Abstract
Linoleic acid (LA) is the precursor of bioactive oxidized linoleic acid metabolites and arachidonic acid, therefore is essential for human growth and plays an important role in good health in general. Because of the low water solubility and sensitivity to oxidation, new ways of LA delivery without compromising the sensory attributes of the enriched products are to be identified. The major whey protein, β-lactoglobulin (β-Lg), is a natural carrier for hydrophobic molecules. The thermal induced changes of the β-Lg-LA complex were investigated in the temperature range from 25 to 85 °C using fluorescence spectroscopy techniques in combination with molecular modeling study and the results were compared with those obtained for β-Lg. Experimental results indicated that, regardless of LA binding, the polypeptide chain rearrangements at temperatures higher than 75 °C lead to higher exposure of hydrophobic residues causing the increase of fluorescence intensity. Phase diagram indicated an all or none transition between two conformations. The LA surface involved in the interaction with β-Lg was about 497 Ǻ2, indicating a good affinity between those two components even at high temperatures. Results obtained in this study provide important details about heat-induced changes in the conformation of β-Lg-LA complex. The thermal treatment at high temperature does not affect the LA binding and carrier functions of β-Lg.
Keywords: β-lactoglobulin, Linoleic acid, Structural changes, Fluorescence spectroscopy, Molecular modeling
Introduction
Current researches in nutraceutical delivery strategies are bringing into attention the binding properties of β-lactoglobulin (β-Lg) (Livney 2010; Perez et al. 2014; Sponton et al. 2014). β-Lg is the major protein in whey and exists as a dimer with each monomer containing 162 aminoacid residues and a molecular mass of 18 kDa. The monomer is a globular protein folded into a calyx called β-barrel that contains eight anti-parallel β-strands (A-H). As member of lipocalin family, it has the ability to bind and transport lipophilic nutrients such as retinol, fatty acids and vitamin D (Sahihi et al. 2011; Mensi et al. 2013; Keppler et al. 2014; Perez et al. 2014). The internal cavity does not possess direct access to the external aqueous environment, so that some sort of protein rearrangement is thought to occur during ligand binding and release. Binding properties could be utilized for the encapsulation of lipophilic bioactive compounds so they may be protected from factors such as oxygen, moisture, temperature etc.
Polyunsaturated fatty acids (PUFA) are involved in the architecture and function of cellular membranes, playing essential roles in several biological processes. Linoleic acid is essential for human growth. It is the precursor of n-6 polyunsaturated fatty acids and their derivatives are involved in complex metabolic pathways. Dietary recommendations for n-6 PUFA are controversial (Choque et al. 2014) and little is known about its metabolism in humans. Food-derived lactobacilli and those present in gut microbial flora of ruminant animals are able to produce bioactive fatty acids, such as conjugated linoleic acid (CLA). It cannot be excluded that bacteria in the gastrointestinal tract of non-ruminants may be able to convert unsaturated fatty acids into CLA, as higher intake of linoleic acid lead to an increment in CLA levels in rats’ tissues (Buccioni et al. 2012). Jiang and Liu (2010) reported that β-Lg-CLA complex has very good stability in in vitro gastrointestinal conditions and can be potentially used as a capsular vehicle of CLA for intracellular transport in colon cancer-targeted therapy. This represents an opportunity for the development of novel functional foods, since CLA seems to have health beneficial effects such as: immunomodulatory activity, antimutagenicity, anticarcinogenic, antiobesity, antidiabetic and antihypertensive (Jelińska et al. 2014; Koba and Yanagita 2014; Bergamo et al. 2014).
Given the structural and conformational particularities of β-Lg, numerous studies exploit the potential of binding of PUFAs, such as to obtain ingredients for food and pharmaceutical applications. However, β-Lg functional properties are easily modified by different processes that include: thermal treatment, high pressure, pH values etc. (Sahihi et al. 2011; Liang and Subirade 2012; Perez et al. 2014). As a consequence, the binding properties are influenced not only by the ligand physicochemical properties but also by the protein structural transitions. Although β-Lg binding properties were intensively studied, there is not enough data about its potential use as carrier of lipophilic bioactive molecules. Thermal treatment could promote important changes in binding properties; therefore information offered in this work may be interesting for the development of new encapsulation strategies and novel products with polyunsaturated fatty acids. In the present study the thermal behavior of β-Lg - linoleic acid complex was investigated by means of fluorescence spectroscopy and molecular modeling. The results were compared with those of protein solutions heated alone in order to get detailed information regarding structural and conformational changes induced by the thermal treatment conjugated with ligand binding.
Materials and methods
Materials
Bovine β-Lg (99 % purity), linoleic acid (LA) (70 %), 1-anilino-8-naphtalenesulphonic acid (ANS), acrylamide and KI were provided by Sigma (Sigma-Aldrich Co., St. Louis, MO). All other chemicals used were of analytical grade. β-Lg was used without further purification. β-Lg solutions were prepared in Tris-HCl pH 8.0 at a protein concentration of 30 mg/mL.
The β-Lg-LA complex was prepared by simple mixing of the two components (Fontana et al. 2013). The LA was added to the protein solutions to reach a final protein/LA molar ratio of 1:10; the mixture was vigorously homogenized for 5 min with a vortex. The excess of LA was removed after centrifugation at 4 °C and 10,000 g for 10 min.
Heat treatment
Solutions were filled in Eppendorf tubes (Eppendorf AG, Hamburg, Germany). Experiments were conducted in a thermostatic water bath (Digibath-2 BAD 4, RaypaTrade, Barcelona, Spain) at temperatures ranging from 25 to 85 °C for 10 min. A heating up time of 10 s was considered for all the heating experiments. The holding time was long enough to ensure structural rearrangements within the complex. The tubes were immediately cooled in ice water to prevent further thermal denaturation. All spectroscopic studies were carried out on the heated-cooled protein, implying that only irreversible/permanent structural changes were detected.
Spectroscopic evaluation of complexes
Intrinsic fluorescence measurements were performed using LS-55 luminescence spectrometer (PerkinElmer Life Sciences, Shelton, CT, USA) with a quartz cell of 10 mm path length. Fluorescence spectra were taken at excitation wavelengths of 295 and 274 nm and emission recorded between 300 and 420 nm.
The intrinsic (phase diagram, fluorescence spectrum, synchronous spectrum and quenching experiments) and extrinsic fluorescence properties (ANS) were performed as described earlier (Stănciuc et al. 2013). All fluorescence spectroscopy experiments were repeated after a 24 h period of storage at 4 °C, in order to determine complex stability.
Molecular modeling
The crystal structure of β-Lg-LA complex (code 4DQ4.pdb) was taken from Brookhaven Protein Data Bank (Loch et al. 2013). The potential energy of the refined model was minimized using AMBER (Assisted Model Building and Energy Refinement) force field and Steepest Descent algorithm, both in vacuum and in explicit solvent environment. The optimized solvated complex was further heated at 25 and 80 °C by means of molecular dynamics simulations and equilibrated long enough to reduce temperature and energy oscillations. Conformational, structural and interaction particularities of β-Lg-LA complex at different temperatures were afterwards checked through VMD 1.9.1, PDBsum, LigPlot + v1.4.5, PISA v1.51 tools (Humphrey et al. 1996; Laskowski and Swindells 2011; Laskowski 2009; Krissinel and Henrick, 2007).
