Abstract
Aim
To investigate the role of 18F–NaF PET/CT and compare it with 99m Tc-MDP whole body bone scintigraphy and 18F-FDG PET/CT in detecting the extent of metastatic bone disease and to present our first experience with 18F–NaF PET/CT in our country.
Materials and methods
A total of 37 histopathologically proven cancer patients (22 male, 15 female) with bone metastasis detected on Tc-99m MDP whole body bone scan were prospectively enrolled Cebeci, following ethics committee approval. 18F–NaF PET/CT was performed to the participants in Ankara University Medical Faculty Nuclear Medicine Department for evaluation of symptomatic skeletal sites which were negative on Tc-99m MDP whole body bone scan. A lesion based comparison was made between 18F–NaF PET/CT and Tc-99m MDP whole body bone scan for each patient and between 18F–NaF PET/CT and 18F-FDG PET/CT in 12/37 patients.
Results
The number of lesions demonstrated by 99m Tc-MDP bone scan and 18F–NaF PET/CT was equal in 4/37 (%11) of the cases. 18F–NaF PET/CT showed a greater number of pathological foci in 89% of participants. 18F–NaF PET/CT was able to show both lytic and blastic lesions and small lesions were better visualized due to the advantage of sectional imaging with much better resolution and higher target/background ratio. 18F–NaF PET/CT demonstrated a greater number of metastases in 10/12 (83%) of the patients when compared to 18F-FDG PET/CT. In the other two patients, bone metastasis could be demonstrated only by 18F–NaF PET/CT. The uptake of 18F-FDG was variable in blastic lesions and cranial bone involvement was missed by 18F-FDG PET/CT in some cases due to physiological brain metabolism.
Conclusion
Although further prospective clinical studies in specific cancer populations are indicated to set the place of 18F–NaF PET/CT in diagnostic scheme, the results of this pilot study from our country support the superiority of 18F–NaF PET/CT in investigation of bone metastasis over 99mTc-MDP bone scan and 18F-FDG PET/CT in various malignancies. 18F–NaF PET/CT is coming forward as a single step bone seeking study, considering all the advantages, but especially potential of detecting occult metastases and reliably directing patient management.
Keywords: 18–NaF PET/CT, Bone metastases, Diagnosis, 99mTc- MDP whole body bone scintigraphy, 18F-FDG PET/CT, Bone oncology
1. Introduction
Metastatic bone disease is the most frequent malignancy of the skeletal system [1]. Early diagnosis of bone metastases is an important step in the management of cancer as they may cause serious endocrinologic, hematologic, neurologic and orthopedic complications and intolerable pain [2], [3].
The most common method for bone scanning is Technetium-99m methylenediphosphonate (99mTc- MDP) bone scintigraphy, because 99mTc- MDP is a cheap and easily available radiopharmaceutical with no toxic effects and whole body bone scintigraphy has an acceptable sensitivity, specificity, positive and negative predictive value.
Fluorine 18-Fluorodeoxyglucose positron emission tomography/computerized tomography (18F-FDG PET/CT) is now used as a useful imaging tool for staging, restaging and evaluation of therapy response for most cancers. The uptake mechanism of the radiopharmaceutical in bone metastases depends on the pathological increase in glycolytic activity of the malignant cells, therefore 18F-FDG shows specifically the malignancy of the bone. 18F-FDG PET/CT also contributes to the true evaluation of bone marrow involvement and soft tissue component of the metastasis [4].
Fluorine 18–Sodium Fluoride (18F–NaF) has been introduced as a bone-seeking agent first in 1962 by Blau et al. [5] and approved by FDA in 1972 for detection of osteogenic activity. However it lost its popularity by the easy availability of Molibdenum-99 (Mo-99) generators and better imaging characteristics of 99mTc- for gamma cameras, with respect to the high energy photons of Fluorine-18. As PET technology spread all around the world in 1990's, 18F–NaF PET/CT regained interest for bone scanning [6]. The kinetics of the radiopharmaceutical is quite useful for imaging. After intravenous injection, it is cleared out from the blood pool fast and it forms fluoroapetite crystals by chemoadsorbtion to the hydroxyapatite crystals [7].
The aim of this study was to investigate the role of 18F–NaF PET/CT and compare it with 99m Tc-MDP whole body bone scintigraphy and 18F-FDG PET/CT in detecting the extent of metastatic bone disease and to present the results of our first experience with 18F–NaF PET/CT practice in our country.
