Abstract
AIM: To investigate the protective effect of magnesium isoglycyrrhizinate (MgIG) on excessive hepatectomy animal model and its possible mechanism.
METHODS: We used the standard 90% hepatectomy model in Sprague-Dawley rats developed using the modified Emond’s method, in which the left, middle, right upper, and right lower lobes of the liver were removed. Rats with 90% liver resection were divided into three groups, and were injected intraperitoneally with 3 mL saline (control group), 30 mg/kg (low-dose group) and 60 mg/kg (high-dose group) of MgIG, respectively. Animals were sacrificed at various time points and blood was drawn from the vena cava. Biochemical tests were performed with an automatic biochemical analyzer for the following items: serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamyl endopeptidase, total bilirubin (TBil), direct bilirubin (DBil), total protein, albumin, blood glucose (Glu), hyper-sensitivity C-reactive protein, prothrombin time (PT), and thrombin time (TT). Postoperative survival time was observed hourly until death. Hepatocyte regeneration was analyzed by immunohistochemistry. Serum inflammatory cytokines (IL-1, IL-6, IL-10, and iNOS) was analyzed by ELISA. STAT3 protein and mRNA were analyzed by Western blot and quantitative reverse-transcription PCR, respectively.
RESULTS: The high-dose group demonstrated a significantly prolonged survival time, compared with both the control and the low-dose groups (22.0 ± 4.7 h vs 8.9 ± 2.0 vs 10.3 ± 3.3 h, P = 0.018). There were significant differences among the groups in ALT, Glu and PT levels starting from 6 h after surgery. The ALT levels were significantly lower in the MgIG treated groups than in the control group. Both Glu and PT levels were significantly higher in the MgIG treated groups than in the control group. At 12 h, ALT, AST, TBil, DBil and TT levels showed significant differences between the MgIG treated groups and the control group. No significant differences in hepatocyte regeneration were found. Compared to the control group, the high-dose group showed a significantly increase in serum inflammatory cytokines IL-1 and IL-10, and a decrease in IL-6. Both STAT3 protein and mRNA levels were significantly lower in the MgIG treated groups than in the control group at 6 h, 12 h, and 18 h after surgery.
CONCLUSION: High-dose MgIG can extend survival time in rats after excessive hepatectomy. This hepatoprotective effect is mediated by inhibiting the inflammatory response through inhibition of the STAT3 pathway.
Keywords: Magnesium isoglycyrrhizinate, Excessive liver resection, Inflammation, Liver regeneration, STAT3
Core tip: Magnesium isoglycyrrhizinate (MgIG), a hepatocyte protective agent, has been shown to have the effects of anti-inflammation, liver cell membrane protection, and liver function improvement. We designed this study, by using the standard 90% hepatectomy model in rats, to clarify the liver protecting function of MgIG and its mechanism. We have researched postoperative survival time, hepatocyte regeneration, liver function, serum inflammatory cytokines, STAT3 protein and mRNA expression. The protective effect of MgIG in standard 90% hepatectomy is limited, which can prolong the survival time. This hepatoprotective effect was not mediated by increasing hepatocyte regeneration but rather by inhibiting the inflammatory response through inhibition of the STAT3 pathway.
INTRODUCTION
Excessive liver resection is the only hope of cure for extra-large occupying liver lesions. Most patients with liver occupying lesions often have other liver abnormalities such as cirrhosis. High surgical risk could lead to fulminant hepatic failure that has a mortality rate up to 70%-90%[1,2]. Protection of the remnant liver function in the first 48 h after surgery remains a great challenge to the surgeons[3]. The efficacy of various existing measures, including the artificial liver and other supporting methods, is limited. Our previous study in rats found that liver regeneration reaches the highest level at 72 h after resection. Animals will most likely survive if the regeneration could compensate for the first 48 h[4]. Therefore, to identify appropriate and effective measures to help the remnant liver to sustain through this risky period is particularly important.
Magnesium isoglycyrrhizinate (MgIG), a hepatocyte protective agent, has been shown to have the effects of anti-inflammation, liver cell membrane protection, and liver function improvement. Efficacy studies showed that protective function of MgIG against acute liver damage is induced by D-galactosamine. MgIG can decrease the serum level of transaminases, prevent liver cell degeneration, and reduce the incidence of necrosis and inflammatory cell infiltration. MgIG is especially effective in the treatment of chronic liver injury induced by carbon tetrachloride in rats, by reducing inflammation and fibrosis, lowering the nitro-monoxide levels, and improving liver function.
Most of previous studies of MgIG have focused on chronic hepatitis, alcoholic cirrhosis, and drug-induced liver injury[5]. A few studies mainly involved surgical ischemia-reperfusion injury[6] and liver regeneration[7]. No publication was found for the anti-inflammatory effect of MgIG after liver resection. In this study, we verified the liver protective effect of MgIG in an animal model of hepatectomy and further investigated its mechanism.
MATERIALS AND METHODS
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institute of Animal Care and Use Committee of Beijing Union Medical College (Permit No.: 2013050125). All surgeries were performed under sodium pentobarbital anesthesia, and all efforts were made to minimize animal suffering.
Experimental animals
Male Sprague-Dawley (SD) rats aged 8 wk (220-260 g) were provided by the Experimental Animal Center of Beijing Union Medical College Hospital.
Reagents
MgIG was provided by JCTT Pharmaceutical Ltd. (Jiangsu Province, China), and 3% (w/v) MgIG in saline was prepared.
Establishment of a rat model with 90% liver resection
The rats were weighted, and anesthetized with an intraperitoneal injection of pentobarbital sodium (60 mg/kg). After routine skin preparation and disinfection, an upper abdominal incision was made. Sequential isolation and resection were performed on the middle lobe, exterior lobe, right lower lobe, and right upper lobe. After confirming that there was no congestion on the caudate lobe, the abdominal cavity was closed.
Experimental design
SD rats were randomly divided into three groups: control (C), low-dose (LD), and high-dose (HD) groups. Hepatectomy was performed by resection of 90% liver tissue, followed by intraperitoneal injection of 3 mL saline (C group), 30 mg/kg (LD group), or 60 mg/kg (HD group) MgIG on the same day of surgery, as well as once daily afterwards.