Results and discussion
Phase diagram
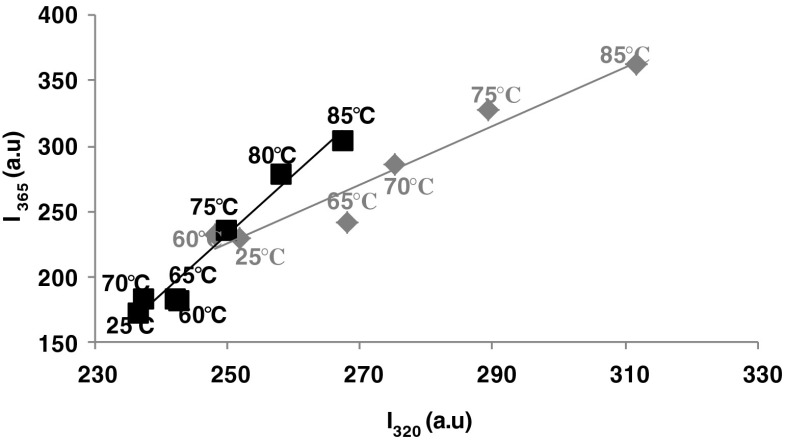
In order to detect the heat-induced conformational changes in β-Lg and β-Lg-LA complex, the phase diagram method developed by Kuznetsova et al. (2004) was employed. This sensitive technique was used in order to highlight the presence of various molecular species. The results presented in Fig. 1 revealed the existence of a linear dependence between I320 versus I365, indicating the presence of two different conformations induced by thermal treatment for both β-Lg heated solutions and β-Lg-LA complexes. Therefore, the structural changes were defined by a two state transition between two molecular species (all or none transition). As it can be seen from Fig. 1, thermal treatment caused significant changes in fluorescence intensity values of β-Lg in the whole studied temperature range. The most important rearrangements of the polypeptide chains occurred at temperatures ranging between 75 and 85 °C and determined an increase in the fluorescence intensity due to a higher exposure of hydrophobic residues to the aqueous environment. Therefore, the unfolding of β-Lg monomers in the whole temperature range studied is a single-phase reaction. The two stage denaturation model for β-Lg was previously reported by Seo et al. (2010). By using Raman spectroscopy experiments, these authors suggested a first denaturation step corresponding to the dissociation of dimers, coupled with the increase of flexibility of the tertiary structure. In the second step, large conformational changes are detected in the secondary structure and described as a loss of α-helix structures and a concomitant formation of β-sheets. However, up to 65 °C, β-Lg seems to form a molten globule state. Qi et al. (1997) suggested that up to 70 °C, a considerable fraction of the β-Lg native regular secondary structure has been lost, virtually counting for all the α-helical content and up to a fifth of the β-sheet. Binding of LA caused conformational changes that stabilized the protein molecules during thermal treatment. Therefore, it seems that β-Lg-LA complex is stable in the temperature range of 25–70 °C, but when temperature increases at 75 °C and higher, the polypeptide chains unfolds.
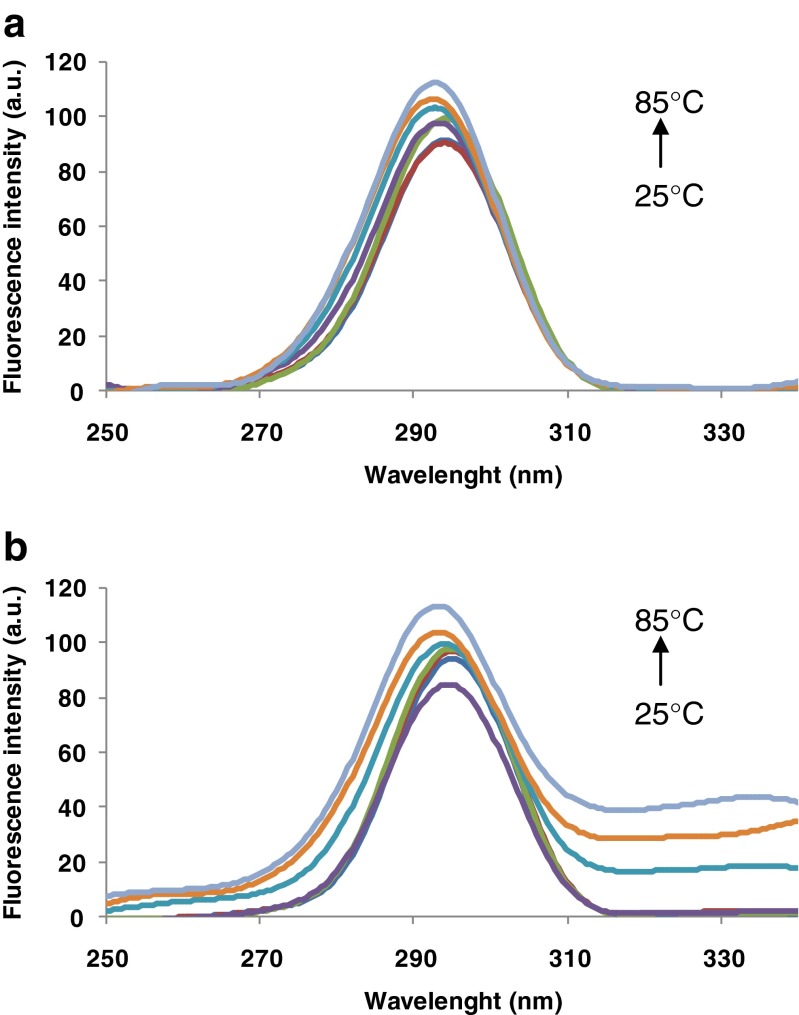
Fig. 1.
