Summary
The recent development of 3D-liver stem cell cultures (hepatic organoids) opens up new avenues for gene and/or stem cell therapy to treat liver disease. To test safety and efficacy, a relevant large animal model is essential but not yet established. Because of its shared pathologies and disease pathways, the dog is considered the best model for human liver disease. Here we report the establishment of a long-term canine hepatic organoid culture allowing undifferentiated expansion of progenitor cells that can be differentiated toward functional hepatocytes. We show that cultures can be initiated from fresh and frozen liver tissues using Tru-Cut or fine-needle biopsies. The use of Wnt agonists proved important for canine organoid proliferation and inhibition of differentiation. Finally, we demonstrate that successful gene supplementation in hepatic organoids of COMMD1-deficient dogs restores function and can be an effective means to cure copper storage disease.
Highlights
-
•
Canine hepatic organoids can be grown long term with chromosomal stability
-
•
Organoids can be derived from liver tissues as small as fine-needle biopsies
-
•
Differentiation and proliferation of canine organoids involve Wnt and Notch
-
•
Successful gene supplementation proves potential for stem cell-based gene therapy
Schotanus and colleagues show that the development of canine hepatic organoids ensures successful use of valuable patient material and demonstrate the use of this cell system for in vitro disease modeling and gene supplementation for gene therapy. This is a step forward for stem cell therapy in liver diseases in human, because numerous (inherited) diseases present in similar molecular biological ways in men and dogs
Introduction
The mammalian liver has a strong regenerative capacity through cell division of mature hepatocytes and cholangiocytes. This is best demonstrated by the response of the liver to partial hepatectomy or acute injury. However, during chronic and severe injury, proliferation of mature cells is impaired. In this situation, hepatic progenitor cells (HPCs) become activated and may contribute to liver regeneration. Despite this remarkable regenerative capacity, treatment for liver failure or genetic liver diseases remains necessary, and the only effective therapeutic approach is orthotopic liver transplantation (Boulter et al., 2013, Duncan et al., 2009, Russo and Parola, 2012). Although whole organ transplantation is often successful, the procedure is highly invasive and faces the problem of liver donor shortage and the risk of graft rejection (Dalgetty et al., 2009). Transplantation of mature hepatocytes has been reported to improve liver function in patients with metabolic disorders and acute liver failure. One major impediment to using adult hepatocytes is their limited supply, because long-term hepatocyte culture with maintenance of their viability and functions has remained difficult (McCarty et al., 2014, Vanhaecke and Rogiers, 2006). The use of cryopreserved hepatocytes showed some clinical success, giving potential for cell banking (Dhawan et al., 2010, Hughes et al., 2012). Bi-potential HPCs that can differentiate into hepatocytes and cholangiocytes have been suggested to be a good alternative source for cell therapy (Dalgetty et al., 2009). The advantages of using HPCs or HPC-derived hepatocytes over mature hepatocytes are that HPCs can be expanded ex vivo and can be cryopreserved, thereby potentially ensuring unlimited cell sources for clinical application. In addition, when using adult stem cells, autologous transplantation may be possible and may abolish the need for lifelong and expensive immunosuppressive drugs (Russo and Parola, 2012). This could be combined with gene correction before autologous transplantation into a patient for the treatment of a genetic disorder. Many attempts have been made to isolate and develop adult liver stem cell culture systems (Guo et al., 2012, He and Feng, 2011, Ichinohe et al., 2013, Kamiya and Inagaki, 2015). However, a two-dimensional culture format does not accurately resemble the three-dimensional (3D) in vivo morphology, and most cell systems are genetically stable for a maximum of 3 months (Scheers et al., 2012). Recently, Huch et al., 2013, Huch et al., 2015 have succeeded in deriving long-term stable liver stem cell cultures in 3D, called hepatic organoids from mouse and human liver tissues. These cultures could be clonally expanded from damage-induced Lgr5+ stem cells or progenitors of mice, which generated de novo hepatocytes and ductal cells during injury in vivo. Both mouse and human organoids differentiated into hepatocytes upon engraftment in injured mouse livers (Huch et al., 2013, Huch et al., 2015). Finally, the in-depth proof of genetic stability of the human hepatic organoids (Huch et al., 2015) lends further credence to this culture system as a logical source of cells for therapeutic repopulation strategies.
To take the development and evaluation of new stem cell-based treatment strategies forward, it is important to use a relevant animal model to provide information on safety and efficacy of treatments. The treatment of pet dogs was reported to be informative in this perspective. Several successful preclinical experiments in rodents have been recapitulated in dogs to better mimic and bridge to human trials (Kamimura et al., 2014, Mata et al., 2014, Roberts et al., 2014). Furthermore, pathologies and their underlying pathways of disease, including the stem cell response, are similar in the liver of men and dogs (Kruitwagen et al., 2014, Schotanus et al., 2009, Spee et al., 2010). The development of a canine hepatic organoid culture system is an essential step to use dogs with liver diseases for the validation of stem cell therapies. Here we describe the establishment of long-term canine hepatic organoids, provide methods of isolation and cryopreservation that ensure successful use of valuable patient material, and show the use of this cell system for in vitro disease modeling and gene supplementation for gene therapy.
Results
Generation of Canine Hepatic Organoids
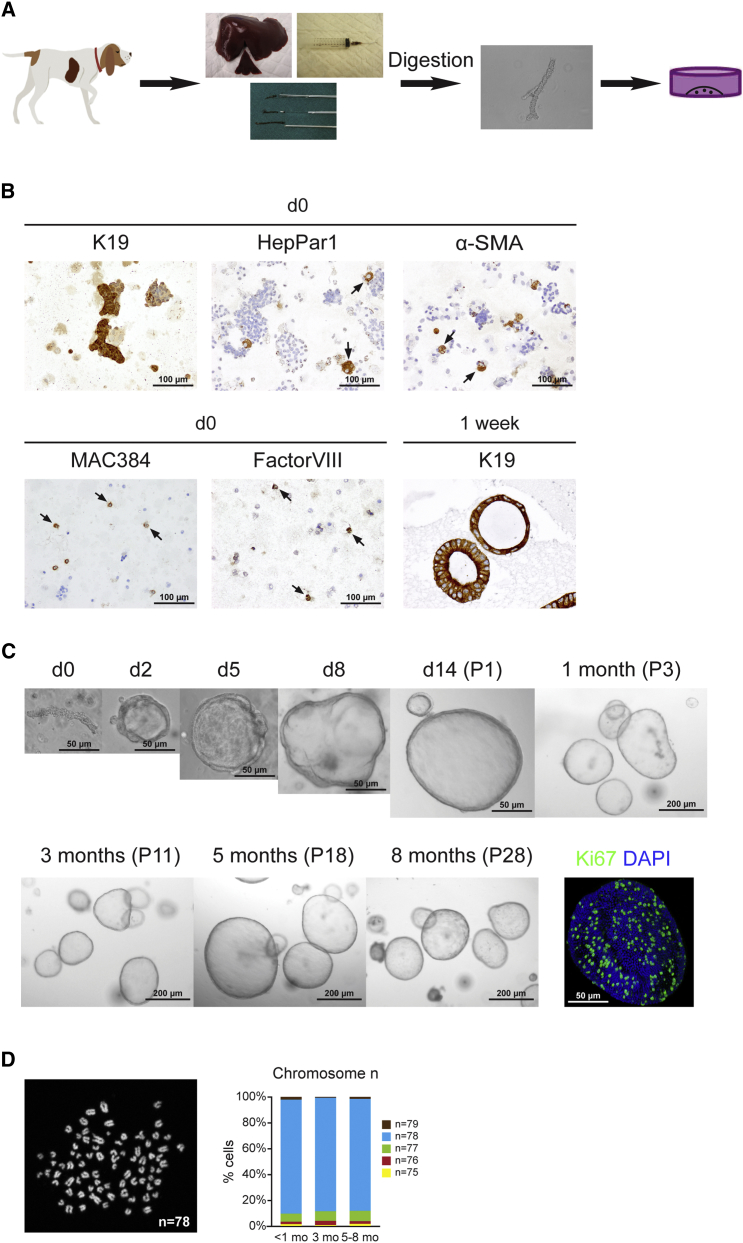
