Abstract
Background
Discovery of potent inhibitors of urease (jack bean) enzyme is the first step in the development of drugs against diseases caused by ureolytic enzyme.
Results
Thirty-two derivatives of barbituric acid as zwitterionic adducts of diethyl ammonium salts were synthesized. All synthesized compounds (4a–z and 5a–s) were screened for their in vitro inhibition potential against urease enzyme (jack bean urease). The compounds 4i (IC50 = 17.6 ± 0.23 µM) and 5l (IC50 = 17.2 ± 0.44 µM) were found to be the most active members of the series, and showed several fold more urease inhibition activity than the standard compound thiourea (IC50 = 21.2 ± 1.3 µM). Whereas, compounds 4a–b, 4d–e, 4g–h, 4j–4r, 4x, 4z, 5b, 5e, 5k, 5n–5q having IC50 values in the range of 22.7 ± 0.20 µM–43.8 ± 0.33 µM, were also found as potent urease inhibitors. Furthermore, Molecular Dynamics simulation and molecular docking studies were carried out to analyze the binding mode of barbituric acid derivatives using MOE. During MD simulation enol form is found to be more stable over its keto form due to their coordination with catalytic Nickel ion of Urease. Additionally, structural–activity relationship using automated docking method was applied where the compounds with high biological activity are deeply buried within the binding pocket of urease. As multiple hydrophilic crucial interactions with Ala169, KCX219, Asp362 and Ala366 stabilize the compound within the binding site, thus contributing greater activity.
Conclusions
This research study is useful for the discovery of economically, efficient viable new drug against infectious diseases.
Graphical abstract:
STD. Thiourea (IC50 = 21.2 ± 1.3 µM)
Electronic supplementary material
The online version of this article (doi:10.1186/s13065-015-0140-1) contains supplementary material, which is available to authorized users.
Keywords: Barbituric acid, Zwitterions, Urease enzyme, Urolitheasis, MD simulation and molecular docking
Background
Urease is a nickel containing enzyme produced by plants, fungi, algae, and bacteria. It is involved in nitrogen turnover and in crop fertilization, as well as in human and animal pathologies. Urease catalyse the hydrolysis of urea in its ammonia and carbon dioxide. Beside its medical, ecological and economical significances as urease has historical significances as it was the first enzyme to be crystallised in 1926 by Sumner [1–3]. Since its discovery in plants [4], Canavalia ensiformis (Fabaceae) urease has been exhaustively investigated [5]. Its activity is strictly dependent on nickel ions (Ni2+) [6]. The first X-ray diffraction based structure of a urease was reported by Jabri and coworkers in 1995 from Klebsiella aerogenes [7]. Later on, other structures for ureases from Bacillus pasteurii [8], Helicobacter pylori [9] and C. ensiformis [10] were reported. The elucidation of the urease structure from a legume (jack bean) was crucial to better understand the requirements for ureolytic activity of this class of enzymes in different organisms [10] were reported.
Urease enzyme is a virulence factor in certain human and animal ailments. It contributes to the development of kidney stones, pyelonephritis, peptic ulcers leading to gastric cancers, and other diseases [11]. It also causes the pathogenesis of hepatic coma urolithiasis, hepatic encephalopathy, pyelonephritis, ammonia and urinary catheter encrustation [12, 13]. The gastric cancer [14, 15] is the fourth most common cancer and the second most common cause of cancer-related deaths worldwide [16]. It is often resulted from pathologies due to Helicobacter pylori. Urease lets bacteria to persist at the low pH of the stomach during colonization and lead to pathogenesis of gastric and peptic ulcers which in the long run may cause cancer [17]. The treatment of infection caused by ureolytic bacteria with antimicrobials, however, often proved to be unsuccessful [13]. The barbiturates possessed a wide range of pharmacological applications, such as anticonvulsant, sedative, anxiolytic, urease inhibition [18], antifungal [19], antimicrobial [20, 21], antitumor, antiviral [13, 22] anti tuberculosis [23], mushroom tyrosinase inhibition [24], radio-sensitization [25], anti-inflammatory, anticancer [26], anesthetic [27], diaminopimelate aminotransferase inhibition [28], and anti-proliferative activities [29].
Based on the therapeutic and pharmacological significances of urease inhibition, our research group is involved in the search of simple but biologically interesting molecules that are easy to synthesize in just fewer steps with high yields. This type of chemistry is easily adopted by the pharmaceutical industry for commercialization. Previously, our research group reported zwitterionic adduct derived from barbituric acid as NO scavenger [30]. In view of these studies; the combined use of green synthetic technology for the high yield production of novel pharmacophoric barbituric acid derivatives and their systematic evalution of biological activities as urease inhibition is discussed in this paper.
Methods
General
All chemicals were purchased from Sigma-Aldrich, Fluka etc., and were used without further purification, unless otherwise stated. All melting points were measured on a Gallenkamp melting point apparatus in open glass capillaries and are uncorrected. IR Spectra were measured as KBr pellets on a Nicolet 6700 FT-IR spectrophotometer. The NMR spectra were recorded on a Varian Mercury Jeol-400 NMR spectrometer. 1H-NMR (400 MHz), and 13C-NMR (100 MHz) were run in deuterated chloroform (CDCl3). Chemical shifts (δ) are referred in terms of ppm and J-coupling constants are given in Hz. Mass spectra were recorded on a Jeol JMS-600 H. Elemental analysis was carried out on Elmer 2400 Elemental Analyzer in CHN mode
Synthesis of 4 and 5 (GP1)
A mixture of 1 (3 mmol) and aldehyde 2 (1.5 mmol), as well as Et2NH (1.5 mmol, 155 μL) were placed in 3 mL of degassed H2O. The reaction mixture was kept at rt up to 5 h under stirring. After completion of the reaction, monitored by TLC, the solid product was filtered, washed with ether (3 × 20 mL) and dried to obtain pure products 4 and 5.
4-(bis(6-Hydroxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)methyl)benzaldehyde diethylaminium salt 4a
4a, as colorless crystal (1.5 g, 2.76 mmol, 92 %). IR (cm−1): 3450, 3000, 2872, 1670, 1582, 1510, 1466, 1384, 1339; 1H-NMR (CDCl3, 400 MHz) 17.58 (s, 1H, OH), 9.90(s, 1H, CHO), 7.73 (d, 2H, J = 8.0 Hz, Ph), 7.29 (d, 2H, J = 8.0 Hz, Ph), 5.93(s, 1H, benzyl-H), 3.33 (s, 12H, 4CH3), 3.06 (q, 4H, J = 7.3 Hz, CH2CH3), 1.27 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, CDCl3): δ = 192.2, 165.3, 164.4, 151.7, 150.3, 134.3, 129.9, 127.3, 91.7, 42.2, 35.1, 29.0, 28.7, 11.5; Anal. for C24H31N5O7; Calcd: C, 57.48; H, 6.23; N, 13.96; Found:C, 57.50; H, 6.25; N, 14.00; LC/MS (ESI): m/z = 501.53 [M]+.
5,5′-(3-Tolylmethylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) diethylaminium salt 4b
4b; rose-colored crystalline materials. m.p.: 135 °C; (97 %, 1.41 g, 2.91 mmol). IR (KBr, cm−1): 3455, 3201, 2988, 1693, 1667, 1611, 1573, 1443; 1H-NMR (400 MHz, CDCl3): δ17.62 (s, 1H, OH), 7.10 (t, 1H, J = 7.3 Hz, Ph), 6.92 (d, 1H, J = 7.3 Hz, Ph), 6.88 (d, 1H, J = 7.3 Hz, Ph), 5.82(s, 1H, benzyl-H), 3.32 (s, 12H, 4CH3), 3.01 (q, 4H, J = 7.3 Hz, CH2CH3), 2.25 (s, 3H, CH3), 1.26 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, CDCl3): δ = 165.3, 164.4, 151.8, 141.7, 137.4, 127.9, 127.1, 126.4, 123.6, 92.1, 42.0, 34.4, 28.9, 28.6, 21.8, 11.4; Anal. for C24H35N5O6; Calcd: C, 59.12; H, 6.82; N, 14.36; Found: C, 59.13; H, 6.81; N, 14.35; LC/MS (ESI): m/z = 487[M]+.
5,5′-((4-Nitrophenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione)diethylaminium salt 4c
4c; a yellow powder; m.p.: 195 °C; (87 %, 1.35 g, 2.61 mmol); IR (KBr, cm−1): 3453, 3205, 2987, 2904, 1675, 1608, 1576, 1511, 1438, 1343, 1254;1H-NMR (400 MHz, CDCl3): δ17.58 (s, 1H, OH), 8.08 (d, 2H, J = 8.8 Hz, Ph), 7.29 (d, 2H, J = 8.8 Hz, Ph), 5.95(s, 1H, benzyl-H), 3.34 (s, 12H, 4CH3), 3.07 (q, 4H, J = 7.3 Hz, CH2CH3), 1.29 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, CDCl3): δ = 165.2, 164.4, 151.6, 150.8, 146.1, 127.5, 123.5, 91.4, 42.2, 34.9, 28.9, 28.7, 11.5; Anal. for C23H30N6O8; Calcd: C, 53.28; H, 5.83; N, 16.21; Found: C, 53.29; H, 5.85; N, 16.23; LC/MS (ESI): m/z = 518[M]+.
A suitable crystal for X-ray diffraction analysis was obtained from DCM/Et2O after 24 h. CCDC-1001798 contains the supplementary crystallographic data for this compound (Additional file 1).
5,5′-((4-Methoxyphenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) diethylaminium salt 4d
4d; rose-colored crystalline materials; m.p.: 160 °C; (90 %, 1.35 g, 2.7 mmol). IR (KBr, cm−1): 3445, 3195, 2977, 2836, 1689, 1664, 1613, 1504, 1447, 1378, 1242; 1H-NMR (400 MHz, CDCl3): δ17.67 (s, 1H, OH), 7.01 (d, 2H, J = 8.8 Hz, Ph), 6.75 (d, 2H, J = 8.8 Hz, Ph), 5.79(s, 1H, benzyl-H), 3.33 (s, 12H, 4CH3), 2.99 (q, 4H, J = 7.3 Hz, CH2CH3), 1.26 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, CDCl3): δ = 165.3, 164.3, 157.4, 151.7, 133.6, 132.0, 127.4, 114.3, 92.1, 55.6, 42.1, 33.8, 28.9, 11.5; Anal. for C24H33N5O7; Calcd: C, 57.25; H, 6.61; N, 13.91; Found: C, 57.26; H, 6.61; N, 13.90; LC/MS (ESI): m/z = 503[M]+.
5,5′-((3-Bromophenyl)methylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) diethylaminium salt 4e
4e; colorless crystalline materials; m.p.: 169 °C; (92 %, 1.5 g, 2.76 mmol). IR (KBr, cm−1): 3450, 3120, 2982, 1694, 1667, 1615, 1577, 1445, 1250; 1H-NMR (400 MHz, CDCl3): δ17.63 (s, 1H, OH), 7.22 (d, 1H, J = 7.3 Hz, Ph), 7.19 (s, 1H,Ph), 7.07 (d, 1H, J = 7.3 Hz, Ph), 7.05 (d, 1H, J = 7.3 Hz, Ph), 5.84(s, 1H, benzyl-H), 3.34 (s, 6H, 2CH3), 3.32 (s, 6H, 2CH3), 3.02 (q, 4H, J = 7.3 Hz, CH2CH3), 1.27 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, CDCl3): δ = 165.2, 164.4, 151.7, 144.7, 129.7,129.6, 128.7, 125.3, 91.5, 42.1, 34.4, 28.9, 28.7, 11.5; Anal. for C23H30BrN5O6; Calcd: C, 50.01; H, 5.47; Br, 14.46; N, 12.68; Found: C, 50.03; H, 5.48; Br, 14.47; N, 12.71; LC/MS (ESI): m/z = 552[M]+.
A suitable crystal for X-ray diffraction analysis was obtained from DCM/Et2O after 24 h. CCDC-1001799 contains the supplementary crystallographic data for this compound.