Observation of survival time
Starting from the closing of the abdomen as 0 h, animal survival time was measured hourly until death. Meanwhile, the general condition of each animal was recorded. We used proper humane endpoints when the rats were in the moribund state or the signs of severe organ system dysfunction non-responsive to treatment appeared and euthanized rats prior to the end of experiments.
Liver function evaluation
Animals were sacrificed at various time points and blood was drawn from the vena cava. Biochemical tests were performed with an automatic biochemical analyzer (HITACHI, Japan) for the following items: serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamyl endopeptidase (GGt), total bilirubin (TBil), direct bilirubin (DBil), total protein (TP), albumin (Alb), blood glucose (Glu), hyper-sensitivity C-reactive protein (hsCRP), prothrombin time (PT), and thrombin time (TT).
Measurement of liver regeneration
The average proportion of liver weight against the body weight was established from 5 rats of same age. It was found that the liver weight was 3.96% ± 0.01% of the body weight. The remnant caudate lobe was removed from the animal model at various time points after surgery and weighed. Liver regeneration rate was calculated by the Okano T formula: Regeneration rate (R, %) = [C-(A-B)]/(A-B) × 100%, where A is preoperative estimation of the rat liver weight, B is the weight of resected liver tissue, and C is the weight of the remnant caudate lobe[8].
Observation of liver damage and regeneration by HE and BrdU staining
BrdU (Sigma, United States) (100 μg/g body weight) was administered intraperitoneally to animals 2 h prior to the removal of remnant caudate lobe. The remaining caudate lobe was removed at 0, 6, 12 and 18 h after surgery, fixed and sectioned, and underwent immunohistochemistry procedure or HE staining. BrdU positive cells were counted under a microscope. At least 3 rats from each group, 3 slides from each rat, and 3 fields from each slide were counted, and the results were presented as the number of positive cells in every 500 total cells being counted.
Expression of proliferating cell nuclear antigen
Paraffin embedded sections of remnant liver tissue were studied by immunohistochemistry for proliferating cell nuclear antigen (PCNA) expression. PCNA antibody was obtained from Abcam, United States. PCNA-positive cells were counted under a light microscope. At least 3 rats from each group, 3 slides from each rat, and 3 fields from each slide were counted, and the results were presented as the number of positive cells in every 500 total cells being counted.
Western blot
Remnant liver tissue was also homogenized and treated with RIPA lysis buffer (Dingguo, China); the extracted proteins were resolved on 4%-12% acrylamide gradient gels. After electrophoresis, proteins were transferred to a PVDF membrane using iBlot fast transfer electric transfer (Invitrogen, United States). The membrane was blocked with 5% milk at room temperature for 1 h, and incubated with primary antibody against PCNA or STAT3 (1:1000, Abcam, United States) 4 °C overnight, followed by TBST washing three times, secondary antibody (1:8000, Abcam United States) incubation at room temperature for 2 h, TBST washing 3 times, and exposure to film with ECL kit (Pierce, United States). Densitometry analysis was performed with BandScan software.
ELISA assay for inflammatory factor detection in serum
Blood was drawn from the vena cava at 0, 6, 12 and 18 h after surgery, and serum was separated and stored -80 °C. ELISA assays to determine IL-1, IL-6, IL-10, and iNOS expression levels were performed according to manufacturer’s instructions (Cusabio, Wuhan, China).
Quantitative real-time PCR
Total RNA was extracted from the remnant liver tissue with Trizol. TAKARA retroviral reverse transcriptase kit (TAKARA, Japan) was used to synthesize cDNA with the reaction condition of 37 °C for 60 min and 95 °C for 3 min. Primers were designed as forward, 5’-CACAACCTGCGAAGAATCAAG-3’ and reverse, 5’-GCTGCTTCTCCGTCACTAC-3’ for STAT3 gene; and forward, 5’-AACGGCTCCGGCATGTGCAA-3’ and reverse, 5’-CTTCTGACCCATGCCCACCA-3 ‘ for β-actin.
Real-time PCR was performed with Applied Biosystems 7500 real-time quantitative PCR instrument (Applied Biosystems 7500, United States) under the following condition: 95 °C for 20 s, 60 °C for 30 s, 72 °C for 30 s for 40 cycles[8].
Statistical analysis
Statistical analyses were performed using SPSS13.0 software (Chicago, IL). Data are expressed as mean ± SD. The difference between groups was compared using one-way ANOVA, and survival analysis was performed using the Kaplan-Meier method. Two-tailed P < 0.05 was considered statistically significant.
RESULTS
Comparison of postoperative survival
Seven out of fifteen (46.7%) rats in the control group did not recover from the anesthesia and died. The remaining rats in the control group exhibited poor condition even though they became awake from anesthesia. No active movement was observed; the hair was dry, and the breathing was slow and laborious. The response to external stimuli was weak, and there was no uptake of water. No animal from the control group survived more than 24 h after surgery. Forty percent (6/15) of the rats in the low-dose MgIG treatment group died before waking up from anesthesia. The remaining rats showed better sign of life than the control group, in that the response to external stimuli was stronger, and some rats could uptake small volume of water. One of the animals survived longer than 24 h. In the high-dose MgIG treatment group, 26.7% (4/15) of the rats died shortly after surgery without waking up from anesthesia. The remaining animals showed slow active movement, uptake of water, and clean hair. Four rats survived longer than 24 h but none exceeded 60 h.
Survival time of the three groups was plotted using Kaplan-Meier survival curves, and the results are shown in Figure 1. Survival time of the control group was 8.9 ± 2.0 h with a median of 6.8 h, low-dose group was 10.3 ± 3.3 h with a median of 5.8 h, and high-dose group 22.0 ± 4.7 h with a median of 17.6 h. There were significant differences in survival time among the three groups (P = 0.018).
Figure 1.