2. Materials-methods
2.1. Patient group
Ankara University Ethics Committee approval was taken for the study. A total of 37 histopathologically proven cancer patients (22 male, 15 female) with bone metastasis detected on Tc-99m MDP whole body bone scan were prospectively enrolled. 18F–NaF PET/CT was performed to the participants in Ankara University Medical Faculty Nuclear Medicine Department for evaluation of symptomatic skeletal sites which were negative on Tc-99m MDP whole body bone scan. Informed consent for 18F–NaF PET/CT procedure was signed by all participants. All patients were over 18 years old with a mean age of 58.91. Also, the results of other imaging modalities concurrently performed with bone scans and 18F–NaF PET/CT for staging, restaging or evaluation of therapy response were also taken under consideration. Twelve patients (32%) also had 18F-FDG PET/CT and 2 patients (0.5%) had In-111 Octreotide whole body scintigraphy and 1 patient had Ga-68 DOTATATE PET/CT. There were 9 breast, 8 lung, 6 prostate, 2 gastric cancer, 1 nasopharynx, 1 cervix, 1 bladder, 1 colon cancer, 1 renal cell carcinoma (RCC), 1 RCC and neuroendocrine tumor (NET), 1 colon and prostate, 1 lung and prostate cancer, 1 pancreas NET, 1 Hodgkin's Lymphoma (HL), 1 non-Hodgkin Lymphoma (NHL) and 1 uterine leiomyosarcoma patients. Fifteen patients have not received any chemo-radiotherapy yet while 10 received only radiotherapy, 6 received only chemotherapy, and 5 received both. One prostate cancer patient received only hormonotherapy (Table 1).
Table 1.
Patient characteristics.
| Patient | Age | Gender | Primary pathology | Therapy history | 18F–NaF PET/CT | Tc-99m MDP Whole body bone scintgraphy | 18F-FDG PET/CT |
|---|---|---|---|---|---|---|---|
| 1 | 64 | F | Hodgkin's Lymphoma | Chemotherapy | + | + | + |
| 2 | 81 | F | Breast cancer | Chemotherapy | + | + | absent |
| 3 | 49 | F | Gastric cancer | Chemotherapy | + | + | absent |
| 4 | 80 | M | Prostate cancer | No history of therapy | + | + | absent |
| 5 | 46 | F | Breast cancer | Chemotherapy, radiotherapy | + | + | + |
| 6 | 60 | M | Lung cancer | Radiotherapy | + | + | absent |
| 7 | 52 | M | Lung cancer | Radiotherapy | + | + | absent |
| 8 | 50 | M | Prostate cancer | Chemotherapy, radiotherapy | + | + | absent |
| 9 | 32 | M | Nasopahrynx cancer | Chemotherapy | + | + | + |
| 10 | 36 | M | Renal cell carcinoma | No history of therapy | + | + | absent |
| 11 | 48 | F | Breast cancer | No history of therapy | + | + | absent |
| 12 | 77 | F | Lung cancer | No history of therapy | + | + | + |
| 13 | 72 | F | Cervix cancer | No history of therapy | + | + | + |
| 14 | 53 | M | Renal cell carcinoma+neuroendocrine tumor | Radiotherapy | + | + | + |
| 15 | 68 | F | Uterine leiomyosarcoma | Radiotherapy | + | + | absent |
| 16 | 66 | M | Lung cancer | No history of therapy | + | + | absent |
| 17 | 80 | M | Urinary bladder cancer | No history of therapy | + | + | absent |
| 18 | 39 | F | Breast cancer | Chemotherapy, radiotherapy | + | + | absent |
| 19 | 55 | M | Lung cancer | No history of therapy | + | + | + |
| 20 | 87 | F | Breast cancer | No history of therapy | + | + | absent |
| 21 | 34 | F | Breast cancer | No history of therapy | + | + | absent |
| 22 | 68 | F | Colon | No history of therapy | + | + | absent |
| 23 | 68 | F | NonHodgkin lymphoma | No history of therapy | + | + | + |
| 24 | 46 | M | Colon+prostate cancer | No history of therapy | + | + | + |
| 25 | 57 | M | Gastric cancer | Radiotherapy | + | + | absent |
| 26 | 60 | F | Breast cancer | Radiotherapy | + | + | absent |
| 27 | 45 | F | Breast cancer | Radiotherapy | + | + | absent |
| 28 | 31 | F | Breast cancer | Radiotherapy | + | + | + |
| 29 | 64 | M | Prostate cancer | No history of therapy | + | + | absent |
| 30 | 37 | M | Lung cancer | No history of therapy | + | + | + |
| 31 | 66 | M | Pancreas neuroendocrine tumor | Radiotherapy | + | + | absent |
| 32 | 59 | M | Lung cancer | Chemotherapy, radiotherapy | + | + | absent |
| 33 | 78 | M | Prostate cancer | Chemotherapy, radiotherapy | + | + | absent |
| 34 | 79 | M | Prostate cancer | Hormonotherapy | + | + | absent |
| 35 | 66 | M | Prostate+lung cancer | Chemotherapy | + | + | absent |
| 36 | 72 | M | Prostate cancer | Radiotherapy | + | + | absent |
| 37 | 55 | M | Lung cancer | Chemotherapy | + | + | + |
2.2. 18F–NaF PET/CT protocol
No special patient preparation was needed except for oral hydration, so that fast clearance from the background and a lower whole body radiation exposure could be obtained. The history of the disease, the chemotherapy, radiotherapy and antihormonal therapy performed were noted for evaluation. The injected doses were 5–10 mCi of 18F–NaF. Voiding was encouraged before imaging. PET scanning started 30 min after injection in supine position from vertex to the midthigh with 3 min/bed position and lower extremities were scanned at about 45 min after injection. CT images from vertex to the toes were obtained for attenuation correction and localization. Low dose CT acquisition was performed with 140 kV, 70 mA, 0.5 s per CT rotation, a pitch of 6 and a section thickness of 5 mm. (Discovery ST PET/CT scanner, General Electric, Milwaukee, Wisconsin, USA was used.)