HPCs have been reported to reside in the biliary tree (Boulter et al., 2013, Cardinale et al., 2011, Duncan et al., 2009). Previously, adult murine and human HPCs were expanded from the ductal compartment under organoid culture conditions (Huch et al., 2013, Huch et al., 2015). To establish canine HPCs cultures, we maintained biliary duct fragments isolated from adult normal dog livers in 3D culture by suspension in Matrigel droplets and R-spondin-1-based culture medium (Figures 1A and 1C). Immunocytochemical characterization of the fraction derived from isolation showed that the cell suspension consisted of hepatocytes (HepPar1), stellate cells or smooth muscle cells (α-smooth muscle actin [α-SMA]), macrophages (MAC384), and endothelial cells (Factor VIII) together with biliary duct fragments (K19). Antibody details are given in Table S1. After 1 week of culture, only K19+ cells remained (Figure 1B), indicating the selection capacity of the expansion medium (EM). The EM for canine hepatic organoids contained generic organoid factors Wnt3A, epidermal growth factor (EGF), Noggin, and R-spondin-1, a potent Wnt agonist. Similar to mouse hepatic organoid cultures, the addition of fibroblast growth factor 10 (FGF10), hepatocyte growth factor (HGF), nicotinamide, and gastrin was required (Huch et al., 2013). Furthermore, culture of canine organoids needed supplementation with a Wnt3A-conditioned medium, Noggin, and a transforming growth factor β (TGF-β) inhibitor throughout culture, and a Rho-kinase inhibitor was provided long term (Figure S1). Under these conditions, canine duct fragments formed cysts within 24–48 hr and later grew out to larger organoids. Rho-kinase inhibitor and TGF-β inhibitor were essential to retain the culture for more than 3 months (Figure S1). The cultures have been maintained for more than 8 months by weekly passaging at a 1:4–1:8 split ratio and retained their characteristics as described later. Ki67 staining showed the strong proliferative activity of organoids (Figure 1C). Karyotype analysis of organoids showed that most cells (>85%) harbored normal chromosome numbers even after 8 months in culture (n = 3 organoid cultures per time point, 100 cells counted per culture) (Figure 1D).
Figure 1.
Generation of Long-Term Canine Hepatic Organoid Cultures Derived from Adult Liver Tissue by Duct Isolation
(A) Scheme of organoid culture. Canine liver tissue obtained from Tru-Cut biopsies, fine-needle aspiration biopsies, or wedge biopsy was enzymatically digested to achieve biliary duct fragments. Isolated ducts were cultured as described. See also Figures S1 and S2.
(B) Representative images of K19, HepPar1, α-SMA, MAC384, and Factor VIII immunostaining in isolated fraction at day (d) 0, showing the presence of biliary duct fragments, hepatocytes, stellate cells, macrophages, and endothelial cells, respectively, in the initial culture fraction, and K19 staining of organoids after 1 week in culture. HepPar1, α-SMA, MAC384, and Factor VIII stainings were negative in organoids 1 week after isolation.
(C) Representative phase contrast images of biliary ducts fragments and growing hepatic organoids in culture at indicated time points and passage numbers. The lower right image is the 3D overlay of Ki67 and DAPI immunofluorescence staining, indicating strong proliferation in organoids.
(D) Representative karyotype of a cell with normal chromosome number n = 78. The graph shows the percentage of cells with chromosome counts in organoids after 1, 3, and 5–8 months in culture (n = 3 independent organoid isolations per time point, 100 cells counted per culture); 1–3 loss (n = 75, 76, and 77); and 1 gain (n = 79).
See also Table S1.
We investigated whether the presence of cells from the in vivo HPC niche (hepatocytes, hepatic stellate cells, macrophages, and endothelial cells) or the size of the ducts, potentially reflecting the anatomical location of the duct in the liver, affected the isolation and growth of canine hepatic organoids. Organoids were cultured from cell fractions immediately obtained after liver digestion-containing ducts and other cell types, as well as cell fractions highly enriched for biliary duct fragments, by manual picking to minimize the presence of other cell types. In addition, smaller or larger duct fragments obtained using a 40 μm cell strainer were separately cultured. None of the different cell compositions showed differences in culture potential, morphology, and gene expression profiles (Figures S2A and S2B). This implied that the growth of organoids is independent from the extent of signaling derived from the non-epithelial cellular niche. For reasons of clinical application, organoids were also derived from Tru-Cut biopsies, fine-needle aspirated livers, and livers frozen up to 5 years in DMSO-based freezing medium. All these methods resulted in the culture of canine hepatic organoids with the same morphological and gene expression characteristics as organoids obtained from freshly explanted livers (Figures S2A and S2B). Established canine hepatic organoids recovered well after cryopreservation and maintained the potential for long-term proliferation under the same culture conditions (data not shown).
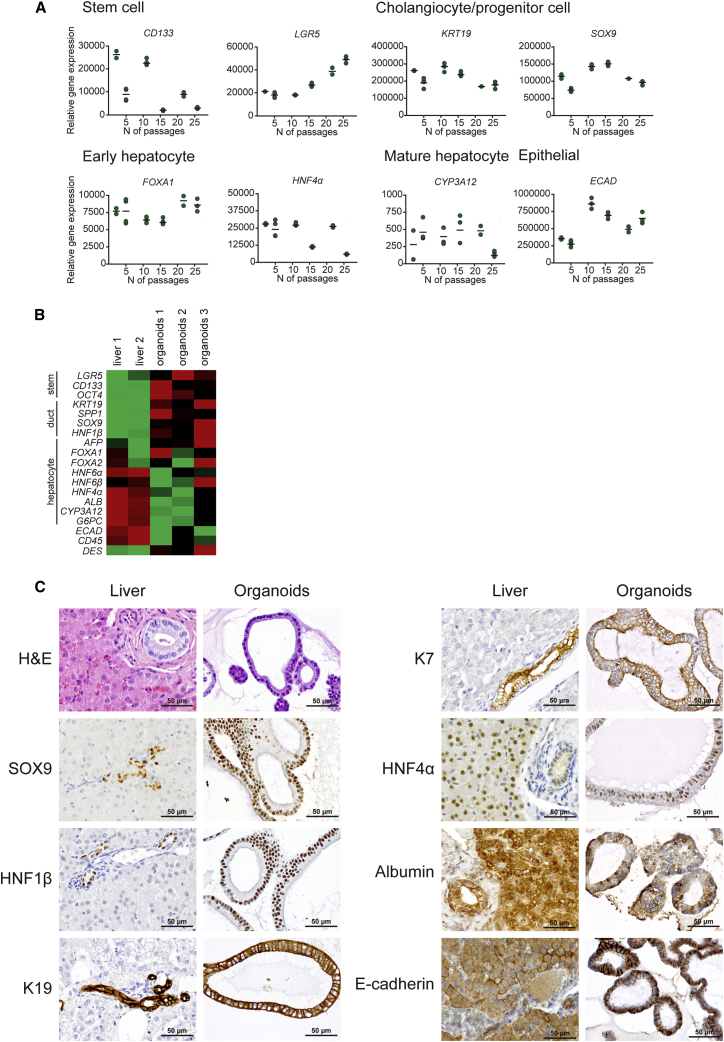
Gene expression analysis of long-term organoid cultures derived from a single dog up to passage P28 (approximately 8 months) showed stable expression of stem cell (CD133 and LGR5), cholangiocyte (KRT19 and SOX9), and early hepatocyte (FOXA1 and HNF4α) markers and very low expression of the mature hepatocyte (CYP3A12) marker over time (Figure 2A). Heat-map (Figure 2B) comparison of the average gene expression from all stages (P2– P26) of the organoid cultures from three dogs and adult normal livers revealed that cells in canine hepatic organoids have a higher expression of the Wnt target gene and stem cell marker Lgr5, the pluripotent stem cell markers CD133 and OCT4, and the bile duct or progenitor cell markers KRT19, SPP1, SOX9, and HNF1β compared to normal liver. Early hepatic marker genes such as alpha-fetoprotein (AFP), FOXA1, FOXA2, and HNF4α mostly had higher expression in organoids compared to normal liver, whereas mature hepatocyte markers such as albumin, CYP3A12, and G6PC had very low expression in organoids. The typical epithelial marker E-cadherin had lower expression in organoids when compared to normal liver, and hematopoietic and mesenchymal cell markers were weakly detected or absent in organoids (Figure 2B and a more detailed depiction in Figure S3). Primer details are shown in Table S2. Overall, these results confirmed the progenitor cell characteristics of the hepatic organoid cultures.
