Abstract
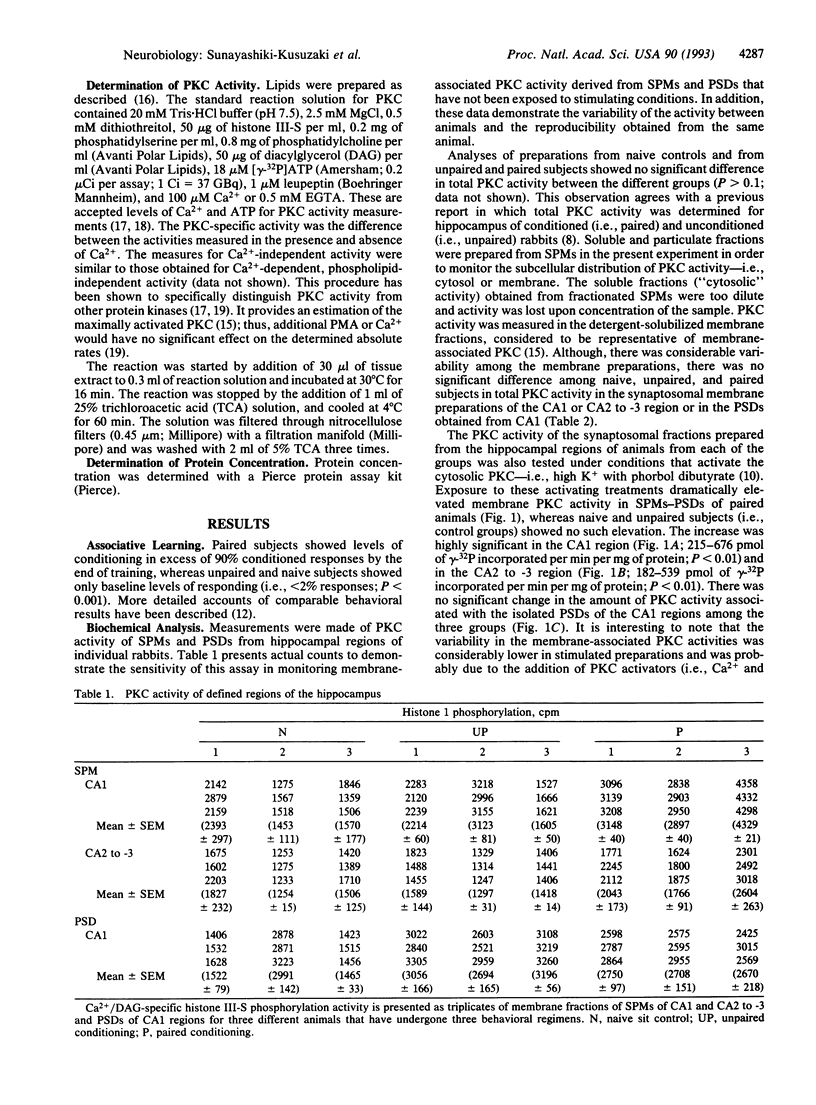
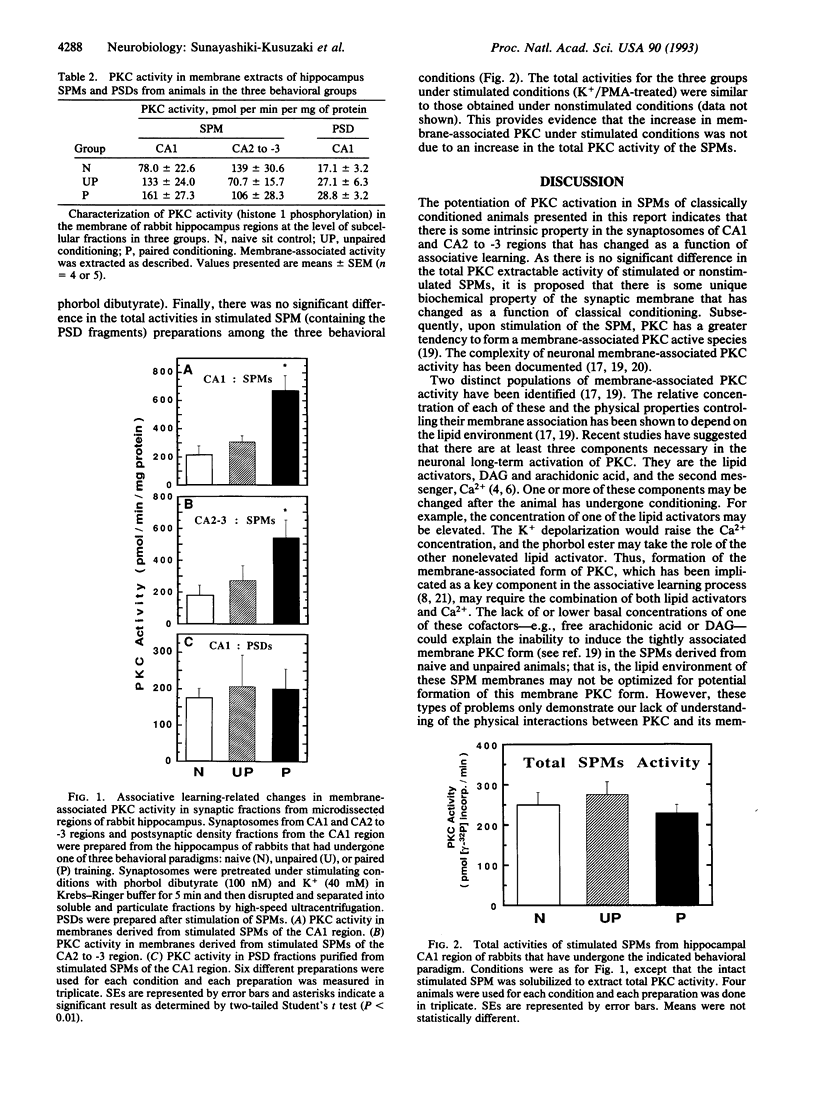
Using electrophysiological, biochemical, and autoradiographic techniques, changes in protein kinase C (PKC) activity in specific regions of the hippocampus have been previously implicated in classical conditioning of the nictitating membrane response of the rabbit. Here we report that activation of PKC is potentiated 2- to 3-fold in synaptosomes of the hippocampal CA1 and CA2 to -3 regions in rabbits that have undergone classical conditioning of the nictitating membrane response. This potentiation is apparently due to a change in the biochemical properties of PKC within the synaptosomes and is not a result of an increase in total PKC activity. This observation correlates a subcellular biochemical change with classical conditioning of a mammal.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L., Kubota M., Neary J. T., Naito S., Coulter D., Rasmussen H. C-kinase activation prolongs Ca2+-dependent inactivation of K+ currents. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1245–1253. doi: 10.1016/0006-291x(86)90384-0. [DOI] [PubMed] [Google Scholar]
- Alkon D. L., Naito S., Kubota M., Chen C., Bank B., Smallwood J., Gallant P., Rasmussen H. Regulation of Hermissenda K+ channels by cytoplasmic and membrane-associated C-kinase. J Neurochem. 1988 Sep;51(3):903–917. doi: 10.1111/j.1471-4159.1988.tb01827.x. [DOI] [PubMed] [Google Scholar]
- Alkon D. L., Rasmussen H. A spatial-temporal model of cell activation. Science. 1988 Feb 26;239(4843):998–1005. doi: 10.1126/science.2830669. [DOI] [PubMed] [Google Scholar]
- Bank B., DeWeer A., Kuzirian A. M., Rasmussen H., Alkon D. L. Classical conditioning induces long-term translocation of protein kinase C in rabbit hippocampal CA1 cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1988–1992. doi: 10.1073/pnas.85.