2.3. 18F–NaF PET/CT image interpretation
Two nuclear medicine specialists made a visual analysis and a consensus was reached in order to avoid inter-observer variability. The readers were blind to the clinical data and to the results of other examinations. First, Maximum intensity projection (MIP) and then sectional images were examined for interpretation. Areas of higher or lower 18F–NaF uptake with respect to the neighboring or symmetrical bone tissue were recorded as pathological and evaluation for metastasis was done by the radiological characteristics of the lesions obtained from the CT data. If changes referring to a benign etiology was detected on CT images overlapping the pathological 18F–NaF uptake, then this uptake was not interpreted as metastatic (for example, activities corresponding to facet joints, vertebra endplates, osteophytes, large joints of the extremities, traumatic fractures etc.).
2.4. Data analysis
A lesion based analysis was made. The pathological uptake sites recorded on both Tc-99m MDP bone scan and 18F–NaF PET/CT scans were directly compared for each patient. For the 12 patients with 18F-FDG PET/CT, 2 patients with In-111 octreotide scintigraphy and 1 patient with Ga-68 DOTATATE PET/CT, similar lesion based comparison was also made between 18F–NaF PET/CT images and other examinations listed. The detectability of Tc-99m MDP, 18F–NaF and 18F-FDG radiopharmaceuticals according to the nature of the metastases, either osteoblastic or osteolytic were also taken in consideration by the help of the radiological data provided by the CT component of the PET/CT images.
3. Results
All 99mTc- MDP bone scintigraphy and 18F–NaF PET/CT results were positive for bone metastasis while 18F-FDG PET/CT results were negative in some of the cases. The results were analyzed with one to one comparison of the examinations for every patient.
3.1. Comparison between 18F–NaF PET/CT and 99mTc- MDP whole body scintigraphy
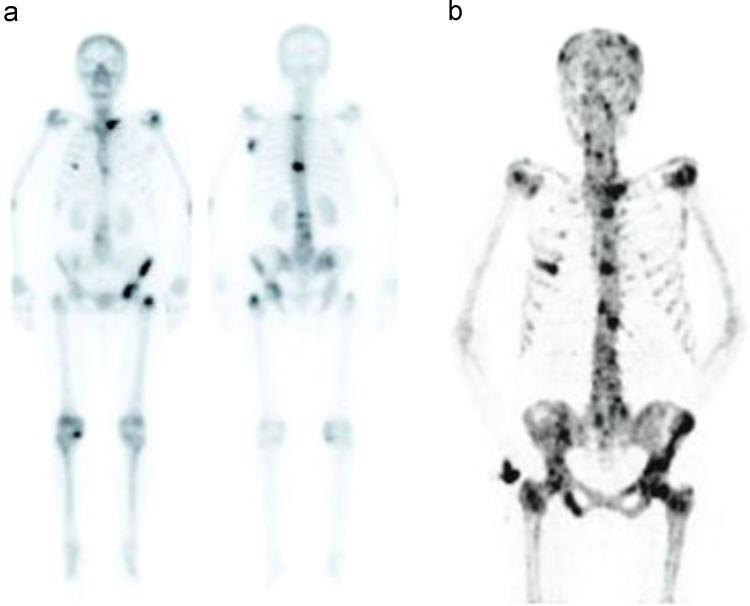
In the comparison results of 18F–NaF PET/CT and 99mTc- MDP whole body scintigraphy of the patients, the number of lesions demonstrated by both examinations was equal in 4/37 (11%) cases and 18F–NaF PET/CT showed multiple pathological foci in 33/37 (89%). This was attributed to the facts that 18F–NaF PET/CT could show both lytic and blastic lesions and small lesions are better visualized with high resolution and high target/background ratio (Fig. 1a and b).
Fig. 1.