5,5′-((4-hydroxyphenyl)methylene)bis(6-hydroxy-1,3-dimethylpyrimidine-2,4(1H,3H)-dione) diethylaminium salt 4f
4f; a yellow powder; m.p.: 180 °C; (88 %, 1.3 g, 2.64 mmol); IR (KBr, cm−1): 3458, 3200, 2980, 2904, 1677, 1620, 1572, 1511, 1438, 1343, 1254;1H-NMR (400 MHz, CDCl3): δ17.62 (s, 1H, OH), 7.31 (d, 2H, J = 8.8 Hz, Ph), 6.99 (d, 2H, J = 8.8 Hz, Ph), 5.79(s, 1H, benzyl-H), 3.33 (s, 12H, 4CH3), 3.03 (q, 4H, J = 7.3 Hz, CH2CH3), 1.27 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, CDCl3): δ = 165.3, 164.4, 151.7, 141.1, 131.2, 128.5, 119.3, 91.7, 42.1, 34.2, 28.9, 28.7, 11.5; Anal. for C23H31N5O7; Calcd: C, 56.43; H, 6.38; N, 14.31; Found: C, 56.44; H, 6.36; N, 14.30; LC/MS (ESI): m/z = 489.52 [M]+.
5,5′-(p-Tolylmethylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) diethylaminium salt 4g
4g; colorless needle materials; m.p.: 152 °C; (97 %, 1.41 g, 2.91 mmol). IR (KBr, cm−1): 3455, 3210, 2984, 2820, 1560, 1449, 1359; 1H-NMR (400 MHz, CDCl3): δ17.64 (s, 1H, OH), 6.99–6.96 (m, 4H, Ph), 5.80(s, 1H, benzyl-H), 3.32 (s, 12H, 4CH3), 3.03 (q, 4H, J = 7.3 Hz, CH2CH3), 2.25 (s, 3H, CH3), 1.28 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, CDCl3): δ = 165.3, 164.3, 151.8, 138.6, 134.8, 128.9, 126.3, 92.1, 42.0, 34.2, 28.9, 28.6, 21.0, 11.4; Anal. for C24H35N5O6; Calcd: C, 59.12; H, 6.82; N, 14.36; Found: C,59.13; H, 6.81; N, 14.35; LC/MS (ESI): m/z = 487[M]+.
A suitable crystal for X-ray diffraction analysis was obtained from DCM/Et2O after 24 h. CCDC-957025 contains the supplementary crystallographic data for this compound.
5,5′-(Naphthalen-2-ylmethylene)bis(1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione) diethylaminium salt 4h
4h; beige powder; m.p.: 146 °C; (94 %, 1.47 g, 2.82 mmol). IR (KBr, cm−1): 3454, 3200, 2967, 1668, 1585, 1438, 1250;1H-NMR (400 MHz, CDCl3): δ17.33 (s, 1H, OH), 8.10 (d, 2H, J = 8.8 Hz, naphthyl-H), 7.99 (d, 2H, J = 8.8 Hz, naphthyl-H), 7.92 (d, 2H, J = 8.8 Hz, naphthyl-H), 7.90 (d, 2H, J = 8.8 Hz, naphthyl-H), 7.84 (d, 2H, J = 8.8 Hz, naphthyl-H), 7.68–7.38 (m, 3H,naphthyl-H), 6.37(s, 1H, benzyl-H), 3.39 (s, 12H, 4CH3), 3.01 (q, 4H, J = 7.3 Hz, CH2CH3), 1.30 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, CDCl3): δ = 164.9, 151.7, 136.8, 135.3, 134.3, 131.5, 129.1, 128.5, 127.0, 125.2 124.9, 123.8, 93.2, 41.8, 33.2, 28.8, 11.4; Anal. for C27H33N5O6; Calcd: C, 61.94; H, 6.35; N, 13.38; Found: C, 61.95; H, 6.34; N, 13.40; LC/MS (ESI): m/z = 523 [M]+.
5,5′-(p-Tolylmethylene)bis(6-hydroxypyrimidine-2,4(1H,3H)-dione) diethylaminium salt 4i
4i; white powder; m.p.: 205 C; (95 %; 1.22 g, 2.85 mmol); IR (KBr, cm−1): 3459, 3120, 2978, 2811, 1689, 1612, 1325, 1252; 1H-NMR (400 MHz, DMSO-d6): δ17.18 (s, 1H, OH), 10.09 (bs, 4H, NH), 6.93 (m, 4H, Ph), 5.90(s, 1H, benzyl-H), 2.79 (q, 4H, J = 7.3 Hz, CH2CH3), 2.20 (s, 3H, CH3), 1.07 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 164.8, 164.1, 151.3, 142.1, 133.5, 128.5, 127.1, 91.6, 42.6, 30.6, 21.1, 13.0; Anal. for C20H25N5O6; Calcd: C, 55.68; H, 5.84; N, 16.23; Found: C, 55.67; H, 5.83; N, 16.22; LC/MS (ESI): m/z = 431[M]+.
5,5′-((4-Chlorophenyl)methylene)bis(6-hydroxypyrimidine-2,4(1H,3H)-dione) diethylaminium salt 4j
4j; a white powder; m.p.: 221 °C; (95 %, 1.28 g, 2.85 mmol); IR (KBr, cm−1): 3435, 3185, 2978, 2830, 1677, 1548, 1448, 1345, 1250;1H-NMR (400 MHz, DMSO-d6): δ17.17 (s, 1H, OH), 10.00 (bs, 4H, NH), 7.18 (m, 4H, Ph), 5.93(s, 1H, benzyl-H), 2.88 (q, 4H, J = 7.3 Hz, CH2CH3), 1.12 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 164.7, 164.0, 151.2, 144.6, 133.5, 129.9, 129.1, 127.8, 91.3, 42.1, 30.7, 11.8; Anal. for C19H22ClN5O6; Calcd C, 50.50; H, 4.91; Cl, 7.85; N, 15.50; Found: C, 50.51; H, 4.90; Cl, 7.83; N, 15.51; LC/MS (ESI): m/z = 451[M]+.
5,5′-((4-Methoxyphenyl)methylene)bis(6-hydroxypyrimidine-2,4(1H,3H)-dione) diethylaminium salt 4K
4k; a beige powder; m.p.: 195 °C; (91 %, 1.22 g, 2.73 mmol); IR (KBr, cm−1): 3449, 3190, 2991, 2835, 1688, 1592, 1505, 1383, 1247;1H-NMR (400 MHz, DMSO-d6): δ17.26 (s, 1H, OH), 9.99 (bs, 4H, NH), 6.92 (d, 2H, J = 8.0 Hz, Ph), 6.72 (d, 2H, J = 8.0 Hz, Ph), 5.88(s, 1H, benzyl-H), 2.90 (q, 4H, J = 7.3 Hz, CH2CH3), 1.14 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 164.6, 164.0, 157.0, 151.2, 137.2, 132.4, 115.1, 91.7, 55.4, 42.1, 30.7, 11.6; Anal. for C20H25N5O7; Calcd C, 53.69; H, 5.63; N, 15.65; Found: C, 53.69; H, 5.63; N, 15.66; LC/MS (ESI): m/z = 447[M]+.
5,5′-(Naphthalen-2-ylmethylene)bis(6-hydroxypyrimidine-2,4(1H,3H)-dione) diethylaminium salt 4l
4 l; a beige powder, m.p.: 192 °C; (93 %, 1.3 g, 2.79 mmol); IR (KBr, cm−1): 3459, 3208, 2994, 1677, 1579, 1448, 1386, 1354;1H-NMR (400 MHz, DMSO-d6): δ16.92 (s, 1H, OH), 10.41 (bs, 4H, NH), 8.13 (d, 1H, J = 8.8 Hz, naphthyl), 7.81(d, 1H, J = 8.8 Hz, naphthyl), 7.63 (d, 1H, J = 8.8 Hz, naphthyl), 7.38–7.32 (m, 4H, naphthyl), 6.46(s, 1H, benzyl-H), 2.79 (q, 4H, J = 7.3 Hz, CH2CH3), 1.08 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 164.9, 151.1,141.5, 135.8, 134.0,132.4, 129.3, 128.7, 126.0,125.8, 125.5, 125.2, 124.9, 123.8, 92.3, 42.5, 29.7, 12.7; Anal. for C23H25N5O6; Calcd C, 59.09; H, 5.39; N, 14.98; Found: C, 59.12; H, 5.40; N, 15.01; LC/MS (ESI): m/z = 467[M]+.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(phenyl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 4m
4m; colorless crystalline material; m.p: 159 °C; (98 %, 671 mg, 1.47 mmol). IR (KBr, cm−1): 3150, 2959, 1667, 1617, 1585, 1422, 1256, 1227;1H NMR (400 MHz, CDCl3): δ 15.28 (s, 1H, OH), 7.17–7.04(m, 5H, Ph), 5.85 (s, 1H, benzyl-H), 3.29 (s, 12H, 4CH3), 2.96(q, 4H, J = 7.3 Hz, CH2CH3), 2.42 (d, 2H, J = 5.1 Hz, CH2), 2.29 (m, 2H, CH2), 1.24(t, 6H, J = 7.3 Hz, CH2CH3), 1.14(s, 3H, CH3), 1.05(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 192.5, 180.8, 152.5, 142.5, 128.0, 126.7, 125.1, 116.3, 90.9, 51.4, 45.9, 42.2, 33.0, 31.5, 29.6, 28.4, 27.6, 11.4; Anal. for C25H35N3O5; calcd: C, 65.62; H, 7.71; N, 9.18;Found: C, 65.61; H, 7.73; N, 9.20; LC/MS (ESI): m/z = 457 [M]+.
A suitable crystal for X-ray diffraction analysis was obtained from CHCl3/Et2O after 24 h. CCDC- 933624 contains the supplementary crystallographic data for this compound.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(p-tolyl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 4n
4n; oily material (97 %; 685 mg, 1.45 mmol). IR (KBr, cm−1): 3150, 2954, 2867, 1675, 1580, 1508, 1447, 1380, 1256, 1145;1H NMR (400 MHz, CDCl3): δ 15.25 (s, 1H, OH), 7.00–6.93(m, 4H, Ph), 5.84 (s, 1H, benzyl-H), 3.28 (s, 12H, 4CH3), 2.90(q, 4H, J = 7.3 Hz, CH2CH3), 2.30 (d, 4H, J = 5.1 Hz, CH2), 2.22 (s, 3H, CH3), 1.20(t, 6H, J = 7.3 Hz, CH2CH3), 1.16(s, 3H, CH3), 1.04(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 196.5, 180.1, 152.8, 140.5, 134.2, 129.8, 128.7, 126.8, 126.7, 115.6, 91.0, 51.4, 45.9, 42.5, 32.6, 31.5, 29.6, 28.4, 27.6, 20.9, 11.9; Anal. for C26H37N3O5; calcd: C, 66.22; H, 7.91; N, 8.91;Found: C, 66.24; H, 7.92; N, 8.87; LC/MS (ESI): m/z = 471 [M]+.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(4-methoxyphenyl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 4o
4o; an oily material (92 %; 672 mg, 1.38 mmol). IR (KBr, cm−1): 3047, 2953, 2866, 2499, 1679, 1577, 1510, 1427, 1373, 1255, 1214;1H NMR (400 MHz, CDCl3): δ 15.26 (s, 1H, OH), 6.98(d, 2H, J = 8.0 Hz, Ph), 6.72(d, 2H, J = 8.0 Hz, Ph), 5.69 (s, 1H, benzyl-H), 3.71 (s, 3H, CH3), 3.29 (s, 12H, 4CH3), 2.87(q, 4H, J = 7.3 Hz, CH2CH3), 2.31 (d, 4H, J = 5.1 Hz, CH2), 1.19(t, 6H, J = 7.3 Hz, CH2CH3), 1.12(s, 3H, CH3), 1.03(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 195.1, 187.2, 157.1, 134.5, 133.9, 127.8, 127.6, 115.6, 113.4, 55.2, 42.6, 31.5, 31.1, 27.9, 12.2; Anal. for C26H37N3O6; calcd: C, 64.05; H, 7.65; N, 8.62;Found: C, 64.11; H, 7.64; N, 8.59; LC/MS (ESI): m/z = 487 [M]+.
