Abstract
Plant associated rhizobacteria prevailing in different agro-ecosystems exhibit multiple traits which could be utilized in various aspect of sustainable agriculture. Two hundred thirty four isolates were obtained from the roots of basmati-385 and basmati super rice varieties growing in clay loam and saline soil at different locations of Punjab (Pakistan). Out of 234 isolates, 27 were able to solubilize zinc (Zn) from different Zn ores like zinc phosphate [Zn3 (PO4)2], zinc carbonate (ZnCO3) and zinc oxide (ZnO). The strain SH-10 with maximum Zn solubilization zone of 24 mm on Zn3 (PO4)2ore and strain SH-17 with maximum Zn solubilization zone of 14–15 mm on ZnO and ZnCO3ores were selected for further studies. These two strains solubilized phosphorous (P) and potassium (K) in vitro with a solubilization zone of 38–46 mm and 47–55 mm respectively. The strains also suppressed economically important rice pathogens Pyricularia oryzae and Fusarium moniliforme by 22–29% and produced various biocontrol determinants in vitro. The strains enhanced Zn translocation toward grains and increased yield of basmati-385 and super basmati rice varieties by 22–49% and 18–47% respectively. The Zn solubilizing strains were identified as Bacillus sp. and Bacillus cereus by 16S rRNA gene analysis.
Keywords: bio fertilizer, PGPR, rhizobacteria, zinc, rice, super basmati, basmati-385, 16S rRNA gene
Introduction
Rice is an important cereal crop growing across the world and is a major staple food of Asian population. In current scenario of intensive farming, soils are continuously depleting in macro and micro nutrients especially in wheat-rice cropping system (Rana et al., 2015). Among micro nutrients, Zn is a highly essential nutrient required throughout the life cycle of rice (Holler et al., 2014).
Farmers use chemical fertilizers to make up the deficiency of essential nutrients and thus achieve high yields. Irrational use of chemical fertilizers has led to severe environmental problems especially contamination of underground water due to leaching and pollution of atmosphere through gaseous emissions (Lockhart et al., 2013; Wu and Ma, 2015). Moreover, fluctuations in price and non-availability of chemical fertilizers due to energy crisis and other reasons pose a major constraint in sustainable crop production. Under these circumstances, plant growth promoting rhizobacteria (PGPR) may offer a valuable alternative to chemical fertilizers.
This is because PGPR live freely in soil, colonize plant roots aggressively and establish symbiotic association with plants. Existence of PGPR with the plant roots is generally classified by two environments viz; rhizosphere and endosphere. Rhizosphere represents the soil volume under the direct influence of root while endosphere represents the internal tissue of root (Timm et al., 2015). The strains inhabiting rhizosphere and endosphere are called rhizobacteria and endophytes respectively.
PGPR enrich soil with major plant nutrients such as nitrogen (N) by fixing it from the atmosphere, phosphorous (P), and potassium (K) by solubilizing them from the soil (Patel et al., 2015; Pii et al., 2015; Zahid et al., 2015). They also assist in bioavailability of Zn by solubilizing it from various ores like Zn3 (PO4)2, ZnCO3, and ZnO (Abaid-Ullah et al., 2015; Sirohi et al., 2015). In addition to provide macro and micronutrients to the plants, PGPR also protect them from pathogens. They suppress the activity of pathogens by producing numerous antifungal metabolites like siderophores, hydrolytic enzymes, and antibiotics (Chowdhury et al., 2015). Therefore, they could be utilized as an alternative to the chemical fertilizers and fungicides and hence may ensure sustainable agriculture production, environmental safety and lower production cost.
This argument is supported by earlier reports where inoculation of plants with PGPR has resulted in the improved nutrition, vigorous plant growth and high yield (El-Sayed et al., 2014; Majeed et al., 2015). Many effective strains have been formulated as biofertilizers. The use of registered biofertilizers and microbial technologies has become a widely accepted strategy in the current intensive agricultural practices prevailing throughout the world (Shen et al., 2015). The PGPR strains identified so far belong to genus Pseudomonas, Ochrobacterum, Bacillus, Azosperillum, Azotobacter, Rhizobium, Stenotrophomonas, Serratia, and Enterobacteria (Hassan et al., 2010; Ma et al., 2011; Abaid-Ullah et al., 2015). Highly positive effects of these species on the growth of various crops such as sugarcane, maize, wheat, rice, canola, sunflower and other vegetables have been observed both in vitro and in vivo under variable climatic conditions (Hassan et al., 2011; El-Sayed et al., 2014). For example, Kosakonia radicincitans increased dry weight and N content of yerba mate by 183 and 30% (Bergottini et al., 2015). Wheat yield was improved by 9% upon inoculation with a consortium of Bacillus thuringiensis and Serratia sp. (Abaid-Ullah et al., 2015; Pereg and McMillan, 2015).
In addition to an increase in yield, PGPR also significantly affect the nutrients uptake by plants. This property of rhizobacteria has drawn the attention of researchers to exploit them in cereals bio fortification. Ramesh et al. (2014) reported that Zn solubilizing strains of Bacillus aryabhattai improved Zn mobilization in wheat and soybean. Recent studies conducted at our laboratory (Abaid-Ullah et al., 2015) reveiled that certain strains of Serratia sp., Pseudomonas sp., and Bacillus sp., enhanced Zn translocation toward wheat grains by 7–12% compared to that of chemical Zn (Abaid-Ullah et al., 2015).
The plant–microbe interaction is quite complex phenomena and effect of this interaction on growth and physiological processes occurring in the life of plant has been explored in detail. It has been widely reported that there is high diversity in origin and function of PGPR and their growth promoting potential may be highly specific to certain soils, plant species, genotypes and cultivars (Lucy et al., 2004; Mehta et al., 2015; Zahid et al., 2015). Hence, a thorough investigation of native bacterial communities, their population and characteristics is required to assess the diversity of indigenous bacteria and their distribution in the rhizoplane of certain crops (Bulgarelli et al., 2013; Piromyou et al., 2013).
Effects of PGPR strains vary for different crops growing in various soil types under variable climatic conditions. Therefore, it is necessary to cultivate region-specific microbial strains for the development of suitable bio inoculum to obtain maximum yield and nutrient content of a specific crop (Farag et al., 2013; Habibi et al., 2014). In view of these facts, present study was designed to isolate indigenous bacterial strains from the rice endosphere growing on clay loam and saline soil at different locations. These strains were screened in vitro for Zn solubilization potential from different Zn ores. The potent strains were characterized for other plant growth promoting traits and inoculated to rice varieties, basmati 385 and super basmati to assess their potential to enhance yield and translocate Zn under net house conditions. The potent strains were identified by 16S rRNA gene analysis.
Materials and methods
Sample collection and isolation of bacteria
Representative plants of five rice varieties viz; super basmati, basmati-385, shaheen basmati, kainat basmati, and basmati-515 growing in two types of soil clay loam and saline were sampled from different locations of Punjab. Three to four fields located in the radius of one kilometer growing in same soil type were identified per location. Four-five plants of variable vigor were selected from each field, uprooted with bulk rhizospheric soil, and pooled up to make a representative sample. The samples were placed individually in paper bags, labeled and transported to lab. Bacterial endophytes were then isolated by following the method of Surette et al. (2003) with certain modifications. Briefly, roots of each plant were separated and thoroughly washed with tap water to remove any adhering soil. The root tissues of each sample were mixed thoroughly and then surface disinfected by a 3 min treatment with commercial bleach (5.25% available chlorine), transferred to a 3% hydrogen peroxide solution for 3 min and finally rinsed three times with sterile milli-Q water followed by air drying in sterile filter paper under the safety cabinet. One gram of the roots was crushed and grinded in sterilized mortar and pestle. The potentially endophytic bacteria were isolated by serial dilution plating of sterilized crushed root on Luria Bertani (LB) agar plates (Hassan et al., 2010). LB agar plates were incubated at 28 ± 2°C for 24–36 h. The individual colonies appearing on the plates were picked and purified by re streaking on LB agar plates. The purified strains were preserved in 20% glycerol at −80°C.
