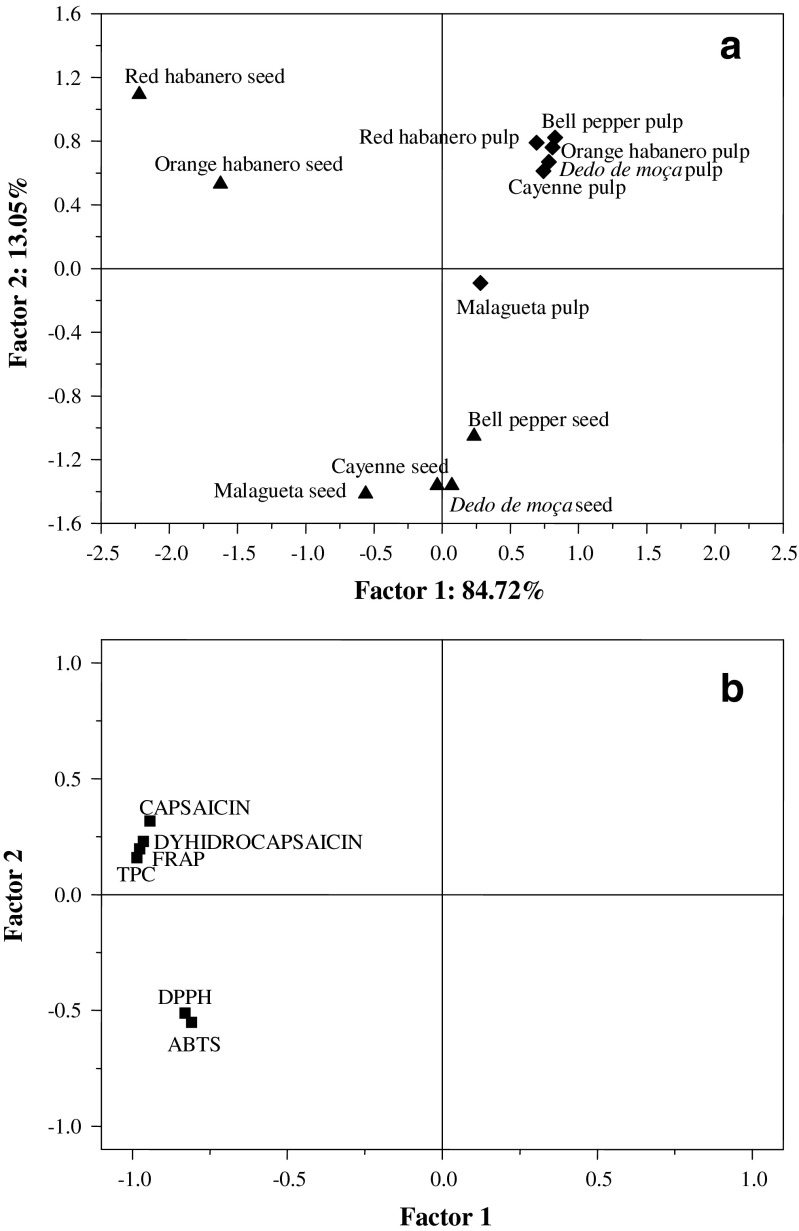
Principal component analysis (PCA) was applied in order to evaluate capsaicin and dyhidrocapsaicin determined by HPLC, total phenolics and the antioxidant activity (DPPH, ABTS•+, and FRAP). The first principal component (PC1) explained up to 84.72 % of the total variance and the second one (PC2) explained 13.05 %. Thus, the two components represented in the 2-D scatter plot explain 97.77 % of the total variance (Fig. 2). Peppers were separated along the PC1 by differences observed in the levels of capsaicin, dihydrocapsaicin, total phenols and antioxidant activity. In the PC2 the samples were separated with respect to DPPH and ABTS. By examining the scatter plots (Fig. 2) it is possible to localize the pulps and seeds and to verify that seeds present higher levels of dihydrocapsaicin, capsaicin, TPC and antioxidant activity, especially the habanero peppers. Samples were separated into three groups being one group formed by red and orange habanero seeds, the second group by other seeds and malagueta pulp, and the third group containing the remaining pulps. The habanero seeds are distinct from the other seeds due to their higher levels of phenolics and antioxidant activity. On the other hand, the malagueta pulp was grouped together with the seeds due to its phenolic content.