Figure 2.
Characterization of Canine Hepatic Organoids
(A) Gene expression analysis by qRT-PCR of different passage numbers of hepatic organoids derived from a single dog. Data are presented as dot plots (n = 2–3 biological replicates). See also Figure S3, which shows the analysis of three additional independent organoid isolations.
(B) Heatmap showing the comparative gene expression of stem cell, cholangiocyte (duct), and hepatocyte markers of three independent organoid isolations (mean of P2–P26 for each isolation, n = 4–6 passages per isolation, n = 2–3 biological replicates per passage) against two adult liver tissues. Red, upregulated; green, downregulated.
(C) Representative images of immunohistochemical or cytochemical stainings of hepatic organoids compared to adult normal liver tissue. Organoids express cholangiocyte markers SOX9, HNF1β, K19, and K7; hepatocyte markers HNF4α and albumin; and epithelial marker E-cadherin.
See also Table S1.
Microscopic morphological examination of H&E-stained organoids revealed that hepatic organoids generally consisted of a single layer of cylindrical or low cuboidal epithelial cells and sometimes a pseudostratified arrangement of the cells. The size of the organoids varied between 50 and 500 μm approximately and sometimes reached a 1 mm diameter. The individual cells measured 10–12 μm (data not shown).
Immunohistochemistry confirmed that cells of the organoids were strongly immunopositive for known liver stem cell or cholangiocyte markers SOX9, HNF1β, K19, and K7. Organoids also expressed the immature hepatocyte marker HNF4A and showed patchy staining for albumin, which is reported to be expressed not only by mature hepatocytes but also by liver stem cells (Koike and Taniguchi, 2012, Sancho-Bru et al., 2009) (Figure 2C). Mature hepatocyte markers ZO1 and MRP2 were negative in organoids grown in EM (Figure 3D). E-cadherin positivity proved the epithelial nature of the organoids (Figure 2C). Antibody details are given in Table S1.
Figure 3.
Hepatocellular Differentiation of Canine Hepatic Organoids
(A) Dot plots show fold change of gene expression of organoids cultured in DM from different passage numbers (P3, P12, and P22) toward organoids cultured in EM in the same passage (n = 3 biological replicates). Expression levels in primary hepatocytes are shown in Figure S3.
(B) Hepatic enzymes and albumin secretion of organoids in DM were measured and presented as fold change toward organoids in EM (n = 3 biological replicates). Albumin secretion in organoids was also compared to HepG2, Huh7, and primary hepatocytes (Hep.). Data presented as average ± SD.
(C) Cyp3a activity was measured in organoids cultured in DM and presented as fold change toward organoids in EM (n = 3 biological replicates). The graph compared Cyp3a activity of organoids in DM to HepG2, Huh7, and Hep. Data presented as average ± SD.
(D) Representative images of immunohistochemical stainings of organoids cultured in EM and DM. Organoids in DM lose cholangiocyte markers such as SOX9 and gain mature hepatocyte markers such as ZO1 and MRP2.
(A)–(D) show the results from one organoid isolation. The experiment has been performed on organoids derived from at least three more different isolations and in different passages, which all show similar results.
Differentiation Potential of Canine Organoids
For induction of hepatocyte maturation of canine hepatic organoids, Wnt agonists were withdrawn and Notch signaling was inhibited. After 14 days of differentiation, gene expression analysis revealed that canine hepatic organoids showed a marked reduction in expression levels of stem cell and cholangiocyte markers (CD133, TERT, LGR5, SPP1, and SOX9) and acquired more characteristics of hepatocytes, illustrated by an induction of hepatocyte markers (AFP, HNF4A, and G6PC). Expression of HNF1β was not changed in differentiated organoids (Figure 3A). Hepatic enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were induced upon differentiation, and differentiated organoids secreted at least 4 times up to 50 times more albumin into the medium compared to organoids cultured in EM and human hepatocellular carcinoma cell lines HepG2 and Huh7. Fresh primary hepatocytes produced 9 times more albumin compared to differentiated organoids (Figure 3B). Hepatocyte cytochrome P450 function (Cyp3a activity) was also induced in differentiated organoids toward the activity of fresh primary hepatocytes (about 6.5 times higher) and was slightly higher than HepG2 and Huh7 (Figure 3C). Immunocytochemical staining of differentiated organoids revealed a clear expression of mature hepatocyte characteristics. Tight junction protein ZO1 was detectable in most organoids after differentiation, and membrane protein MRP2 was detected in some organoids. SOX9, a marker of bile duct and stem cells, was clearly lost upon differentiation (Figure 3D). The gene expression and functional studies indicated no difference in differentiation potential between organoids from lower (P3), middle (P12), and higher (P22) passage numbers (Figures 3A–3C). Overall, the results showed that canine hepatic organoids can be differentiated toward mature hepatocytes with comparable potential as mouse and human organoids and retain their differentiation potential over time in culture (Huch et al., 2013, Huch et al., 2015).