Phase diagram analysis of heat-induced conformational changes of β-Lg (grey diamonds) and β-Lg-LA complex (black squares) based on intrinsic fluorescence intensity values measured at wavelengths 320 and 365 nm. The temperature values are indicated in the vicinity of the corresponding symbol. Three independent tests were carried out in each case and SD was lower than 2.5 %
Intrinsic fluorescence
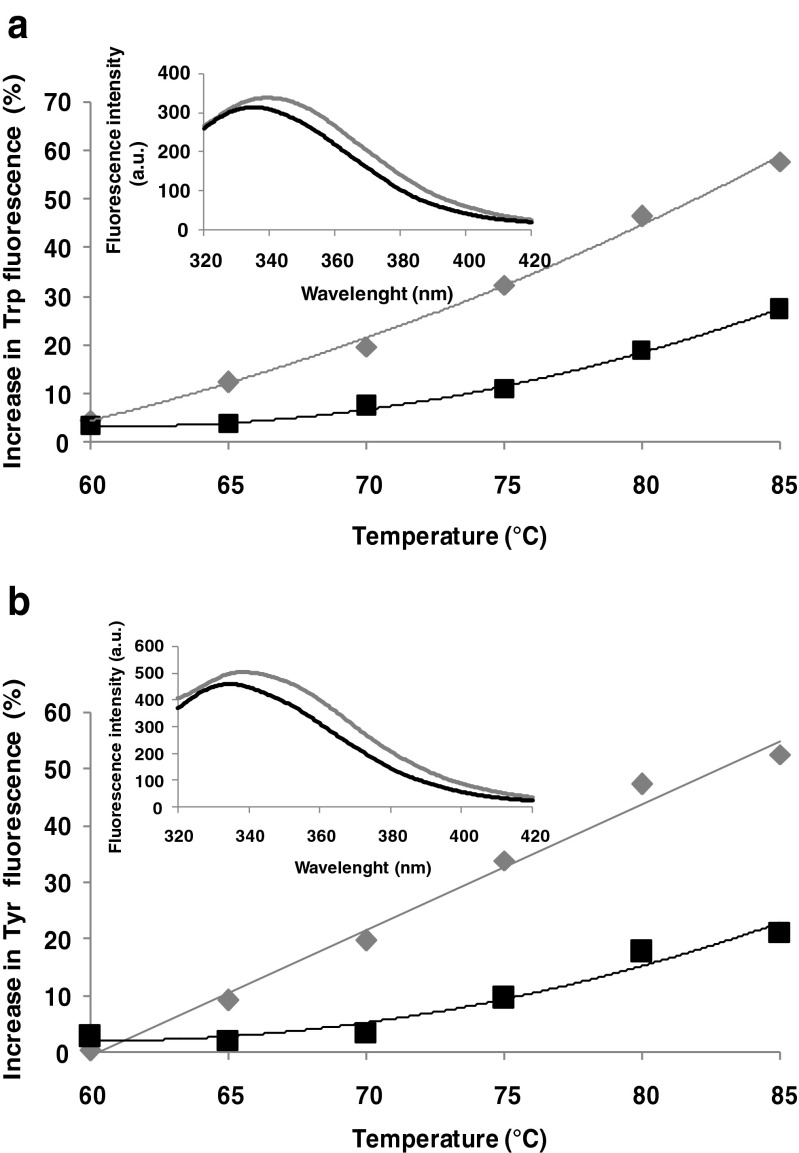
Intrinsic fluorescence allows sensitive assessment of protein conformational changes in response to changes in environmental conditions (pH, temperature, etc.) (Perez et al. 2014). Thermal treatment caused a significant increase in the fluorescence intensity (Fig. 2). When β-Lg solutions were thermally treated in the absence of LA, a significant increases in fluorescence intensity was recorded both when excited at 292 nm (up to 58 % at 85 °C with respect to 25 °C) (Fig. 2a) and at 274 nm (up to 53 % at 85 °C with respect to 25 °C) (Fig. 2b). β-Lg possess two Trp residues, in position 19 and 61. Trp19 is located in a non-polar region in the main cavity of the protein, whereas Trp61 is found on the surface of the molecule, near the disulfide brigde Cys66-Cys160, who quenched its fluorescence. Two lipophilic ligand binding sites have been reported for β-Lg: a central hydrophobic β-barrel and a superficial pocket (Wang et al. 1998, 1999). It seems that fatty acids bind at the superficial pocket, while other molecules bind to the calyx (Frapin et al. 1993; Wang et al. 1998, 1999). These studies also report that the β-Lg fluorescence intensity increased when fatty acids bound to the superficial pocket. Contrarily, in our study no significant differences in fluorescence intensity were observed when LA was added to the protein solutions (Fig. 2a inset). The in silico observations comply with experimental results obtained for the complex. The exposure of the Trp and Tyr residues to the solvent was computed by means of PISA after performing molecular dynamics simulations. When increasing the temperature from 25 to 80 °C the total accessible surface area (ASA) of Trp residues increased by 1.42 Å2/mol whereas the total ASA of Tyr residues increased by 4.42 Å2/mol. In particular, when compared to room temperature higher exposure was obtained at 80 °C for Trp61 (ASA increased from 95.47 to 99.1 Å2), Tyr20 (ASA increased from 51.11 to 56.11 Å2) and Tyr99 (ASA increased from 27.11 to 31.39 Å2). The detailed atomic level check indicated that Trp19, Tyr42 and Tyr102 residues contributed to a lower extent to the fluorescence due to their lower exposure, which decreased with the temperature increase. The buried surface area of Tyr and Trp residues upon β-Lg-LA complex formation is zero. Taheri-Kafrani et al. (2010) suggested that the transfer of Trp to a hydrophilic environment and increase in polar interactions lead to a red shift in wavelength and to an increase in intensity of the emission maximum. When excited at 292 nm, the maximum of fluorescence intensity (λmax) was at 339 nm for the β-Lg solutions and at 335 nm for the complex. The heat treatment resulted in a 10 nm red-shift in both protein and complex solutions. Similar behavior was observed when samples were excited at 274 nm, with a red-shift of 7.5 nm for protein solutions and 9.5 nm for the complex when heated at 85 °C. It can be concluded that due to thermally treatment, the molecules unfolds with an exposure of hydrophobic regions to the aqueous environment. Red-shifts in the λmax were associated with unfolding of polypeptides chains in the tertiary structure of β-Lg from the complex (Perez et al. 2014).
Fig. 2.