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D. A role for membrane-inserted protein kinase C in cellular memory? Trends Biochem Sci. 1989 Mar;14(3):87–88. doi: 10.1016/0968-0004(89)90126-6. [DOI] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Cohen R. S., Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980 Sep;86(3):831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker L. V., De Graan P. N., Gispen W. H. Transmitter release: target of regulation by protein kinase C? Prog Brain Res. 1991;89:209–233. doi: 10.1016/s0079-6123(08)61724-0. [DOI] [PubMed] [Google Scholar]
- Dunkley P. R., Heath J. W., Harrison S. M., Jarvie P. E., Glenfield P. J., Rostas J. A. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: homogeneity and morphology of subcellular fractions. Brain Res. 1988 Feb 16;441(1-2):59–71. doi: 10.1016/0006-8993(88)91383-2. [DOI] [PubMed] [Google Scholar]
- Kitano T., Go M., Kikkawa U., Nishizuka Y. Assay and purification of protein kinase C. Methods Enzymol. 1986;124:349–352. doi: 10.1016/0076-6879(86)24026-4. [DOI] [PubMed] [Google Scholar]
- Lester D. S., Alkon D. L. Activation of protein kinase C phosphorylation pathways: a role for storage of associative memory. Prog Brain Res. 1991;89:235–248. doi: 10.1016/s0079-6123(08)61725-2. [DOI] [PubMed] [Google Scholar]
- Lester D. S., Collin C., Etcheberrigaray R., Alkon D. L. Arachidonic acid and diacylglycerol act synergistically to activate protein kinase C in vitro and in vivo. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1522–1528. doi: 10.1016/0006-291x(91)91745-x. [DOI] [PubMed] [Google Scholar]
- Lester D. S. High-pressure extraction of membrane-associated protein kinase C from rat brain. J Neurochem. 1989 Jun;52(6):1950–1953. doi: 10.1111/j.1471-4159.1989.tb07284.x. [DOI] [PubMed] [Google Scholar]
- Lester D. S. In vitro linoleic acid activation of protein kinase C. Biochim Biophys Acta. 1990 Sep 24;1054(3):297–303. doi: 10.1016/0167-4889(90)90100-r. [DOI] [PubMed] [Google Scholar]