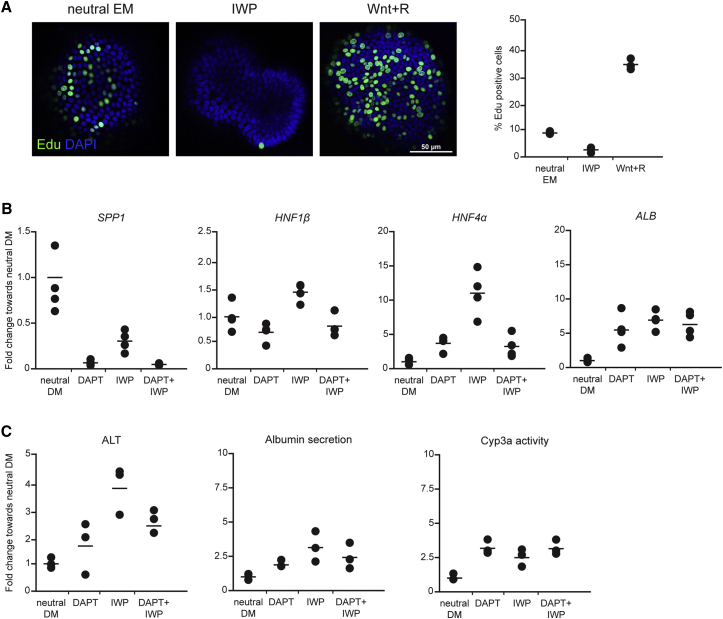
The Role of Wnt on HPC Biology
As opposed to mouse and human organoid cultures, the culture of canine hepatic organoids partly relied on the addition of Wnt3a. Wnt is a known regulator of stem cell proliferation and differentiation during embryogenesis (Nusse et al., 2008, Thompson and Monga, 2007), and it provides maintenance of stem cell characteristics (Clevers et al., 2014), which is essential for long-term stable cell cultures. To investigate the role of Wnt in promoting long-term canine organoid cultures, we investigated its effect on proliferation and differentiation. Organoids were grown in neutral EM (without Wnt agonists), medium supplemented with Wnt agonists (Wnt3a-conditioned medium and R-spondin-1-conditioned medium), or medium supplemented with the Wnt inhibitor IWP-2 for 24 hr. Using a 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay to measure DNA replication, we showed that activation of Wnt signaling strongly enhances proliferation, whereas inhibition of Wnt suppressed proliferation of canine hepatic organoids (Figure 4A). To address the role of Wnt in differentiation status of the organoids, we treated cultures with Notch inhibitor N-[(3,5-difluorophenyl)acetyl]-l-alanyl-2-phenylglycine-1,1-dimethylethyl ester (DAPT, a standard differentiation medium [DM]) or with Wnt inhibitor IWP-2 in neutral DM (DM without DAPT). This showed that Wnt inhibition-induced hepatocytic differentiation to the same degree as Notch inhibition, as shown by downregulation of gene expression levels of SPP1 and upregulation of HNF4A and Albumin gene expression, as well as upregulation of the hepatocytic enzyme ALT, albumin production, and cytochrome P450 activity compared to neutral DM. Next, we assessed whether the combined inhibition of Wnt and Notch signaling further increased the degree of differentiation of the cells (IWP-2 and DAPT in neutral DM). This co-inhibition did not result in an additive differentiation effect (Figures 4B and 4C). However, gene expression level of SPP1 in one of the three experiments showed synergistic downregulation under DAPT and IWP-2 treatment (data not shown). Taken together, the results corroborate the importance of Wnt signaling in the proliferation and maintenance of the stemness of canine hepatic organoids.
Figure 4.
Role of Wnt in Proliferation and Differentiation of Canine Hepatic Organoids
(A) Representative images of EdU and DAPI immunofluorescent whole-mount staining of organoids (3D overlay) cultured in neutral EM or with Wnt inhibitor (IWP) or Wnt3a-conditioned medium with R-spondin (Wnt+R) added. The graph shows the percentage of EdU-positive cells within an organoid (n = 3 biological replicates of at least 20 organoids).
(B) Gene expression analysis by qRT-PCR of organoids cultured in neutral DM with and without Wnt inhibitor (IWP), Notch inhibitor (DAPT), or both (n = 4 biological replicates). Results are presented as fold change toward neutral DM.
(C) ALT, albumin secretion, and Cyp3a activity of organoids cultured in neutral DM with or without Wnt inhibitor (IWP), Notch inhibitor (DAPT), or both (n = 3 biological replicates). Results are presented as fold change toward neutral DM.
The figure shows the results from one organoid isolation representative for a total of three independent organoid isolations with comparable experimental outcomes.
See also Table S2.
Organoid Disease Modeling
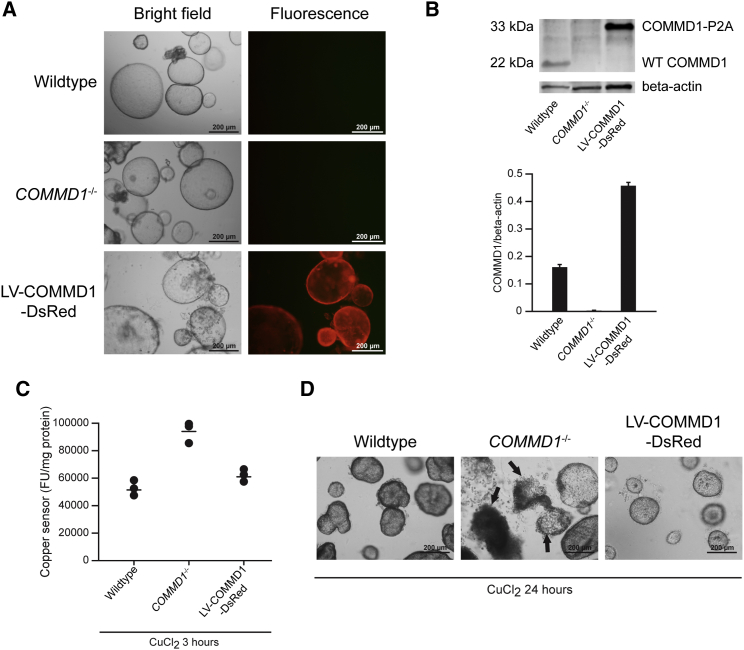
To evaluate the use of canine hepatic organoids for disease modeling, we isolated hepatic organoids from dogs with an autosomal recessive COMMD1 deficiency (Figure 5A). Our previous longitudinal studies demonstrated that these dogs develop copper-induced hepatitis highly similar to Wilson’s disease in humans (Favier et al., 2012, Favier et al., 2015). Intracellular copper levels of 14-day-differentiated organoids subjected to high copper levels (500 μM CuCl2) for 3 hr in the media were measured using a fluorescence-based copper sensor (Cotruvo et al., 2015, Miller et al., 2006, Zeng et al., 2006). This demonstrated that COMMD1−/− organoids had a higher intracellular accumulation of copper compared to normal organoids, similar to the copper excretion defect in the in vivo situation (Figure 5C). This finding clearly demonstrates the potential to use the canine hepatic organoid technology for (translational) disease modeling purposes, such as the COMMD1-deficient copper storage disease as an important model for human Wilson’s disease. We next assessed the potential for gene correction as a basis for autologous gene therapy. By using DsRed-expressing puromycin-selectable lentiviral vectors, the COMMD1 gene was transferred to COMMD1-deficient organoids. Five passages after transduction, all cells in organoids showed DsRed positivity, reflecting COMMD1 gene transfer (Figure 5A). Western blot analysis confirmed the accomplishment of COMMD1 gene insertion and protein expression in COMMD1−/− organoids (Figure 5B). To determine a functional effect of COMMD1 gene supplementation, we again measured intracellular copper levels in differentiated COMMD1−/−, LV-COMMD1-DsRed, and wild-type organoids after 3 or 24 hr of CuCl2 treatment. The results revealed that COMMD1 gene supplementation normalized cellular copper content in LV-COMMD1-DsRed organoids to wild-type levels. Twenty-four hours after exposure to high copper levels, the COMMD1-deficient organoids showed reduced viability due to copper accumulation, whereas wild-type organoids and LV-COMMD1-DsRed organoids remained normal (Figure 5D). These data suggest that LV-COMMD1-DsRed and wild-type organoids were superior in copper excretion compared to COMMD1-deficient organoids and that gene transfer can successfully rescue the phenotype in vitro.