5-((4-Chlorophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 4p
4p; oily material (97 %; 715 mg, 1.45 mmol). IR (KBr, cm−1): 3151, 2955, 2868, 2497, 1675, 1580, 1481, 1444, 1379, 1258, 1206;1H NMR (400 MHz, CDCl3): δ 15.02 (s, 1H, OH), 7.12–6.95(m, 4H, Ph), 5.87 (s, 1H, benzyl-H), 3.30 (s, 12H, 4CH3), 2.90(q, 4H, J = 7.3 Hz, CH2CH3), 2.38 (s, 4H, CH2), 1.20(t, 6H, J = 7.3 Hz, CH2CH3), 1.16(s, 3H, CH3), 1.04(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 198.1, 181.0, 152.5, 141.5, 130.6, 128.3, 128.2, 128.0, 127.9, 115.2, 90.7, 65.9, 49.8, 42.3, 32.4, 31.5, 31.2, 29.6, 28.4, 27.6, 15.3, 11.4; Anal. for C25H34ClN3O5; calcd: C, 61.03; H, 6.97; Cl, 7.21; N, 8.54;Found: C, 61.06; H, 7.00; Cl, 7.18; N, 8.57; LC/MS (ESI): m/z = 492 [M]+.
5-((4-Bromophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 4q
4q; an oily material (95 %, 761 mg, 1.42 mmol). IR (KBr, cm−1): 3155, 2955, 2867, 2500, 1674, 1579, 1430, 1376, 1204;1H NMR (400 MHz, CDCl3): δ 15.20 (s, 1H, OH), 7.34 (d, 2H, J = 8.0 Hz, Ph), 6.98 (d, 2H, J = 8.0 Hz, Ph), 5.79 (s, 1H, benzyl-H), 3.27 (s, 12H, 4CH3), 2.99(q, 4H, J = 7.3 Hz, CH2CH3), 2.40 (d, 2H, J = 5.1 Hz, CH2), 2.28(m, 2H, CH2), 1.29(t, 6H, J = 7.3 Hz, CH2CH3), 1.18(s, 3H, CH3), 1.04(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 199.1, 191.2, 164.8, 152.4, 142.8, 132.5, 131.0, 129.9, 128.7, 128.6, 118.9, 115.9, 90.6, 51.2, 45.8, 42.3, 32.7, 31.5, 29.5, 28.5, 28.3, 27.6, 11.4; Anal. for C25H34BrN3O5; calcd: C, 55.97; H, 6.39; Br, 14.89; N, 7.83;Found: C, 56.00; H, 6.40; Br, 14.86; N, 7.82; LC/MS (ESI): m/z = 536 [M]+.
5-((3-Bromophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 4r
4r; oily material (93 %, 745 mg, 1.39 mmol). IR (KBr, cm−1): 3050, 2955, 2868, 2500, 1675, 1581, 1444, 1378, 1255, 1205; 1H NMR (400 MHz, CDCl3): δ 15.63 (s, 1H, OH), 7.22 (d, 1H, J = 7.3 Hz, Ph), 7.19 (s, 1H, Ph), 7.07 (d, 1H, J = 7.3 Hz, Ph), 7.05 (d, 1H, J = 7.3 Hz, Ph), 5.84 (s, 1H, benzyl-H), 3.34(s, 6H, 2CH3), 3.32(s, 6H, 2CH3), 2.98(q, 4H, J = 7.3 Hz, CH2CH3), 2.31 (d, 4H, J = 5.1 Hz, CH2), 1.24(t, 6H, J = 7.3 Hz, CH2CH3), 1.12(s, 3H, CH3), 1.03(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 190.8, 186.4, 165.2, 164.4, 151.7, 144.7, 129.7,129.6, 128.7, 125.3, 91.5, 42.1, 34.4, 28.9, 28.7, 11.5; Anal. for C25H34BrN3O5; calcd: C, 55.97; H, 6.39; Br, 14.89; N, 7.83;Found: C, 56.01; H, 6.41; Br, 14.86; N, 7.84; LC/MS (ESI): m/z = 536 [M]+.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(1-nitrophenyl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 4s
4s; a beige material; m.p: 146 °C; (92 %, 690 mg, 1.37 mmol). IR (KBr, cm−1): 3054, 2953, 2865, 2500, 1673, 1580, 1510, 1427, 1373, 1255, 1214;1H NMR (400 MHz, CDCl3): δ 15.33 (s, 1H, OH), 7.01-7.35 (m, 3H, Ph), 5.65 (s, 1H, benzyl-H), 3.70 (s, 12H, 4CH3), 2.89(q, 4H, J = 7.3 Hz, CH2CH3), 2.30(d, 4H, J = 14.7 Hz, CH2), 1.15(t, 6H, J = 7.3 Hz, CH2CH3), 1.10(s, 3H, CH3), 1.00(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 161.6, 153.2, 145.5, 141.6, 129.1, 128.2, 127.8, 125.8, 88.5, 49.1, 41.9, 27.5, 11.5; Anal. for C25H34N4O7; calcd: C, 59.75; H, 6.82; N, 11.15; Found: C, 59.72; H, 6.80; N, 11.17; LC/MS (ESI): m/z = 502[M]+.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(4-(dimethylamino)phenyl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 4t
4t; a beige material; m.p: 165 °C; (73 %, 550 mg, 1.1 mmol). IR (KBr, cm−1): 3055, 2950, 2865, 2500, 1669, 1580, 1510, 1427, 1373, 1255, 1214;1H NMR (400 MHz, CDCl3): δ 15.33 (s, 1H, OH), 7.02 (d, 2H, J = 8.0 Hz, Ph), 6.75 (d, 2H, J = 8.8 Hz, Ph), 5.69 (s, 1H, benzyl-H), 3.70 (s, 12H, 4CH3), 3.01 (s, 6H, N(CH3)2), 2.89(q, 4H, J = 7.3 Hz, CH2CH3), 2.31(d,4H, J = 14.7 Hz, CH2), 1.15(t, 6H, J = 7.3 Hz, CH2CH3), 1.12(s, 3H, CH3), 1.00(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 161.6, 153.2, 145.5, 141.6, 129.1, 128.2, 127.8, 125.8, 88.5, 49.1, 41.9, 41.8, 27.5, 11.5; Anal. for C27H39N4O5; calcd: C, 64.91; H, 7.87; N, 11.21;Found: C, 64.90; H, 7.87; N, 11.23; LC/MS (ESI): m/z = 499.29[M]+.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(4-hydroxyphenyl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 4v
4v; a white solid material; m.p: 162 °C; (91 %, 645 mg, 1.36 mmol). IR (KBr, cm−1): 23097, 2939, 2884, 2828, 2498, 1747, 1574, 1530, 1506, 1466, 1384, 1241;1H NMR (400 MHz, DMSO-d6): δ 14.52 (s, 1H, OH), 8.50 (brs, 1H, OH), 6.76(d, 2H, J = 8.0 Hz, Ph), 6.50(d, 2H, J = 8.0 Hz, Ph), 6.04(s, 1H, benzyl-H), 3.07 (s, 12H, 2CH3), 3.14(q, 4H, J = 7.3 Hz, CH2CH3), 2.92 (q, 4H, J = 13.9 Hz, CH2), 206 (s, 4H, CH2), 1.12(t, 6H, J = 7.3 Hz, CH2CH3), 0.98(s, 3H, CH3); 13C NMR (100 MHz, DMSO-d6): δ = 198.0, 188.5, 154.1, 136.6, 128.3, 115.3, 114.3, 90.1, 50.9, 45.5, 42.1, 31.6, 30.7, 29.7, 11.7; Anal. for C25H35N3O6; calcd: C, 63.41; H, 7.45; N, 8.87;Found: C, 63.40; H, 7.43; N, 8.85; LC/MS (ESI): m/z = 473 [M]+.
4-((6-hydroxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)benzaldehyde diethylaminium salt 4x
4x; as solid (1.26 g, 90 %). IR (cm−1): 3156, 2950, 2872, 1678, 1590, 1508, 1375, 1256, 1232, 1167; 1H-NMR (CDCl3, 400 MHz): 14.16 (s, 1H, OH), 9.80 (s, 1H, CHO), 8.01 (brs, 2H, NH), 6.98 (d, 2H, J = 7.3 Hz, Ph), 6.75 (d, 2H, J = 7.3 Hz, Ph), 5.61(s, 1H, benzyl-H), 3.73 (s, 6H, CH3), 2.92 (q, 4H, J = 7.3 Hz, CH2CH3), 2.31 (m, 4H, 2CH2), 1.26(t, 6H, J = 7.3 Hz, CH2CH3), 1.05(s, 3H, CH3), 1.00(s, 3H, CH3); 13C-NMR (100 MHz, CDCl3): δ = 193.0, 188.1, 165.0, 157.2, 127.8, 115.7,113.8, 91.6, 55.2, 48.8, 48.6, 42.4, 31.5, 29.4, 27.7, 11.7; Anal. for C26H35N3O6; Calcd: C, 64.31; H, 7.27; N, 8.65; Found:C, 64.30; H, 7.26; N, 8.63; LC/MS (ESI): m/z = 485.57 [M]+.
5-((2,4-Dichlorophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 4w
4w; a beige solid material; m.p: 164 °C; (90 %, 710 mg, 1.35 mmol). IR (KBr, cm−1): 3059, 2995, 2867, 2114, 1741, 1658, 1591, 1463, 1429, 1370, 1341, 1256, 12011H-NMR (400 MHz, CDCl3): δ 14.80 (s, 1H, OH), 7.29 (d, 1H, J = 8.0 Hz, Ph), 7.19 (s, 1H, Ph), 7.12(d, 2H, J = 8.0 Hz, Ph), 5.76 (s, 1H, benzyl-H), 3.28 (s, 12H, 4CH3), 3.07(q, 4H, J = 7.3 Hz, CH2CH3), 2.37 (s, 2H, CH2), 2.27 (d, 2H, J = 5.1 Hz, CH2), 1.34 (t, 6H, J = 7.3 Hz, CH2CH3), 1.04(s, 3H, CH3), 1.01 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 199.1, 165.4, 164.4, 152.5, 139.8, 133.6, 131.7, 131.2, 129.3, 126.4, 115.7, 89.8, 51.2, 45.7, 41.9, 32.4, 31.2, 28.3, 28.2, 11.3; Anal. for C25H33Cl2N3O5; calcd: C, 57.04; H, 6.32; Cl, 13.47; N, 7.98;Found: C, 57.09; H, 6.31; Cl, 13.44; N, 8.01; LC/MS (ESI): m/z = 526 [M]+.
5-((2,6-Dichlorophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 4y
4y an oily material (89 %, 702 mg, 1.33 mmol). IR (KBr, cm−1): 3048, 2955, 2869, 2728, 2494, 1676, 1575, 1428, 1372, 1238, 1196;1H NMR (400 MHz, CDCl3): δ 14.80 (s, 1H, OH), 7.36 (d, 2H, J = 8.0 Hz, Ph), 7.29 (t, 1H, J = 8.0 Hz, Ph), 7.12(d, 2H, J = 8.0 Hz, Ph), 5.98 (s, 1H, benzyl-H), 3.26 (s, 12H, 4CH3), 2.92(q, 4H, J = 7.3 Hz, CH2CH3), 2.37 (s, 2H, CH2), 2.27 (d, 2H, J = 5.1 Hz, CH2), 1.24(t, 6H, J = 7.3 Hz, CH2CH3), 1.094(s, 3H, CH3), 1.04(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 192.8, 188.9, 165.3, 164.3, 152.5, 149.7, 137.4, 131.5, 129.8, 126.5, 124.2, 115.5, 114.7, 89.9, 53.5, 41.4, 31.9, 28.7, 28.2, 11.4; Anal. for C25H33Cl2N3O5; calcd: C, 57.04; H, 6.32; Cl, 13.47; N, 7.98; Found: C, 57.08; H, 6.30; Cl, 13.45; N, 8.00; LC/MS (ESI): m/z = 526 [M]+.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(naphthalen-2-yl)methyl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 4z
4z; a white solid material; m.p: 170 °C; (94 %, 715 mg, 1.41 mmol). IR (KBr, cm−1): 2994, 2948, 2866, 2506, 1742, 1651, 1603, 1570, 1526, 1473, 1431, 1362, 1245;1H NMR (400 MHz, CDCl3): δ 14.26 (s, 1H, OH), 7.46–7.22 (m, 7H, naphthyl), 6.20 (s, 1H, benzyl-H), 3.26 (s, 6H, 2CH3), 3.23 (s, 6H, 2CH3), 3.14(q, 4H, J = 7.3 Hz, CH2CH3), 2.41 (q, 4H, J = 5.1 Hz, CH2), 2.23 (s, 2H, CH2), 1.37(t, 6H, J = 7.3 Hz, CH2CH3), 1.07(s, 3H, CH3), 1.01(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 199.0, 180.5, 165.3, 164.3, 152.5, 149.7, 136.8, 131.5, 129.9, 126.5, 124.2, 115.5, 114.7, 89.9, 50.9, 45.5, 41.7, 31.3, 30.7, 28.2, 11.1; Anal. for C29H37N3O5; calcd: C, 68.62; H, 7.35; N, 8.28; Found: C, 68.65; H, 7.34; N, 8.30; LC/MS (ESI): m/z = 507 [M]+.