Fig. 2.
Scatter plots Factor 2 vs. Factor 1 of the phenolic compounds and in vitro antioxidant activity among peppers pulps and seeds. a Scores and b loading plots
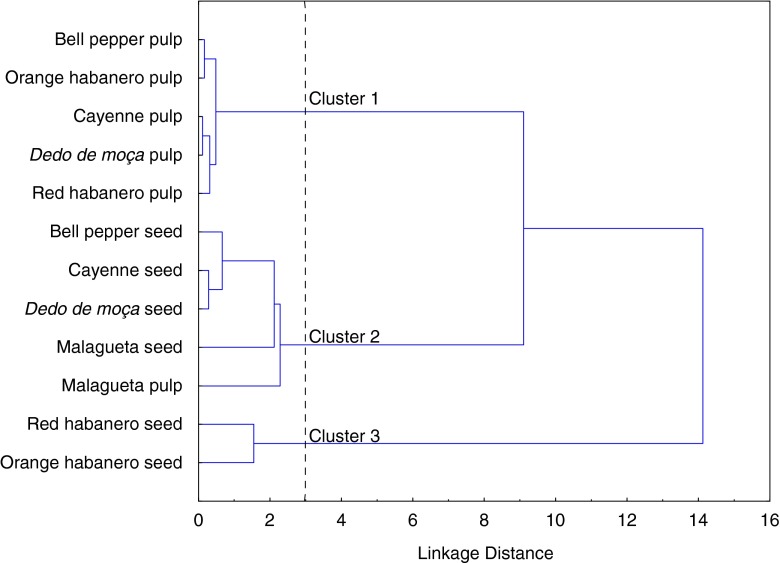
Similarities among the samples were evaluated using hierarchical cluster analysis (HCA). Three clusters were divided (Fig. 3) which corroborate the results of the PCA. Cluster 1 comprises the pulps of bell pepper, orange habanero, Cayenne, dedo de moça and red habanero, which present the lowest levels of the compounds that were quantified. Most seed samples were grouped in cluster 2 (bell pepper, cayenne, dedo de moça and malagueta) together with the malagueta pulp. Cluster 3 is formed by orange and red habanero seeds, containing the highest levels of all compounds.
Fig. 3.
Dendrogram for peppers obtained by hierarchical cluster analysis (HCA)
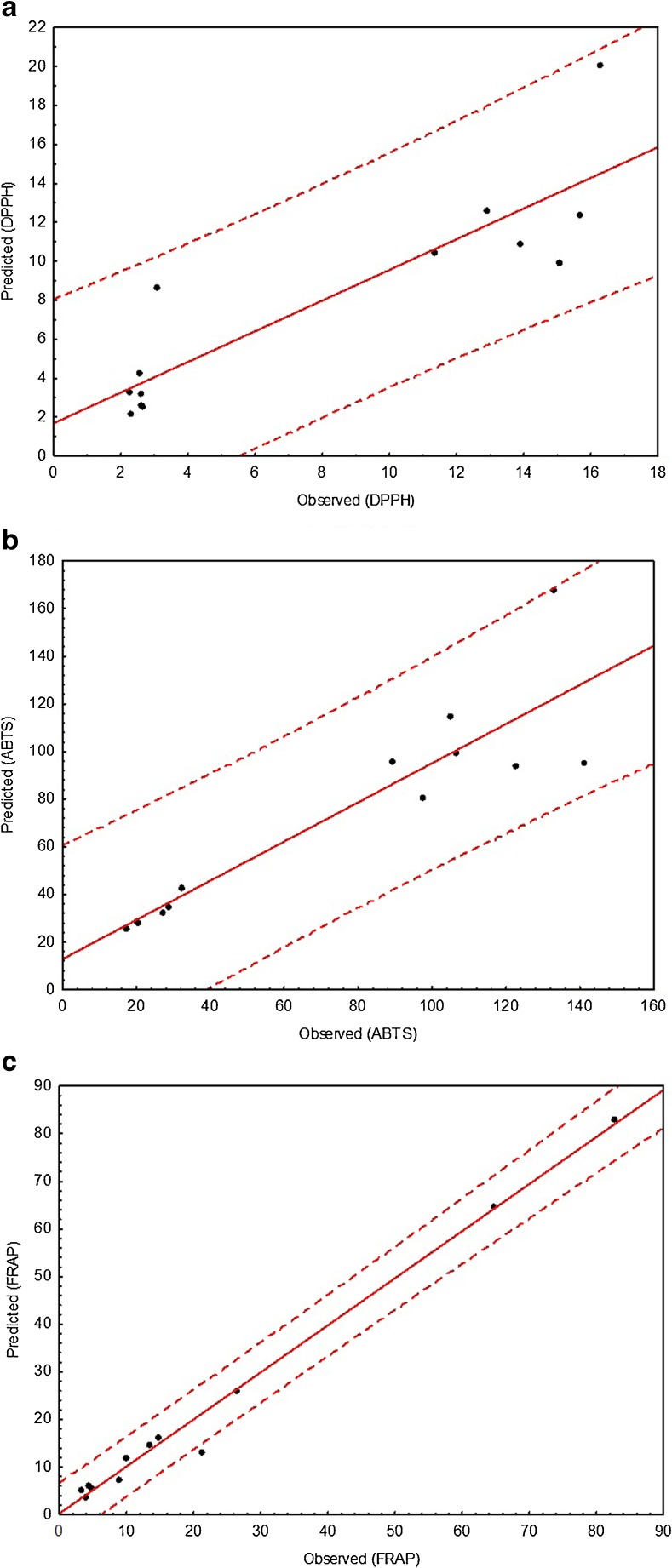
Multiple linear regression (MLR) was used to propose models for predicting the antioxidant activity based on the contents of capsaicin, dihydrocapsaicin, and total phenols (Fig. 4). All the proposed models were statistically significant (p < 0.002) and indicated that the majority of the predicted values were within the 95 % confidence interval (Fig. 4). Models constructed using the DPPH and ABTS assays showed the lowest values of the adjusted regression coefficient (R2Adj > 0.71) and the highest values of accuracy and bias factor. The model constructed using the FRAP assay showed the highest regression coefficient (R2Adj = 0.9888) and the accuracy and bias factor more proximate to 1.00 (Table 3). Thus, the contents of capsaicin, dihydrocapsaicin, and total phenols are more adequate to predict the antioxidant activity measured by the FRAP assay.