- LoTurco J. L., Coulter D. A., Alkon D. L. Enhancement of synaptic potentials in rabbit CA1 pyramidal neurons following classical conditioning. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1672–1676. doi: 10.1073/pnas.85.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T., Shearman M. S., Nishizuka Y. Synaptosomal protein kinase C subspecies: B. Down-regulation promoted by phorbol ester and its effect on evoked norepinephrine release. J Neurochem. 1991 Apr;56(4):1263–1269. doi: 10.1111/j.1471-4159.1991.tb11420.x. [DOI] [PubMed] [Google Scholar]
- Olds J. L., Anderson M. L., McPhie D. L., Staten L. D., Alkon D. L. Imaging of memory-specific changes in the distribution of protein kinase C in the hippocampus. Science. 1989 Aug 25;245(4920):866–869. doi: 10.1126/science.2772638. [DOI] [PubMed] [Google Scholar]
- Orr N., Yavin E., Lester D. S. Identification of two distinct populations of protein kinase C in rat brain membranes. J Neurochem. 1992 Feb;58(2):461–470. doi: 10.1111/j.1471-4159.1992.tb09744.x. [DOI] [PubMed] [Google Scholar]
- Orr N., Yavin E., Shinitzky M., Lester D. S. Application of high-pressure to subfractionate membrane protein-lipid complexes: a case study of protein kinase C. Anal Biochem. 1990 Nov 15;191(1):80–85. doi: 10.1016/0003-2697(90)90391-l. [DOI] [PubMed] [Google Scholar]
- Schreurs B. G., Sanchez-Andres J. V., Alkon D. L. Learning-specific differences in Purkinje-cell dendrites of lobule HVI (Lobulus simplex): intracellular recording in a rabbit cerebellar slice. Brain Res. 1991 May 10;548(1-2):18–22. doi: 10.1016/0006-8993(91)91100-f. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Siekevitz P. Properties of a protein kinase C activity in synaptic plasma membrane and postsynaptic density fractions isolated from canine cerebral cortex. J Neurochem. 1989 Dec;53(6):1751–1762. doi: 10.1111/j.1471-4159.1989.tb09240.x. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R., Lester D. S. The mechanism of activation of protein kinase C: a biophysical perspective. Biochim Biophys Acta. 1992 Apr 7;1134(3):261–272. doi: 10.1016/0167-4889(92)90185-e. [DOI] [PubMed] [Google Scholar]