2-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(phenyl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5a
5a; as solid (1.26 g, 95 %). IR (cm−1): 2955 (s), 1586 (s), 1382 (s), 776 (s), 576 (s), 480 (s); 1H-NMR (CDCl3, 400 MHz) δ 13.91 (s, OH), 8.25 (bs, 1H. NH2), 7.01–7.21 (m, 5H. ArH), 5.74 (s, 1H, PhCH), 2.84 (q, J = 6.6 Hz, 4H, NHCH2CH3), 2.31 (s, 8H, CH2 + COCH2), 1.18 (t, J = 6.6 Hz, 6H, NHCH2CH3), 0.95–1.14 (m, 12H, CH3);13C-NMR (CDCl3, 100 MHz): δ 199.1, 179.3, 142.4, 128.0, 126.8, 125.2, 115.5, 50.6, 45.9, 42.3, 34.2, 32.0, 11.4; Anal. Calcd.for C27H37NO4: C, 73.36; H, 8.98; N, 3.07; O, 14.57; Found: C, 73.43; H, 8.90; N, 3.17; O, 14.49; LC/MS (ESI): m/z = 441.29 [M]+.
2-((4-Chlorophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5b
3c; as solid (92 %, 1.31 g). IR (cm−1): 2956 (s), 1706 (s), 1573 (s), 1486 (s), 1382 (s), 1263 (s), 732 (s), 605 (s), 485 (s); 1H-NMR (CDCl3, 400 MHz) δ 13.59 (s, OH), 8.51 (bs, 2H. NH2), 6.89–7.21 (m, 4H. ArH), 5.70 (s, 1H, PhCH), 2.90 (q, J = 7.3 Hz, 4H, NHCH2CH3), 2.30 (s, 8H, CH2 + COCH2), 1.21 (t, J = 7.3 Hz, 6H, NH2CH2CH3), 0.91–1.16 (m, 12H, CH3); 13C-NMR (CDCl3, 100 MHz): δ197.3, 188.6, 139.5, 130.8, 128.3, 128.1, 115.2, 49.7, 44.9, 42.2, 34.3, 33.1, 31.5, 11.3; Anal. Calcd. forC27H38ClNO4: C, 68.23; H, 8.19; N, 2.97; O, 13.34; Found: C, 68.12; H, 8.05; N, 2.90; O, 13.44; LC/MS (ESI): m/z = 475.25 [M]+.
2-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(p-tolyl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5c
5c; as solid (93 %, 1.2 g). IR (cm−1): 2957 (s), 1571 (s), 1483 (s), 1383 (s), 1267 (s), 739 (s), 488 (s); 1H-NMR (CDCl3, 400 MHz) δ 13.73 (s, OH), 7.83 (bs, 2H. NH2), 6.91–7.05 (m, 4H. ArH), 5.73 (s, 1H, PhCH), 2.84 (q, J = 7.3 Hz, 4H, NHCH2CH3), 2.31 (s, 8H, CH2 + COCH2), 2.23 (s, 3H, PhCH3), 1.18 (t, J = 7.3 Hz, 6H, NH2CH2CH3), 0.94–1.16 (m, 12H, CH3), 13C-NMR (CDCl3, 100 MHz): δ195.8, 187.3, 144.4, 134.0, 128.6, 126.8, 115.6, 51.8, 46.1, 42.7, 34.9, 32.7, 31.4, 20.9,12.4; Anal. Calcd. forC28H41NO4: C, 73.79; H, 9.14; N, 3.09; O, 13.91; Found: C, 73.81; H, 9.07; N, 3.07; O, 14.05; LC/MS (ESI): m/z = 455.30 [M]+.
2-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(m-tolyl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5d
5d; as solid (91 %, 1.24 g). IR (cm−1): 2952 (s), 1572 (s), 1483 (s), 1381 (s), 1227 (s), 1143 (s), 787 (s), 463 (s); 1H-NMR (CDCl3, 400 MHz) δ 13.78 (s, OH), 7.85 (bs, 2H. NH2), 6.88–7.03 (m, 4H. ArH), 5.71 (s, 1H, PhCH), 2.91 (q, J = 7.4 Hz, 4H, NHCH2CH3), 2.38 (s, 8H, CH2 + COCH2), 2.28 (s, 3H, PhCH3), 1.16 (t, J = 7.4 Hz, 6H, NH2CH2CH3), 0.91–1.12 (m, 12H, CH3); 13C-NMR (CDCl3, 100 MHz): δ195.9, 187.5, 144.7, 134.1, 128.4, 126.9, 115.8, 51.9, 46.3, 42.6, 34.8, 32.8, 31.2, 20.6, 12.3; Anal. Calcd.for C28H41NO4: C, 73.85; H, 9.09; N, 3.13; O, 13.79; Found: C, 73.81; H, 9.07; N, 3.07; O, 14.05; LC/MS (ESI): m/z = 455.30 [M]+.
2-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(4-methoxyphenyl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5e
5e; as solid (89 %, 1.26 g). IR (cm−1): 3121 (s), 1668 (s), 1614 (s), 1578 (s), 1446 (s), 778 (s), 608 (s), 457 (s); 1H-NMR (CDCl3, 400 MHz) δ 14.67 (s, OH), 8.22 (bs, 2H. NH2), 6.97 (d, J = 7.4 Hz, 2H. ArH), 6.72 (d, J = 7.4 Hz, 2H, ArH), 5.72 (s, 1H, PhCH), 3.72 (s, 3H, OCH3), 2.85 (q, J = 7.4 Hz, 4H, NHCH2CH3), 2.30 (s, 8H, CH2 + COCH2), 1.20 (t, J = 7.4 Hz, 6H, NH2CH2CH3), 0.96–1.16 (m, 12H, CH3); 13C-NMR (CDCl3, 100 MHz): δ 194.1, 187.5, 157.6, 133.1, 127.8, 115.7, 113.4, 55.2, 50.7, 45.3, 42.5, 34.1, 31.5, 31.1,11.9; Anal. Calcd.for C28H41NO5: C, 71.19; H, 8.79; N, 3.05; O, 17.11; Found: C, 71.31; H, 8.76; N, 2.97; O, 16.96; LC/MS (ESI): m/z = 471.30 [M]+.
2-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(4-nitrophenyl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5f
5f; as solid (90 %, 1.26 g). IR (cm−1): 2872 (s), 1582 (s), 1510 (s), 1466 (s), 1384 (s), 1339 (s), 757 (s), 487 (s); 1H-NMR (CDCl3, 400 MHz) δ15.12 (s, OH), 8.32(bs, 2H. NH2), 8.01 (m, J = 8.8 Hz, 2H.ArH), 7.21 (d, J = 8.8 Hz, 2H, ArH), 5.92 (s, 1H, PhCH), 2.94 (q, J = 7.3 Hz, 4H, NHCH2CH3), 2.29 (s, 8H, CH2 + COCH2), 1.21 (t, J = 7.3 Hz, 6H, NH2CH2CH3), 0.91–1.06 (m, 12H, CH3); 13C-NMR (CDCl3, 100 MHz): δ 194.9, 186.8, 151.9, 145.5, 127.7, 123.2, 114.8, 50.3, 42.5, 45.2, 34.1, 32.2, 31.6, 11.4; Anal. Calcd. forC27H38N2O6: C, 66.74; H, 7.98; N, 5.55; O, 19.91; Found: C, 66.64; H, 7.87; N, 5.76; O, 19.73; LC/MS (ESI): m/z = 468.27 [M]+.
2-((2,6-Dichlorophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5g
5g; as solid (91 %, 1.39 g). IR (cm−1): 2953 (s), 2869 (s), 1711 (s), 1575 (s), 1497 (s), 1367 (s), 1220 (s), 776 (s), 448 (s); 1H-NMR (CDCl3, 400 MHz) δ 14.78 (s, OH), 8.71 (bs, 2H. NH2), 7.24(s, J = 14.4 Hz, 1H, ArH), 7.16 (m, 1H, ArH), 6.95 (d, J = 14.4 Hz, 1H, ArH), 5.89 (s, 1H, PhCH), 2.90 (q, J = 7.4 Hz, 4H, NHCH2CH3), 2.19 (bs, 8H, CH2 + COCH2), 1.17 (t, J = 7.4 Hz, 6H, NH2CH2CH3), 0.88–1.03 (bs, 12H, CH3); 13C-NMR (DMSO-d6, 100 MHz): δ 198.3, 189.1, 139.1, 134.9, 128.2, 125.9, 114.2, 51.1, 47.6, 42.5, 34.3, 31.8, 30.3, 11.9; Anal. Calcd.for C27H37Cl2NO4: C, 63.46; H, 7.55; N, 2.43; O, 12.91; Found: C, 63.52; H, 7.31; N, 2.74; O, 12.54; LC/MS (ESI): m/z = 509.21 [M]+.
2-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(3-nitrophenyl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5h
5h; as solid (90 %, 1.26 g). IR (cm−1): 2872 (s), 1582 (s), 1510 (s), 1466 (s), 1384 (s), 1339 (s), 757 (s), 487 (s); 1H-NMR (CDCl3, 400 MHz) δ 15.12 (s, OH), 8.32(bs, 2H. NH2), 8.01 (m, J = 8.8 Hz, 2H.ArH), 7.21 (d, J = 8.80 Hz, 2H, ArH), 5.92 (s, 1H, PhCH), 2.94 (q, J = 7.3 Hz, 4H, NHCH2CH3), 2.29 (s, 8H, CH2 + COCH2), 1.21 (t, J = 7.3 Hz, 6H, NH2CH2CH3), 0.91–1.06 (m, 12H, CH3); 13C-NMR (CDCl3, 100 MHz): δ194.9, 186.8, 151.9, 145.5, 127.7, 123.2, 114.8, 50.3, 45.2, 42.5, 34.1, 32.2, 31.6, 11.4; Anal. Calcd. forC27H38N2O6: C, 66.74; H, 7.98; N, 5.55; O, 19.91; Found: C, 66.64; H, 7.87; N, 5.76; O, 19.73; LC/MS (ESI): m/z = 468.27 [M]+.
2-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(2-nitrophenyl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5i
5i; as solid (87 %, 1.22 g). IR (cm−1): 3096 (s), 2938 (s), 2869 (s), 1580 (s), 1539 (s), 1506 (s), 1384 (s), 1241 (s), 1033 (s), 778 (s), 604 (s), 524 (s); 1H-NMR (CDCl3, 400 MHz) δ 14.27 (s, OH), 8.74 (bs,2H. NH2), 7.10 (m, 4H, ArH), 6.22 (s, 1H, PhCH), 2.03 (q, J = 7.3 Hz, 4H, NHCH2CH3), 2.20 (bs, 8H, CH2 + COCH2), 1.29 (t, J = 7.3 Hz, 6H, NH2CH2CH3), 0.99 (bs, 12H, CH3);13C-NMR (CDCl3, 100 MHz): δ 198.9, 181.9, 149.7, 137.4, 131.3, 130.2, 125.9, 124.1, 114.5, 49.9, 44.8, 42.0, 33.6, 31.4, 29.4, 11.2; Anal. Calcd. forC27H38N2O6: C, 66.94; H, 7.87; N, 5.43; O, 19.96; Found: C, 66.64; H, 7.87; N, 5.76; O, 19.73; LC/MS (ESI): m/z = 468.27 [M]+.