Demonstration of a greater number of lesions in 18F–NaF PET/CT (b) study than 99mTc- MDP whole body bone scintigraphy (a) in a patient with Non-Hodgkin Lymphoma.
The sectional images provided by 18F–NaF PET/CT was advantageous over planar bone scintigraphy in the way that some lesions that could not be recognized or localized by 99mTc-MDP bone scan were easily detected by PET. (Figs. 2a and b, 3a and b).
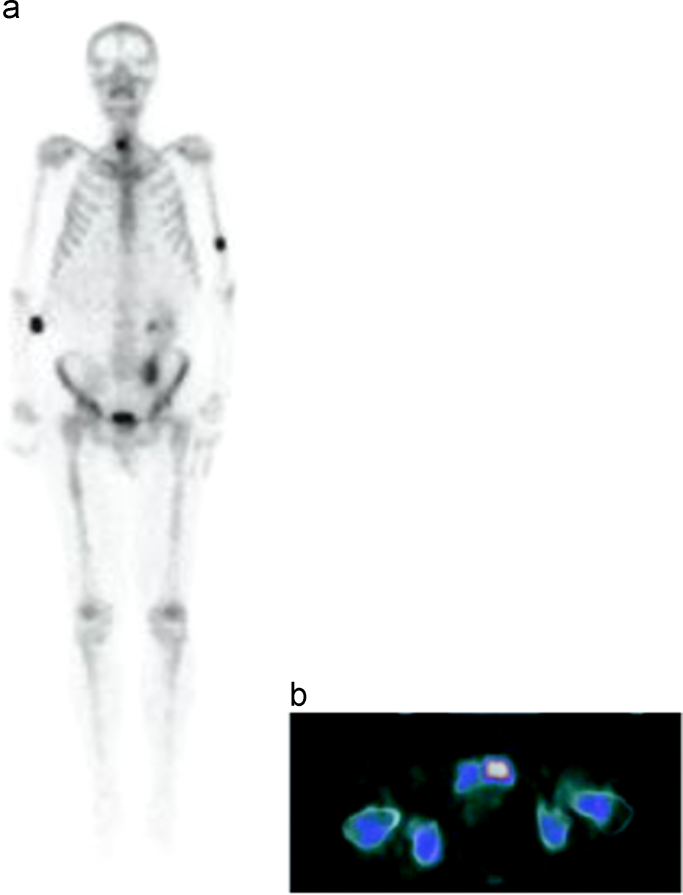
Fig. 2.
In the RCC patient, the lesion cannot be distinguished from bladder activity in 99mTc- whole body bone scintigraphy (a) while it can be demonstrated on axial images of the pelvis on 18F–NaF PET/CT (b).
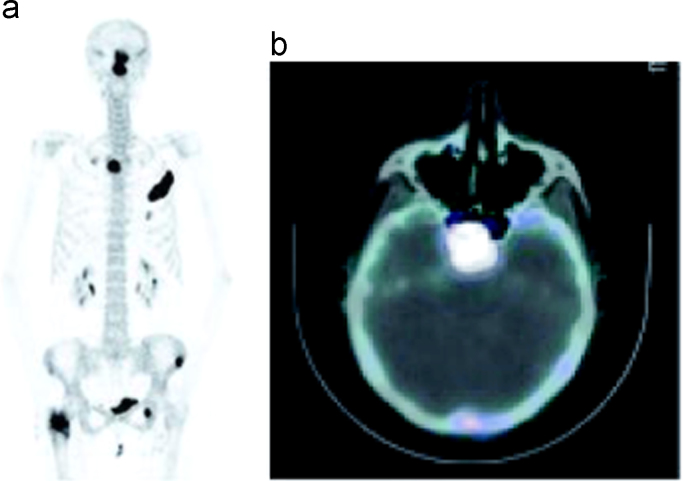
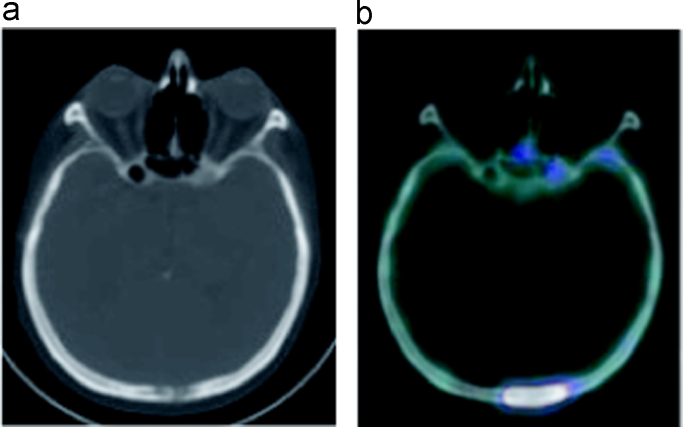
Fig. 3.