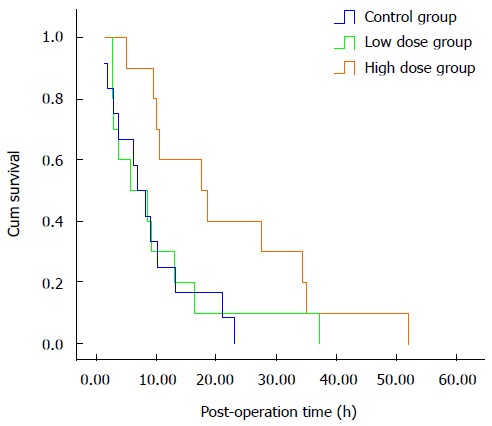
Kaplan-Meier survival curves of the three experimental animal groups.
Liver function assessment
Liver function of the animals at various time points after hepatectomy was assessed by studying a variety of serum biomarkers including ALT, AST, GGT, TBIL, DBIL, TP, ALB, Glu, hsCRP, PT and TT.
As shown in Table 1, there were significant differences among the groups in ALT, Glu and PT levels starting from 6 h after surgery. The ALT levels were significantly lower in the MgIG treated groups than in the control group. Both Glu and PT levels were significantly higher in the MgIG treated groups than in the control group. At 12 h, ALT, AST, TBil, DBil and TT levels showed significant differences between the MgIG treated groups and the control group (P < 0.05 for TBIL and P < 0.01 for all the rest). We also tested serum ALB and hsCRP at various time points after hepatectomy and found no significant difference.
Table 1.
Serological test results of experimental animals after excessive liver resection
|
Time point (mean ± SD) |
||||||
| Item | Group | 0 h | 6 h | 12 h | 18 h | P value1 |
| Control | 165.8 ± 118.0 | 864.0 ± 471.7 | 1927.5 ± 1079.0 | 1969.6 ± 1201.6 | 0.000 | |
| ALT (U/L) | Low dose | 86.4 ± 23.4 | 357.2 ± 119.8 | 705.1 ± 341.5 | 1597.7 ± 998.6 | 0.000 |
| High dose | 145.6 ± 106.8 | 524.7 ± 182.5 | 1234.8 ± 784.2 | 1321.2 ± 797.4 | 0.000 | |
| P value2 | 0.133 | 0.002 | 0.003 | 0.3 | ||
| Control | 228.4 ± 110.4 | 929.3 ± 477.7 | 1779.5 ± 495.9 | 1673.2 ± 668.8 | 0.000 | |
| AST (U/L) | Low dose | 192.6 ± 37.5 | 664.7 ± 171.5 | 1027.5 ± 396.9 | 1904.3 ± 794.7 | 0.000 |
| High dose | 193.1 ± 99.5 | 694.6 ± 278.7 | 1370.6 ± 502.7 | 1355.2 ± 671.5 | 0.000 | |
| P value | 0.556 | 0.158 | 0.001 | 0.192 | ||
| Control | 1.11 ± 0.93 | 1.50 ± 0.85 | 2.14 ± 1.51 | 1.55 ± 0.93 | 0.197 | |
| GGT (U/L) | Low dose | 0.70 ± 0.48 | 0.88 ± 0.35 | 1.27 ± 1.27 | 2.89 ± 2.42 | 0.008 |
| High dose | 0.72 ± 0.47 | 1.00 ± 0.71 | 2.36 ± 1.21 | 2.00 ± 1.08 | 0.001 | |
| P value | 0.316 | 0.154 | 0.147 | 0.158 | ||
| Control | 50.7 ± 4.9 | 47.3 ± 3.6 | 46.3 ± 4.8 | 45.5 ± 3.6 | 0.031 | |
| TP (g/L) | Low dose | 48.3 ± 2.9 | 45.0 ± 2.9 | 44.9 ± 3.2 | 44.6 ± 3.8 | 0.039 |
| High dose | 51.6 ± 3.3 | 45.5 ± 2.4 | 48.5 ± 3.6 | 45.6 ± 5.8 | 0.001 | |
| P value | 0.101 | 0.212 | 0.094 | 0.849 | ||
| Control | 1.95 ± 0.62 | 6.68 ± 1.32 | 16.59 ± 2.16 | 20.13 ± 4.19 | 0.000 | |
| TBil (U/L) | Low dose | 1.20 ± 0.30 | 4.35 ± 0.77 | 7.45 ± 4.66 | 14.82 ± 2.06 | 0.000 |
| High dose | 1.67 ± 0.43 | 6.23 ± 2.22 | 14.31 ± 2.16 | 12.81 ± 2.29 | 0.000 | |
| P value | 0.539 | 0.532 | 0.011 | 0.206 | ||
| Control | 1.22 ± 0.37 | 4.27 ± 0.64 | 13.56 ± 1.83 | 16.33 ± 3.46 | 0.000 | |
| DBil (U/L) | Low dose | 0.65 ± 0.15 | 2.88 ± 0.47 | 5.25 ± 1.01 | 11.15 ± 1.72 | 0.000 |
| High dose | 0.99 ± 0.26 | 4.62 ± 1.82 | 10.95 ± 1.59 | 9.72 ± 1.80 | 0.000 | |
| P value | 0.353 | 0.527 | 0.004 | 0.14 | ||
| Control | 7.41 ± 1.27 | 4.92 ± 2.54 | 6.83 ± 2.33 | 4.21 ± 1.40 | 0.001 | |
| Glu (mmol/L) | Low dose | 6.50 ± 1.21 | 6.30 ± 1.62 | 6.09 ± 1.84 | 4.11 ± 2.13 | 0.012 |
| High dose | 7.12 ± 2.05 | 8.54 ± 3.69 | 5.25 ± 2.19 | 5.21 ± 1.02 | 0.002 | |
| P value | 0.395 | 0.016 | 0.162 | 0.149 | ||
| Control | 42.7 ± 6.1 | 42.0 ± 4.6 | 47.8 ± 10.1 | 41.2 ± 10.0 | 0.754 | |
| TT (s) | Low dose | 48.9 ± 3.5 | 49.7 ± 6.6 | 49.5 ± 5.3 | 48.4 ± 0.4 | 0.984 |
| High dose | 38.2 ± 6.1 | 46.2 ± 2.0 | 11.5 ± 1.1 | 43.2 ± 1.1 | 0.000 | |
| P value | 0.127 | 0.226 | 0.001 | 0.36 | ||
| Control | 8.9 ± 0.6 | 10.3 ± 0.3 | 11.7 ± 1.1 | 12.4 ± 0.4 | 0.001 | |
| PT (s) | Low dose | 9.9 ± 0.6 | 10.9 ± 0.5 | 12.6 ± 1.9 | 14.2 ± 1.6 | 0.016 |
| High dose | 9.1 ± 0.1 | 12.6 ± 0.7 | 11.5 ± 1.1 | 14.8 ± 0.8 | 0.000 | |
| P value | 0.077 | 0.003 | 0.618 | 0.076 | ||
Comparison among time points of same group;
Comparison among groups at same time point. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; PT: Prothrombin time; TT: Thrombin time; TBil: Total bilirubin; Glu: Glucose; DBil: Direct bilirubin.