Screening of Zn solubilizing bacteria
Potential of isolates to solubilize Zn from various ores such as zinc sulfide (ZnS), ZnO, Zn3(PO4)2, and ZnCO3 was tested on Bunt and Rovira agar medium (Bunt and Rovira, 1955). The bacterial strains were grown in LB overnight. Five microliter of each bacterial suspension having optical density (OD) normalized to 0.5 was inoculated on specific plates containing 0.1% of the respective ore. The inoculated plates were incubated at 30°C for 36–96 h. Appearance of halo zone around the colonies indicated their potential to solubilize Zn which was estimated by measuring the zone diameter. There were three biological replicates and the experiment was repeated twice.
Morphological and biochemical characterization of potent Zn solubilizing strains
Two strains exhibiting maximum potential to solubilize Zn from various ores were selected for morphological and biochemical characterization. For each test, the strains were freshly grown in LB broth overnight and normalized to the OD-600 of 0.5 before inoculation on respective plate.
Gram reaction and antibiotic resistance
The Zn solubilizing strains were characterized for Gram reaction and intrinsic resistance to antibiotics following the method of Vincent (1970). Briefly, the strains were inoculated on agar plates and spreaded by swab. Antibiotic discs of levofloxacin (5 μg), streptomycin (10 μg), piperociline (100 μg), amoxyciline (10 μg), tetracycline (30 μg), kanamycine (30 μg), vanlomycine (30 μg), and minocycline (30 μg) were placed on the plate of each strain and incubated at 28 ± 2°C for 24–48 h. Inhibition of bacterial growth was observed around each antibiotic disc and strains were designated as highly resistant, moderate resistant, moderate susceptible and highly susceptible on the basis of inhibition zone diameter as recommended by the manufacturer.
Phosphorus (P) and potassium (K) solubilizing activity
Ability of strains to solubilize the major plant nutrients (P, K) were tested on Pikovskaya agar containing tricalcium phosphate as insoluble phosphate source and Aleksandrov agar having potassium aluminum silicate as source of insoluble inorganic potassium respectively (Pikovskaya, 1948; Kumar et al., 2012). Each bacterial culture was spot inoculated in the center of respective agar plates. The plates were incubated at 28 ± 2°C for 7–10 days and observed for the appearance of halo zone around the colonies. Size of the zone diameter around the colonies provided a semi quantitative potential of P and K solubilization of the strains. The experiment was repeated twice with three biological replicates.
Determination of indole-3-acetic acid and siderophores
Indole-3-acetic acid (IAA) production by the Zn solubilizers was qualitatively determined by growing the strains on LB agar plates supplemented with 100 μg mL−1 of tryptophan (Shrivastava and Kumar, 2011). A 0.5 cm deep cavity of 1–2 cm diameter was made by sterile cork borer. A 100 μL of freshly grown culture was inoculated in each cavity. The inoculated plates were incubated at 28 ± 2°C. After 16 h incubation, the bacterial colonies were removed from the cavities with the help of sterile cotton swab and approximately 200 μL of IAA reagent consisting of (1 mL of 0.5 M FeCl3 mixed in 50 mL of 35% HClO4) was added in the cavity and observed for change in color. Appearance of pink halo zone around the cavity indicated production of IAA by the bacterial culture. Qualitative production of siderophores by the bacterial strains was detected on the Chrome-azurol S (CAS) medium (Schwyn and Neilands, 1987). Each bacterial culture was inoculated separately on CAS agar plates and incubated at 28 ± 2°C for 72 h. The plates were observed for the change in color i.e., orange to yellow and zone diameter was measured to estimate the production of siderophores semi quantitatively.
Antagonism against economically important pathogens of rice
Two economically important pathogens of rice, Pyricularia oryzae causing rice blast and Fusarium moniliforme causing bakanae disease of rice were used as test strains to study the antagonistic potential of potent Zn solubilizers. The antagonism was tested by dual culture assay as described by Spence et al. (2014) with certain modifications. The fungal disc of diameter 6 mm was placed at the center of potato dextrose agar (PDA) petri plate. The bacterial culture was spotted at a distance of 4 cm from fungal disc. LB broth instead of bacterial culture was used in mocked (control). The PDA plates were sealed with parafilm and incubated at 28 ± 2°C for 8–10 days. The mycelial diameter of fungus growing out from the edge of bacterial colony was measured. Percentage inhibition of fungal mycelium was calculated by using the following formula:
Where C = mycelium diameter (cm) of the fungus growing in the control plate and T = mycelium diameter (cm) of the fungus growing in the bacterial treated plates. The experiment was repeated twice with three biological replicates each time.
Determination of HCN (Hydrogen Cyanide)
Production of HCN was determined by following the method of Miller and Higgins (1970) with certain modifications. The bacterial cells were inoculated on LB agar plates amended with 4.4 g glycine L−1. A piece of filter paper having diameter 6 cm was dipped in solution consisting of 0.5% picric acid, 1% Na2CO3 and placed in the upper lid of each petri plates. The plates were wrapped with parafilm and incubated at 28 ± 2°C for 48–72 h. The change in color of filter paper from yellow to brown was used as indicator for HCN production.
Determination of hydrolytic enzymes
Hydrolytic enzymes production such as protease, cellulase, and glucanase were detected on the agar plates containing skim milk, carboxy methylcellulase and laminarin respectively (Hassan et al., 2011; Kumar et al., 2012; Abraham et al., 2013). The bacterial strains were inoculated on the respective agar plates and incubated at 28 ± 2°C for 4–7days. Development of halo zone around the colonies indicated enzyme production. Zone of exo β-1, 3-glucanase was observed after staining with congo red (Nagpure et al., 2014).
Molecular identification of Zn solubilizing bacteria
Zn solubilizing bacteria were identified at molecular level by sequencing 16S rRNA gene. The genomic DNA of bacterial strains was extracted by CTAB extraction (Wilson, 1987). A 1500 bp 16S rRNA gene was amplified by using the primers P1 (5′-AGAGTTTGATCCTGGTCAGAACGAACGCT-3′) and P6 (TACGGCTACCTTGTTACGACTTCACCCC - 3′) as described by Tan et al. (1997). PCR reaction mixture consisting of 10–15 ng DNA, 1.5 mM MgCl2,1 X PCR buffer, 200 μM of each, dATP, dCTP, dGTP, and dTTP (Fermentas), 10 mM of each primer, and 1.0–1.5 U of Taq polymerase (Fermentas) was amplified in thermocycler (Peq lab Germany) with the amplifying conditions; initial denaturation at 95°C for 5 min, 25 cycles (94°C for 1 min, 56°C for 1 min, 72°C for 1.75 min) followed by final extension at 72°C for 5 min (Tan et al., 1997; Hassan et al., 2010). The amplified 16S rRNA gene was analyzed on 1% agarose gel and compared with 1 kb DNA ladder (Fermentas). Specific band of 16S rRNA gene was eluted from the gel and purified by using the Gel Extraction Kit (Qiagen). The purified PCR product was sequenced commercially by Macrogen Inc. (Korea). The 16S rRNA gene sequence was annotated, analyzed on BLAST and identified on the basis of closest homologous strain.