Demonstration of the uptake seen in the nasal region on 18F–NaF PET/CT MIP images (a) belonging to sphenoid bone metastases on axial images (b) in a metastatic colon cancer patient.
3.2. Comparison between 18F–NaF PET/CT and 18F-FDG PET/CT
The comparison results of 18F–NaF PET/CT and 18F-FDG PET/CT revealed that 18F–NaF PET/CT demonstrated a greater number of metastases in 10/12 (83%) patients. 18F-FDG PET/CT was unable to demonstrate bone metastasis in the other two cases (HL and cervix carcinoma).
18F–NaF PET/CT could show both blastic and sclerotic lesions (Figs. 4a and b, 5a and b). 18F–NaF PET/CT was commonly more successful in blastic metastasis than 18F-FDG PET/CT.
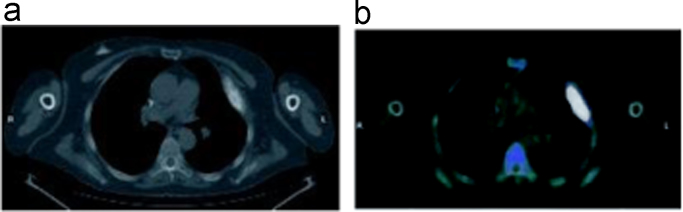
Fig. 4.
Axial CT image of sclerotic metastastases on the 6. left costa (a) and intense pathological 18F–NaF uptake (b).
Fig. 5.
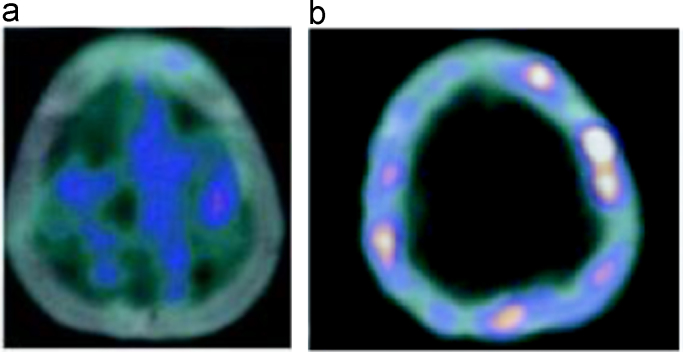
Axial CT image of a lytic metastatic lesion of the cranium (a) with intense pathological 18F–NaF uptake (b).
Additionally, 18F–NaF PET/CT could easily detect cranial bone involvement that 18F-FDG PET/CT missed because physiological brain metabolism masked (Fig. 6a and b).
Fig. 6.
Cranial bone involvement cannot be distinguished on 18F-FDG PET/CT axial sections (a) due to the physiological brain metabolism, but it is demonstrated well on 18F–NaF PET/CT axial images (b).
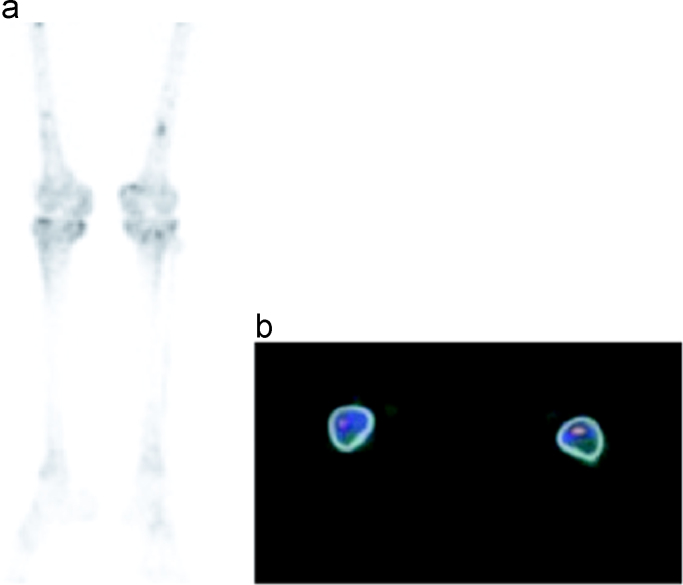
Bone marrow involvement could not be demonstrated in the 99mTc MDP bone scans but was positive in 5 patients (13%) in 18F–NaF PET/CT (Fig. 7a and b).
Fig. 7.
Demonstration of focal 18F–NaF uptake sites seen on 18F–NaF PET/CT MIP images (a) at the distal femur bilaterally belonging to bone marrow involvement on axial sections (b).
In the RCC patient while only two metastatic bone lesions could be demonstrated on 18F-FDG PET/CT, 15 lesions were seen on bone scan and countless, extensive metastases throughout the skeleton were detected on 18F–NaF PET/CT.