Liver regeneration
Figure 2 shows the liver regeneration status at various time points after surgery. Proportion of weight of the remnant liver was found to be higher immediately after hepatectomy surgery compared with the normal proportion of liver to the body weight in all the three groups, and the weight of remnant liver tissue increased over time. In the control group, a 2.71% ± 0.58% increase was found at 0 h, and 11.95% ± 1.14% at the time of 18 h. However, there were no significant differences among the three groups (Figure 2).
Figure 2.
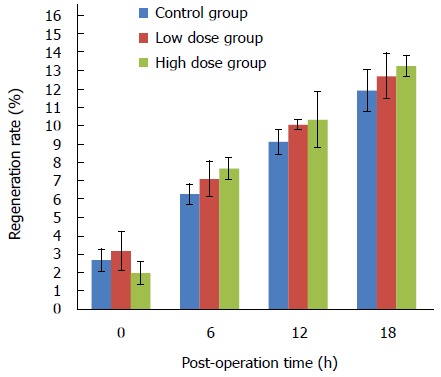
Regeneration rates at various time points in three groups.
Liver cell degeneration and necrosis were observed after HE staining of the remnant liver sections (magnification, × 100) in all animals. Fatty degeneration was found in animals of both the low-dose and high-dose MgIG treatment groups, but was more prominontin the high-dose group. The representative HE staining of liver sections of the animals in the high-dose group at 0 h, 6 h, 12 h and 18 h is shown in Figure 3. Hepatocytes had normal size and shape, and no obvious damage was found in the nucleus at 0 h (Figure 3A). At 6 h, cells showed little change in size or shape. Cell number increased, and the arrangement was slightly disordered (Figure 3B). At 12 h, a more increase in cell number as well as more disordered arrangements was observed. There was a small number of cells with fatty degeneration, but no obvious liver cell necrosis was visible (Figure 3C). More fatty degeneration and necrosis were observed at 18 h. The number of cells decreased, and the cell arrangement became irregular (Figure 3D). No obvious generative nodule was observed under a microscope. Obvious fatty degeneration and necrosis were observed in all three groups at 12 h and 18 h.
Figure 3.
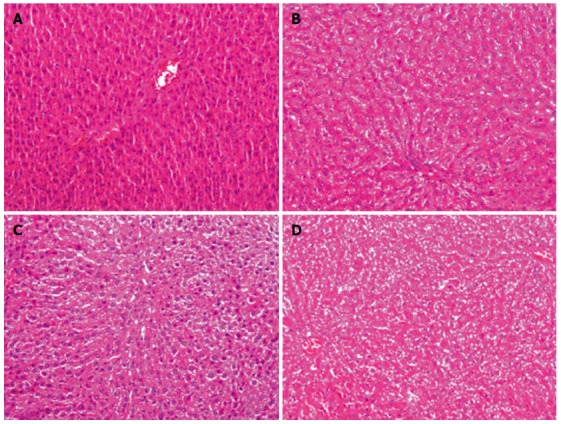
Hematoxylin-eosin staining in the high-dose group at 0 h (A), 6 h (B), 12 h (C) and 18 h (D) (magnification × 100).
As a thymidine analogue, BrdU can substitute thymidine to incorporate into the double-stranded DNA during DNA synthesis. Therefore, cells that have gone through S phase (DNA synthesis phase) would become BrdU positive, an indication of cell proliferation. As shown in Figure 4, the number of BrdU-positive cells among the three groups showed no significant difference. No obvious change was noted in BrdU-positive cells in the control group at 0 h, 6 h and 12 h, although there was a slight but not statistically significant increase in BrdU-positive cells at 18 h. Similar patterns were observed in both the high-dose and low-dose MgIG treated groups.
Figure 4.
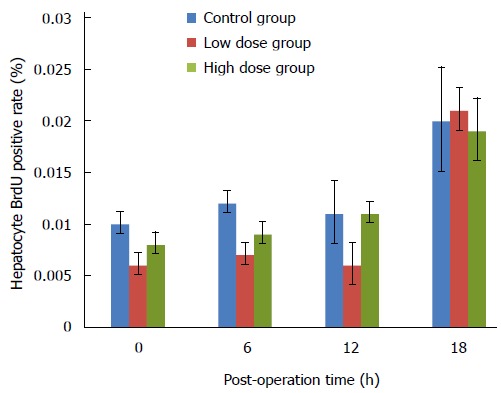
BrdU positive rates of the caudate lobe hepatocytes at various time points in three groups.
PCNA is an auxiliary protein for DNA synthase δ whose expression is cell cycle dependent. Its expression starts at late G1 phase and reaches the peak level at S phase. PCNA has been used as an indicator for cell proliferation. In this study, we used both immunohistochemistry to detect the percentage of PCNA-positive cells in the remnant liver tissue, as well as Western blot to assess the overall expression levels of PCNA in the liver. Figure 5 shows that there was no significant difference in the percentage of PCNA-positive cells among the three groups. Over the course of 18 h, the number of PCNA-positive cells in all groups remained at a relative low level. As shown in Figure 6, there were no significant differences in the overall expression levels of PCNA among three groups as indicated by Western blot analysis.