Fig. 4.
Predicted and observed values for a DPPH, b ABTS, and c FRAP
Table 3.
Models, regression coefficients (R2) and errors in the prediction of the antioxidant activity by multiple linear regression
| Antioxidant assay | Equation | R2 | R2 adj | AF | BF |
|---|---|---|---|---|---|
| DPPH | DPPH = −1.3238–0.0404* CAP - 0.0126*DIH + 0.0292*TPC | 0.7871 | 0.7162 | 1.30 | 1.10 |
| ABTS | ABTS = − 3.8609–0.3900*CAP - 0.0741*DIH + 0.2461*TPC | 0.8225 | 0.7633 | 1.27 | 1.10 |
| FRAP | FRAP = 0.5070 + 0.0064*CAP + 0.0016*DIH + 0.0278*TPC | 0.9945 | 0.9888 | 1.18 | 1.06 |
CAP capsaicin, DIH dihydrocapsaicin, TPC total phenolic compounds, AF accuracy factor, BF bias factor
Conclusion
In general, the contents of phenolics and capsainoids as well as the antioxidant activities were higher in seeds than in pulps, confirming previous reports. The results presented here revealed high correlation between antioxidant activity and capsainoid contents and between antioxidant activity and phenolic contents. PCA explained 97.77 % of the total variance of the data, and allowed to separate the samples into three groups. HCA also allowed to separate the samples into three different clusters, corroborating PCA, MLR indicated that the values of capsaicin, dihydrocapsaicin, and total phenols are more adequate to predict the antioxidant activity measured by the FRAP assay. From the extracts of seed and pulp examined in the present work the two with the highest capsainoid and phenolic contents and antioxidant activity were those of the red and orange habanero seeds.