Effect of Zn solubilizing bacteria on rice plants
Effect of Zn solubilizing strains inoculation on plant growth, yield and grain Zn concentration on two rice varieties basmati 385 and basmati super was examined in cleaned earthen pots (20 cm × 30 cm) under net house conditions. The pots were filled with 5 Kg sterilized clay loam soil. NPK fertilizer was applied at the rate of 40, 30, and 20 mg kg−1 of soil in the form of urea, single super phosphate and potassium sulfate respectively. Phosphorous (P) and K were applied in single dose before sowing the plants while N was applied in three split doses. The experiment was laid out in a completely randomized design (CRD) with three replications per treatment. Zinc sulfate (ZnSO4) at the rate of 7.5 mg kg −1 of soil was used as Zn in respective treatments. There were eight treatments viz. T1 = un-inoculated plants (Negative control), T2 = Zn (positive control), T3 = Zn solubilizing strains SH-10, T4 = Zn solubilizing strains SH-17, T5 = Consortium of Zn solubilizing strains SH-10 and SH-17, T6 = Zn solubilizing strains SH-10 + Zn, T7 = Zn solubilizing strains SH-17 + Zn, T8 = Consortium of Zn solubilizing strains + Zn.
Roots of 30 days old rice plants obtained from nursery were surface sterilized and transferred aseptically to the pots. The Zn solubilizing rhizobacteria were inoculated as soil drenching near the plant roots after 2 days of seedling transplant. The bacterial strains were grown in LB broth in a 250 mL Erlenmeyer flask on a shaking incubator at 100 rev min−1, 28 ± 2°C for overnight. The cells were pelleted by centrifugation and dissolved in 0.85% saline with a cell OD = 0.45 (~109 CFU mL−1). One mL of this cell suspension was applied near the root of each seedling. Sterile saline without bacteria was applied in negative control. The pots were kept in net house during the months of July to October (Natural season of crop). The plants were irrigated when needed until maturity. A second dose of bio inoculants was applied after 45 days of 1st inoculation. The plants were harvested during the month of October and observed for all agronomic traits like number of tillers, plant height, panicle length, thousand grain weight and yield except the leaf chlorophyll content which was measured at anthesis stage. Three leaves per plant were randomly selected and chlorophyll content of each leaf was measured by SPAD meter (Minolta, Tokyo, Japan) from different places (Ranganathan et al., 2006). Plant height was measured from ground level to the tip of panicle by using a measuring rod. The number of tillers were counted by uprooting each plant. The plants of each pot were threshed to separate grains and straw which were weighed separately. Average values of respective parameters were computed and expressed per pot.
Determination of grain Zn content and Zn translocation index (ZTI)
Plants of four treatments viz un-inoculated plants (T1), Zn (T2), consortium of strains (T5) and consortium of strains along with Zn (T8) were selected for determining the Zn content in shoot and grains. The Zn analysis was carried-out commercially by the Nuclear Institute for food and Agriculture (NIFA), Peshawar, KPK, Pakistan. Zn translocation index (ZTI) toward rice grains was calculated by using the formula (Rengel and Graham, 1996).
Statistical analysis
The data were subjected to analysis of variance using statistical package Genstat 9.2 (VSN International Ltd., Hemel Hempstead, Hertfordshire, UK, Abaid-Ullah et al., 2015). The differences among various treatment means were compared using the Fisher's protected least significant differences test (LSD) at probability level (P ≤ 0.05) (Steel and Torrie, 1980).
Results
Prevalence of Zn solubilizing bacteria in rice endosphere
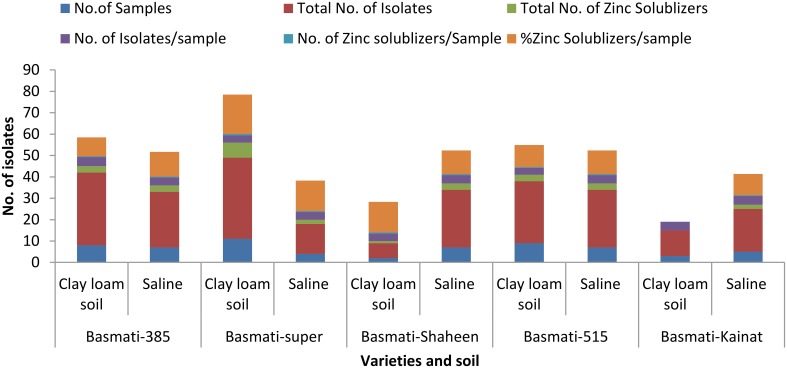
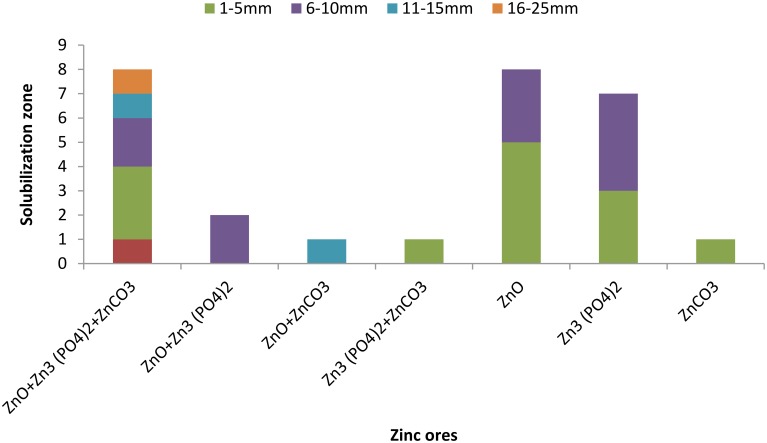
A total of 234 isolates were obtained from different rice varieties growing at varying locations. Number of isolates in rice endosphere were found to be variable i.e., 3–4 isolates per sample (Figure 1). Twenty seven isolates solubilized Zn either from one or more Zn ores with a solubilization zone of 1–24 mm (Figure 2, Table S1). Distribution of Zn solubilizers associated with rice endosphere was highly variable i.e., 0–18% per sample depending upon the variety and soil conditions (Figure 1). Two strains SH-10 and SH-17 showing maximum solubilizing zone on respective Zn ores were designated as potent Zn solubilizers and selected for further studies.
Figure 1.
Prevalence of zinc solubilizing bacteria in the endosphere of different rice varieties.
Figure 2.
Number of isolates capable to solubilize various zinc ores with different solubilization zone.
Morphological and plant growth promoting (PGP) traits of the potent Zn solubilizers
The Zn solubilizers were Gram positive rod. The strain SH-10 resisted all the antibiotics except levofloxacin (5 μg), tetracycline (30 μg), vanlomycine (30 μg), and minocycline (30 μg) while strain SH-17 showed resistance against all the antibiotics. The strains solubilized P and K with a solubilization zone of 38–46 mm and 47–55 mm respectively but did not produce IAA and HCN. They also inhibited the growth of P. oryzae and F. moniliforme by 22–30% and produced the antifungal metabolites protease, cellulase and glucanase. The strain SH-10 did not produce siderophores (Table 1).
Table 1.
Plant growth promoting traits of Zn solubilizing bacteria isolated from the rice varieties endosphere.
| Traits | Strains | |
|---|---|---|
| Nutrient solubilization* | Bacillus sp. SH-10 | Bacillus cereus SH-17 |
| Phosphorous | 38.0b | 46.0a |
| Potassium | 47.0b | 55.0a |
| ANTAGONISM** | ||
| P. oryzea | 23.7b | 30.0a |
| F. moniliforme | 22.0a | 22.3a |
| ANTIFUNGAL METABOLITES*** | ||
| Glucanase | 42.2a | 141.1a |
| Protease | 10.9b | 16.9a |
| Cellulase | 15.2b | 74.5a |
| Siderphore | 0.0b | 53.7a |
Values are mean of three replicates and bearing different letters in the same row are significantly different from each other according to the analysis of variance (p < 0.05).
The values are solubilization zones in mm.
The values are percent inhibition of fungal mycelium.
Effect of Zn solubilizers on yield and yield components of rice varieties
Basmati-385
Zn solubilizing strains significantly increased the yield and yield components of rice variety basmati-385 (Table 2). Maximum effect on yield (45.8 g/pot) and yield components i.e., plant height (97.1 cm), number of tillers (31.4/pot), panicle length (20.6 cm), chlorophyll content (34.5) was observed in co-inoculation of the Zn solubilizers along with the Zn followed by the Zn treatment (Table 2). The strain SH-17 and Zn resulted a 35.4 g grain yield/pot which was statistically at par with that of consortium of Zn solubilizers and Zn (45.8 g grain yield/pot) and only Zn treatment (34.5 g grain yield/pot).