Figure 5.
Restoration of Cellular Copper Excretion by COMMD1 Gene Supplementation
(A) Representative phase contrast and fluorescence images of wild-type, COMMD1−/−, and LV-COMMD1-DsRed organoids, showing successful transduction in LV-COMMD1-DsRed organoids
(B) COMMD1 immunoblot of protein extracts from wild-type, COMMD1−/−, and LV-COMMD1-DsRed organoids, showing successful protein expression (n = 3 biological replicates). Beta-actin was used as the loading control. The COMMD1 protein in LV-COMMD1-DsRed organoids is larger due to the remaining amino acids of the cleavage site P2A added to the vector. Quantification of protein presented as average ± SD.
(C) Copper sensor measurement before and after CuCl2 treatment of wild-type, COMMD1−/−, and LV-COMMD1-DsRed organoids (n = 3 biological replicates), showing that LV-COMMD1-DsRed organoids successfully restored copper excretion.
(D) Representative phase contrast images of wild-type, COMMD1−/−, and LV-COMMD1-DsRed organoids after CuCl2 treatment. Deterioration of the culture and cell death (arrows) are presented in COMMD1−/− organoids.
This figure represents results of three repeated experiments with independent COMMD1−/− organoids.
Discussion
Three-dimensionally grown liver stem cells, so-called hepatic organoids, have value in the field of regenerative medicine, both for knowledge development and for therapeutic strategies such as (autologous) cell transplantation. Finding methods to generate organoids from tissues derived by minimally invasive techniques is therefore critical. In the current study, we describe the successful growth of organoids derived from a large piece of fresh explanted liver, Tru-Cut liver biopsy (14G needle), fine-needle aspiration biopsy (22G needle), DMSO-based frozen livers, and even tissues that were kept in cold DMEM with 1% fetal bovine serum (FBS) for up to 2 days (data not shown). In all situations, organoids formed within 1 week after isolation, could be split at a ratio of 1:4–1:8 from the start, and remained normal in chromosome number for more than 8 months, in line with mouse and human hepatic organoids (Huch et al., 2013, Huch et al., 2015). This flexibility of the tissue sampling method, combined with the quick formation of organoids in culture, proves their usefulness for the clinic and the potential to use patient-derived tissues to circumvent the need for donor livers.
The organoids in this study were derived from dog livers. The establishment of canine organoids is important because dogs can fulfill a critical role as a clinically relevant animal model in the translation from mouse to man. A recent study elegantly showed that the treatment of dogs with a new therapy can facilitate and improve the translation to human practice. The promising anticancer outcomes and risk-benefit profile obtained from a comparative canine trial, in conjunction with the results observed in rats, have encouraged Roberts et al. (2014) to move forward to successful phase 1 studies in human patients. Genetic studies have shown that many canine diseases are similar to human diseases in terms of causative gene mutation and phenotypic presentation. In particular, the pathologies of spontaneous liver diseases are largely shared by humans and dogs with respect to the histological and molecular characteristics of the underlying pathways (Arends et al., 2009, Kruitwagen et al., 2014, Schotanus et al., 2009, Spee et al., 2010). In addition, the size of dogs is similar to that of human pediatric metabolic liver patients, allowing the optimization of technical aspects such as route of injection or benefit of repetitive injection of cells for transplantation or novel therapeutic approaches.
Canine hepatic organoids show characteristics of the putative liver stem or progenitor cells in the liver based on their expression of biliary and hepatocellular markers and their potential to differentiate toward hepatocytes. The differentiated organoids obtained hepatocyte-specific functions higher than those of human liver cell lines, HepG2 and Huh7, although primary isolated hepatocytes still showed higher expression of albumin, comparable to previous studies (Banas et al., 2007, Huch et al., 2013). The inability to induce full hepatocyte maturation in vitro is well known in the field, and perhaps this only occurs after transplantation into a liver, as was shown for mouse- and human-derived hepatic organoids or stem cells that differentiated into mature hepatocytes upon engraftment in the liver (Huch et al., 2013, Huch et al., 2015, Lu et al., 2015). The HPC niche plays an important role in the determination of fate decision and probably supports in vivo differentiation of transplanted HPCs (Boulter et al., 2012, Español-Suñer et al., 2012, Van Hul et al., 2011). Hepatocyte transplantation is currently more efficient than stem cell transplantation (Azuma et al., 2007, Huch et al., 2013, Yovchev et al., 2008). Therefore, further improvement of hepatocyte differentiation in vitro, for example, by altering the composition of the culture matrix, may improve stem cell transplantation strategies. Moreover, it will assist the use of patient-derived cells to model liver diseases that require full hepatocyte differentiation for disease manifestation, toxicology studies, or personalized treatment strategies.
To culture genetically stable canine organoids for more than 8 months, we modified murine hepatic organoid EM (Huch et al., 2013). Continuous supply of Wnt agonists, inhibitors of TGF-β, and Rho-associated protein kinase (ROCK) inhibitors was necessary for long-term viable canine cultures. Different species require specific and slightly different intervention with pathways involved in hepatic cell fate control and differentiation (Lemaigre, 2009, Si-Tayeb et al., 2010) to obtain long-term stable cultures. Neither mouse nor human hepatic organoids need the addition of Wnt3a for long-term culture (Huch et al., 2013, Huch et al., 2015). Recent work reported on a pericentral hepatocyte population in mice that contributes to maintenance of hepatocytes in a Wnt-dependent fashion (Wang et al., 2015). Our data demonstrate that for canine hepatic organoids, Wnt is important for proliferation and for maintenance of long-term stability, while the inhibition of Wnt enhanced differentiation. This information is interesting in view of the role of Wnt in canine HPCs during liver disease and may partly explain the lack of differentiation during chronic hepatitis or lobular dissecting hepatitis in dogs (Schotanus et al., 2014). It shows that organoid cultures are a useful tool to study stem cell biology and may serve to find potential therapeutic targets to stimulate endogenous adult HPCs to repopulate the liver during diseases.
Transplantation of wild-type differentiated mouse hepatic organoids into Fah−/− mice resulted in full maturation into hepatocytes and the extended lifetime of the mice (Huch et al., 2013). This study demonstrated the potential of organoids for transplantation goals, although engraftment efficiency needs to be enhanced to obtain a complete liver function rescue (Grompe, 2014). An important therapeutic approach could be the autologous transplantation of genetically corrected organoids, derived from metabolically diseased liver patients. For that purpose, successful gene correction must be performed in vitro. In this study we used our dog model of COMMD1-deficiency-induced copper storage disease. This gene mutation causes copper toxicosis with a phenotype similar to Wilson’s disease in humans (Favier et al., 2012, Favier et al., 2015, Fieten et al., 2014). COMMD1-deficient organoids showed enhanced copper storage in culture. Upon transduction with a COMMD1 construct, successful restoration of copper excretion and enhanced viability upon copper exposure was achieved. These experiments demonstrated the feasibility to perform successful gene correction in hepatic organoids, a key prerequisite for autologous transplantation therapy. Moreover, they confirm that organoids represent a powerful tool for disease modeling of canine liver diseases that serve as a robust translational model for human liver diseases such as Wilson’s disease (Favier et al., 2012). The first functional restoration experiments have been performed in human cystic fibrosis patient-derived intestinal organoids using CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 genome editing. This corrected disease-causing mutations (Schwank et al., 2013) and illustrated the amazing therapeutic potential of combining organoids and genome editing technology. Two main challenges that remain for the use of genetically corrected organoids in clinical application are graft rejection and tumorigenicity. First, immune reaction due to non-self protein in transduced organoids may occur despite autologous transplantation; therefore, (short term) immunosuppressive therapy will be necessary. Second, genetic instability may be introduced during culture upon transduction and increase the risk of tumor formation. This could be assessed in long-term experiments using large translational models such as the COMMD1−/− dog.