Acknowledgments
This study was supported by UEM (Universidade Estadual de Maringá), UTFPR (Universidade Tecnológica Federal do Paraná), CNPq (Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico), and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior)
References
- Alberti A, Zielinski AAF, Zardo DM, Demiate IM, Nogueira A, Mafra LI. Optimisation of the extraction of phenolic compounds from apples using response surfasse methodology. Food Chem. 2014;149:151–158. doi: 10.1016/j.foodchem.2013.10.086. [DOI] [PubMed] [Google Scholar]
- Alvarez-Parrilla E, de La Rosa L, Amarowicz R, Shahidi FF. Antioxidant activity of fresh and processed jalapeño and serrano peppers. J Agric Food Chem. 2011;59:163–173. doi: 10.1021/jf103434u. [DOI] [PubMed] [Google Scholar]
- Andrew J (1994) Peppers: The Domesticated Capsicums, New Edition. University of Texas Press; New sub edition, p.50. ISBN 978-0-2927-0467-1
- Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127:183–198. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- Apak R, Guçlu K, Demirata B, Ozyurek M, Çelik SE, Bektasoglu B, Berker KI, Ozyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with CUPRAC assay. Molecules. 2007;12:1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora R, Gill NS, Chauhan G, Rana AC. An Overview about versatile molecule capsaicin. Int J Pharm Sci Drug Res. 2011;4(3):280–286. [Google Scholar]
- Asnin L, Park SW. Isolaion and analysis of bioactive compounds in Capsicum peppers. Crit Rev Food Sci Nutr. 2015;55:254–289. doi: 10.1080/10408398.2011.652316. [DOI] [PubMed] [Google Scholar]
- Bae H, Jayaprakasha GK, Jifon J, Patil BS. Extraction efficiency and validation of an HPLC method for flavonoid analysis in peppers. Food Chem. 2012;130:751–758. doi: 10.1016/j.foodchem.2011.07.041. [DOI] [Google Scholar]
- Barbero GF, Ruiz AG, Liazid A, Palma M, Vera JC, Barroso CG. Evolution of total and individual capsaicinoids in peppers during ripening of the Cayenne pepper plant (Capscicum annuum L.) Food Chem. 2014;153:200–206. doi: 10.1016/j.foodchem.2013.12.068. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brewer MS. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci F. 2011;10:221–247. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- Cano A, Acosta M, Arnao MB. A method to measure antioxidant activity in organic media: application to lipophilic vitamins. Redox Rep. 2000;5:365–370. doi: 10.1179/135100000101535933. [DOI] [PubMed] [Google Scholar]
- Carvalho SI, Bianchetti LB. Botânica e Recursos Genéticos. In: da Ribeiro CSC, Lopes CA, Carvalho SIC, Henz GP, Reifschneider FJB, editors. Pimentas Capsicum. Brasília: Embrapa Hortaliças; 2008. pp. 39–54. [Google Scholar]
- Cheema SK, Pant MR. Estimation of capsaicin in seven cultivated varieties of Capsicum annuum L. Res J Pharm Biol Chem Sci. 2011;2:701–706. [Google Scholar]
- Deepa N, Kaur C, Singh B, Kapoor HC. Antioxidant activity in some red sweet pepper cultivars. J Food Compos Anal. 2006;19:572–578. doi: 10.1016/j.jfca.2005.03.005. [DOI] [Google Scholar]
- Deepa N, Kaura C, George B, Singh B, Kapoor H. Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity.LWT- Food. Sci Technol. 2007;40:121–129. [Google Scholar]
- Estrada B, Pomar F, Díaz J, Merino F, Bernal MA. Pungency level in fruits of the padron pepper with different water supply. Hortil Sci. 1999;81:385–396. doi: 10.1016/S0304-4238(99)00029-1. [DOI] [Google Scholar]
- Haminiuk CWI, Plata-Oviedo MSV, Guedes AR, Stafussa AP, Bona E, Carpes ST. Chemical, antioxidant and antibacterial study of Brazilian fruits. Intern J Food Sci Technol. 2011;46:1529–1537. doi: 10.1111/j.1365-2621.2011.02653.x. [DOI] [Google Scholar]