Table 2.
Effect of zinc solubilizing bacteria on yield and yield components of rice variety basmati -385 under net house conditions.
| Treatments | Plant height (cm) | No. Tiller/Pot | Chlorophyll content | Panicle Length(cm) | Grain Yield/Pot (g) | Straw weight/Pot (g) | Biological Yield/pot (g) | Percent increase over control | |
|---|---|---|---|---|---|---|---|---|---|
| Grain yield | Biological yield | ||||||||
| Control | 52.7e | 18.7c | 13.7e | 7.4d | 23.3c | 48.9d | 72.2e | – | – |
| Zinc | 88.2b | 22.4bc | 30.7b | 18.2ab | 34.5b | 68.1bc | 102.6bc | 32.5 | 29.6 |
| Bacillus sp. SH-10 | 69.2c | 20.8bc | 20.2d | 12.3c | 32.8bc | 55.8d | 88.5d | 29.0 | 18.4 |
| B. cereus SH-17 | 61.1d | 19.4c | 19.1d | 8.7d | 30.0bc | 55.5d | 85.5de | 22.0 | 15.5 |
| Consortium of Bacillus sp. SH-10 and B. cereus SH-17 | 71.1c | 21.8bc | 24.7c | 17.2b | 33.2bc | 59.3cd | 92.6cd | 30.0 | 22.0 |
| Bacillus sp. SH-10 + Zinc | 92.5ab | 25.8b | 32.5ab | 20.4a | 32.0bc | 76.0ab | 108.0b | 27.2 | 33.1 |
| B. cereus SH-17 + Zinc | 92.1ab | 24.8b | 31.3b | 18.8ab | 35.4ab | 68.5bc | 103.9bc | 34.2 | 30.5 |
| Consortium of Bacillus sp. SH-10 and B. cereus SH-17 + Zinc | 97.1a | 31.4a | 34.5a | 20.6a | 45.8a | 83.9a | 129.7a | 49.1 | 44.3 |
| P- value | 43.5 | 6.3 | 80.9 | 27.7 | 3.1 | 11.8 | 16.9 | – | |
| F-value | < 0.001 | 0.002 | < 0.001 | < 0.001 | 0.034 | < 0.001 | < 0.001 | ||
Control, non-inoculated; Zinc, Zinc sulfate (ZnSO4) at the rate of 7.5 mg kg−1 of soil. Values are mean of three replicates and bearing different letters in the same column are significantly different from each other according to the analysis of variance (p < 0.05).
Super basmati
Effect of Zn solubilizing bacteria on the yield and yield components of rice variety super basmati was significant (Table 3). Highest grain yield/pot (38.6 g) and yield components i.e., plant height (100.9 cm), tillers/pot (33.8), chlorophyll content (34.9) and 1000 grain weight (23.2 g) were observed in super basmati plants treated with the consortium of strains and Zn followed by that of treated with strain SH-10 and Zn with grain yield/pot (32.3 g), plant height (92.3 cm), tillers/pot (30.6), chlorophyll content (32.9), and 1000 grain weight (21.4 g). Moreover, effect of treatments i.e., strain SH-10 and Zn, SH-17 and Zn and only Zn on basmati rice was statistically same but different from that of other treatments (Table 3).
Table 3.
Effect of zinc solubilizing bacteria on yield and yield components of rice variety super basmati under net house conditions.
| Treatments | Plant Height (cm) | No. Tiller/Pot | Chlorophyll Content | Panicle Length*(cm) | 1000 Grain Weight (g) | Grain Yield/pot (g) | Straw weight/pot (g) | Bio Yield/pot(g) | Percent increase over control | |
|---|---|---|---|---|---|---|---|---|---|---|
| Grain yield | Biological yield | |||||||||
| Control | 66.8e | 18.2d | 20.1e | 20.1 | 12.2f | 20.3d | 41.0f | 61.6f | – | – |
| Zinc | 79.1c | 28.5b | 31.7abc | 25.9 | 19.1c | 30.4b | 57.1c | 88.2c | 33.2 | 30.2 |
| Bacillus sp. SH-10 | 72.5de | 21.3d | 27.6cd | 22.4 | 15.5e | 24.8c | 46.2e | 71.7e | 18.1 | 14.1 |
| B. cereus SH-17 | 71.2de | 20.1d | 26.2d | 21.3 | 15.1e | 24.6c | 46.0e | 71.6e | 17.5 | 14.0 |
| Consortium of Bacillus sp. SH-10 and B. cereus SH-17 | 76.7cd | 25.2c | 30.0bcd | 24.7 | 17.4d | 26.4c | 52.2d | 79.3d | 23.1 | 22.3 |
| Bacillus sp. SH-10 + Zinc | 92.3b | 30.6ab | 32.9ab | 28.2 | 21.4b | 32.3b | 61.0b | 94.0b | 37.2 | 34.5 |
| B. cereus SH-17 + Zinc | 81.8c | 29.9b | 32.2ab | 26.5 | 20.2bc | 31.9b | 59.1bc | 91.3bc | 36.4 | 32.5 |
| Consortium of Bacillus sp. SH-10 and B. cereus SH-17 + Zinc | 100.9a | 33.8a | 34.9a | 29.7 | 23.2a | 38.6a | 66.1a | 105.4a | 47.4 | 41.6 |
| P-value | 32.6 | 27.7 | 10.8 | 1.8 | 71.1 | 64.3 | 70.9 | 145.6 | – | |
| F-value | < 0.001 | < 0.001 | < 0.001 | 0.172 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
Control, non-inoculated; Zinc, Zinc sulfate (ZnSO4) at the rate of 7.5mg kg−1 of soil. Values are mean of three replicates and bearing different letters in the same column are significantly different from each other according to the analysis of variance (p < 0.05).
Non-significant.
Zn translocation index of rice varieties
Effect of Zn solubilizers on the ZTI of both varieties was similar (Table 4). Highest ZTI was observed in the rice plants treated with consortium of strains (ZTI = 1.6–1.7) followed by the plants treated with only Zn (ZTI = 1.3–1.4) or consortium of strains and Zn (ZTI = 1.3–1.4). The lowest ZTI was observed in the un-inoculated plants (ZTI = 0.9–1.1). This clearly shows the role of Zn solubilizers in Zn translocation toward rice grains.
Table 4.
Effect of zinc solubilizing bacteria on zinc translocation index in rice varieties basmati-385 and super basmati.
| Treatment | Straw (mg/Kg) | Husk (mg/Kg) | Grains (mg/Kg) | Harvest index |
|---|---|---|---|---|
| BASMATI-385 | ||||
| Control | 19.0b | 22.7d | 17.7b | 0.9c |
| Zinc | 20.3b | 28.0c | 27.3a | 1.3ab |
| Consortium of Bacillus sp. SH-10 and B. cereus SH-17 | 19.0b | 34.3a | 30.7a | 1.6a |
| Consortium of Bacillus sp. SH-10 and B. cereus SH-17 + Zinc | 24.0a | 31.7b | 30.0a | 1.3bc |
| P-value | 0.011 | < 0.001 | 0.002 | 0.015 |
| SUPER BASMATI | ||||
| Control | 19.7b | 22.0b | 22.3b | 1.1c |
| Zinc | 23.0a | 31.0a | 31.7a | 1.4b |
| Consortium of Bacillus sp. SH-10 and B. cereus SH-17 | 18.7b | 30.0a | 32.3a | 1.7a |
| Consortium of Bacillus sp. SH-10 and B. cereus SH-17 + Zinc | 22.0a | 34.0a | 30.7a | 1.4b |
| P-value | 0.006 | 0.004 | < 0.001 | 0.002 |
Control, non-inoculated; Zinc, Zinc sulfate (ZnSO4) at the rate of 7.5 mg kg−1 of soil.