The increase in Trp (a) and Tyr (b) fluorescence of β-Lg (grey) and β-Lg-LA complex (black) as a function of temperature. The excitation wavelength was 292 and 274 nm respectively. Three independent tests were carried out in each case and SD was lower than 3.5 %. Inset: The emission spectrum of un-treated β-Lg (grey) and β-Lg-LA complex (black)
ANS binding
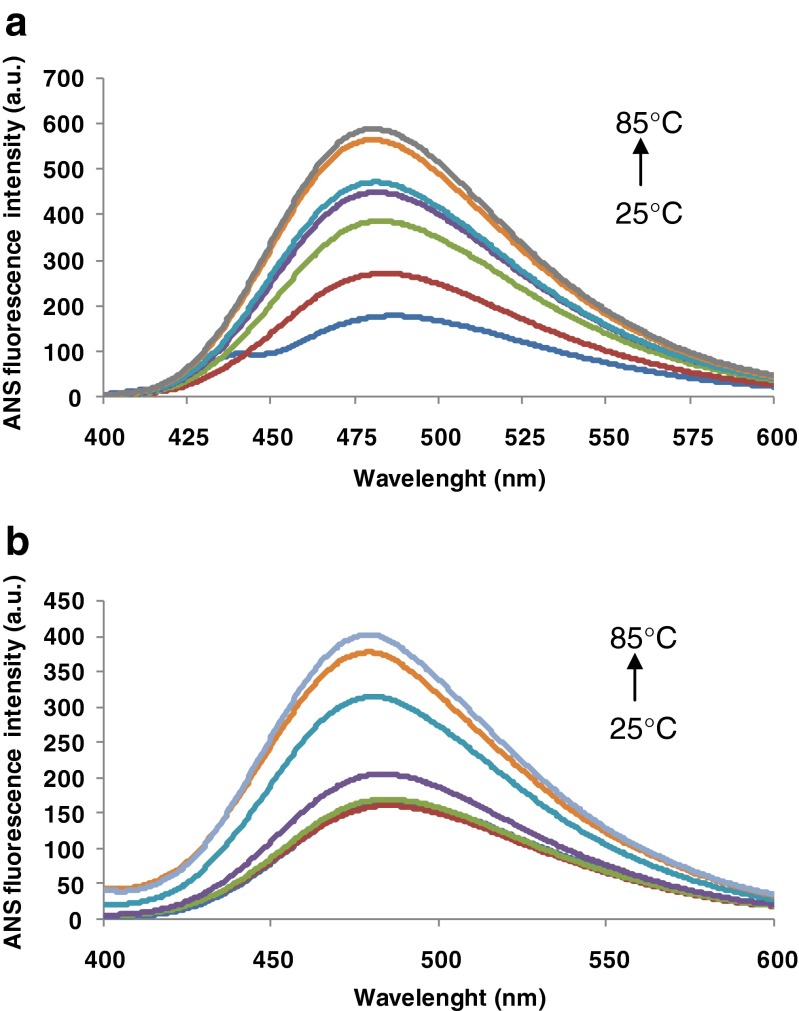
ANS binds to the protein molecule through non-covalent hydrophobic or electrostatic interactions. ANS binding to the hydrophobic domains of protein surface leads to an increase in fluorescence intensity (Sponton et al. 2014). ANS molecules can interact with the hydrophobic patches or clefts present on the native protein surface. The extrinsic fluorescence emission spectra of the β-Lg and β-Lg-LA complex are presented in Fig. 3. The temperature dependent fluorescence increase suggests an increase of the exposed hydrophobic area. The ANS binding was highly influenced by temperature, especially above 70 °C, when initially buried hydrophobic regions became more accessible. Thus, the thermal treatment at 85 °C caused the increase by 144 % of the fluorescence intensity of β-Lg-LA, and by ~178 % in case of β-Lg with respect to room temperature.
Fig. 3.
Heat-induced structural changes of β-Lg solutions (a) and β-Lg-LA complex (b) at different temperature monitored by ANS fluorescence intensity. Three independent tests were carried out in each case and SD was lower than 3.5 %
Matulis and Lovrien (1998) suggested that ANS binding depends primarily on the electrostatic interaction between negatively charged sulfonate group of ANS and positively charged amino acid residues of the protein. After the initial binding, hydrophobic interaction between some of the bound ANS and the protein may occur (Rade-Kukic et al. 2011). The increase in ANS fluorescence observed at higher temperatures can be explained also by the less compact structure of β-Lg in these conditions, resulting from intramolecular electrostatic repulsions (Santambrogio and Grandori 2008).
The λmax values of the untreated solutions were 486 nm for β-Lg-LA complex, and 487 nm for β-Lg. This small blue-shift of the emission maxima indicates that at least part of the Trp residues is transferred into more hydrophobic environment during the interaction of β-Lg with the LA. A blue-shift of 7 nm in the emission maximum was observed at 85 °C, indicating that heat-treated β-Lg and β-Lg-LA bind ANS in a less polar environment compared to native state.
Synchronous fluorescence spectroscopy
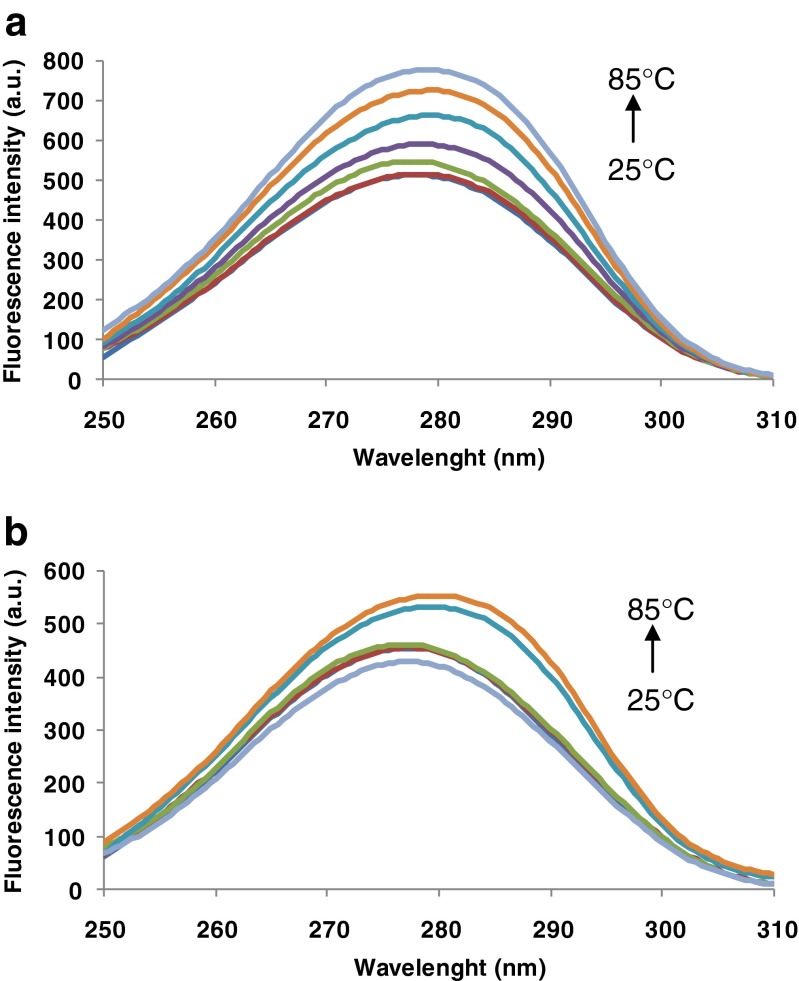
Synchronous fluorescence measurements provide information about the microenvironment in the vicinity of the fluorophore groups (Zhu and Liu 2012). Changes of polarity around the chromophore molecule can be explored by measurement of the possible shift in the maximum emission wavelength (Bi et al. 2012). Therefore, the heat-induced changes in Trp and Tyr microenvironment were obtained by setting the excitation and emission wavelength interval (∆λ) to 60 and 15 nm, respectively (Ehteshami et al. 2013). Synchronous fluorescence spectrum (SFS) at ∆λ of 60 nm for β-Lg (a) and β-Lg-LA (b) are shown in Fig. 4. In case of both samples, the SFS had a single peak around 278–280 nm. A small 2.5 red-shift of the maximum emission of Trp residues can be observed in Fig. 4b. A significant increase in fluorescence intensity was recorded in both cases with temperature increase from 25 to 85 °C. When β-Lg was heated alone, an increase in fluorescence intensity between 0.46 and 51.5 % in the temperature range of 60–85 °C was observed. Addition of LA led to a more heat stable complex. The heat treatment induced limited unfolding of polypeptide chains with an increase in fluorescence intensity to approx. 23 % at 85 °C.
Fig. 4.