The HL patient had no skeletal involvement on 18F-FDG PET/CT, only a heterogeneous nonspecific uptake in the vertebral column on bone scan was detected but marked 18F–NaF uptake around the lytic lesions were identified on CT images of the lumbar vertebrae. Similarly, in the cervix carcinoma patient, while there were no metastatic lesions on 18F-FDG PET/CT, multiple pathological foci were detected by 99mTc- MDP whole body bone scintigraphy and 18F–NaF PET/CT.
In NHL and nasopharynx cancer patients, findings were compatible in both three modalities showing extensive skeletal metastases.
3.3. Comparison between 18F–NaF PET/CT and other modalities in NET patients
The pancreas NET case showed 6 bone metastases on In-111 octreotide scintigraphy, 15 lesions on Ga-68 DOTA-Tyr3-Octreotate (DOTATATE) PET/CT and 19 lesions on 18F–NaF PET/CT.
4. Discussion
The aim of this study was to evaluate the role of 18F–NaF PET/CT in detecting the extent of metastatic bone disease in comparison with 99mTc- MDP whole body bone scintigraphy and 18F-FDG PET/CT and to present our first experience with 18F–NaF.
Since 1990's, when PET/CT cameras started to gain prevalence around the world, the studies published about the utility of 18F–NaF PET/CT in metastatic bone disease were carried on specific patient groups like prostate, breast, thyroid and hepatocellular cancer. Although the diversity of primary diagnosis of the patients in our patient group interrupts homogeneity, a vast majority of our patients (62%) have the most commonly studied cancer types (prostate, breast and lung cancer).
The general statement that can be concluded from the studies comparing the utility of 18F–NaF PET or PET/CT with 99mTc- MDP whole body bone scintigraphy is that 18F–NaF PET/CT generally has a higher sensitivity and specificity than bone scan. Higher uptake of 18F–NaF than 99mTc- MDP in the skeleton and a faster blood clearance, yield a better target/background ratio in a shorter time period. 18F–NaF uptake in both lytic and blastic metastasis, sectional imaging advantage of the whole body and easy detection of small lesions with improved resolution of PET technology, better visualization of bone marrow lesions are all contributing factors to the success of 18F–NaF PET/CT [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. This is important in the way that 18F–NaF PET/CT may diagnose metastatic lesions while other modalities are found normal and thus alter the stage and management of the cancer patients. In our study, compatibly with the literature data, we observed 18F–NaF uptake in both lytic and blastic metastases. Small lesions, which could not be detected by 99mTc- MDP whole body bone scintigraphy, were easily visualized by the high-resolution power of PET/CT in 18F–NaF PET/CT studies. Although no bone marrow involvement could be demonstrated on 99mTc- MDP whole body bone scintigraphy, in some patients 18F–NaF showed an ability of true distinguishing of marrow lesions especially when focal uptake sites are close the joints where high uptake can be seen due to degenerative process.
In studies comparing 18F–NaF PET/CT with 99mTc- MDP whole body bone scan and SPECT, although no statistically significant difference between the accuracy of SPECT and 18F–NaF PET was found, the investigators argued that 18F–NaF PET was still the best imaging tool for bone scanning since SPECT imaging of the entire vertebral column took quite a long time [14], [17]. SPECT was beyond the scope of our study, but if could be performed, some other lesions, like the one missed in 99mTc- MDP whole body bone scintigraphy in the symphysis pubis due to bladder activity and the sphenoid bone metastasis which could not be differentiated in the anterior view of the MIP images, would possibly be detected also by bone scintigraphy on SPECT images.
There are only a few studies directly comparing the effectiveness of 18F-FDG PET/CT and 18F–NaF PET/CT in the literature. Although the method of choice is 18F-FDG PET/CT for bone imaging in malignancies, which commonly present lytic bone metastases, it has been reported that the diagnostic accuracy of 18F–NaF PET/CT is higher than 18F-FDG PET/CT in various cancers [18], [19], [20], [21], [22], [23], [24], [25]. In our study, 18F-FDG PET/CT and 18F–NaF PET/CT could be compared in 12 patients. 18F–NaF PET/CT showed a greater number of lesions in 10/12 patients. 18F-FDG PET/CT was negative for bone metastasis in the other 2 cases.
The uptake of 18F-FDG in metastatic bone disease depends on the glycolytic activity of the tumoral bone lesions. Although it seems to be tumor specific, 18F-FDG has a limited sensitivity in detection of low-grade tumors growing slowly and have low glycolytic activity [22], [23].
One reason for detecting a greater number of lesions in 18F–NaF PET/CT in our study was th at cranial bone involvement could be demonstrated by 18F–NaF PET/CT. In some of these cases, the physiological brain metabolism masked the possible uptake of 18F-FDG in the cranial bones. In the literature, there were no comments on these distinctive characteristics of these two radiopharmaceuticals.