Values are mean of three replicates and bearing different letters in the same column are significantly different from each other according to the analysis of variance (p < 0.05).
Molecular identification of potent Zn solubilizers
The potent Zn solubilizers were identified as Bacillus sp. and Bacillus cereus on the basis of 16S rRNA gene analysis. The sequences of strains were submitted to NCBI Gene Bank database under accession numbers KT380823 and KT380824.
Discussion
PGPR inhabit wide range of crops growing under varying agricultural practices and commonly used as bio inoculants. In addition to their synergistic effect on plant's growth and yield, they have strong potential to enhance the Zn content of cereals (Sharma et al., 2013; Wang et al., 2014; Abaid-Ullah et al., 2015). Utilization of such PGPR to enhance (Zn) content of rice grains could be a promising strategy to minimize the Zn deficiency in human beings. Keeping in view the specific advantages of indigenous strains such as host adaptability and field efficacy, certain potent strains were screened in vitro and in vivo to enhance the growth, yield and Zn content of rice varieties.
Among the various potentially endophytic isolates associated with rice varieties, a significant difference in the number of Zn solubilizers was observed in different varieties and soil types. Maximum Zn solubilizers were enumerated from the endosphere of variety super basmati growing in clay loam soil while minimum strains were obtained from the endosphere of basmati kainat. However, in saline soil growing plants, prevalence of Zn solubilizers in the endosphere of all rice varieties was almost similar. The significant difference in the number of Zn solubilizers among different varieties and soil types may be due to the fact that plant microbe interaction is highly dependent on soil conditions and plant genotype (Schreiter et al., 2014; Sugiyama and Yazaki, 2014; Belimov et al., 2015). Variation in quantity and composition of microbes associated with the rhizosphere and endosphere of different plants, species and even varieties within same species have already been well documented (Beneduzi et al., 2013; Lagos et al., 2014; Ling et al., 2014). In a recent study, Hameed et al. (2015) has reported that the diversity of bacteria inhabiting rice endosphere and their distribution as well as PGP characteristics were dependent on multiple factors such as host's genotype, soil characteristics and nutrients. The PGPR strains have been screened from the rhizosphere of rice grown in different countries (de Souza et al., 2013) but less attention has been paid to explore the Zn solubilizers associated with rice, a crop which grows in flooded conditions and numerous factors affect the Zn availability in such conditions (Lefèvre et al., 2014; Abaid-Ullah et al., 2015).
In this study, a large number of potentially endophytic isolates were recovered from the rice endosphere but a very few strains depicted Zn solubilization potential. The strains capable to solubilize maximum Zn from respective ores were further tested for their morphological traits like Gram's reaction, cell shape and colony morphology. These traits are essential to recognize specific bacterial strain and tentative identification (Mohamad et al., 2014).
A potent strain exhibiting multiple PGPR traits must be able to resist extreme environmental conditions so that it may survive and maintain optimum population throughout the life cycle of specific crop. Competitive ability of strain to survive in environment is strongly correlated with its intrinsic antibiotic resistance. The Zn solubilizing strains resisted most of the important antibiotics which depicted their ability to tolerate the environmental stress. PGPR especially belonging to genus Bacillus sp. are able to survive in adverse environmental conditions due to their ability to form endospores and change the fatty acid patterns depending on variable colonizing niches (Checinska et al., 2015; Diomande et al., 2015). Thus, the Zn solubilizers screened in this study could maintain their population in rice rhizosphere throughout the crop cycle.
Phosphorous (P) and K are the macro nutrients required by rice and the other field crops for growth and optimum yield (Saleque et al., 2013; Wang et al., 2013; Damon et al., 2014; Zorb et al., 2014). Uptake of these macro (N, P, K) and micronutrients (Zn) by the plants from soil is mutually dependent (Bouain et al., 2014). They experience a complex processes in soil and exhibit dynamic equilibrium between insoluble and soluble forms under the influence of soil pH. This equilibrium could be affected by the acid secretion and other activities of soil microbiota, thereby enhancing their availability to plant roots for absorption (Saravanan et al., 2004). Zn application enhanced the uptake of macronutrients (N, P, K) in rice and influenced the biological properties of soil (Pooniya et al., 2012). These facts further advocate the worth of Zn solubilizers screened in this study as they also solubilize the mineral P and K.
Ability of rhizobacteria to solubilize Zn, K, and (PO4)−3 is dependent on secretion of acids such as gluconic acids, lactic acid, malic acid and oxalic acid etc (Estrada et al., 2013; Zhang and Kong, 2014; Abaid-Ullah et al., 2015). These three elements [Zn, K, and (PO4)−3] are released from their insoluble compounds by following the same basic mechanism of acidification and thus correlate the metabolism of each other.
A PGPR could be an ideal candidate for the development of bioformulation provided it possesses multiple characteristics for plant growth promotion. The Zn solubilizers suppress growth of economically important pathogens of rice P. oryzae and F. moniliforme and produce several secondary metabolites involved in antagonistic activity of the PGPR. These metabolites include siderophores which chelate iron and deprive the pathogen from an important source of nutrition, thereby inhibiting the pathogen by creating a competitive environment (Aznar and Dellagi, 2015). The strains also produce hydrolytic enzymes like glucanase, cellulase and protease which hydrolyze the various components of cell wall, thereby paralyzing the pathogen which ultimately leads to death of the pathogen (Nagpure et al., 2014). Thus, Zn solubilizing rhizobacteria exhibit multiple PGP traits in vitro which are similar to the earlier findings (El-Sayed et al., 2014; Abaid-Ullah et al., 2015). Suppression of pathogens by Zn solubilizers augment their potential as an effective bioinoculant because they can protect the plants from devastating diseases along with providing nutrition (Abaid-Ullah et al., 2015). In certain bacteria, the properties of Zn solubilization and pathogen suppression has been found to be interlinked. As reported by Saravanan et al. (2007), presence of solubilized Zn in the culture filtrate enhanced the antagonistic activity of Gluconacetobacter diazotrophicus. The siderophores produced by the antagonistic bacteria chelate iron and play major role in their antagonistic activity. On the other hand, Fe +3 oxidation under aerobic soil conditions limits the Zn availability (Gao, 2007). This fact advocates the production of siderophores as a mechanism adopted by PGPR for enhancing Zn availability.
In vivo evaluation of the strains exhibiting multiple plant growth promoting properties in laboratory conditions is necessary to develop an effective bio inoculum. Hence, the potent strains were inoculated on two rice varieties, basmati 385 and super basmati grown in net house under the natural growth conditions. The strains inoculated in consortium along with chemical Zn significantly enhanced the yield and related parameters in present research. Effect of PGPR on rice yield has already been reported (Estrada et al., 2013; Ji et al., 2014) but they synergize the effect of chemical Zn on rice basmati varieties, is being firstly reported. As the strains showed their potential to increase yield and yield related traits in vivo they may serve as useful bio inoculum.
In addition to increase yield, the Zn solubilizing rhizobacteria also enhanced Zn translocation to the rice grains in similar way as that of chemical Zn. These findings were similar to earlier studies where PGPRs translocated Zn toward rice grains (Tariq et al., 2007; Sharma et al., 2014; Vaid et al., 2014; Wang et al., 2014). The Zn translocation toward rice grains may depend upon the ability of rhizobacteria to enhance Zn availability by executing multiple mechanisms such as mineralization, solubilization and induction of physiological processes in rice involved in Zn uptake just like the induction of systemic resistance in rice against pathogens (Lucas et al., 2014). However, it needs to explore in future studies. There was no significant increase in Zn translocation toward rice grains when consortium of Zn solubilizers was inoculated along with the chemical Zn. This may be due to the inherent Zn uptake potential of the tested rice varieties, i.e., basmati 385 and super basmati. These findings suggest potential use of strains in rice bio fortification with Zn.