Synchronous fluorescence spectra at ∆λ of 60 nm for β-Lg (a) and β-Lg-LA (b). Three independent tests were carried out in each case and SD was lower than 3.0 %
The SFS at ∆λ of 15 nm for β-Lg and β-Lg-LA are shown in Fig. 5 (a and b, respectively). Similarly, the SFS had a single peak around 294–295 nm. When heat-treated at 85 °C the fluorescence intensity of β-Lg increased by 23 %, and by 21 % in case of β-Lg-LA with respect to the samples tempered at 25 °C.
Fig. 5.
Synchronous fluorescence spectra at ∆λ of 15 nm for β-Lg (a) and β-Lg-LA (b). Three independent tests were carried out in each case and SD was lower than 3.0 %
The results presented in Fig. 5 indicate that the polarity around Tyr residues decreased with the temperature increase, and the overall microenvironment of Tyr was also altered. Moreover, the polarity around Trp residues increases, being located in a more hydrophilic environment, whereas Trp19 is located on the LA binding site (Shen et al. 2014). Taking into account the location of the two Trp residues in β-Lg, it is most likely that the conjugated effect of LA and heat treatment was to change the microenvironment and polarity around Trp19, which is originally buried in the hydrophobic core of protein.
3D fluorescence spectroscopy
Three-dimensional fluorescence spectra have become a popular fluorescence analysis technique, offering details about conformational changes in proteins (Shi et al. 2010). The characteristic parameters of heat-treated β-Lg and β-Lg-LA complex are presented in Table 1. Peak 1 reveals the spectral characteristics of Trp and Tyr residues whereas peak 2 refers to the fluorescence characteristics of polypeptide backbone structure (Shen et al. 2013). The fluorescence positions of peak 1 for β-Lg and β-Lg-LA complex were at 280/335 nm and respectively at 280/336 nm, whereas the Stokes shifts were 55 and 56 nm. The thermal treatment of both solutions revealed that the peak positions of Trp and Tyr residues red-shifted with 9 nm for β-Lg at 75 °C, with increasing the Stokes shifts, followed by blue-shifts at higher temperatures (Table 1). This phenomenon indicates that thermal treatment significantly increased the microenviroment polarity of Trp and Tyr residues, which are likely to be located in more hydrophilic regions of the molecules, therefore increasing the extent of their exposure. The large fluorescence increase (~95 %) for the heat-treated β-Lg solutions is of the same order as reported by Manderson et al. (1999) and Croquennec et al. (2004), for the heat-treated β-Lg at pH 6.7. This increase in fluorescence intensity may results also from the decrease in Trp quenching, due to the increase in the distance between Trp61 and Cys66-Cys160 disulfide bond.
Table 1.
Three-dimensional fluorescence spectral characteristics of the heat-treated solutions
| Temperature (°C) | β-Lg solutions | β-Lg-OA complex | ||
|---|---|---|---|---|
| FImax | λmax | FImax | λmax | |
| Peak 1 | ||||
| 25 | 362.47 ± 10.65 | 335.0 ± 0.5 | 452.49 ± 29.46 | 336.0 ± 1.5 |
| 60 | 503.09 ± 19.34 | 336.5 ± 0.5 | 454.36 ± 19.09 | 334.0 ± 0.0 |
| 65 | 522.71 ± 15.40 | 340.5 ± 1.0 | 453.46 ± 15.67 | 335.0 ± 1.5 |
| 70 | 577.71 ± 23.43 | 342.0 ± 0.5 | 417.77 ± 32.27 | 335.0 ± 1.5 |
| 75 | 635.38 ± 18.77 | 344.0 ± 0.5 | 482.73 ± 6.60 | 338.0 ± 1.5 |
| 80 | 705.38 ± 25.67 | 342.5 ± 1.0 | 530.32 ± 13.26 | 343.5 ± 0.5 |
| 85 | 531.51 ± 35.78 | 341.0 ± 0.5 | 540.91 ± 13.29 | 343.0 ± 2.0 |
| Peak 2 | ||||
| 25 | 234.01 ± 11.26 | 334.5 ± 0.5 | 230.04 ± 11.76 | 335.0 ± 0.0 |
| 60 | 258.97 ± 10.67 | 336.5 ± 1.5 | 220.90 ± 8.15 | 334.0 ± 1.5 |
| 65 | 270.78 ± 10.37 | 336.5 ± 1.0 | 227.32 ± 8.51 | 335.0 ± 1.5 |
| 70 | 279.83 ± 15.79 | 340.5 ± 0.5 | 225.84 ± 5.68 | 334.5 ± 1.7 |
| 75 | 330.11 ± 12.29 | 345.0 ± 0.5 | 235.91 ± 0.0 | 334.5 ± 1.06 |
| 80 | 371.04 ± 15.49 | 340.0 ± 1.0 | 252.07 ± 1.46 | 342.5 ± 2.5 |
| 85 | 74.168 ± 10.56 | 346.5 ± 0.5 | 217.89 ± 0.0 | 342.5 ± 0.5 |
The linoleic acid binding is possible due to well-known Tanford transition, which implies that at pH higher than 7.0, in addition to dimer dissociation; β-Lg molecules open their calyx, thus favoring the binding of ligands. The formed complex is much more stable at heating, with a small blue-shift of 2 nm at temperatures ranging from 60 to 70 °C. Further increased of the temperature caused a red-shift of 7 nm and an increase in fluorescence intensity of only 20 % at 85 °C. These conformational changes indicate sequential changes in the polarity of Trp residues microenvironment from a less polar to a more polar environment.
The fluorescence intensity of peak 2 slightly increases after heat-treatment, indicating that the protein backbone was changed. In the case of β-Lg solutions, a red-shift of 10.5 nm was observed at 75 °C, followed by a blue-shift of 5 nm at 80 °C, and a further red-shift of 6.5 nm at 85 °C. We may therefore assume that the heat-treatment caused a sequential process of unfolding and folding of the polypeptide chain, which resulted in conformational changes and increased hydrophobicity. The complex was significantly stable to heat treatment up to 75 °C, whereas a red-shift of 7 nm was measured at 80–85 °C.
Combining these observations with the synchronous fluorescence spectra results, we can conclude that the thermal treatment induced unfolding of the protein, resulting in conformational changes that increased the exposure of some hydrophobic regions which were previously buried. Moreover, the phenomenon described above and the analysis of the characteristic fluorescence of the peaks revealed that thermal treatment induced some microenvironmental and conformational changes of the protein even when in complex with LA.
Acrylamide and KI quenching
Fluorescence quenching is a powerful tool in the analysis of protein dynamics, conformational changes, binding constants and solvent exposure (Matyus et al. 2006; Callis 2014). In order to characterize the microenvironment of the fluorophores, the acrylamide was used to quench fully and partially exposed Trp residues (Bódis et al. 2013). KI quenching was used to obtain more detailed information about Trp residues accessibility. Both acrylamide and KI quenching showed linear plots indicating that all Trp residues were equally accessible to the quencher (data not shown) and therefore a single quenching mechanism occurs. The quenching constants had significantly higher values for β-Lg solutions compared to those of the complex. Heating caused an increase in Ksv values as a consequence of the unfolding (Table 2). When compared to the samples tempered at 25 °C, an increase by 85 % can be observed for the heat-treated β-Lg solutions at 85 °C, whereas heating the complex at the same temperature led to an increase of approx. 55 %, as a result of boosting the accessibility of the fluorophore to the quencher.