The types of the primary tumor of 12 patients who have also undergone 18F-FDG PET/CT were heterogeneous (2 breast, 4 lung, 1 nasopharynx, 1 cervix cancer, 1 RCC, 1 HL, 1 NHL and 1 uterine leiomyosarcoma). There exists a case report showing the effectiveness of 18F–NaF PET/CT in RCC [26]. In our series, while there were only two bone lesions on 18F-FDG PET/CT of the RCC patient, 18F–NaF PET/CT revealed extensive skeletal metastasis. There are no studies specifically investigating the role of 18F–NaF PET/CT in lymphoma, nasopharynx cancer, NET or gynecological tumors. We demonstrated that in these cases 18F–NaF PET/CT was superior to 99mTc-MDP bone scan, 18F-FDG PET/CT and to Ga-68 DOTATATE PET/CT in NET. Although the number of patients is limited, our pilot findings may still contribute to the literature.
This was a prospective pilot study performed in our department in order to examine the role of 18F–NaF PET/CT by comparing the results of 18F–NaF PET/CT primarily with Tc-99m MDP bone scan and 18F-FDG PET/CT, the two imaging modalities frequently used to assess skeletal involvement in oncology. To the best of our knowledge, our results also reflect the first experience of 18F–NaF PET/CT practice in our country. There were some limitations of this study including the heterogeneity of the study population in terms of diagnosis and received therapies, bias towards patients with known bone metastasis, unavailability of SPECT imaging and impossibility of histopathological confirmation of pathological sites due to ethical concerns. Further prospective studies are needed in specific malignancies in order to demonstrate the clinical benefits of 18F–NaF PET/CT over other imaging tools.
5. Conclusion
Our pilot study supports the superiority of 18F–NaF PET/CT over Tc-99m MDP bone scintigraphy and 18F-FDG PET/CT. It has a potential to replace them both as a bone seeking study, considering all the advantages and ability of detecting occult bone metastasis and therefore directing the staging of the patients, but further prospective studies should be enrolled to set the role and usage of 18F–NaF PET/CT in routine clinical practice.
Acknowledgments
Authors have no acknowledgments to declare.
References
- 1.Hage W.D., Aboulafia A.J., Aboulafia D.M. Incidence, location, and diagnostic evaluation of metastatic bone disease. Orthop. Clin. North Am. 2000;31:515–528. doi: 10.1016/s0030-5898(05)70171-1. [DOI] [PubMed] [Google Scholar]
- 2.Coleman R.E. Skeleteal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 4.Fogelman I., Cook G., Israel O., Van der Wall H. Positron emission tomography and bone metastases. Semin. Nucl. Med. 2005;35:135–142. doi: 10.1053/j.semnuclmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Blau M., Nagler W., Bender M.A. Fluorine-18: a new isotope for bone scanning. J. Nucl. Med. 1962;3:332–334. [PubMed] [Google Scholar]
- 6.Eberlein U., Bröer J.H., Vandevoorde C., Santos P., Bardiès M., Bacher K. Biokinetics and dosimetry of commonly used radiopharmaceuticals in diagnostic nuclear medicine-a review. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:2269–2281. doi: 10.1007/s00259-011-1904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blake G.M., Park-Holohan S.J., Cook G.J., Fogelman I. Quantitative studies of bone with the use of F18-Fluoride and Tc99m-methylene diphosphanate. Semin. Nucl. Med. 2001;1:28–49. doi: 10.1053/snuc.2001.18742. [DOI] [PubMed] [Google Scholar]
- 8.Jadvar H., Desai B., Ji L., Conti P.S., Dorff T.B., Groshen S.G., Gross M.E. Prospective evaluation of 18F–NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin. Nucl. Med. 2012;37:637–643. doi: 10.1097/RLU.0b013e318252d829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook G.J., Fogelman I. The role of positron emission tomography in skeletal disease. Semin. Nucl. Med. 2001;31:50–61. doi: 10.1053/snuc.2001.18746. [DOI] [PubMed] [Google Scholar]
- 10.Schiepers C., Nuyts J., Bormans G., Dequeker J., Bouillon R., Mortelmans L. Fluoride kinetics of the axial skeleton measured in vivo with fluorine -18-fluoride PET. J. Nucl. Med. 1997;38:1970–1976. [PubMed] [Google Scholar]