Figure 5.
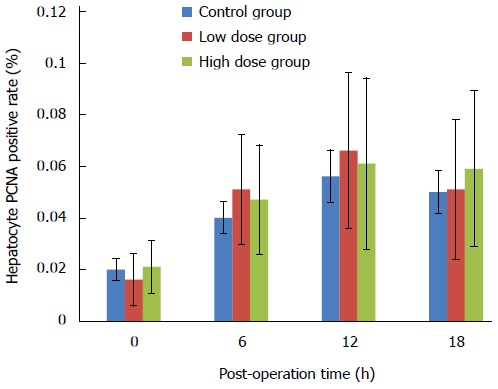
Proliferating cell nuclear antigen positive rates of the caudate lobe hepatocytes at various time points in three groups. PCNA: Proliferating cell nuclear antigen.
Figure 6.
Expression of proliferating cell nuclear antigen in the caudate lobe detected by Western blot at various time points in three groups. PCNA: Proliferating cell nuclear antigen.
Inflammatory cytokines
Inflammatory cytokines IL-1, IL-6, IL-10 and iNOs in serum were detected by ELISA. As shown in Figure 7, IL-1 level at 6 h after surgery was significantly higher in the serum of rats in the MgIG treated groups than in the control group (P < 0.05; Figure 7A). IL-6 level at 12 h was significantly lower in the low-dose group than in the control group, and in the high-dose group than in the low-dose group (P < 0.05; Figure 7B). The level of IL-10 was found to be significantly higher in the high-dose group at 18 h than in the other groups (P < 0.05; Figure 7C). No significant difference was found among the groups in iNOs expression at any time (Figure 7D).
Figure 7.
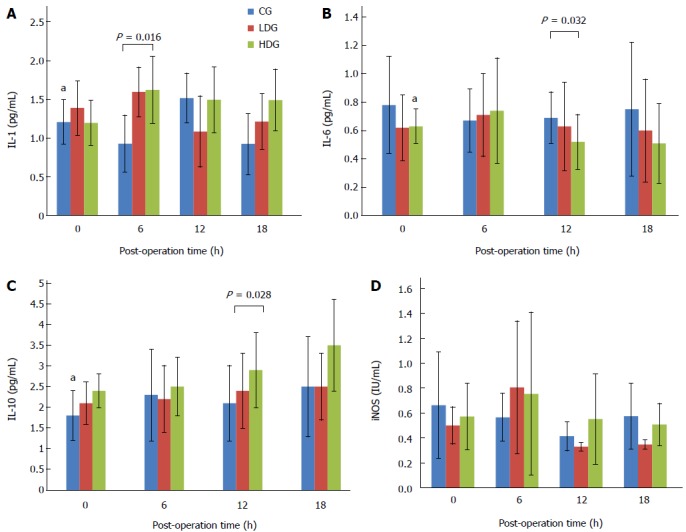
Expression of cytokines IL-1, IL-6, IL-10 and iNOS at various time points in three groups. aP < 0.05.
STAT3 mRNA and protein levels
STAT3 is an important transcription factor that can be activated in response to a variety of cytokines and growth factors. Our finding of the elevated serum levels of IL-1 and IL-10 as well as reduced level of IL-6 in the rats of the high-dose group suggests that MgIG may modulate the inflammation response. To further clarify the mechanism, we studied the STAT3 protein expression in the remnant liver tissue by Western blot. At least 6 rats from each group were studied, and Figure 8 shows one representative Western blot. Densitometry analysis was performed to calculate relative protein expression levels based on the level of β-actin. There was no significant difference in STAT3 levels among all three groups at 0 h. The expression level of STAT3 in the high-dose group at 6 h was found to be significantly lower than those of the control group and low-dose group (P < 0.05). At 12 h, the high-dose group still exhibited lower STAT3 level compared with the control and low-dose groups, but there was no statistically significant difference. At 18h, STAT3 expression in the low-dose and high-dose groups was lower than that in the control group. The expression of STAT3 in the control group was found to be increasing gradually over the course of 18 h after hepatectomy, and significant differences existed between each time points (P < 0.01). No statistically significant differences in STAT3 levels, however, were found among different time points in the low-dose group. In the high-dose group, STAT3 protein was inhibited initially, followed by a gradual increase, and at 18 h reached 4.5 times of the level at 0 h. This may explain the suppression of inflammatory reaction in the high-dose group.
Figure 8.
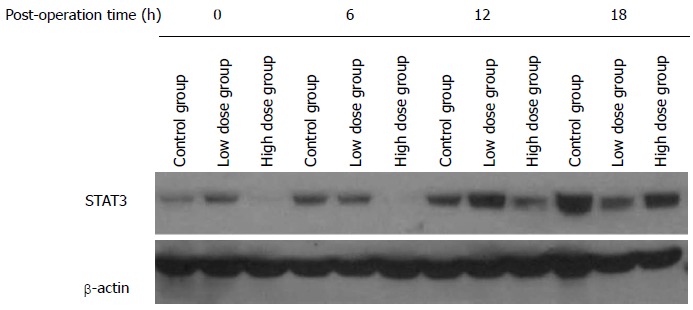
Expression of STAT3 in caudate lobe detected by Western blot at various time points in three groups.
Quantitative real-time PCR was used to detect the relative levels of STAT3 mRNA, and results are shown in Table 2. At 0 h, no significant differences in STAT3 mRNA levels were seen among three groups. At both 6 h and 12 h, the levels of STAT3 mRNA in the high-dose group were significantly lower than those in the control and low-dose groups (P < 0.05). At 18 h, STAT3 mRNA levels in rat livers of the low-dose and high-dose groups were lower than that of the control group, but there was no statistically significant difference.
Table 2.