Table 2.
The Stern Volmer quenching constants (K sv) in the different conformational stages of solutions
| Temperature (°C) | KSV × 103(mol L−1) | |||
|---|---|---|---|---|
| Acrylamide | KI | |||
| β-Lg | β-Lg-OA | β-Lg | β-Lg-OA | |
| 25 | 3.44 ± 0.87a | 2.80 ± 0.10 | 0.92 ± 0.02 | 0.80 ± 0.01 |
| 60 | 3.72 ± 0.58 | 2.82 ± 0.07 | 0.93 ± 0.07 | 0.90 ± 0.02 |
| 65 | 3.88 ± 0.69 | 3.06 ± 0.18 | 1.32 ± 0.12 | 0.96 ± 0.31 |
| 70 | 4.80 ± 0.71 | 3.03 ± 0.05 | 1.59 ± 0.69 | 1.02 ± 0.08 |
| 75 | 5.54 ± 0.98 | 3.67 ± 0.19 | 1.67 ± 0.43 | 1.04 ± 0.03 |
| 80 | 6.14 ± 0.94 | 4.39 ± 0.02 | 2.05 ± 0.47 | 0.91 ± 0.01 |
| 85 | 6.38 ± 0.97 | 4.33 ± 0.27 | 1.89 ± 0.34 | 0.78 ± 0.0 |
aStandard deviations
When quenching with KI, the Stern-Volmer constant for β-Lg solutions at 25 °C was 0.92 ± 0.02·103 mol L−1, having a significant increase of approx. 124 % at 85 °C. An increase in Ksv values from 0.80 ± 0.01·103 mol L−1 for the untreated complex, up to 1.04 ± 0.03·103 mol L−1 at 75 °C was also observed. The corresponding Ksv values reflect a higher accessibility of KI to the complex up to 75 °C, probably due to the increased Trp residues exposure, followed by a lower accessibility due to the folding of polypeptide chains at higher temperature. These values highlight a sequential denaturation process involving unfolding of molecules in the temperature range 25–75 °C, followed by folding at higher temperatures. The quenching constants were higher for acrylamide compared to the KI, as expected for the amino terminal Trp residues.
In silico analysis of thermally induced changes in β-Lg-LA complex
The β-Lg model from 4DQ4.pdb shares 100 % sequence similarity with the 3NPO.pdb which was previously used for investigating the heat-induced conformational and structural changes in β-Lg. When simulating the thermal behavior of the ligand-free β-Lg a significant reduction of the strand content was obtained; the temperature increase up to 80 °C caused strands decrease with 13.2–13.8 % with respect to the room temperature (Stănciuc et al. 2012). On the other hand both strands motifs and total helical content were well preserved in the β-Lg-LA complex treated at high temperatures (42.4 and 15.8 %, respectively at 80 °C). With respect to the initial crystal structure, a new G type helix between aminoacids Pro38 and Arg40 was observed in the β-Lg model equilibrated at 80 °C in the presence of LA, whereas the Gln159 – His161 sequence lost the specific hydrogen bonding responsible for the helical conformation. The most important changes in the protein structure are related to the beta turn, gamma turns and beta bulge motifs. The thermal treatment up to 80 °C destabilized the Ala37- Arg40, Trp61- Gly64 and Thr76- Pro79 beta turns and all four gamma turns from the initial model, potentially affecting the flexibility of some loops surrounding the β-barrel where is located the LA binding site (Loch et al. 2013). The molecular rearrangements favored formation of new native-like motifs such as seven beta turns (Pro79-Phe82, Ile84-Glu89, Asp85-Asn90, Asn109-Glu114, Ser110-Gln115, Lys141-Pro144 and Glu158-His161), two inverse gamma turns (Gly9-Asp11 and Glu127-Asp129) and one antiparallel classic beta bulge (involving Ile84, Glu89 and Asn90).
Even though the total number of residues at protein surface does not change significantly with the temperature (150 residues at 27 °C and 149 residues at 80 °C), an increase of ~166 Ǻ2 of the total solvent accessible area was obtained, mostly because of the rearrangements of the side chains of the exposed aminoacids. The geometry of the β-Lg-LA complex is not only due to the contacts established between the two components, but also to the interaction with the solvent molecules (Krissinel and Henrick 2007). Formation of β-Lg-LA complex has been shown to be driven by both enthalpy, associated to the interaction between fatty acid, solvent and protein, and entropy, given by conformational changes of the ligand or of the binding site in direct correlation with water displacement while binding the ligand (Loch et al. 2013). As a consequence of the heat induced structural changes, the solvation energy value, estimated as the surface hydrophobic residue-solvent interaction (ΔG) increased from −38.8 kcal/mol at 27 °C to −32.4 kcal/mol at 80 °C, whereas the solvation energy of protein folding decreases from −137.7 to −143.6 kcal/mol. Anyway, no important change appears in the surface of the ligand binding site. The total solvent accessible surface area which gets buried upon β-Lg-LA complex formation increased from 364.8 to 365.4 Ǻ2.
In addition to the protein component, the temperature increase also affects the conformation of the LA causing the reduction of the total surface from 597.68 to 566.52 Ǻ2 and of solvation energy from 5.6 to 5.0 kcal/mol. Previous studies indicate the almost linear geometry of the LA when bound by different types of proteins (Loch et al. 2013), with potential alternative conformation for the region defined by the atoms C3-C6. Indeed, the comparative analysis of LA conformation after performing molecular dynamics equilibration steps at 25 and 80 °C indicates the fatty acid chain U bending and shortening (Fig. 6). Anyway, regardless of simulated temperature, the LA surface involved in the interaction with β-Lg is about 497 Ǻ2, indicating a rather good affinity between the two components of the complex.
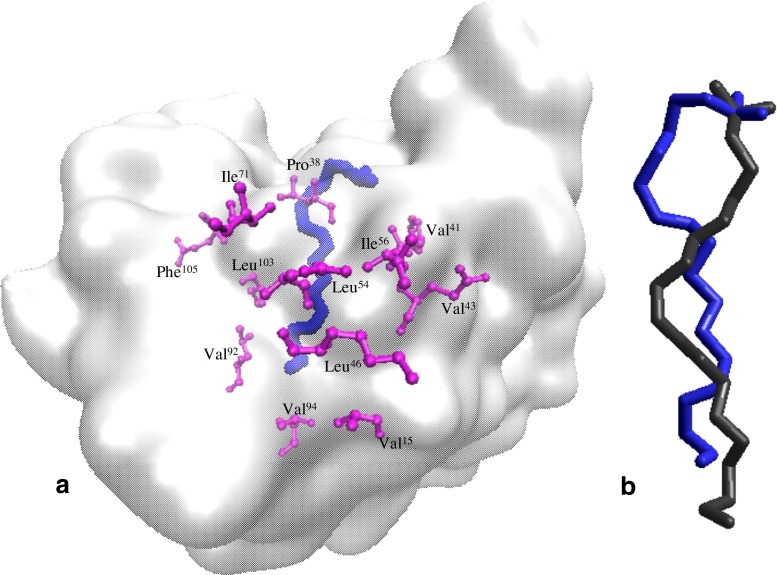
Fig. 6.