Taken together, the ability to culture hepatic organoids obtained directly from patients opens several new research and therapeutic avenues. First, they can be used as a system to investigate fundamental research questions in relevant patient material of different species. Second, it provides a platform for pre-clinical modeling of liver diseases. Third, these cells have great potential as an alternative source for autologous stem cell therapies. Methods described in this paper pave the way to culture organoids from fresh and frozen patient samples derived with low-invasive techniques. The prospect of using hepatic organoids in cell therapy is encouraging but does require strong validation in the clinical setting. The development of liver stem cell cultures from the dog as a large animal model is an important step to overcome the challenges of moving from basic translational research toward application in human patients. Treatment of pet dogs in an academic clinical environment has the benefit to serve both veterinary and human medicine. The next step is therefore to perform transplantation studies in dogs to assess long-term safety, efficacy, and tumorigenicity of organoids for treatment of liver diseases.
Experimental Procedures
Isolation of Canine Biliary Duct Fragments and Culture of Hepatic Organoids
The use of liver tissues from dogs was approved by Utrecht University’s ethical committee. Fresh canine adult normal liver tissues were taken from liver explants (unless otherwise noted), 14G Tru-Cut needle biopsies, or fine-needle aspiration (22G) from dogs used in non-liver-related research (the reduce, reuse, recycle policy of Utrecht University). Liver tissues from COMMD1-deficient dogs were collected and frozen in a previous experiment approved by Utrecht University’s ethical committee, as required under Dutch legislation (ID2007.III.06.080). No animals were harmed or killed for the purpose of this study. Liver tissues were used for isolation immediately or frozen in DMSO-based freezing medium (Gibco) under a slow-freezing method and thawed rapidly in a 37°C water bath before isolation. Visible bile ducts were removed before isolation. After mechanical dissection, liver tissues were enzymatically digested in DMEM (Gibco) with 1% FBS (Gibco) containing 0.3 mg/ml of type II collagenase (Gibco) and 0.3 mg/ml of dispase (Gibco) at 37°C for a total of 3–5 hr until biliary duct fragments appeared. The isolated ducts were then mixed with Matrigel (BD Biosciences) and seeded. Culture medium was added after gelation of the Matrigel. EM was based on AdvDMEM/F12 (Invitrogen) supplemented with 1% B27 (Invitrogen), 1% N2 (Invitrogen), 1.25 mM N-acetylcysteine (Sigma-Aldrich), 10 nM gastrin (Sigma-Aldrich), 200 ng/ml of EGF (Invitrogen), 5% R-spondin-1-conditioned medium (provided by Calvin J. Kuo), 100 ng/ml of FGF10 (PeproTech), 10 mM nicotinamide (Sigma-Aldrich), 25 ng/ml of HGF (PeproTech), 100 ng/ml of Noggin (PeproTech), 30% Wnt3a-conditioned medium (prepared as in Willert et al., 2003), 10 μM Y-27632 2HCl (ROCK inhibitor, Selleckchem), and 0.5 μM A83-01 (TGFβ inhibitor, Tocris Bioscience). Organoids were split by removal from Matrigel using cold AdvDMEM/F12, mechanical dissociation into smaller fragments, and transfer into fresh Matrigel. Passage was performed weekly at a 1:4–1:8 split ratio. The medium was changed every second day.
Karyotyping of Hepatic Organoids
Organoids (after 1, 3, and 5–8 months in culture) in the exponential growth phase were arrested in metaphase with 0.05 μg/ml of colcemid (Gibco) for 6 hr at 37°C. Next, organoids were washed and dissociated into single cells using TrypLE select enzyme (Gibco). Cell pellets were then incubated for 10 min in 56 mM KCl and fixed with methanol:acetic acid (3:1) after KCl was discarded. Finally, cells were dropped on slides and chromosomes from at least 100 metaphase arrested cells per organoid culture (three organoid cultures per time point) were stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich) and counted. Three independent organoid cultures derived from different dogs were used at each time point. Photographs were taken using a confocal microscope (Leica).
Hepatocyte Differentiation
For induction of hepatocyte maturation, hepatic organoids were seeded in Matrigel and kept 2 days under EM, as described previously. Then Wnt3a-conditioned medium, R-spondin-1, HGF, FGF10, gastrin, nicotinamide, and ROCK inhibitor were withdrawn from the medium and 10 nM DAPT (Selleckchem), a Notch inhibitor, was added (day 0). From day 6, 30 μM dexamethasone (Sigma-Aldrich) was supplemented in the medium. The medium was changed every other day for a period of 14 days.
Hepatocyte Functional Studies
For determination of albumin secretion, a culture medium from differentiated organoids was collected at day 14 of differentiation. Protein in the medium was concentrated using Amicon Ultra centrifugal filters (Millipore), and the amount of albumin was measured using a DxC-600 Beckman (Beckman Coulter). The values were normalized for total cell number. For measurement of cytochrome P450 activity, 14-day-differentiated organoids were removed from Matrigel and incubated in 50 μM luciferin-PFBE substrate (Promega) in hepatozyme medium (Gibco) containing 10% FBS for 8 hr at 37°C. Cyp3a activity was then measured with a luminometer using the P450-Glo cytochrome P450 assay kit according to the manufacturer’s instructions (Promega). For comparative analysis, freshly isolated canine hepatocytes were used (Arends et al., 2009). Furthermore, human cell lines HepG2 (cultured in DMEM with 10% FBS) and Huh 7 (cultured in DMEM with 10% FBS) were included for comparative functional analysis.
For measurement of the expression of hepatic enzymes in differentiated organoids, cells were lysed in milliQ at day 14 and stored at −20°C. ALT and AST were measured using the DxC-600 Beckman (Beckman Coulter) standard protocols, and values were corrected for total cell counts.
Wnt Interference Experiments
For assessment of the role of Wnt signaling pathway in organoid proliferation, neutral EM consisting of standard EM without Wnt3a-conditioned medium and R-spondin-1-conditioned medium was compared to neutral EM containing 10 μM of Wnt inhibitor IWP-2 (Stemgent) and standard culture medium (neutral EM plus Wnt3a-conditioned medium and R-spondin-1-conditioned medium). Organoids were fed with neutral EM for 4 days upon seeding. Then Wnt agonists or Wnt inhibitor was added for 24 hr. The EdU incorporation assay was used to assess proliferative activity within the organoids, performed as described earlier. Images were taken using a confocal microscope (Leica). Quantitative analysis of EdU-positive cells was performed by Fiji Is Just ImageJ and ImageJ macros.
For assessment of the role of Wnt and Notch in hepatocellular differentiation, neutral DM consisting of DM without DAPT was compared to neutral DM plus 10 μM of Wnt inhibitor IWP-2 (Stemgent) with or without the presence of 10 nM DAPT (Selleckchem). IWP-2 was added to the medium from the day organoids were seeded. DAPT was started after 2 days in culture according to the normal differentiation protocol. The medium was changed every other day for a period of 14 days.