2-((4-Formylphenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5j
5j; as solid (75 %, 1.01 g). IR (cm−1): 3150 (s), 1586 (s), 1519 (s), 1469 (s), 1381 (s), 1339 (s), 779 (s), 495 (s); 1H-NMR (DMSO-d6, 400 MHz) δ16.45 (s, OH), 8.39 (bs, 2H. NH2), 6.78 (m, J = 8.04 Hz, 2H. ArH), 6.49 (d, J = 8.04 Hz, 2H, ArH), 6.08 (s, 1H, PhCH), 3.00 (s, 6H, N(CH3)2), 2.89 (q, J = 7.32 Hz, 4H, NHCH2CH3), 2.10 (s, 8H, CH2 + COCH2), 1.15 (t, J = 7.32 Hz, 6H, NH2CH2CH3), 0.88–1.01 (m, 12H, CH3); 13C-NMR (DMSO-d6,100 MHz): δ196.1, 183.6, 154.1, 136.1, 128.3, 115.3, 114.3,50.9,45.6, 42.0, 41.7, 34.2, 31.9, 29.8, 11.8; Anal. Calcd.for C28H39NO5: C, 71.61; H, 8.37; N, 2.98; Found: C, 71.61; H, 8.37; N, 2.98; LC/MS (ESI): m/z = 69.28 [M]+.
2-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(4-hydroxyphenyl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5k
5k; as solid (88 %, 1.01 g). IR (cm−1): 3157 (s), 1584 (s), 1519 (s), 1469 (s), 1381 (s), 1339 (s), 779 (s), 495 (s); 1H-NMR (DMSO-d6, 400 MHz) δ 16.41 (s, OH), 8.32 (bs, 2H. NH2), 6.75 (m, J = 8.0 Hz, 2H. ArH), 6.45 (d, J = 8.0 Hz, 2H, ArH), 6.04 (s, 1H, PhCH), 2.88 (q, J = 7.3 Hz, 4H, NHCH2CH3), 2.50 (s, 1H, PhOH), 2.06 (s, 8H, CH2 + COCH2), 1.12 (t, J = 7.32 Hz, 6H, NH2CH2CH3), 0.85–0.97 (m, 12H, CH3); 13C-NMR (DMSO-d6, 100 MHz): δ 196.1, 183.6, 154.1, 136.1,128.3, 115.3, 114.3, 50.9, 45.6, 42.0, 34.2, 31.9, 29.8, 11.8; Anal. Calcd.for C27H39NO5: C, 70.74; H, 8.89; N, 3.13; O, 17.61; Found: C, 70.87; H, 8.59; N, 3.06; O, 17.48; LC/MS (ESI): m/z = 383.19 [M]+.
4-((6-Hydroxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)(6-hydroxy-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)methyl)benzaldehyde diethylaminium salt 5l
5l; as white solid (88 %, 1.20 g). IR (cm−1): 3455, 3305, 3000, 2910, 1677, 1582, 1510, 1466, 1384, 1339; 1H-NMR (CDCl3, 400 MHz) 17.30 (s, 1H, OH), 9.90 (s, 1H, CHO), 8.23 (brs, 2H, NH), 7.56 (d, 2H, J = 8.0 Hz, Ph), 7.11 (d, 2H, J = 8.0 Hz, Ph), 5.85(s, 1H, benzyl-H), 3.34 (s, 12H, 4CH3), 3.03 (q, 4H, J = 7.3 Hz, CH2CH3), 1.25 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, CDCl3): δ = 192.1, 165.2, 164.1, 151.2, 150.0, 134.1, 129.5, 127.5, 91.6, 42.2, 35.1, 29.0, 28.7, 11.5; Anal. for C22H27N5O7; Calcd: C, 55.81; H, 5.75; N, 14.79; Found:C, 55.83; H, 5.76; N, 14.81; LC/MS (ESI): m/z = 473.48 [M]+.
5-((4-Chlorophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 5m
5m; an oily product (90 %, 625 mg, 1.35 mmol). IR (KBr, cm−1): 3049, 2954, 2865, 2499, 1738, 1699, 1590, 1483, 1375, 1292, 1258, 1225, 1205;1H NMR (400 MHz, CDCl3): δ 13.32 (s, 1H, OH), 8.83 (brs, 2H, NH), 7.27(d, 2H, J = 8.0 Hz, Ph), 7.00(d, 2H, J = 8.0 Hz, Ph), 5.89 (s, 1H, benzyl-H), 2.88(q, 4H, J = 7.3 Hz, CH2CH3), 2.31 (d, 4H, J = 5.1 Hz, CH2), 1.19(t, 6H, J = 7.3 Hz, CH2CH3), 1.09(s, 3H, CH3), 1.03(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 190.9, 141.0, 134.8, 131.0, 129.5, 128.3, 115.3, 91.1, 47.1, 42.7, 31.6, 31.5, 29.1, 28.2, 27.8, 11.3; Anal. for C23H30ClN3O5; calcd: C, 59.54; H, 6.52; Cl, 7.64; N, 9.06;Found: C, 59.57; H, 6.51; Cl, 7.60; N, 9.02; LC/MS (ESI): m/z = 463 [M]+.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(phenyl)methyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 5n
5n; a white solid material; m.p: 215 °C; (93 %, 598 mg, 1.39 mmol). IR (KBr, cm−1): 3027, 2948, 2867, 2156, 1683, 1593, 1451, 1374, 1291, 1257, 11411H-NMR (400 MHz, CDCl3): δ 12.26 (s, 1H, OH), 9.31(brs, 2H, NH), 7.12(m, 5H, Ph), 5.52 (s, 1H, benzyl-H), 2.99(q, 4H, J = 7.3 Hz, CH2CH3), 2.45 (d, 4H, J = 5.1 Hz, CH2), 1.24(t, 6H, J = 7.3 Hz, CH2CH3), 1.09(s, 3H, CH3), 1.03(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 198.5, 180.8, 152.5, 142.5, 128.0, 126.7, 125.1, 116.3, 90.9, 51.4, 45.9, 42.2, 33.0, 28.4, 27.6, 11.3; Anal. for C23H31N3O5; calcd: C, 64.32; H, 7.27; N, 9.78;Found: C, 64.29; H, 7.29; N, 9.80; LC/MS (ESI): m/z = 429[M]+.
5-((4-Bromophenyl)(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 5o
5o; a white solid material; m.p: 208 °C; (89 %, 678 mg, 1.33 mmol); IR (KBr, cm−1): 3093, 2939, 2885, 2829, 2551, 1746, 1686, 1576, 1506, 1466, 1416, 1268, 1241; 1H NMR (400 MHz, CDCl3): δ 13.31 (s, 1H, OH), 8.67 (brs, 2H, NH), 7.05(m, 4H, Ph), 5.79 (s, 1H, benzyl-H), 2.79(q, 4H, J = 7.3 Hz, CH2CH3), 2.35 (d, 4H, J = 5.1 Hz, CH2), 1.21(t, 6H, J = 7.3 Hz, CH2CH3), 1.11(s, 3H, CH3), 1.03(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 198.5, 180.1, 152.8, 140.5, 131.4, 130.7, 128.7, 128.6, 118.5, 115.6, 91.0, 50.9, 42.8, 31.6, 31.5, 29.2, 28.3, 27.8, 11.3; Anal. for C23H30BrN3O5; calcd: C, 54.34; H, 5.95; Br, 15.72; N, 8.27;Found: C, 54.35; H, 5.96; Br, 15.69; N, 8.30; LC/MS (ESI): m/z = 508 [M]+.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(p-tolyl)methyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 5p
5p; a white solid material; m.p: 213 °C; (91 %, 604 mg, 1.36 mmol). IR (KBr, cm−1): 3150, 2955, 2867, 1690, 1592, 1508, 1375, 1256, 1232, 1167;1H NMR (400 MHz, CDCl3): δ 13.31 (s, 1H, OH), 8.83 (brs, 2H, NH), 7.27(d, 2H, J = 8.0 Hz, Ph), 7.00(d, 2H, J = 8.0 Hz, Ph), 5.88 (s, 1H, benzyl-H), 2.83(q, 4H, J = 7.3 Hz, CH2CH3), 2.31 (d, 4H, J = 5.1 Hz, CH2), 2.23 (s, 3H, CH3), 1.19(t, 6H, J = 7.3 Hz, CH2CH3), 1.04(s, 3H, CH3), 1.02(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 196.5, 180.1, 152.8, 140.5, 131.4, 130.7, 128.7, 128.6, 118.5, 115.6, 91.0, 50.9, 42.8, 31.6, 31.5, 29.2, 28.3, 27.8, 20.9, 11.3; Anal. for C24H33N3O5; calcd: C, 64.99; H, 7.50; N, 9.47;Found: C, 64.95; H, 7.49; N, 9.50; LC/MS (ESI): m/z = 443 [M]+.
2-((4-Formylphenyl)(6-hydroxy-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5q
5q; a white solid material; m.p: 205 °C; (87 %, 594 mg, 1.3 mmol). IR (KBr, cm−1): 3145, 2950, 2870, 1677, 1550, 1510, 1375, 1256, 1232, 1167; 1H NMR (400 MHz, CDCl3): δ 13.35 (s, 1H, OH), 9.92 (s, 1H, CHO), 8.80 (brs, 2H, NH), 7.30(d, 2H, J = 8.0 Hz, Ph), 7.05(d, 2H, J = 8.0 Hz, Ph), 5.85 (s, 1H, benzyl-H), 2.89(q, 4H, J = 7.3 Hz, CH2CH3), 2.30 (d, 4H, J = 5.1 Hz, CH2), 2.26(s, 3H, CH3), 1.22(t, 6H, J = 7.3 Hz, CH2CH3), 1.08(s, 3H, CH3), 1.05(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 198, 181.3, 152.8, 140.5, 131.4, 130.7, 128.7, 128.6, 118.5, 115.6, 91.0, 50.9, 42.8, 31.6, 31.5, 29.2, 28.3, 27.8, 20.9, 11.3; Anal. for C24H31N3O6; calcd: C, 63.00; H, 6.83; N, 9.18;Found: C, 63.01; H, 6.84; N, 9.18; LC/MS (ESI): m/z = 457 [M]+.
5-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(naphthalen-2-yl)methyl)-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate diethylaminium salt 5r
5r; an oily product (90 %, 646 mg, 1.35 mmol). IR (KBr, cm−1): 3049, 2948, 2863, 2725, 1685, 1594, 1508, 1371, 1252, 1216; 1H NMR (400 MHz, CDCl3): δ 14.25 (s, 1H, OH), 7.46-7.22(m, 7H, naphthyl), 6.21 (s, 1H, benzyl-H), 3.27 (s, 6H, 2CH3), 3.25 (s, 6H, 2CH3), 3.14(q, 4H, J = 7.3 Hz, CH2CH3), 2.41 (q, 4H, J = 5.1 Hz, CH2), 2.23 (s, 2H, CH2), 1.37(t, 6H, J = 7.3 Hz, CH2CH3), 1.07(s, 3H, CH3), 1.01(s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ = 199.1, 180.5, 165.5, 164.2, 152.5, 149.7, 136.8, 131.5, 129.9, 126.5, 124.2, 115.5, 114.7, 89.9, 50.9, 45.5, 41.7, 31.3, 30.7, 28.2, 11.3; Anal. for C27H33N3O5; calcd: C, 67.62; H, 6.94; N, 8.76; Found: C, 67.65; H, 6.96; N, 8.80; LC/MS (ESI): m/z = 479 [M]+.