- 11.Schirrmeister H., Guhlmann A., Kotzerke J., Santjohanser C., Kühn T., Kreienberg R. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J. Clin. Oncol. 1999;17:2381–2389. doi: 10.1200/JCO.1999.17.8.2381. [DOI] [PubMed] [Google Scholar]
- 12.Bombardieri E., Aktolun C., Baum R., Bishof-Delaloye A., Buscombe J., Chatal J.F. Bone scintigraphy procedures guidelines for tumor imaging. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:B99–B106. doi: 10.1007/s00259-003-1347-2. [DOI] [PubMed] [Google Scholar]
- 13.G. Segall, D. Delbeke, M.G. Stabin, E. Even-Sapir, J. Fair, R. Sajdak et al. SNM Guideline for Sodium 18F-Fluoride PET/CT Bone Scans, 2010. [DOI] [PubMed]
- 14.Schirrmeister H., Glatting G., Hetzel J., Nüssle K., Arslandemir C., Buck A.K. Prospective evaluation of the clinical value of planar bone scans, SPECT, and (18)F-labeled NaF PET in newly diagnosed lung cancer. J. Nucl. Med. 2001;42:1800–1804. [PubMed] [Google Scholar]
- 15.Petrén-Mallmin M., Andréasson I., Ljunggren O., Ahlström H., Bergh J., Antoni G. Skeletal metastases from breast cancer: uptake of 18F-fluoride measured with positron emission tomography in correlation with CT. Skelet. Radiol. 1998;27:72–76. doi: 10.1007/s002560050340. [DOI] [PubMed] [Google Scholar]
- 16.Even-SapirE Mishani E., Flusser G., Metser U. 18F-Fluoride positron emission tomography and positron emission tomography/computed tomohraphy. Semin. Nucl. Med. 2007;37:462–469. doi: 10.1053/j.semnuclmed.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Hetzel M., Arslandemir C., König H.H., Buck A.K., Nüssle K., Glatting G. H F–18 NaF PET for detection of bone metastases in lung cancer: accuracy, cost-effectiveness, and impact on patient management. J. Bone Miner. Res. 2003;18:2206–2214. doi: 10.1359/jbmr.2003.18.12.2206. [DOI] [PubMed] [Google Scholar]
- 18.Yen R.F., Chen C.Y., Cheng M.F., Wu Y.W., Shiau Y.C., Wu K., Hong R.L. The diagnostic and prognostic effectiveness of F-18 sodium fluoride PET-CT in detecting bone metastases for hepatocellular carcinoma patients. Nucl. Med. Commun. 2010;31:637–645. doi: 10.1097/MNM.0b013e3283399120. [DOI] [PubMed] [Google Scholar]
- 19.Krüger S., Buck A.K., Mottaghy F.M., Hasenkamp E., Pauls S., Schumann C. Detection of bone metastases in patients with lung cancer: 99mTc-MDP planar bone scintigraphy, 18F-fluoride PET or 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2009;36(11):1807–1812. doi: 10.1007/s00259-009-1181-2. [DOI] [PubMed] [Google Scholar]
- 20.Even-Sapir E., Metser U., Flusser G., Zuriel L., Kollender Y., Lerman H. Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J. Nucl. Med. 2004;45:272–278. [PubMed] [Google Scholar]
- 21.Schirrmeister H., Guhlmann A., Elsner K., Kotzerke J., Glatting G., Rentschler M. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J. Nucl. Med. 1999;40(10):1623–1629. [PubMed] [Google Scholar]
- 22.Histed S.N., Lindenberg M.L., Mena E., Turkbey B., Choyke P.L., Kurdziel K.A. Review of functional/anatomical imaging in oncology. Nucl. Med. Commun. 2012;33:349–361. doi: 10.1097/MNM.0b013e32834ec8a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chua S., Gnanasegaran G., Cook G.J. Miscellaneous cancers (lung, thyroid, renal cancer, myeloma, and neuroendocrine tumors): role of SPECT and PET in imaging bone metastases. Semin. Nucl. Med. 2009;39:416–430. doi: 10.1053/j.semnuclmed.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Krüger S., Buck A.K., Mottaghy F.M., Hasenkamp E., Pauls S., Schumann C. Detection of bone metastases in patients with lung cancer: 99mTc-MDP planar bone scintigraphy, 18F-fluoride PET or 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2009;36(11):1807–1812. doi: 10.1007/s00259-009-1181-2. [DOI] [PubMed] [Google Scholar]
- 25.Damle N., Bal C., Bandopadhyaya G., Kumar L., Kumar P., Malhotra A. Role of 18F-fluoride PET/CT in the detection of bone metastases in breast cancer patients (abstract) J. Nucl. Med. 2007;48(Suppl. 2):142P. [Google Scholar]
- 26.Bhargava P., Hanif M., Nash C. Whole-body F-18 sodium fluoride PET-CT in a patient with renal cell carcinoma. Clin. Nucl. Med. 2008;33:894–895. doi: 10.1097/RLU.0b013e31818ca43c. [DOI] [PubMed] [Google Scholar]