The potent Zn solubilizers capable to enhance growth, yield and grain Zn content of rice were identified as Bacillus sp. and Bacillus cereus. These findings are similar to that of earlier reports in which zinc solubilizers have been found to be recurrent among various bacterial taxa (He et al., 2011; Abaid-Ullah et al., 2015). Certain strains of B. cereus are opportunistic human pathogens but numerous strains isolated from plant rhizoplane exhibit PGPR traits and their potential as bio fertilizer is well documented (Niu et al., 2011; Chun Juan et al., 2012; Ramesh et al., 2014; Chowdhury et al., 2015). Thus, the Bacillus sp. capable to solubilize nutrients, produce siderophores and antagonize the pathogens by different mechanisms could be used as effective bio inoculants.
Conclusion
The Zn solubilizing bacteria associated with indigenous host enhance the growth, yield and Zn content by providing nutrition as well as disease protection. Such strains could be ideal candidate to develop bioformulation to improve not only rice yield but also produce Zn fortified rice grains. Moreover, these findings could help researchers to explore the mechanisms involved in PGPR mediated Zn translocation in cereals. The Zn solubilizing strains reported in this study would be made available upon request as per institutional material transfer agreement policy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Higher Education Commission (HEC), Pakistan for providing funds under NRPU grant no. 20-1982, National institute for food and agriculture (NIFA), Peshawar for Zn analysis of rice samples, Mr. Sajid (Lab attendant) for his help in growing rice plants and all the technical staff involved in maintaining the net/green house. We are highly thankful to Dr. Samina Nadeem (Professor, Department of humanities, CIIT Islamabad) and Dr. Saqib Mumtaz (Assistant Professor, Department of biosciences, CIIT Islamabad) for improving the English of manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2015.01286
References
- Abaid-Ullah M., Hassan M. N., Jamil M., Brader G., Shah M. K. N., Sessitsch A., et al. (2015). Plant growth promoting rhizobacteria: an alternate way to improve yield and quality of wheat (Triticum aestivum). Int. J. Agri. Biol. 17, 51–60. [Google Scholar]
- Abraham A., Narayanan S. P., Philip S., Nair D. G., Chandrasekharan A., Kochupurackal J. (2013). In silico characterization of a novel β-1, 3-glucanase gene from Bacillus amyloliquefaciens a bacterial endophyte of Hevea brasiliensis antagonistic to Phytophthora meadii. J. Mol. Model. 19, 999–1007. 10.1007/s00894-012-1645-3 [DOI] [PubMed] [Google Scholar]
- Aznar A., Dellagi A. (2015). New insights into the role of siderophores as triggers of plant immunity: what can we learn from animals? J. Exp. Bot. 66, 3001–3010. 10.1093/jxb/erv155 [DOI] [PubMed] [Google Scholar]
- Belimov A. A., Puhalsky I. V., Safronova V. I., Shaposhnikov A. I., Vishnyakova M. A., Semenova E. V., et al. (2015). Role of plant genotype and soil conditions in symbiotic plant-microbe interactions for adaptation of plants to cadmium-polluted soils. Water Air Soil Pollut. 226, 1–15. 10.1007/s11270-015-2537-9 [DOI] [Google Scholar]
- Beneduzi A., Moreira F., Costa P. B., Vargas L. K., Lisboa B. B., Favreto R., et al. (2013). Diversity and plant growth promoting evaluation abilities of bacteria isolated from sugarcane cultivated in the South of Brazil. Appl. Soil Ecol. 63, 94–104. 10.1016/j.apsoil.2012.08.010 [DOI] [Google Scholar]
- Bergottini V. M., Otegui M. B., Sosa D. A., Zapata P. D., Mulot M., Rebord M., et al. (2015). Bio-inoculation of yerba mate seedlings (Ilex paraguariensis St. Hill.) with native plant growth-promoting rhizobacteria: a sustainable alternative to improve crop yield. Biol. Fert. Soils 51, 749–755. 10.1007/s00374-015-1012-5 [DOI] [Google Scholar]
- Bouain N., Shahzad Z., Rouached A., Khan G. A., Berthomieu P., Abdelly C., et al. (2014). Phosphate and zinc transport and signalling in plants: toward a better understanding of their homeostasis interaction. J. Exp. Bot. 65, 5725–5741. 10.1093/jxb/eru314 [DOI] [PubMed] [Google Scholar]
- Bulgarelli D., Schlaeppi K., Spaepen S., Ver Loren van Themaat E., Schulze-Lefert P. (2013). Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 64, 807–838. 10.1146/annurev-arplant-050312-120106 [DOI] [PubMed] [Google Scholar]
- Bunt J., Rovira A. (1955). Microbiological studies of some subantarctic soils. J. Soil Sci. 6, 119–128. 10.1111/j.1365-2389.1955.tb00836.x [DOI] [Google Scholar]
- Checinska A., Paszczynski A., Burbank M. (2015). Bacillus and other spore-forming genera: variations in responses and mechanisms for survival. Annu. Rev. Food Sci. Technol. 6, 351–369. 10.1146/annurev-food-030713-092332 [DOI] [PubMed] [Google Scholar]
- Chowdhury P. S., Hartmann A., Gao X., Borriss R. (2015). Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42-a review. Front. Microbiol. 6:780. 10.3389/fmicb.2015.00780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun Juan W., YaHui G., Chao W., HongXia L., DongDong N., YunPeng W., et al. (2012). Enhancement of tomato (Lycopersicon esculentum) tolerance to drought stress by plant-growth-promoting rhizobacterium (PGPR) Bacillus cereus AR156. J. of Agr. Biotechnol. 20, 1097–1105. [Google Scholar]
- Damon P. M., Bowden B., Rose T., Rengel Z. (2014). Crop residue contributions to phosphorus pools in agricultural soils: a review. Soil Biol. Biochem. 74, 127–137. 10.1016/j.soilbio.2014.03.003 [DOI] [Google Scholar]
- de Souza R., Beneduzi A., Ambrosini A., Da Costa P. B., Meyer J., Vargas L. K., et al. (2013). The effect of plant growth promoting rhizobacteria on the growth of rice (Oryza sativa L.) cropped in southern Brazilian fields. Plant Soil 366, 585–603. 10.1007/s11104-012-1430-1 [DOI] [Google Scholar]
- Diomande S. E., Nguyen-The C., Guinebretiere M. H., Broussolle V., Brillard J. (2015). Role of fatty acids in Bacillus environmental adaptation. Front. Microbiol. 6:813. 10.3389/fmicb.2015.00813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed W. S., Akhkha A., El-Naggar M. Y., Elbadry M. (2014). In vitro antagonistic activity, plant growth promoting traits and phylogenetic affiliation of rhizobacteria associated with wild plants grown in arid soil. Front. Microbiol. 5:651. 10.3389/fmicb.2014.00651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada G. A., Baldani V. L. D., de Oliveira D. M., Urquiaga S., Baldani J. I. (2013). Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant Soil 369, 115–129. 10.1007/s11104-012-1550-7 [DOI] [Google Scholar]
- Farag M. A., Zhang H., Ryu C. M. (2013). Dynamic chemical communication between plants and bacteria through airborne signals: induced resistance by bacterial volatiles. J. Chem. Ecol. 39, 1007–1018. 10.1007/s10886-013-0317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X. (2007). Bioavailability of Zinc to Aerobic Rice. Ph.D. dissertation, Wageningen University and Research Centre. [Google Scholar]