2-((2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)(naphthalen-2-yl)methyl)-5,5-dimethyl-3-oxocyclohex-1-enolate diethylaminium salt 5s
5s; as solid (93 %, 1.33 g). IR (cm−1): 3053 (s), 2943 (s), 2866 (s), 1688 (s), 1566 (s), 1511 (s), 1383 (s), 1241 (s), 1035 (s), 774 (s), 482 (s), 554 (s); 1H-NMR (CDCl3, 400 MHz) δ 1.01 (bs, 12H, CH3), 1.19 (t, J = 7.3 Hz, 6H, NH2CH2CH3), 2.29 (bs, 8H, CH2 + COCH2), 2.88 (q, J = 7.3 Hz, 4H, NHCH2CH3), 6.32 (s, 1H, PhCH), 7.55–7.64 (m, 2H, ArH), 7.69 (t, J = 7.4 Hz, 1H, ArH), 7.91 (d, J = 8.8 Hz, 1H, ArH), 7.99 (d, J = 6.6 Hz, 1H, ArH), 8.10 (d, J = 8.1 Hz, 1H, ArH), 9.25 (d, J = 8.0 Hz, 1H, ArH), 1039 (s,2H. NH2), 14.25 (s, OH); 13C-NMR (CDCl3, 100 MHz): δ 193.6, 182.8, 136.8, 135.4, 133.8, 131.5, 124.7, 116.8, 50.5, 130.6, 128.6, 129.1, 127.0, 45.3, 42.2, 33.9, 31.4, 29.8, 11.7; Anal. Calcd.for C30H39NO4: C, 75.83; H, 8.05; N, 3.03; O, 13.29; Found: C, 75.71; H, 8.23; N, 2.91; O, 13.40; LC/MS (ESI): m/z = 477.29 [M]+.
Procedure for In vitro Urease Inhibiton Assay
Reaction mixture comprising of 25μL of enzyme (jack bean urease) (1 unit/well) solution and 55 μL of phosphate buffers (4 mM) containing 100 mM urea were incubated with 5 μL of test compounds dissolved in methanol (0.5 mM concentration) at 30 °C for 15 min in 96-well plates. Urease activity was determined by measuring ammonia production using the indophenol method as described by Weather burn [30]. Briefly, 45 μl each phenol reagent (1 % w/v phenol and 0.005 % w/v sodium nitroprussside) and 70 μL of alkali reagent(0.5 % w/v NaOH and 0.1 % active chloride NaOCl) were added to each well. The increasing absorbance at 630 nm was measured afther 50 min, using a microplate reader (Molecular Device, USA). All reactions were performed in triplicate in a final volume of 200 μL. The results (change in absorbance per min) were processed by using softMax Pro software (molecular Device, USA). The entire assays were performed at pH 6.8. Percentage inhibitions were calculated from the formula 100 − (ODtestwell/ODcontrol) × 100. Thiourea was used as the standard inhibitor of urease [31, 32].
Materials and methods for MD simulation and molecular docking studies
Receptor and ligand preparation
The crystal structure of helicobacter pylori (HP) urease in complex with acetohydroxamic acid, (PDB entry code 1E9Y) was retrieved from the protein data bank [33]. All the water molecules were removed from the PDB crystal structure and hydrogen atoms were added. This structure was followed by energy minimization with amber99 force field (http://www.chempcomp.com) in the molecular operating environment (MOE) Software packages [34]. The three dimensional structure of the compounds were constructed via Builder module implemented in MOE. Subsequently all the compounds structures were minimized by using MMFF94 force field [35] in MOE preceding to molecular docking studies.
Protocol selection
Initially docking was performed for both the isomers i.e. keto and enol form. For docking purpose, default docking parameters of MOE is used such as Triangle Matcher Algorithm with two different rescoring functions. London dG and GBVI/WSA dG were used to generate 30 poses of each ligand and were saved in MOE database. Finally, docking results were analyzed by visualizing several interactions of compounds within binding pocket of proteins.
Molecular dynamic simulation
The keto and enol complexes were energy-minimized to eliminate possible steric strain up to 0.1 gradients by using AMBER99 force field. The relaxed complexes were then subjected to MD simulations using MOE 2013.0801 software. Each complex was gradually simulated at 300 K for 100 ps, in order to simulate the physiological conditions, system is allowed to maintain at physiological temperature of 300 K. The temperature is attained gradually, to avoid protein destruction, over a period of 100 ps. Initially, protein is heated from 0 to 50 K, followed by its ramping to 100, 200 and finally 300 K and then equilibrated at 300 K for even distribution of water molecules keeping protein molecule constrained. After equilibration step MD simulation was performed for 5 ns by using the Nose-Poincare-Anderson (NPA) method [36]. To make ensemble trajectories NVT ensemble was used.
The trajectory output files were saved after every 1 ps for future analysis. Equilibration was monitored by convergence in terms of the temperature, energy, density and the RMSD (root-mean-squared deviations) of the backbone atoms as compared to the crystal structure of both complexes.
Results and discussion
Chemistry
In our continued interest [30, 37–47] in the development of highly expedient methods for the synthesis of diverse heterocyclic compounds of biological importance via one-pot multi-component reactions (MCRs) and avoiding organic solvents during the reactions in organic synthesis leads to efficient, environmentally benign reagents, clean, and economical technology (Green Chemistry Concepts). In the present investigation, reaction of equimolar amounts of barbituric acid 1a,b dimedone 2 with aldehyde 3 in presence of aqueous diethylamine medium at RT afforded zwitterionic adducts 4a–z and 5a–s in quantitative yields by simple filtration (Scheme 1).
Scheme 1.
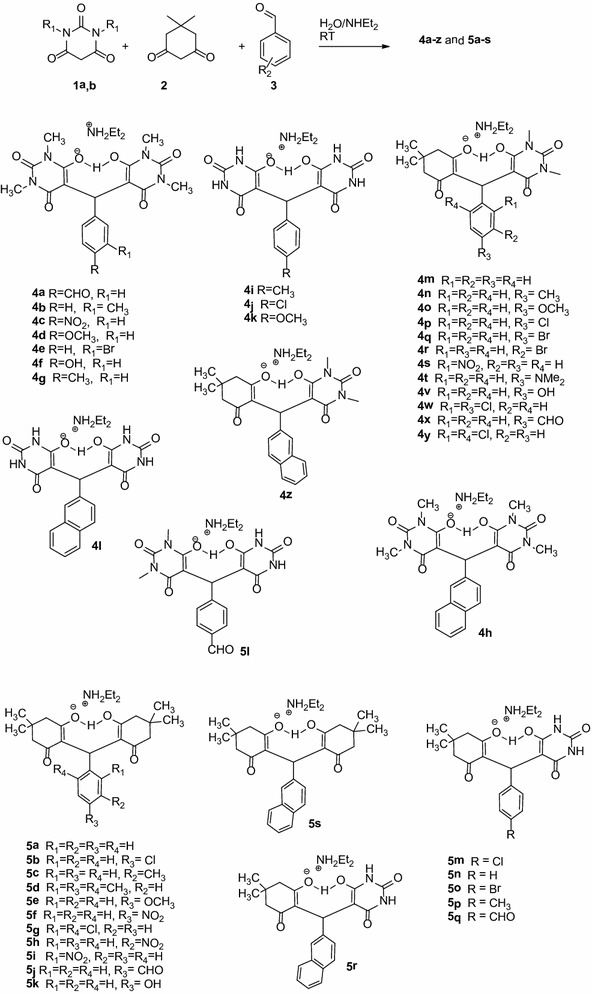
Synthesis of compounds 4a–z and 5a–s
Biological activity
Thirty-two new derivatives of barbituric acid as zwitterionic adducts of diethyl ammonium salts having bis(6-hydroxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl) (4a–h), bis-(6-hydroxypyrimidine-2,4(1H,3H)-dione) (4i–4l), (2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (4m–4z), 4-((6-Hydroxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)(6-hydroxy-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)methyl) benzaldehyde (5l), (2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate (5m–5r) and twelve derivatives of dimedone as zwitterionic adducts of diethyl ammonium salts having bis-(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en (5a–k and 5s) as basic nucleus were screened in vitro for their ureas enzyme inhibition potential against thiourea (IC50 = 21.2 ± 1.3 µM), as an standard tested compounds (Table 1).
Table 1.
In vitro urease inhibiton activity of compounds 4a–z and 5a–s
| Compound | Urease inhibition IC50 ± SEM [µM] | Compound | Urease inhibition IC50 ± SEM [µM] |
|---|---|---|---|
| 4a | 39.3 ± 0.36 | 4x | 38.5 ± 0.28 |
| 4b | 34.4 ± 1.57 | 4y | 83.4 ± 1.00 |
| 4c | 54.2 ± 0.47 | 4z | 39.8 ± 1.38 |
| 4d | 31.6 ± 0.79 | 5a | 74.5 ± 0.88 |
| 4e | 27.5 ± 0.12 | 5b | 29.7 ± 0.67 |
| 4f | 54.2 ± 0.83 | 5c | 61.4 ± 1.12 |
| 4g | 28.5 ± 0.41 | 5d | 51.3 ± 0.45 |
| 4h | 40.3 ± 0.32 | 5e | 39.8 ± 0.75 |
| 4i | 17.6 ± 0.23 | 5f | 106.4 ± 1.49 |
| 4j | 22.3 ± 0.73 | 5g | 170.7 ± 1.55 |
| 4k | 25.8 ± 0.23 | 5h | 49.0 ± 0.55 |
| 4l | 22.7 ± 0.20 | 5i | 210.1 ± 0.29 |
| 4m | 39.3 ± 0.79 | 5j | 72.6 ± 0.59 |
| 4n | 41.2 ± 0.58 | 5k | 43.8 ± 0.33 |
| 4o | 83.0 ± 0.66 | 5l | 17.2 ± 0.44 |
| 4p | 39.7 ± 0.70 | 5m | 65.9 ± 0.61 |
| 4q | 24.6 ± 0.42 | 5n | 23.7 ± 0.57 |
| 4r | 27.5 ± 0.19 | 5o | 34.6 ± 0.79 |
| 4s | 109.7 ± 1.10 | 5p | 27.4 ± 0.54 |
| 4t | 142.1 ± 0.64 | 5q | 41.6 ± 0.41 |
| 4v | 52.2 ± 1.26 | 5r | 82.8 ± 0.72 |
| 4w | 59.4 ± 0.98 | 5s | 123.2 ± 0.37 |
| STD. Thiourea | 21.2 ± 1.3 |
Among barbituric acid zwitterionic adducts (4a–h) having bis(6-Hydroxy-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl) ring as basic nucleus, all ccompounds 4a, 4b, 4d, 4e, 4g and 4f showed IC50 values 39.3 ± 0.36, 34.4 ± 1.57, 31.6 ± 0.79, 27.5 ± 0.12, 28.5 ± 0.41, and 40.3 ± 0.32 µM respectively, and were found to be the potent urease inhibitors except compounds 4c (IC50 = 54.2 ± 0.47 µM) and 4f (IC50 = 54.2 ± 0.83 µM), while compared with the standard compound thiourea (IC50 = 21.2 ± 1.3 µM).
Among the barbituric acid derived derivatives (4i–4l), having bis(6-hydroxypyrimidine-2,4(1H,3H)-dione) as backbone, all tested compounds i.e.4i (IC50 = 17.6 ± 0.23 µM), 4j (IC50 = 22.3 ± 0.73 µM), 4 k (IC50 = 25.8 ± 0.23 µM) and 4 l (IC50 = 22.7 ± 0.20 µM) were found to be potent inhibitors of urease enzyme. Methyl substituted phenyl ring containing compound 4i (IC50 = 17.6 ± 0.23 µM) was the most active candidate of the series.
Third series of the derivatives of barbituric acids having (2-Hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)-1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate ring as basic nucleus (4m–4z) were also evaluated for their urease enzyme inhibition. Compounds 4m (IC50 = 39.3 ± 0.79 µM), 4n (IC50 = 41.2 ± 0.58 µM), 4p (IC50 = 39.7 ± 0.70 µM), 4q (IC50 = 24.6 ± 0.42 µM), 4r (IC50 = 27.5 ± 0.19 µM), 4x (IC50 = 38.5 ± 0.28 µM), and 4z (IC50 = 39.8 ± 1.38 µM) was found to be potent urease inhibitors against the standard thiourea.