STAT3 mRNA levels at various time points after excessive liver resection in rats
|
STAT3 mRNA expression (mean ± SD) |
|||||||||
| Group | 0 h | n | 6 h | n | 12 h | n | 18 h | n | P value1 |
| Control | 0.33 ± 0.11 | 3 | 0.43 ± 0.21 | 3 | 0.57 ± 0.24 | 3 | 1.07 ± 0.47 | 3 | 0.021 |
| Low dose | 0.41 ± 0.15 | 3 | 0.44 ± 0.19 | 3 | 0.78 ± 0.34 | 3 | 0.89 ± 0.45 | 3 | 0.043 |
| High dose | 0.38 ± 0.24 | 3 | 0.11 ± 0.07 | 3 | 0.30 ± 0.14 | 3 | 0.87 ± 0.36 | 3 | 0.025 |
| P value2 | 0.401 | 0.012 | 0.044 | 0.849 | |||||
Comparison among time points of same group;
Comparison among groups at same time point.
DISCUSSION
Glycyrrhizin is commonly used clinically as a liver protective medicine. MgIG is the fourth generation of glycyrrhizin preparations. It has better affinity with target cell receptors, and stronger anti-inflammatory and anti-oxidation effects. It has been shown to stabilize hepatocyte membrane and improve liver function[7,9]. MgIG has shown the effects of lowering the liver toxicity of free fatty acid by preventing mitochondrial damage[10,11] and protecting hepatocytes from ischemia and reperfusion induced injury[12,13].
Employing an improved version of Emond’s method[14], we generated an animal model of 90% hepatectomy with 100% mortality within 24 h[15], for the purpose of evaluating appropriate treatments.
Our results showed significantly longer survival time of rats in the treatment group than in the control group. And the survival time seemed to be MgIG dose-dependent. Also, general appearance in the treated groups was also superior to that in the control group. By examining liver functions, it was found that MgIG demonstrated a liver protective effect, resulting in low ALT, AST, TBil, and DBil levels in the treated groups. This study found that postoperative ALT, AST, TBil, and TBil gradually increased to reach the peak at 18 h, which may be due to direct physical injury and surgery factors such as ischemia and reperfusion injury. At the postoperative 0 h, the liver function and coagulation parameters had no significant differences among the three groups, implying that MgIG protects the liver function, but cannot have an immediate impact. It was found that the magnitude of increase in transaminases in the treatment group was significantly lower than that in the control group at postoperative time points of 6 h and 12 h, with the high-dose group being more obvious. These findings demonstrated a protective effect of MgIG on the remnant liver after hepatectomy. Comparing serum biochemical markers of normal rats and early death rats, we found that a rapid liver cell deterioration and significant raise of liver enzymes. This reflects an excessive inflammatory response and a severe necrosis of the residual liver cells after 90% hepatectomy, and confirms the correspondence between early death and excessive inflammation in rats.
Liver regeneration after hepatectomy is one of the major mechanisms for compensating liver volume loss, and maintaining sufficient liver function[16-18]. In this study, however, we did not find obvious regeneration, even though the weight of remnant liver tissue did increase over time, which could be caused by physical response towards surgery, such as edema and congestion, and short time period of the study. It thus implies that MgIG improves the survival time of the rats with 90% hepatectomy mainly through the decreased inflammatory response rather than regeneration.
Excessive inflammatory reaction plays an important role in remaining liver damage in the postoperative outcome[19-21]. Inhibition of inflammatory reaction would be beneficial to the hepatocyte regeneration[22-25]. MgIG may prolong the survival time after hepatectomy by enhancing liver regeneration and/or suppressing excessive inflammatory response[21,26,27]. We found that compared to the control group, serum IL-10 levels were significantly increased in the MgIG treated groups, while IL-6 levels were significantly decreased. This indicates that MgIG may modulate inflammatory response in rats after hepatectomy, in which the inflammatory response in the MgIG treated groups was inhibited. This may explain the reason that the MgIG treated groups had a prolonged survival time after hepatectomy.
The JAK/STAT3 pathway, which plays a critical role in the inflammation response, can be activated by various cytokines including IL-6[28-30]. We found that the expression levels of STAT3 in remnant livers of the animals treated with MgIG were decreased compared to the ones treated with saline. This could be one of the possible mechanisms for the inflammation inhibition. Further study is required to assess STAT3 function, as well as the expression and function of other related proteins.
More fatty degeneration was found in the high-dose MgIG treatment group with visible necrosis, indicating that MgIG might affect the fatty acid metabolism in severely injured liver. It is not known whether high doses of MgIG could have any side effect, since this is not observed in the low-dose MgIG treatment group. The clinical significance of the fatty degeneration remains unclear. The prolonged survival time that the high-dose group demonstrated is a collective result of the administration of the MgIG; however, since no animals survived longer than 60 h in this study, it is not clear whether there is any drawback of the use of the drug in the long run.
There were certain limitations in this study. The animal model was generated to an extreme of 90% resection, which probably will never occur in human beings. Therefore, it would be hard to relate our results to clinical practice. Excessive hepatectomy might also limit the effect of MgIG. In addition, the dose-effect relationship is not clear, besides the high dose resulted in longer survival time. This creates more questions than answers such as: whether MgIG functions through receptor binding on cell surface or directly on cellular proteins; what is the mechanisms for the decreased levels of ALT, AST, TBil, and DBil resulting from MgIG treatment.
In conclusion, MgIG application in excessive hepatectomy animals resulted in prolonged survival time, reduced transaminases, total bilirubin, as well as the inflammation response. The STAT3 pathway was inhibited in a way that the expression of STAT3 protein was decreased. The prolonged survival time could be potentially critical and lifesaving, because it creates a valuable time window for other treatment applications.
COMMENTS
Background
Excessive liver resection is the only hope of cure for large occupying liver lesions. Protection of the remnant liver function in first 48 h after surgery remains a great challenge to the liver surgeons. The efficacy of various existing measures, including the artificial liver and other supporting methods, is often limited. Anti-inflammation in the remnant liver after hepatectomy may be of benefit for the patients.