Details on the β-Lg – LA model equilibrated at 80 °C. a The protein is represented in QuickSurf style, the LA molecule (blue) in Licorice style, and the aminoacid residues directly involved in the interaction with LA (violet) in CPK style using VMD software. b Comparison of LA molecules equilibrated at 25 °C (black) and at 80 °C (blue) by superimposing the C1 atoms of the two molecules
The β-Lg structural changes and variations registered at the interface with LA highly influence the interaction properties within the complex. As expected, the interaction energy, estimated by subtracting the individual energy of each component of the complex from the total energy, increased from −6680.16 kcal/mol at 25 °C to −6590.32 kcal/mol at 80 °C, suggesting a slight decrease of protein affinity for the fatty acid at high temperature. Indeed, a detailed atomic level check of the binding site indicates some differences between models equilibrated at 25 and 80 °C. At low temperature, our results comply with crystallography data, indicating that the carboxyl group of LA is directed towards Lys60, Glu62 and Lys69 (Loch et al. 2013). The thermally induced molecular events within and in the vicinity of the LA binding pocket appear to affect the β-Lg-LA interface properties. Concerning the carboxyl group, at high temperature Pro38 establishes new hydrophobic contacts and one hydrogen bond of 2.76 Å with the oxygen atom, in addition to the hydrophobic contacts with C3 and C4. The 2.86 Å-long interface hydrogen bond involving Glu62 stabilizing the β-Lg-LA complex at room temperature (Loch et al. 2013) was destroyed due to local conformational changes. At high temperature new hydrophobic contacts were established between Val15 and C16 atom, whereas Ile56 migrates towards C18 of the distorted LA molecule.
Conclusions
Combining in silico and fluorescence based experimental approaches allowed comprehensive prediction of thermal induced behavior of the β-Lg-LA complex. The β-Lg-LA complex was thermally treated at different temperatures ranging from 25 to 85 °C. Experimental results suggested that the formed complexes were stable in the temperature range 25–75 °C and that at higher temperatures the polypeptide chain unfolded. Detailed analysis of the simulation datasets suggested a good thermodynamic stability of the β-Lg-LA complex even at 80 °C. This study offers valuable insights on the heat induced changes in β-Lg-LA complex, providing the knowledge base for developing new strategies for nanoencapsulation of bioactive compounds using β-Lg as a carrier.
Acknowledgments
The work has been funded by the Sectoral Operational Programme Human Resources Development 2007–2013 of the Ministry of European Funds through the Financial Agreement POSDRU/159/1.5/S/132397.
References
- Bergamo P., Luongo D., Miyamoto J., Cocca E., Kishino S., Ogawa J., Tanabe S., Rossi M. Immunomodulatory activity of a gut microbial metabolite of dietary linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, associated with improved antioxidant/detoxifying defences. J Funct Foods. 2014;II:192–202. doi: 10.1016/j.jff.2014.10.007. [DOI] [Google Scholar]
- Bi S., Yan L., Pang B., Wang Y. Investigation of three flavonoids binding to bovine serum albumin using molecular fluorescence technique. J Lumin. 2012;132:132–140. doi: 10.1016/j.jlumin.2011.08.014. [DOI] [Google Scholar]
- Bódis E., Raics K., Nyitrai M., Majer Z., Lukács A. Fluorescence lifetime distributions report on protein destabilisation in quenching experiments. J Photochem Photobiol B Biol. 2013;129:108–114. doi: 10.1016/j.jphotobiol.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Buccioni A., Decandia M., Minieri S., Molle G., Cabiddu A. Lipid metabolism in the rumen: new insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim Feed Sci Technol. 2012;174:1–25. doi: 10.1016/j.anifeedsci.2012.02.009. [DOI] [Google Scholar]
- Callis P. Binding phenomena and fluorescence quenching. I: descriptive quantum principles of fluorescence quenching using a supermolecule approach. J Mol Struct. 2014;1077:14–21. doi: 10.1016/j.molstruc.2014.04.050. [DOI] [Google Scholar]
- Choque B., Catheline D., Rioux V., Legrand P. Linoleic acid: between doubts and certainties. Biochimie. 2014;96:14–21. doi: 10.1016/j.biochi.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Croquennec T., Molle D., Mehra R., Bouhallab S. Spectroscopic characterization of heat-induced nonnative β-lactoglobulin monomers. Protein Sci. 2004;13:1340–1346. doi: 10.1110/ps.03513204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehteshami M., Rasoulzadeh F., Mahboob S., Rashidi M. R. Characterization of 6-mercaptopurine binding to bovine serum albumin and its displacement from the binding sites by quercetin and rutin. J Lumin. 2013;135:164–169. doi: 10.1016/j.jlumin.2012.10.044. [DOI] [Google Scholar]
- Fontana A., Spolaore B., Polverino de Laureto P. The biological activities of protein/oleic acid complexes reside in the fatty acid. Biochim Biophys Acta. 2013;1834:1125–1143. doi: 10.1016/j.bbapap.2013.02.041. [DOI] [PubMed] [Google Scholar]
- Frapin D., Dufour E., Haertle T. Probing the fatty acid binding site of β-lactoglobulins. J Protein Chem. 1993;12:443–449. doi: 10.1007/BF01025044. [DOI] [PubMed] [Google Scholar]
- Humphrey W., Dalke A., Schulten K. VMD - visual molecular dynamics. J Mol Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Jelińska M., Bialek A., Mojska H., Gielecińska I., Tokarz A. Effect of conjugated linoleic acid mixture supplemented daily after carcinogen application on linoleic and arachidonic acid metabolites in rat serum and induced tumours. Biochim Biophys Acta. 2014;1842:2230–2236. doi: 10.1016/j.bbadis.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Jiang H.R., Liu N. Self-assembled beta-lactoglobulin-conjugated linoleic acid complex for colon cancer-targeted substance. J Dairy Sci. 2010;93:3931–3939. doi: 10.3168/jds.2010-3071. [DOI] [PubMed] [Google Scholar]