Among fourth series of the derivatives of barbituric acid having (2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en-1-yl)methyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidin-4-olate) ring as basic nucleus (5m–5r), compound 5n (IC50 = 23.7 ± 0.57 µM), 5o (IC50 = 34.6 ± 0.79 µM), 5p (IC50 = 27.4 ± 0.54 µM), and 5q (IC50 = 41.6 ± 0.41 µM), showed poetnt urease inhibiton. All other compounds found to be weak urease inhibitors.
Similarly dimedone derivatives, bis-(2-hydroxy-4,4-dimethyl-6-oxocyclohex-1-en) ring conatining compounds 5a–s were also evaluated for their in vitro urease enzyme inhibition potential. Compounds 5b (IC50 = 29.7 ± 0.67 µM), 5e (IC50 = 39.8 ± 0.75 µM), and 5k (IC50 = 43.8 ± 0.33 µM), showed good enzyme inhibtion. All other compounds found to be significant to weak urease inhibitors (IC50 = 49.0 ± 0.55–210.1 ± 0.29 µM).
On the basis of the evaluated urease inhibition abilities of the above five different series of barbituric acid and dimedone derivatives as zwitter ion adduct compounds 4i (IC50 = 17.6 ± 0.23 µM) and 5 l (IC50 = 17.2 ± 0.44 µM) found to be the most active compounds and showed more urease inhibiton poetntial than the standard compound thiourea.
Molecular modeling and docking studies
In order to obtain deep insight into the binding mechanism of barbituric acid derivatives within the active site of urease enzyme and to obtain further validations of experimental results, MD simulation studies were performed.
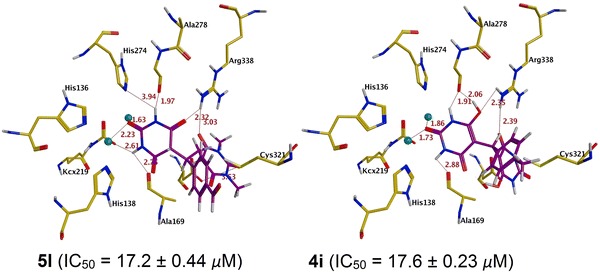
Forty-four barbituric acid derivatives (1–44) were docked into the binding pocket of urease. All the compounds were observed to accept analogous conformations with similar binding mode around the binding site of urease and these compounds were found to interact with nickel metal ions and the hotspot binding pocket residues (His137, His138, Ala169, KCX219, Asp362, Ala366 etc.) Visual inspection for predicted binding conformations of most potent compounds 5l and 4i (IC50 = 17.2 ± 0.44 M and IC50 = 17.6 ± 0.23 M) revealed that both compounds can adopt conformation for a better fit into the binding groove of urease. Further analysis of the top ranked poses of these compounds revealed that these compounds involved in multiple hydrogen bonding interactions with His138, Ala169, KCX219, Gly279, Asp362 and Arg338 residues. Compound 5i was found to be the least potent among the active ligands with IC50value of 210.1 ± 0.29 μM. Additionally; compounds 4a–4h, 4j–4r, and 4v–5e, 5m–5q, 5h and 5k showed a good urease inhibitory activity. In case of most active compound 5l the amine moiety adjacent to the carbonyl group formed hydrogen bond with the side chain oxygen of modified lysine KCX 219 at a distance of 2.61 Å. Another hydrophillic interaction found between carbonyl moiety of aldehyde group at para position of benzene ring and NH of His323 at a distance of 2.82 Å. The compound is further stabilized by the numerous hydrophilic interactions provided by catalytic residues Ala169 (2.75 Å), Gly279 (1.97 Å) and Asp362 (3.14 Å) NH moiety with carbonyl oxygen of compound 5l. Another important residue Arg338 formed two hydrogen bonds with carbonyl oxygen of pyrimidine ring at a distance of 2.32 and 3.03 Å, respectively. The best docked conformation of compound 5l predicted by MOE showed that the compound is deeply inserted within the urease binding site which is further stabilize by multiple hydrophilic and hydrophobic interactions, contributing to the higher activity of this compound. The binding mode of 5l is represented in Fig. 1a.
Fig. 1.
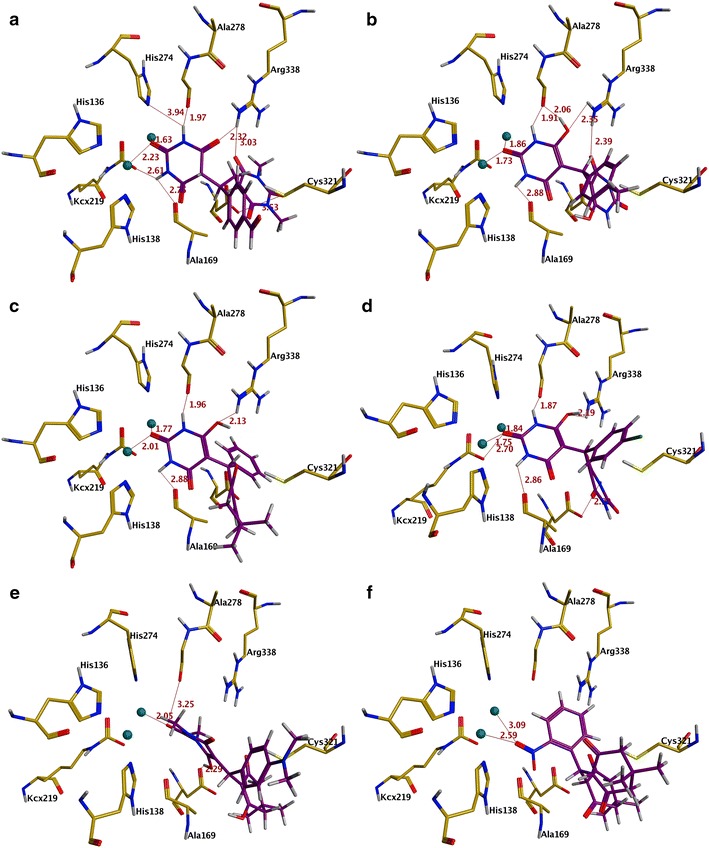
The docked poses of urease inhibitors: most active 5l (a), 4i (b), active 5n (c), 4j (d) and least active 4t (e), 5g (f). The interacting residues are presented in yellow stick while the ligands are shown in purple sticks
The binding mechanism of compound 4i reveals that multiple hydrogen bonding interactions found between ligand and hotspot residues. The carbonyl of pyrimidine moiety engaged in hydrogen bonding interaction with the NH of KCX219 and Arg338 at a distance of 2.71 and 2.35 Å, respectively. The NH of pyrimidine ring form hydrogen bond with the oxygen of Ala169, Gly279 and Asp362 at a distance of 2.88, 1.91 and 3.38 Å, respectively. Moreover these interactions are further stabilized by polar interactions with the His138. Due to the absence of aldehyde group at meta position of compound 4i, it is unable to make hydrophillic interaction with His323. The binding pattern of 4i in the ligand binding site of urease is shown in Fig. 1b.
Compounds 4j and 5n adopted a similar binding mechanism to 5l with some minor changes. The carbonyl oxygen of these compounds are hydrogen bonded to NH of Arg338 (2.19 and 2.13 Å) and KCX219 (2.7 and 2.91 Å), while NH of pyrimidine moiety of these compounds are engaged in hydrogen bond interactions with Ala169 (2.86 and 2.88 Å), Gly279 (1.87 and 1.96 Å) and Asp 362 (3.01 and 3.33 Å) as observed in 5l (Fig. 1). The non substituted benzene ring of the molecule is exposed to the surface and not found to be involved in such interactions. The docked orientation of 4j and 5n is shown in Fig. 1c, d.
To explain the inactivity of compounds 4s–4t, 5f–5g, 5i and 5s all the inactive compounds were also docked in the urease binding cavity by using MOE. By docking pose analysis, it is evident that all the inactive compounds were poorly occupied in the binding site. The carbonyl oxygen of 4t, 5g and 5i is replaced by an alkyl group as a result, hydrogen bonding interactions with catalytic residues are lost. The docked poses are shown in Fig. 1e, f. Similarly, the compounds 4s, 5i containing nitro group at R1 position instead of electron donating group may be one of the reasons for the inactivity of these compounds. These compounds interact with nickel ions but interactions with the catalytic residues are not as effective as observed in case of active compounds and shown in Fig. 1e, f). Consequently, absence of hydrogen bond interactions of these inactive compounds with crucial residues Ala169, Arg338 and Asp362 might be the reason for the inactivity of these compounds in the in vitro assay.
Molecular dynamic simulations
In order to understand the binding mechanism of barbituric acid derivatives molecular dynamic (MD) simulation was performed. The enol form of barbituric acid derivatives is found to be more stable during MD simulation as compared to its keto form. The keto form established only a weak interaction with nickel as compared to the enol. Interaction with nickel ion is crucial for inhibitory mechanism of urease inhibitors. The two forms disagree after 500 ps simulation as the distance between Ni and keto form increases gradually. To obtain further interaction pattern for two different form of barbituric acid derivatives, docking was also performed with both the possible forms. In the docking experiment, similar interactions with catalytic residues were observed except interaction with nickel metal. The obtained conformation explained the three dimensional structure of protein, which can be changed without fluctuating covalent bonds. The RMSD plot of protein conformation verses time for both the complexes is given in Fig. 2, which support the stability of enol form as nickel complex.
Fig. 2.
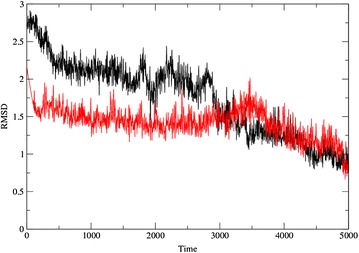
The RMSD plot of barbituric acid derivatives enol and keto complexes. Red color represents the enol form and black color represents the keto form RMSD
Conclusion
This study conclude that a simple one step chemistry can generate extra-ordinary array bioactive compounds. During this study, we synthesized barbituric acid derivatives by simple filtration and evaluated for their urease inhibitory activity. Compounds (4a–4z and 5a–s) were evaluated for their urease inhibition potential in vitro against the standard compound thiourea (IC50 = 128.8 ± 2.1 µM). Compounds 4i (IC50 = 17.6 ± 0.23 µM) and 5l (IC50 = 17.2 ± 0.44 µM) were found to be the most active members of the series with several fold more urease inhibition activity than the standard compound thiourea. The promising result of the current study indicates that barbituric acid derivatives can be investigated for the treatment of urease associated complications, such as peptic ulcer.
Authors’ contributions
AB proposed and designed research subject; GL performed research; FA carried out the assay of urease inhibition; SJ carried out the computation studies; AB, SY, and ZUH wrote the paper. AMA and MIC helped in the result and discussion and edit the final manuscript; All authors read and approved the final manuscript.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this Research group NO (RG -257-1436-1437).
Competing interests
The authors declare that they have no competing interests.
Additional file
10.1186/s13065-015-0140-1 Supplementary information containing the spectra of the synthesized compounds.
Footnotes
Assem Barakat, Abdullah Mohammed Al-Majid, Gehad Lotfy, Fiza Arshad, Sammer Yousuf, M. Iqbal Choudhary, Sajda Ashraf and Zaheer Ul-Haq contributed equally
Contributor Information
Assem Barakat, Phone: +966-11467-5884, Email: ambarakat@ksu.edu.sa.
Abdullah Mohammed Al-Majid, Email: amajid@ksu.edu.sa.
Gehad Lotfy, Email: lotfygehad@yahoo.com.
Fiza Arshad, Email: Arshad@hotmail.com.
Sammer Yousuf, Email: dr.sammer.yousuf@gmail.com.
M. Iqbal Choudhary, Email: iqbalhej@yahoo.com.
Sajda Ashraf, Email: Ashrafsajda@hotmail.com.
Zaheer Ul-Haq, Email: zaheer.qasmi@iccs.edu.