Research frontiers
A previous study in rats found that liver regeneration reached the highest level at 72 h after resection. Animals will most likely survive if the regeneration could compensate for the first 48 h. Therefore, to identify appropriate and effective measures to help the remnant liver to sustain through this risky period is particularly important. Magnesium isoglycyrrhizinate (MgIG), a hepatocyte protective agent, has been shown to have the effects of anti-inflammation, liver cell membrane protection, and liver function improvement. Most of the previous studies of MgIG have focused on chronic hepatitis, alcoholic cirrhosis, and drug-induced liver injury. A few studies mainly involved surgical ischemia-reperfusion injury and liver regeneration. This study was designed to investigate the protective effect of MgIG on excessive hepatectomy animal model and its possible mechanism.
Innovations and breakthroughs
This is the first to report the anti-inflammatory effect of MgIG after liver resection. The protective effect of MgIG has been shown to prolong the survival time following standard 90% hepatectomy. This hepatoprotective effect was not via an increase in hepatocyte regeneration, rather through inhibition in the inflammatory response through via STAT3 pathway.
Applications
The protective effect of MgIG in standard 90% hepatectomy can prolong the survival time. This study provides experimental evidence with potential benefits for the further mechanism research or clinical studies.
Terminology
Liver regeneration rate was calculated by the Okano T formula: Regeneration rate (R, %) = [C-(A-B)]/(A-B) × 100%, where A is preoperative estimation of the rat liver weight, B is the weight of resected liver tissue, and C is the weight of the remnant caudate lobe.
Peer-review
This is an interesting study which was designed to investigate whether magnesium isoglycyrrhizinate inhibits inflammatory response through STAT3 pathway to protect remnant liver function. In this study, Sprague-Dawley rats with 90% liver resection were divided into three groups. The postoperative survival time, hepatocyte regeneration, liver function, serum inflammatory cytokines and STAT3 protein were analyzed. They found that high-dose MgIG can extend survival time in rats after excessive hepatectomy. And the hepatoprotective effect was not by increasing hepatocyte regeneration but rather by inhibiting the inflammatory response through inhibition of the STAT3 pathway. The study is well designed and conducted, and the results are reliable and interesting.
Footnotes
Supported by a grant from the State Science and Technology Ministry, No. 2012BAI06B01.
Institutional review board statement: The study protocol was approved by the Ethics Committee of the Peking Union Medical College Hospital.
Institutional animal care and use committee statement: All animal experiments were reviewed and approved by the Ethics Committee of the Peking Union Medical College Hospital.
Conflict-of-interest statement: The authors have none to declare.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 5, 2015
First decision: June 3, 2015
Article in press: September 15, 2015
P- Reviewer: Ward J S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Miao R, Luo H, Zhou H, Li G, Bu D, Yang X, Zhao X, Zhang H, Liu S, Zhong Y, et al. Identification of prognostic biomarkers in hepatitis B virus-related hepatocellular carcinoma and stratification by integrative multi-omics analysis. J Hepatol. 2014;61:840–849. doi: 10.1016/j.jhep.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 2.Yu Z, Mao Y. Current status on investigation of the inflammatory response after liver resection. Shiyong Waike Zazhi. 2004;24:124–125. [Google Scholar]
- 3.Lu H, Lu L, Xu ZC, Lu YJ, Zhao B, Zhuang L, Hao BB, Zhang F. Tauroursodeoxycholic acid and 4-phenyl butyric acid alleviate endoplasmic reticulum stress and improve prognosis of donation after cardiac death liver transplantation in rats. Hepatobiliary Pancreat Dis Int. 2014;13:586–593. doi: 10.1016/s1499-3872(14)60269-1. [DOI] [PubMed] [Google Scholar]
- 4.Mao Y, Yu Z, Sang X, Lu X, Zhong S. A study on prolonging survival time of rats following 90% hepatectomy. Shiyong Waike Zazhi. 2004;19:301–303. [Google Scholar]
- 5.Bao Q, Yang L, Wang L, Cui D. Protective effect of Magnesium Isoglycyrrhizinate on acute liver injury induced by CCl4. Shijie Huaren Xiaohua Zazhi. 2008;16:1004–1007. [Google Scholar]
- 6.Fei X, Zhai Y, Zhang H, Ye Q. Effect of magnesium isoglycyrrhizinate on hepatocyte apoptosis and bcl-2 expression after ischemia reperfusion injury in rat liver. Shiyong Linchuang Yiyao Zazhi. 2011;15:5–7. [Google Scholar]
- 7.Zhao D, Liang L, Yin X. Magnesium Isoglycyrrhizinate provides positive effect on regeneration and liver function of resected liver with cirrhosis of in rats. Shiyong Waike Zazhi. 2010;25:988–991. [Google Scholar]
- 8.Fan YD, Praet M, Van Huysse J, Lelie B, De Hemptinne B. Effects of portal vein arterialization on liver regeneration after partial hepatectomy in the rat. Liver Transpl. 2002;8:146–152. doi: 10.1053/jlts.2002.30887. [DOI] [PubMed] [Google Scholar]
- 9.Yan Y, Mo Y, Zhang D. [Magnesium isoglycyrrhizinate prevention of chemotherapy-induced liver damage during initial treatment of patients with gastrointestinal tumors] Zhonghua Ganzangbing Zazhi. 2015;23:204–208. doi: 10.3760/cma.j.issn.1007-3418.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Y, Zhang J, Shang J, Zhang L. Prevention of free fatty acid-induced hepatic lipotoxicity in HepG2 cells by magnesium isoglycyrrhizinate in vitro. Pharmacology. 2009;84:183–190. doi: 10.1159/000235873. [DOI] [PubMed] [Google Scholar]