- Hwang IG, Shin YJ, Lee J, Yoo SM. Effect of different cooking methods on the activity properties of red pepper (Capsicum annuum L.) Prev Nutr Food Sci. 2012;17:286–292. doi: 10.3746/pnf.2012.17.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari SL, Joshi A, Kachhwaha S, Ochoa-Alejo N. Chilli peppers—A review on tissue culture and transgenesis. Biotechnol Adv. 2010;28:35–48. doi: 10.1016/j.biotechadv.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Kuskoski EM, Asuero AG, Troncoso AM, Mancini-Filho J, Fett R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciênc Technol Alim. 2005;25:726–732. doi: 10.1590/S0101-20612005000400016. [DOI] [Google Scholar]
- Materska M, Perucka I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.) J Agric Food Chem. 2005;53:1750–1756. doi: 10.1021/jf035331k. [DOI] [PubMed] [Google Scholar]
- Medina-Juarez LA, Molina-Quyada DMA, Del Toro-Sanchez CL, Gonzalez-Aguilar GA, Gamez-Meza N. Antioxidant activity of peppers (Capsicum annuum L) extracts and characterization of their phenolic constituints. Interciencia. 2012;37:588–593. [Google Scholar]
- Menichini F, Tundis R, Bonesi M, Loizzo MR, Conforti F, Statti G, Cindio BD, Houghton PJ, Menichini F. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chem. 2009;114:553–560. doi: 10.1016/j.foodchem.2008.09.086. [DOI] [Google Scholar]
- Mensor LL, Menezes FS, Leitão GG. Screening of brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- Pandhair V, Sharma S. Accumulation of capsaicin in seed, pericarp and placenta of Capsicum annuum L fruit. J Plant Biochem Biotechnol. 2008;17:23–27. doi: 10.1007/BF03263255. [DOI] [Google Scholar]
- Patras A, Tiwari BK, Brunton NP, Butler F. Modelling the effect of different sterilisation treatments on antioxidant activity and colour of carrot slices during storage. Food Chem. 2009;114:484–491. doi: 10.1016/j.foodchem.2008.09.104. [DOI] [Google Scholar]
- Reyes-Escogido ML, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E. Chemical and pharmacological aspects of capsaicin. Molecules. 2011;16:1253–1270. doi: 10.3390/molecules16021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala KD, Crapiste G. Drying kinetics and quality changes during drying of red pepper. LWT-Food Sci Technol. 2008;41:789–795. doi: 10.1016/j.lwt.2007.06.007. [DOI] [Google Scholar]
- Sharma SK, Vy AS, Sharma M. Mechanisms and clinical uses of capsaicin. Eur J Pharm. 2013;720:55–62. doi: 10.1016/j.ejphar.2013.10.053. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Srinivasan K. Biological activities of pepper alkaloids. Nat Prod. 2013;45:1397–1437. doi: 10.1007/978-3-642-22144-6_184. [DOI] [Google Scholar]
- Sung Y, Chang YY, Ting NL. Capsaicin biosynthesis in water-stressed hot pepper fruits. Bot Bull Acad Sin. 2005;46:35–42. [Google Scholar]
- Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH. Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- Vega-Gálvez A, Scala KD, Rodríguez K, Lemus-Mondaca R, Miranda M, López J, Perez-Won M. Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian) Food Chem. 2009;11:647–653. doi: 10.1016/j.foodchem.2009.04.066. [DOI] [Google Scholar]
- Yaldiz G, Ozguven M, Sekeroglu N. Variation in capsaicin contents of different Capsicum species and lines by varying drying parameters. Ind Crops Prod. 2010;32:434–438. doi: 10.1016/j.indcrop.2010.06.013. [DOI] [Google Scholar]
- Zhuang Y, Chen L, Sun L, Cao J. Bioactive characteristics and antioxidant activities of nine peppers. J Funct Foods. 2012;4:331–338. doi: 10.1016/j.jff.2012.01.001. [DOI] [Google Scholar]
- Zielinski AAF, Haminiuk CWI, Alberti A, Nogueira A, Demiate IM, Granato D. A comparative study of the phenolic compounds and the in vitro antioxidant activity of different Brazilian teas using multivariate statistical techniques. Food Res Int. 2014;60:246–254. doi: 10.1016/j.foodres.2013.09.010. [DOI] [Google Scholar]
- Zielinski AAF, Ávila S, Ito V, Nogueira A, Wosiacki G, Haminiuk CWI. The Association between Chromaticity, Phenolics, Carotenoids, and In Vitro Antioxidant Activity of Frozen Fruit Pulp in Brazil: An Application of Chemometrics. J Food Sci. 2014;79(4):C510–C516. doi: 10.1111/1750-3841.12389. [DOI] [PubMed] [Google Scholar]