References
- 1.Krajewska B, Ureases I. Functional, catalytic and kinetic properties: a review. J Mol Catal B Enzym. 2009;59(1):9–21. doi: 10.1016/j.molcatb.2009.01.003. [DOI] [Google Scholar]
- 2.Follmer C. Ureases as a target for the treatment of gastric and urinary infections. J Clin Pathol. 2010;63(5):424–430. doi: 10.1136/jcp.2009.072595. [DOI] [PubMed] [Google Scholar]
- 3.Maroney MJ, Ciurli S. Nonredox nickel enzymes. Chem Rev. 2013;114(8):4206–4228. doi: 10.1021/cr4004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi T. On the occurrence of urease in higher plants. J Coll Agric Tokyo Imp Univ. 1909;1:1–14. [Google Scholar]
- 5.Sumner JB. The isolation and crystallization of the enzyme urease preliminary paper. J Biol Chem. 1926;69(2):435–441. [Google Scholar]
- 6.Dixon NE, Gazzola C, Blakeley RL, Zerner B. Jack bean urease (EC 3.5. 1.5). Metalloenzyme. Simple biological role for nickel. J Am Chem Soc. 1975;97(14):4131–4133. doi: 10.1021/ja00847a045. [DOI] [PubMed] [Google Scholar]
- 7.Jabri E, Carr MB, Hausinger RP, Karplus PA. The crystal structure of urease from Klebsiella aerogenes. Science. 1995;268(5213):998–1004. doi: 10.1126/science.7754395. [DOI] [PubMed] [Google Scholar]
- 8.Benini S, Rypniewski WR, Wilson KS, Miletti S, Ciurli S, Mangani S. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: why urea hydrolysis costs two nickels. Structure. 1999;7(2):205–216. doi: 10.1016/S0969-2126(99)80026-4. [DOI] [PubMed] [Google Scholar]
- 9.Ha NC, Oh ST, Sung JY, Cha KA, Lee MH, Oh BH. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat Struct Mol Biol. 2001;8(6):505–509. doi: 10.1038/88563. [DOI] [PubMed] [Google Scholar]
- 10.Balasubramanian A, Ponnuraj K. Crystal structure of the first plant urease from jack bean: 83 years of journey from its first crystal to molecular structure. J Mol Biol. 2010;400:274–283. doi: 10.1016/j.jmb.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Mobley HL, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Rev. 1995;59(3):451–480. doi: 10.1128/mr.59.3.451-480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayerdörffer E, Ottenjann R. The role of antibiotics in Campylobacter pylori associated peptic ulcer disease. Scand J Gastroenterol. 1988;23(S142):93–100. doi: 10.3109/00365528809091721. [DOI] [PubMed] [Google Scholar]
- 13.Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–2053. doi: 10.1002/(SICI)1097-0142(19981115)83:10<2049::AID-CNCR1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8(1):1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- 15.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37:4–66. doi: 10.1016/S0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 16.López-Muñoz F, Ucha-Udabe R, Alamo C. The history of barbiturates a century after their clinical introduction. Neuropsychiatr Dis Treat. 2005;1(4):329. [PMC free article] [PubMed] [Google Scholar]
- 17.Mobley HL, Hausinger RP. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauf A, Ahmed F, Qureshi AM, Khan A, Qadir MI, Choudhary MI, Haddad TB. Synthesis and urease inhibition studies of barbituric and thiobarbituric acid derived sulphonamides. J Chin Chem Soc. 2011;58(4):528–537. doi: 10.1002/jccs.201190017. [DOI] [Google Scholar]
- 19.Lee JH, Lee S, Park MY, Myung H. Characterization of thiobarbituric acid derivatives as inhibitors of hepatitis C virus NS5B polymerase. Virology journal. 2011;8(1):18. doi: 10.1186/1743-422X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidwai M, Thakur R, Mohan R. Ecofriendly synthesis of novel antifungal (thio) barbituric acid derivatives. Acta Chim Slov. 2005;52:88–92. [Google Scholar]
- 21.Dabholkar VV, Ravi DT. Synthesis of Biginelli products of thiobarbituric acids and their antimicrobial activity. J Serb Chem Soc. 2010;75(8):1033–1040. doi: 10.2298/JSC090106060D. [DOI] [Google Scholar]
- 22.Balas VI, Verginadis II, Geromichalos GD, Kourkoumelis N, Male L, Hursthouse MB, Hadjikakou SK. Synthesis, structural characterization and biological studies of the triphenyltin (IV) complex with 2-thiobarbituric acid. Eur J Med Chem. 2011;46(7):2835–2844. doi: 10.1016/j.ejmech.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Laxmi SV, Reddy YT, Kuarm BS, Reddy PN, Crooks PA, Rajitha B. Synthesis and evaluation of chromenyl barbiturates and thiobarbiturates as potential antitubercular agents. Bioorg Med Chem Lett. 2011;21(14):4329–4331. doi: 10.1016/j.bmcl.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 24.Yan Q, Cao R, Yi W, Yu L, Chen Z, Ma L, Song H. Synthesis and evaluation of 5-benzylidene (thio) barbiturate-β-d-glycosides as mushroom tyrosinase inhibitors. Bioorg Med Chem Lett. 2009;19(15):4055–4058. doi: 10.1016/j.bmcl.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Reddy YT, Sekhar KR, Sasi N, Reddy PN, Freeman ML, Crooks PA. Novel substituted (Z)-5-((N-benzyl-1H-indol-3-yl) methylene) imidazolidine-2, 4-diones and 5-((N-benzyl-1H-indol-3-yl) methylene) pyrimidine-2, 4, 6 (1H, 3H, 5H)-triones as potent radio-sensitizing agents. Bioorg Med Chem Lett. 2010;20(2):600–602. doi: 10.1016/j.bmcl.2009.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penthala NR, Ponugoti PR, Kasam V, Crooks PA. 5-((1-Aroyl-1H-indol-3-yl) methylene)-2-thioxodihydropyrimidine-4, 6 (1H, 5H)-diones as potential anticancer agents with anti-inflammatory properties. Bioorg Med Chem Lett. 2013;23(5):1442–1446. doi: 10.1016/j.bmcl.2012.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orhan DD, Küpeli E, Yesilada E, Ergun F. Anti-inflammatory and antinociceptive activity of flavonoids isolated from Viscum album ssp. album. Z Naturforsch C J Biosci. 2006;61(1–2):26–30. doi: 10.1515/znc-2006-1-205. [DOI] [PubMed] [Google Scholar]
- 28.Fan C, Clay MD, Deyholos MK, Vederas JC. Exploration of inhibitors for diaminopimelate aminotransferase. Bioorg Med Chem. 2010;18(6):2141–2151. doi: 10.1016/j.bmc.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Madadi NR, Penthala NR, Janganati V, Crooks PA. Synthesis and anti-proliferative activity of aromatic substituted 5-((1-benzyl-1H-indol-3-yl) methylene)-1, 3-dimethylpyrimidine-2, 4, 6 (1H, 3H, 5H)-trione analogs against human tumor cell lines. Bioorg Med Chem Lett. 2014;24(2):601–603. doi: 10.1016/j.bmcl.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barakat A, Al-Majid AM, Al-Najjar HJ, Mabkhot YN, Javaid S, Yousuf S, Choudhary MI. Zwitterionic pyrimidinium adducts as antioxidants with therapeutic potential as nitric oxide scavenger. Eur J Med Chem. 2014;84:146–154. doi: 10.1016/j.ejmech.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Weatherburn MW. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39(8):971–974. doi: 10.1021/ac60252a045. [DOI] [Google Scholar]
- 32.Mohammed Khan K, Saify ZS, Arif Lodhi M, Butt N, Perveen S, Murtaza Maharvi G, Atta-Ur-Rahman S. Piperidines:1 a new class of urease inhibitors. Nat Product Res. 2006;20(6):523–530. doi: 10.1080/1478641500059383. [DOI] [PubMed] [Google Scholar]
- 33.Ha NC, Oh ST, Sung JY, Cha KA, Lee MH, Oh BH Supramolecular assembly and acid resistance of Helicobacter pylori urease. 2001; 480–8 [DOI] [PubMed]
- 34.Molecular Operating Environment (MOE), 2013.08; Chemical Computing Group Inc., 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 2015
- 35.Halgren TA. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. Wiley Online Library, Vol 17 1996. p. 490–519
- 36.Bond SD. The Nose Poincare method for constant temperature molecular dynamics. J Comput Phys. 1999;151:114–134. doi: 10.1006/jcph.1998.6171. [DOI] [Google Scholar]
- 37.Al-Majid AM, Barakat A, Al-Najjar HJ, Mabkhot YN, Ghabbour HA, Fun HK. Tandem Aldol-Michael reactions in aqueous diethylamine medium: a greener and efficient approach to bis-pyrimidine derivatives. Int J Mol Sci. 2013;14(12):23762–23773. doi: 10.3390/ijms141223762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barakat A, Al-Majid AM, Al-Ghamdi AM, Mabkhot YN, Siddiqui MRH, Ghabbour HA, Fun HK. Tandem Aldol-Michael reactions in aqueous diethylamine medium: a greener and efficient approach to dimedone-barbituric acid derivatives. Chem Cent J. 2014;8(1):9. doi: 10.1186/1752-153X-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Najjar HJ, Barakat A, Al-Majid M, Mabkhot YN, Weber M, Ghabbour HA, Fun HK. A greener, efficient approach to michael addition of barbituric acid to nitroalkene in aqueous diethylamine medium. Molecules. 2014;19(1):1150–1162. doi: 10.3390/molecules19011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Majid AM, Islam MS, Barakat A, Al-Qahtani NJ, Yousuf S, Choudhary MI. Tandem Knoevenagel-Michael reactions in aqueous diethylamine medium: A greener and efficient approach towards bis-dimedone derivatives. Arab J Chem. 2014 [Google Scholar]
- 41.Barakat A, Al-Najjar HJ, Al-Majid AM, Soliman SM, Mabkhot YN, Ghabbour HA, Fun H-K. Synthesis, and Molecular characterization, of 5,5′-((2,4-dichlorophenyl)methylene)bis(1,3-dimethylpyrimidine 2,4,6(1H,3H,5H)-trione) J Mol Struct. 2015;1084:207–215. doi: 10.1016/j.molstruc.2014.12.030. [DOI] [Google Scholar]
- 42.Barakat A, Al-Najjar HJ, Al-Majid AM, Soliman SM, Mabkhot YN, Rafi Shaik M, Ghabbour HA, Fun H-K. Synthesis, NMR, FT-IR, X-ray structural characterization, DFT analysis and Isomerism aspects of 5-(2,6-dichlorobenzylidene)pyrimidine-2,4,6(1H,3H,5H)-trione. Spectrochim Acta. 2015;147:107–115. doi: 10.1016/j.saa.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Barakat A, Al-Majid AM, Al-Najjar HJ, Choudhary MI, Yousuf S. Crystal Structure of 1,3-Dimethyl-5-(2,4,6-trimethylbenzylidene)pyrimidine-2,4,6(1H,3H,5H)-trione. Zeitschrift für Kristallographie –NCS, 2014; 269–270
- 44.Barakat A, Al-Najjar HJ, Al-Majid AM, Adil SF, Ali M, Masand VH, Ghabbour HA, Fun H-K. Synthesis, X-ray diffraction, thermogravimetric and DFT analyses of pyrimidine Derivatives. Molecules. 2014;19:17187–17201. doi: 10.3390/molecules191117187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Najjar HJ, Barakat A, Al-Majid AM, Mabkhot YN, Weber M, Ghabbour HA, Fun H-K. A greener and efficient approach to Michael addition of barbituric acid to nitroalkene in aqueous diethylamine medium. Molecules. 2014;19:1150–1162. doi: 10.3390/molecules19011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Islam MS, Barakat A, Al-Majid AM, Ghabbour HA, Fun H-K, Siddiqui MR. Stereoselective synthesis of spiro[5.5]undecane derivatives via base promoted [5 + 1] double michael addition of N, N-dimethylbarbituric acid to dienones. Arab J Chem. 2015 [Google Scholar]
- 47.Barakat A, Soliman SM, Al-Majid AM, lofty G, Ghabbour HA, Fun H-K, Yousuf S, Choudhary MI, Abdul Wadood. Synthesis and structure investigation of novel pyrimidine-2,4,6-trione derivatives of highly potential biological activity as anti-diabetic agent. J Mol Struct 2015; 1098:365–376