- Keppler J.K., Sӧnnichsen F.D., Lorenzen P.C., Schwarz K. Differences in heat stability and ligand binding among β-lactoglobulin genetic variants A, B and C using 1H NMR and fluorescence quenching. Biochim Biophys Acta. 2014;1844:1083–1093. doi: 10.1016/j.bbapap.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Koba K., Yanagita T. Health benefits of conjugated linoleic acid (CLA) Obes Res Clin Pract. 2014;8(6):525–532. doi: 10.1016/j.orcp.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Krissinel E., Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372(3):774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Kuznetsova M., Turoverov K. K., Uversky V. N. Use of the phase diagram method to analyze the protein unfolding–refolding reactions: fishing out the “invisible” intermediates. J Proteome Res. 2004;3:485–494. doi: 10.1021/pr034094y. [DOI] [PubMed] [Google Scholar]
- Laskowski R. A. PDBsum new things. Nucleic Acids Res. 2009;37:D355e–DD359. doi: 10.1093/nar/gkn860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski R. A., Swindells M. B. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- Liang L., Subirade M. Study of the acid and thermal stability of β-lactoglobulin-ligand complexes using fluorescence quenching. Food Chem. 2012;132:2023–2029. doi: 10.1016/j.foodchem.2011.12.043. [DOI] [Google Scholar]
- Livney Y.D. Milk proteins as vehicles for bioactives. COCIS. 2010;15:73–83. [Google Scholar]
- Loch J.I., Bonarek P., Polit A., Ričs D., Dziedzicka-Wasylewska M., Lewinski K. Binding of 18-carbon unsaturated fatty acids to bovine β-lactoglobulin–structural and thermodynamic studies. Int J Biol Macromol. 2013;57:226–231. doi: 10.1016/j.ijbiomac.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Manderson G.A., Hardman M.J., Creamer L.K. Effect of heat treatment on bovine β-lactoglobulin a, B, and C explored using thiol availability and fluorescence. J Agric Food Chem. 1999;47:3617–3627. doi: 10.1021/jf990591g. [DOI] [PubMed] [Google Scholar]
- Matulis D., Lovrien R. 1-anilino-8-naphthalene sulfonate anion-protein binding depends primarily on ion pair formation. Biophysica. 1998;74:422–429. doi: 10.1016/S0006-3495(98)77799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyus L., Szollosi J., Jenei A. Steady-state fluorescence quenching applications for studying protein structure and dynamics. J Photochem Photobiol B Biol. 2006;83:223–236. doi: 10.1016/j.jphotobiol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Mensi A., Choiset Y., Rabesona H., Haertlé T., Borel P., Chobert J.-M. Interactions of β-lactoglobulin variants A and B with vitamin A. Competitive binding of retinoids and carotenoids. J Agric Food Chem. 2013;61:4114–4119. doi: 10.1021/jf400711d. [DOI] [PubMed] [Google Scholar]
- Perez A., Andermatten R., Rubiolo A., Santiago L. β-lactoglobulin heat-induced aggregates as carriers of polyunsaturated fatty acids. Food Chem. 2014;158:66–72. doi: 10.1016/j.foodchem.2014.02.073. [DOI] [PubMed] [Google Scholar]
- Qi X.L., Holt C., McNulty D., Clarke T., Brownlow S., Jones G. Effect of temperature on the secondary structure of β-lactoglobulin at pH 6.7, as determined by CD and IR spectroscopy: a test of the molten globule hypothesis. Biochem J. 1997;324:341–346. doi: 10.1042/bj3240341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rade-Kukic K., Schmitt C., Rawel H.M. Formation of conjugates between β-lactoglobulin and allyl isothiocyanate: effect on protein heat aggregation, foaming and emulsifying properties. Food Hydrocoll. 2011;25:694–706. doi: 10.1016/j.foodhyd.2010.08.018. [DOI] [Google Scholar]
- Sahihi M., Bordbar A.K., Ghayeb Y. Thermodynamic stability and retinol binding property of β-lactoglobulin in the presence of cationic surfactants. J Chem Thermodyn. 2011;43:1185–1191. doi: 10.1016/j.jct.2011.03.004. [DOI] [Google Scholar]
- Santambrogio C., Grandori R. Monitoring the tanford transition in betalactoglobulin by 8-anilino-1-naphthalene sulfonate and mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:4049–4054. doi: 10.1002/rcm.3824. [DOI] [PubMed] [Google Scholar]
- Seo J-A, Hédoux A, Guinet Y, Paccou L, Affouard F, Lerbret A, Descamps M. Thermal denaturation of beta-lactoglobulin and stabilization mechanism by trehalose analyzed from Raman spectroscopy investigations. J Phys Chem B. 2010;114:6675–6684. doi: 10.1021/jp1006022. [DOI] [PubMed] [Google Scholar]
- Shen H., Gu Z., Jian K., Qi J. In vitro study on the binding gemcitabine to bovine serum albumin. J Pharm Biomed Anal. 2013;75:86–93. doi: 10.1016/j.jpba.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Shen L-L, Xu H, Huang F, Li Y, Xiao J, Xiao Z, He Z, Zhou K. Study on interaction of ligupurpuroside A with bovine serum albumin by multi-spectroscopic methods. J Lumin. 2014;154:80–88. doi: 10.1016/j.jlumin.2014.04.009. [DOI] [Google Scholar]
- Shi X., Li X., Gui M., Zhou H., Yang R., Zhang H., Jin Y. Studies on interaction between flavonoids and bovine serum albumin by spectral methods. J Lumin. 2010;130:637–644. doi: 10.1016/j.jlumin.2009.11.008. [DOI] [Google Scholar]
- Sponton O.E., Perez A.A., Carrara C., Santiago L.G. Effect of limited enzymatic hydrolysis on linoleic acid binding properties of β-lactoglobulin. Food Chem. 2014;146:577–582. doi: 10.1016/j.foodchem.2013.09.089. [DOI] [PubMed] [Google Scholar]
- Stănciuc N., Aprodu I., Râpeanu G., Bahrim G. Fluorescence spectroscopy and molecular modelling investigations on the thermally induced structural changes of bovine b-lactoglobulin. Innovative Food Sci Emerg Technol. 2012;15:50–56. doi: 10.1016/j.ifset.2012.03.001. [DOI] [Google Scholar]
- Stănciuc N., Aprodu I., Râpeanu G., Bahrim G. pH and heat-induced structural changes of bovine α-lactalbumin in response to oleic acid binding. Eur Food Res Technol. 2013;236:257–266. doi: 10.1007/s00217-012-1882-9. [DOI] [Google Scholar]
- Taheri-Kafrani A., Asgari-Mobarakeh E., Bordbar A.K., Haertlé T. Structure-function relationship of β-lactoglobulin in the presence of dodecyltrimethyl ammonium bromide. Colloids Surf B Biointerfaces. 2010;75:268–274. doi: 10.1016/j.colsurfb.2009.08.045. [DOI] [PubMed] [Google Scholar]
- Wang Q., Allen J.C., Swaisgood H.E. Protein concentration dependence of palmitate binding to β-lactoglobulin. J Dairy Sci. 1998;81:76–81. doi: 10.3168/jds.S0022-0302(98)75553-5. [DOI] [Google Scholar]
- Wang Q, Allen JC, Swaisgood HE. Binding of lipophilic nutrients to β-lactoglobulin prepared by bioselective adsorbtion. J Dairy Sci. 1999;82:257–264. doi: 10.3168/jds.S0022-0302(99)75231-8. [DOI] [PubMed] [Google Scholar]
- Zhu S.Z., Liu Y. Spectroscopic analyses on interaction of naphazoline hydrochloride with bovine serum albumin. Spectrochim Acta. 2012;98:142–147. doi: 10.1016/j.saa.2012.08.040. [DOI] [PubMed] [Google Scholar]