COMMD1-DsRed Construct Production and Viral Transduction
For production of the viral construct, lentivirus was produced using HEK293T cells. Linear polyethylenimine (PEI) at 1 mg/ml (Polysciences) was diluted in DMEM and incubated for 5 min at room temperature (RT). The DNA solution containing HDM-Hgpm2, RC-CMV-Rev1b, HDM-tat1b, HDM-VSV-G, and pHAGE2-EF1a-COMMD1-DsRed-PuroR was then mixed with PEI solution (1 μg DNA:5 μg PEI) and incubated for 10–20 min at RT. The obtained transfection mix was added to complete mouse embryonic fibroblast media (cultured in DMEM with 10% FBS), supplied to the HEK293T cells, and incubated overnight. Media-containing virus was harvested on days 2–4. Media were ultracentrifuged at 72,000 g for 1.5–2 hr at 4°C. The pellet was resuspended in 150 μl sterile PBS + 1% BSA, and aliquots were stored at −80°C until use.
For viral transduction, COMMD1-deficient canine hepatic organoids were isolated from frozen liver derived from COMMD1-deficient dogs and cultured as described earlier. Organoids were removed from Matrigel and underwent mechanical dissociation. Organoid fragments were incubated with TrypLE select enzyme (Gibco) for 5 min at 37°C to break the fragments into small cell clusters. The cell clusters were then resuspended in infection medium containing 8 μg/ml of Polybrene (Sigma-Aldrich) with lentivirus (1:10,000). Suspensions were transferred to a Costar ultra-low-attachment 24-well plate (Corning Life Sciences). Spinoculation was performed for 60 min at 32°C at 600 g. After spinoculation, the plate with organoids was incubated for 6 hr at 37°C before continuing in Matrigel culture as described earlier. Two days after transduction, 2 μg/ml of puromycin (Life Technologies) was added to the medium to select for the transduced cells. A standard of 0.5 μg/ml of puromycin was used in the culture medium from 3 days post-transduction. The organoids after viral transduction are referred as LV-COMMD1-DsRed organoids.
Intracellular Copper Measurement
COMMD1-deficient, LV-COMMD1-DsRed, and wild-type organoids were differentiated as described earlier. At day 14 of differentiation, organoids were removed from Matrigel and incubated in DM containing 500 μM CuCl2 (Sigma-Aldrich) for 3 or 24 hr at 37°C. Controls did not contain CuCl2. After washing with PBS, organoids were incubated with copper fluorescent probe CS1 (Cotruvo et al., 2015, Miller et al., 2006, Zeng et al., 2006) for 20 min at 37°C. Organoids were then lysed with radioimmunoprecipitation assay buffer, and fluorescence was measured at 488 nm. Between every step, organoids were washed with PBS and centrifuge at 100 g for 5 min. Fluorescence signals were normalized for protein concentration (Lowry assay, Bio-Rad).
Acknowledgments
The authors would like to acknowledge Christopher J. Chang for providing the copper sensor 1 probe (Department of Chemistry, University of California, Berkeley), Lenie C. Boswijk-Appelhof (UVDL, Utrecht University) for her technical assistance on the AST/ALT and albumin measurements, and Carla Zijlstra (Department of Biochemistry and Cell Biology, Utrecht University) for her help with metaphase spread. M.H. is a Wellcome Trust Sir Henry Dale Fellow and is jointly funded by the Wellcome Trust and the Royal Society (104151/Z/14/Z). This work was funded by the Dutch Research Counsel NWO ZON/MW (92003538 and 16004121).
Published: October 8, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, three figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.09.002.
Supplemental Information
References
- Arends B., Spee B., Schotanus B.A., Roskams T., van den Ingh T.S., Penning L.C., Rothuizen J. In vitro differentiation of liver progenitor cells derived from healthy dog livers. Stem Cells Dev. 2009;18:351–358. doi: 10.1089/scd.2008.0043. [DOI] [PubMed] [Google Scholar]
- Azuma H., Paulk N., Ranade A., Dorrell C., Al-Dhalimy M., Ellis E., Strom S., Kay M.A., Finegold M., Grompe M. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat. Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banas A., Teratani T., Yamamoto Y., Tokuhara M., Takeshita F., Quinn G., Okochi H., Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- Boulter L., Govaere O., Bird T.G., Radulescu S., Ramachandran P., Pellicoro A., Ridgway R.A., Seo S.S., Spee B., Van Rooijen N. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter L., Lu W.Y., Forbes S.J. Differentiation of progenitors in the liver: a matter of local choice. J. Clin. Invest. 2013;123:1867–1873. doi: 10.1172/JCI66026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale V., Wang Y., Carpino G., Cui C.B., Gatto M., Rossi M., Berloco P.B., Cantafora A., Wauthier E., Furth M.E. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- Clevers H., Loh K.M., Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- Cotruvo J.A., Jr., Aron A.T., Ramos-Torres K.M., Chang C.J. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 2015;44:4400–4414. doi: 10.1039/c4cs00346b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgetty D.M., Medine C.N., Iredale J.P., Hay D.C. Progress and future challenges in stem cell-derived liver technologies. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G241–G248. doi: 10.1152/ajpgi.00138.2009. [DOI] [PubMed] [Google Scholar]
- Dhawan A., Puppi J., Hughes R.D., Mitry R.R. Human hepatocyte transplantation: current experience and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2010;7:288–298. doi: 10.1038/nrgastro.2010.44. [DOI] [PubMed] [Google Scholar]
- Duncan A.W., Dorrell C., Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Español-Suñer R., Carpentier R., Van Hul N., Legry V., Achouri Y., Cordi S., Jacquemin P., Lemaigre F., Leclercq I.A. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–1575.e7. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- Favier R.P., Spee B., Schotanus B.A., van den Ingh T.S., Fieten H., Brinkhof B., Viebahn C.S., Penning L.C., Rothuizen J. COMMD1-deficient dogs accumulate copper in hepatocytes and provide a good model for chronic hepatitis and fibrosis. PLoS ONE. 2012;7:e42158. doi: 10.1371/journal.pone.0042158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier R.P., Spee B., Fieten H., van den Ingh T.S., Schotanus B.A., Brinkhof B., Rothuizen J., Penning L.C. Aberrant expression of copper associated genes after copper accumulation in COMMD1-deficient dogs. J. Trace Elem. Med. Biol. 2015;29:347–353. doi: 10.1016/j.jtemb.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Fieten H., Penning L.C., Leegwater P.A., Rothuizen J. New canine models of copper toxicosis: diagnosis, treatment, and genetics. Ann. N Y Acad. Sci. 2014;1314:42–48. doi: 10.1111/nyas.12442. [DOI] [PubMed] [Google Scholar]
- Grompe M. Liver stem cells, where art thou? Cell Stem Cell. 2014;15:257–258. doi: 10.1016/j.stem.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Guo J., Li J., Lu Y., Xin J., Jiang L., Wu W., Cao H., Qiu Y. A novel technique for hepatic progenitor cell isolation from normal adult rat livers. ASAIO J. 2012;58:73–78. doi: 10.1097/MAT.0b013e318239fce5. [DOI] [PubMed] [Google Scholar]