- 11.Xie C, Li X, Wu J, Liang Z, Deng F, Xie W, Zhu M, Zhu J, Zhu W, Geng S, et al. Anti-inflammatory Activity of Magnesium Isoglycyrrhizinate Through Inhibition of Phospholipase A2/Arachidonic Acid Pathway. Inflammation. 2015;38:1639–1648. doi: 10.1007/s10753-015-0140-2. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Qin J, Lu S. Magnesium isoglycyrrhizinate protects hepatic L02 cells from ischemia/reperfusion induced injury. Int J Clin Exp Pathol. 2014;7:4755–4764. [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao ZW, Zhang W, Ma L, Qiu ZW. Therapeutic effect of magnesium isoglycyrrhizinate in rats on lung injury induced by paraquat poisoning. Eur Rev Med Pharmacol Sci. 2014;18:311–320. [PubMed] [Google Scholar]
- 14.Emond J, Capron-Laudereau M, Meriggi F, Bernuau J, Reynes M, Houssin D. Extent of hepatectomy in the rat. Evaluation of basal conditions and effect of therapy. Eur Surg Res. 1989;21:251–259. doi: 10.1159/000129034. [DOI] [PubMed] [Google Scholar]
- 15.Shu H, Liu K, He Q, Zhong F, Yang L, Li Q, Liu W, Ye F, Huang W. Ulinastatin, a protease inhibitor, may inhibit allogeneic blood transfusion-associated pro-inflammatory cytokines and systemic inflammatory response syndrome and improve postoperative recovery. Blood Transfus. 2014;12 Suppl 1:s109–s118. doi: 10.2450/2013.0224-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jourdainne V, Péan N, Doignon I, Humbert L, Rainteau D, Tordjmann T. The Bile Acid Receptor TGR5 and Liver Regeneration. Dig Dis. 2015;33:319–326. doi: 10.1159/000371668. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Li J, Ou YJ, Zhu XX, Yin XY, Zhu YX, Tang D. Hepatoprotective effect of ulinastatin in a rat model of major hepatectomy after obstructive jaundice. Dig Dis Sci. 2015;60:1680–1689. doi: 10.1007/s10620-015-3543-x. [DOI] [PubMed] [Google Scholar]
- 18.Ben Mosbah I, Duval H, Mbatchi SF, Ribault C, Grandadam S, Pajaud J, Morel F, Boudjema K, Compagnon P, Corlu A. Intermittent selective clamping improves rat liver regeneration by attenuating oxidative and endoplasmic reticulum stress. Cell Death Dis. 2014;5:e1107. doi: 10.1038/cddis.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du Z, Zhou Y, Lu X, Li L, Lu C, Li L, Li B, Bu H, Yang J, Shi Y. Octreotide prevents liver failure through upregulating 5’-methylthioadenosine in extended hepatectomized rats. Liver Int. 2015:Epub ahead of print. doi: 10.1111/liv.12863. [DOI] [PubMed] [Google Scholar]
- 20.Li CH, Zhang X, Ge XL, Huang X, Zhang AQ, Gu WQ. Effects of combined anisodamine and neostigmine treatment on the inflammatory response and liver regeneration of obstructive jaundice rats after hepatectomy. Biomed Res Int. 2014;2014:362024. doi: 10.1155/2014/362024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schon HT, Weiskirchen R. Immunomodulatory effects of transforming growth factor-β in the liver. Hepatobiliary Surg Nutr. 2014;3:386–406. doi: 10.3978/j.issn.2304-3881.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan R, Zhang L, Xia N, Liu Q, Sun H, Guo H. Knockdown of augmenter of liver regeneration in HK-2 cells inhibits inflammation response via the mitogen-activated protein kinase signaling pathway. Inflamm Res. 2015;64:453–462. doi: 10.1007/s00011-015-0825-x. [DOI] [PubMed] [Google Scholar]
- 23.Yan R, Li Y, Zhang L, Xia N, Liu Q, Sun H, Guo H. Augmenter of liver regeneration attenuates inflammation of renal ischemia/reperfusion injury through the NF-kappa B pathway in rats. Int Urol Nephrol. 2015;47:861–868. doi: 10.1007/s11255-015-0954-8. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, Zhan Q, Liu HX, Chau T, Li Y, Yvonne Wan YJ. Accelerated partial hepatectomy-induced liver cell proliferation is associated with liver injury in Nur77 knockout mice. Am J Pathol. 2014;184:3272–3283. doi: 10.1016/j.ajpath.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kampschulte M, Stöckl C, Langheinrich AC, Althöhn U, Bohle RM, Krombach GA, Stieger P, Churin Y, Kremer S, Dierkes C, et al. Western diet in ApoE-LDLR double-deficient mouse model of atherosclerosis leads to hepatic steatosis, fibrosis, and tumorigenesis. Lab Invest. 2014;94:1273–1282. doi: 10.1038/labinvest.2014.112. [DOI] [PubMed] [Google Scholar]
- 26.Best J, Manka P, Syn WK, Dollé L, van Grunsven LA, Canbay A. Role of liver progenitors in liver regeneration. Hepatobiliary Surg Nutr. 2015;4:48–58. doi: 10.3978/j.issn.2304-3881.2015.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng MZ, Zhuang PY, Hei ZY, Lin PY, Chen ZS, Liu YB, Quan ZW, Tang ZH. ZBTB20 is involved in liver regeneration after partial hepatectomy in mouse. Hepatobiliary Pancreat Dis Int. 2014;13:48–54. doi: 10.1016/s1499-3872(14)60006-0. [DOI] [PubMed] [Google Scholar]
- 28.van Lienden KP, Hoekstra LT, van Trigt JD, Roelofs JJ, van Delden OM, van Gulik TM. Effect of hepatic artery embolization on liver hypertrophy response in a rabbit liver VX2 tumor model. Hepatobiliary Pancreat Dis Int. 2013;12:622–629. doi: 10.1016/s1499-3872(13)60098-3. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Lee JS, Hyun J, Kim J, Kim SU, Cha HJ, Jung Y. Tumor necrosis factor-inducible gene 6 promotes liver regeneration in mice with acute liver injury. Stem Cell Res Ther. 2015;6:20. doi: 10.1186/s13287-015-0019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikolaou K, Sarris M, Talianidis I. Molecular pathways: the complex roles of inflammation pathways in the development and treatment of liver cancer. Clin Cancer Res. 2013;19:2810–2816. doi: 10.1158/1078-0432.CCR-12-1961. [DOI] [PubMed] [Google Scholar]