- Habibi S., Djedidi S., Prongjunthuek K., Mortuza M. F., Ohkama-Ohtsu N., Sekimoto H., et al. (2014). Physiological and genetic characterization of rice nitrogen fixer PGPR isolated from rhizosphere soils of different crops. Plant Soil 379, 51–66. 10.1007/s11104-014-2035-7 [DOI] [Google Scholar]
- Hameed A., Yeh M. W., Hsieh Y. T., Chung W. C., Lo C. T., Young L. S. (2015). Diversity and functional characterization of bacterial endophytes dwelling in various rice (Oryza sativa L.) tissues, and their seed borne dissemination into rhizosphere under gnotobiotic P- stress. Plant Soil 394, 177–197. 10.1007/s11104-015-2506-5 [DOI] [Google Scholar]
- Hassan M. N., Afghan S., Hafeez F. Y. (2010). Suppression of red rot caused by Colletotrichum falcatum on sugarcane plants using plant growth promoting rhizobacteria. Biocontrol. 55, 531–542. 10.1007/s10526-010-9268-z [DOI] [Google Scholar]
- Hassan M. N., Afghan S., Hafeez F. Y. (2011). Biological control of red rot in sugarcane by native pyoluteorin producing Pseudomonas putida strain NH-50 under field conditions and its potential mode of actions. Pest Manage. Sci. 67, 1147–1154. 10.1002/ps.2165 [DOI] [PubMed] [Google Scholar]
- He Z. L., Van Nostrand J. D., Deng Y., Zhou J. Z. (2011). Development and applications of functional gene microarrays in the analysis of the functional diversity, composition, and structure of microbial communities. Front. Environ. Sci. Engin. China 5, 1–20. 10.1007/s11783-011-0301-y [DOI] [Google Scholar]
- Holler S., Meyer A., Frei M. (2014). Zinc deficiency differentially affects redox homeostasis of rice genotypes contrasting in ascorbate level. J. Plant Physiol. 171, 1748–1756. 10.1016/j.jplph.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Ji S. H., Gururani M. A., Chun S. C. (2014). Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol. Res. 169, 83–98. 10.1016/j.micres.2013.06.003 [DOI] [PubMed] [Google Scholar]
- Kumar P., Dubey R. C., Maheshwari D. K. (2012). Bacillus strains isolated from rhizosphere showed plant growth promoting and antagonistic activity against phytopathogens. Microbiol. Res. 167, 493–499. 10.1016/j.micres.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Lagos L. M., Navarrete O. U., Maruyama F., Crowley D. E., Cid F. P., Mora M. L., et al. (2014). Bacterial community structures in rhizosphere microsites of ryegrass (Lolium perenne var. Nui) as revealed by pyrosequencing. Biol. Fert. Soils 50, 1253–1266. 10.1007/s00374-014-0939-2 [DOI] [Google Scholar]
- Lefèvre I., Vogel-Mikuš K., Jeromel L., Vavpetič P., Planchon S., Arčon I., et al. (2014). Differential cadmium and zinc distribution in relation to their physiological impact in the leaves of the accumulating Zygophyllum fabago L. Plant Cell Environ. 37, 1299–1320. 10.1111/pce.12234 [DOI] [PubMed] [Google Scholar]
- Ling N., Deng K., Song Y., Wu Y., Zhao J., Raza W., et al. (2014). Variation of rhizosphere bacterial community in watermelon continuous mono-cropping soil by long-term application of a novel bioorganic fertilizer. Microbiol. Res. 169, 570–578. 10.1016/j.micres.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Lockhart K. M., King A. M., Harter T. (2013). Identifying sources of groundwater nitrate contamination in a large alluvial groundwater basin with highly diversified intensive agricultural production. J. Contam. Hydrol. 151, 140–154. 10.1016/j.jconhyd.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Lucas J. A., García-Cristobal J., Bonilla A., Ramos B., Gutierrez-Manero J. (2014). Beneficial rhizobacteria from rice rhizosphere confers high protection against biotic and abiotic stress inducing systemic resistance in rice seedlings. Plant Physiol. Biochem. 82, 44–53. 10.1016/j.plaphy.2014.05.007 [DOI] [PubMed] [Google Scholar]
- Lucy M., Reed E., Glick B. R. (2004). Applications of free living plant growth-promoting rhizobacteria. Anton van Leeuwenhoek 86, 1–25. 10.1023/B:ANTO.0000024903.10757.6e [DOI] [PubMed] [Google Scholar]
- Ma Y., Rajkumar M., Luo Y., Freitas H. (2011). Inoculation of endophytic bacteria on host and non-host plants-effects on plant growth and Ni uptake. J. Hazard. Mater. 195, 230–237. 10.1016/j.jhazmat.2011.08.034 [DOI] [PubMed] [Google Scholar]
- Majeed A., Abbasi M. K., Hameed S., Imran A., Rahim N. (2015). Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front. Microbiol. 6:198. 10.3389/fmicb.2015.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., Walia A., Kulshrestha S., Chauhan A., Shirkot C. K. (2015). Efficiency of plant growth-promoting P-solubilizing Bacillus circulans CB7 for enhancement of tomato growth under net house conditions. J. Basic Microbiol. 55, 33–44. 10.1002/jobm.201300562 [DOI] [PubMed] [Google Scholar]
- Miller R. L., Higgins V. J. (1970). Association of cyanide with infection of Birdsfoot Trefoil by Stemphylium loti. Phytopathol. 60, 104–110. 10.1094/Phyto-60-104 [DOI] [Google Scholar]
- Mohamad N. A., Jusoh N. A., Htike Z. Z., Win S. L. (2014). Bacteria identification from microscopic morphology: a survey. Int. J. Soft Comp. Art. Intellig Appl. 3, 2319–1015. 10.5121/ijscai.2014.3201 [DOI] [Google Scholar]
- Nagpure A., Choudhary B., Gupta R. K. (2014). Mycolytic enzymes produced by Streptomyces violaceusniger and their role in antagonism towards wood-rotting fungi. J. Basic Microbiol. 54, 397–407. 10.1002/jobm.201200474 [DOI] [PubMed] [Google Scholar]
- Niu D. D., Liu H. X., Jiang C. H., Wang Y. P., Wang Q. Y., Jin H. L., et al. (2011). The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate-and jasmonate/ethylene-dependent signaling pathways. Mol. Plant Microbe Interact. 24, 533–542. 10.1094/MPMI-09-10-0213 [DOI] [PubMed] [Google Scholar]
- Patel R. R., Thakkar V. R., Subramanian B. R. (2015). A Pseudomonas guariconensis strain capable of promoting growth and controlling collar rot disease in Arachis hypogaea L. Plant Soil 390, 369–381. 10.1007/s11104-015-2436-2 [DOI] [Google Scholar]
- Pereg L., McMillan M. (2015). Scoping the potential uses of beneficial microorganisms for increasing productivity in cotton cropping systems. Soil Biol. Biochem. 80, 349–358. 10.1016/j.soilbio.2014.10.020 [DOI] [Google Scholar]
- Pii Y., Mimmo T., Tomasi N., Terzano R., Cesco S., Crecchio C. (2015). Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fert. Soils 51, 403–415. 10.1007/s00374-015-0996-1 [DOI] [Google Scholar]
- Pikovskaya R. I. (1948). Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiol. 17, 362–370. [Google Scholar]
- Piromyou P., Noisangiam R., Uchiyama H., Tittabutr P., Boonkerd N., Teaumroong N. (2013). Indigenous microbial community structure in rhizosphere of Chinese kale as affected by plant growth-promoting rhizobacteria inoculation. Pedosphere 23, 577–592. 10.1016/S1002-0160(13)60051-X [DOI] [Google Scholar]
- Pooniya V., Shivay Y. S., Rana A., Nain L., Prasanna R. (2012). Enhancing soil nutrient dynamics and productivity of basmati rice through residue incorporation and zinc fertilization. Eur. J. Agron. 41, 28–37. 10.1016/j.eja.2012.03.004 [DOI] [Google Scholar]