- He Z., Feng M. Activation, isolation, identification and culture of hepatic stem cells from porcine liver tissues. Cell Prolif. 2011;44:558–566. doi: 10.1111/j.1365-2184.2011.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M., Dorrell C., Boj S.F., van Es J.H., Li V.S., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M.J. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M., Gehart H., van Boxtel R., Hamer K., Blokzijl F., Verstegen M.M., Ellis E., van Wenum M., Fuchs S.A., de Ligt J. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/j.cell.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R.D., Mitry R.R., Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012;93:342–347. doi: 10.1097/TP.0b013e31823b72d6. [DOI] [PubMed] [Google Scholar]
- Ichinohe N., Tanimizu N., Ooe H., Nakamura Y., Mizuguchi T., Kon J., Hirata K., Mitaka T. Differentiation capacity of hepatic stem/progenitor cells isolated from D-galactosamine-treated rat livers. Hepatology. 2013;57:1192–1202. doi: 10.1002/hep.26084. [DOI] [PubMed] [Google Scholar]
- Kamimura K., Kanefuji T., Yokoo T., Abe H., Suda T., Kobayashi Y., Zhang G., Aoyagi Y., Liu D. Safety assessment of liver-targeted hydrodynamic gene delivery in dogs. PLoS ONE. 2014;9:e107203. doi: 10.1371/journal.pone.0107203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A., Inagaki Y. Stem and progenitor cell systems in liver development and regeneration. Hepatol. Res. 2015;45:29–37. doi: 10.1111/hepr.12349. [DOI] [PubMed] [Google Scholar]
- Koike H., Taniguchi H. Characteristics of hepatic stem/progenitor cells in the fetal and adult liver. J. Hepatobiliary Pancreat. Sci. 2012;19:587–593. doi: 10.1007/s00534-012-0544-4. [DOI] [PubMed] [Google Scholar]
- Kruitwagen H.S., Spee B., Viebahn C.S., Venema H.B., Penning L.C., Grinwis G.C., Favier R.P., van den Ingh T.S., Rothuizen J., Schotanus B.A. The canine hepatic progenitor cell niche: molecular characterisation in health and disease. Vet. J. 2014;201:345–352. doi: 10.1016/j.tvjl.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Lemaigre F.P. Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology. 2009;137:62–79. doi: 10.1053/j.gastro.2009.03.035. [DOI] [PubMed] [Google Scholar]
- Lu W.Y., Bird T.G., Boulter L., Tsuchiya A., Cole A.M., Hay T., Guest R.V., Wojtacha D., Man T.Y., Mackinnon A. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 2015;17:971–983. doi: 10.1038/ncb3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata M., Vera J.F., Gerken C., Rooney C.M., Miller T., Pfent C., Wang L.L., Wilson-Robles H.M., Gottschalk S. Toward immunotherapy with redirected T cells in a large animal model: ex vivo activation, expansion, and genetic modification of canine T cells. J. Immunother. 2014;37:407–415. doi: 10.1097/CJI.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty W.J., Usta O.B., Luitje M., Bale S.S., Bhushan A., Hegde M., Golberg I., Jindal R., Yarmush M.L. A novel ultrathin collagen nanolayer assembly for 3-D microtissue engineering: layer-by-layer collagen deposition for long-term stable microfluidic hepatocyte culture. Technology (Singap. World Sci.) 2014;2:67–74. doi: 10.1142/S2339547814500083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.W., Zeng L., Domaille D.W., Chang C.J. Preparation and use of Coppersensor-1, a synthetic fluorophore for live-cell copper imaging. Nat. Protoc. 2006;1:824–827. doi: 10.1038/nprot.2006.140. [DOI] [PubMed] [Google Scholar]
- Nusse R., Fuerer C., Ching W., Harnish K., Logan C., Zeng A., ten Berge D., Kalani Y. Wnt signaling and stem cell control. Cold Spring Harb. Symp. Quant. Biol. 2008;73:59–66. doi: 10.1101/sqb.2008.73.035. [DOI] [PubMed] [Google Scholar]
- Roberts N.J., Zhang L., Janku F., Collins A., Bai R.Y., Staedtke V., Rusk A.W., Tung D., Miller M., Roix J. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med. 2014;6:249ra111. doi: 10.1126/scitranslmed.3008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo F.P., Parola M. Stem cells in liver failure. Best Pract. Res. Clin. Gastroenterol. 2012;26:35–45. doi: 10.1016/j.bpg.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Sancho-Bru P., Najimi M., Caruso M., Pauwelyn K., Cantz T., Forbes S., Roskams T., Ott M., Gehling U., Sokal E. Stem and progenitor cells for liver repopulation: can we standardise the process from bench to bedside? Gut. 2009;58:594–603. doi: 10.1136/gut.2008.171116. [DOI] [PubMed] [Google Scholar]
- Scheers I., Maerckx C., Khuu D.N., Marcelle S., Decottignies A., Najimi M., Sokal E. Adult-derived human liver progenitor cells in long-term culture maintain appropriate gatekeeper mechanisms against transformation. Cell Transplant. 2012;21:2241–2255. doi: 10.3727/096368912X639026. [DOI] [PubMed] [Google Scholar]
- Schotanus B.A., van den Ingh T.S., Penning L.C., Rothuizen J., Roskams T.A., Spee B. Cross-species immunohistochemical investigation of the activation of the liver progenitor cell niche in different types of liver disease. Liver Int. 2009;29:1241–1252. doi: 10.1111/j.1478-3231.2009.02024.x. [DOI] [PubMed] [Google Scholar]
- Schotanus B.A., Kruitwagen H.S., van den Ingh T.S., van Wolferen M.E., Rothuizen J., Penning L.C., Spee B. Enhanced Wnt/β-catenin and Notch signalling in the activated canine hepatic progenitor cell niche. BMC Vet. Res. 2014;10:309. doi: 10.1186/s12917-014-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwank G., Koo B.K., Sasselli V., Dekkers J.F., Heo I., Demircan T., Sasaki N., Boymans S., Cuppen E., van der Ent C.K. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–658. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K., Lemaigre F.P., Duncan S.A. Organogenesis and development of the liver. Dev. Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Spee B., Carpino G., Schotanus B.A., Katoonizadeh A., Vander Borght S., Gaudio E., Roskams T. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2010;59:247–257. doi: 10.1136/gut.2009.188367. [DOI] [PubMed] [Google Scholar]
- Thompson M.D., Monga S.P. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- Van Hul N., Lanthier N., Español Suñer R., Abarca Quinones J., van Rooijen N., Leclercq I. Kupffer cells influence parenchymal invasion and phenotypic orientation, but not the proliferation, of liver progenitor cells in a murine model of liver injury. Am. J. Pathol. 2011;179:1839–1850. doi: 10.1016/j.ajpath.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaecke T., Rogiers V. Hepatocyte cultures in drug metabolism and toxicological research and testing. Methods Mol. Biol. 2006;320:209–227. doi: 10.1385/1-59259-998-2:209. [DOI] [PubMed] [Google Scholar]
- Wang B., Zhao L., Fish M., Logan C.Y., Nusse R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–185. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K., Brown J.D., Danenberg E., Duncan A.W., Weissman I.L., Reya T., Yates J.R., 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Yovchev M.I., Grozdanov P.N., Zhou H., Racherla H., Guha C., Dabeva M.D. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology. 2008;47:636–647. doi: 10.1002/hep.22047. [DOI] [PubMed] [Google Scholar]
- Zeng L., Miller E.W., Pralle A., Isacoff E.Y., Chang C.J. A selective turn-on fluorescent sensor for imaging copper in living cells. J. Am. Chem. Soc. 2006;128:10–11. doi: 10.1021/ja055064u. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.