- Ramesh A., Sharma S. K., Sharma M. P., Yadav N., Joshi O. P. (2014). Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in vertisols of central India. Appl. Soil Ecol. 73, 87–96. 10.1016/j.apsoil.2013.08.009 [DOI] [Google Scholar]
- Rana A., Kabi S. R., Verma S., Adak A., Pal M., Shivay Y. S., et al. (2015). Prospecting plant growth promoting bacteria and cyanobacteria as options for enrichment of macro and micronutrients in grains in rice wheat cropping sequence. Cog. Food Agric. 1, 10373–10379. 10.1080/23311932.2015.1037379 [DOI] [Google Scholar]
- Ranganathan S., Suvarchala V., Rajesh Y. B. R. D., Prasad M. S., Padmakumari A. P., Voleti S. R. (2006). Effects of silicon sources on its deposition, chlorophyll content, and disease and pest resistance in rice. Biol. Plant. 50, 713–716. 10.1007/s10535-006-0113-2 [DOI] [Google Scholar]
- Rengel Z., Graham R. D. (1996). Uptake of zinc from chelate buffered nutrient solutions by wheat genotypes differing in Zn efficiency. J. Exp. Bot. 47, 217–226. 10.1093/jxb/47.2.217 [DOI] [Google Scholar]
- Saleque M. A., Uddin M. K., Ferdous A. K. M., Rashid M. H. (2013). Potassium constrained high yields in irrigated rice. J. Plant Nutr. 36, 1829–1840. 10.1080/01904167.2013.815767 [DOI] [Google Scholar]
- Saravanan V. S., Kalaiarasan P., Madhaiyan M., Thangaraju M. (2007). Solubilization of insoluble zinc compounds by Gluconacetobacter diazotrophicus and the detrimental action of zinc ion (Zn2+) and zinc chelates on root knot nematode Meloidogyne incognita. Lett. Appl. Microbiol. 44, 235–241. 10.1111/j.1472-765X.2006.02079.x [DOI] [PubMed] [Google Scholar]
- Saravanan V. S., Subramoniam S. R., Raj S. A. (2004). Assessing in vitro solubilization potential of different zinc solubilizing bacterial (zsb) isolates. Braz. J. Microbiol. 35, 121–125. 10.1590/S1517-83822004000100020 [DOI] [Google Scholar]
- Schreiter S., Sandmann M., Smalla K., Grosch R. (2014). Soil type dependent rhizosphere competence and biocontrol of two bacterial inoculant strains and their effects on the rhizosphere microbial community of field-grown lettuce. PLoS ONE 9:e103726. 10.1371/journal.pone.0103726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. (1987). Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160, 47–56. 10.1016/0003-2697(87)90612-9 [DOI] [PubMed] [Google Scholar]
- Sharma A., Shankhdhar D., Shankhdhar S. C. (2013). Enhancing grain iron content of rice by the application of plant growth promoting rhizobacteria. Plant Soil Environ. 59, 89–94. [Google Scholar]
- Sharma A., Shankhdhar D., Shankhdhar S. C. (2014). Growth promotion of the rice genotypes by PGPRs isolated from rice rhizosphere. J. Soil Sci. Plant. Nutr. 14, 505–517. 10.4067/s0718-95162014005000040 [DOI] [Google Scholar]
- Shen Z., Ruan Y., Chao X., Zhang J., Li R., Shen Q. (2015). Rhizosphere microbial community manipulated by 2 years of consecutive biofertilizer application associated with banana Fusarium wilt disease suppression. Biol. Fert. Soils 51, 553–562. 10.1007/s00374-015-1002-7 [DOI] [Google Scholar]
- Shrivastava U. P., Kumar A. (2011). A simple and rapid plate assay for the screening of Indole-3-acetic Acid (IAA) producing microorganisms. Int. J. Appl. Biol. Pharm. Technol. 2, 120–123. [Google Scholar]
- Sirohi G., Upadhyay A., Srivastava P. S., Srivastava S. (2015). PGPR mediated Zinc biofertilization of soil and its impact on growth and productivity of wheat. J. Soil Sci. Plant Nutr. 15, 202–216. 10.4067/s0718-95162015005000017 [DOI] [Google Scholar]
- Spence C., Alff E., Johnson C., Ramos C., Donofrio N., Sundaresan V., et al. (2014). Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Boil. 14:130. 10.1186/1471-2229-14-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel R. G., Torrie J. H. (1980). Principles and Procedures of Statistics. New York, NY: McGraw. [Google Scholar]
- Sugiyama A., Yazaki K. (2014). Flavonoids in plant rhizospheres: secretion, fate and their effects on biological communication. Plant Biotech. 31, 431–443. 10.5511/plantbiotechnology.14.0917a [DOI] [Google Scholar]
- Surette M. A., Sturz A. V., Lada R. R., Nowak J. (2003). Bacterial endophytes in processing carrots (Daucus carota L. var. sativus): their localization, population density, biodiversity and their effects on plant growth. Plant Soil 253, 381–390. 10.1023/A:1024835208421 [DOI] [Google Scholar]
- Tan Z. Y., Xiao-Dong X., En-Tao W., Jun-Lian G., Esperanza M., Wen-Xin C. (1997). Phylogenetic and genetic relationships Mesorhizobium tianshanense and related rhizobia. Int. J. Syst. Bacteriol. 47, 874–879. 10.1099/00207713-47-3-874 [DOI] [PubMed] [Google Scholar]
- Tariq M., Hameed S., Malik K. A., Hafeez F. Y. (2007). Plant root associated bacteria for zinc mobilization in rice. Pak. J. Bot. 39, 245–253. [Google Scholar]
- Timm C. M., Campbell A. G., Utturkar S. M., Jun S. R., Parales R. E., Tan W. A., et al. (2015). Metabolic functions of Pseudomonas fluorescens strains from Populus deltoides depend on rhizosphere or endosphere isolation compartment. Front. Microbiol. 6:1118. 10.3389/fmicb.2015.01118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaid S. K., Kumar B., Sharma A., Shukla A. K., Srivastava P. C. (2014). Effect of zn solubilizing bacteria on growth promotion and zn nutrition of rice. J. Soil Sci. Plant Nutr. 14, 889–910. 10.4067/s0718-95162014005000071 [DOI] [Google Scholar]
- Vincent J. M. (1970). A Manual for the Practical Study of Root-nodule Bacteria: IBP Handbook. Oxford: Blackwell; Scientific, 15. [Google Scholar]
- Wang M., Zheng Q., Shen Q., Guo S. (2013). The critical role of potassium in plant stress response. Int. J. Mol. Sci. 14, 7370–7390. 10.3390/ijms14047370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yang X., Zhang X., Dong L., Zhang J., Wei Y., et al. (2014). Improved plant growth and Zn accumulation in grains of rice (Oryza sativa L.) by inoculation of endophytic microbes isolated from a Zn hyperaccumulator, Sedum alfredii H. J. Agric. Food Chem. 62, 1783–1791. 10.1021/jf404152u [DOI] [PubMed] [Google Scholar]
- Wilson K. (1987). Preparation of genomic DNA from bacteria. Curr. Protoc. Mol. Biol. Chapter 2: Unit 2.4. 10.1002/0471142727.mb0204s56 [DOI] [PubMed]
- Wu W., Ma B. (2015). Integrated nutrient management (INM) for sustaining crop productivity and reducing environmental impact: a review. Sci. Total Environ. 512, 415–427. 10.1016/j.scitotenv.2014.12.101 [DOI] [PubMed] [Google Scholar]
- Zahid M., Abbasi M. K., Hameed S., Rahim N. (2015). Isolation and identification of indigenous plant growth promoting rhizobacteria from Himalayan region of Kashmir and their effect on improving growth and nutrient contents of maize (Zea mays L.). Front. Microbiol. 6:207. 10.3389/fmicb.2015.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Kong F. (2014). Isolation and identification of potassium solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl. Soil Ecol. 82, 18–25. 10.1016/j.apsoil.2014.05.002 [DOI] [Google Scholar]
- Zorb C., Senbayram M., Peiter E. (2014). Potassium in agriculture status and perspectives. J. Plant Physiol. 171, 656–669. 10.1016/j.jplph.2013.08.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.