Abstract
AIM: To establish an ultrasonographic classification based on a large sample of patients with confirmed hepatic alveolar echinococcosis (AE).
METHODS: Clinical data and ultrasonography (US) findings of 185 patients (100 males; 85 females; mean age at diagnosis: 51.4 ± 17.6 years; mean age at time of US examination: 58.7 ± 18.2 years) were retrospectively reviewed with respect to the US morphology of hepatic AE lesions. The sonomorphological findings were grouped according to a five-part classification scheme.
RESULTS: Application of the new classification resulted in the following distribution of sonomorphological patterns among the patients examined: hailstorm (54.1%); pseudocystic (13.5%); ossification (13.0%); hemangioma-like (8.1%); and metastasis-like (6.5%). Only 4.9% of lesions could not be assigned to a sonomorphological pattern.
CONCLUSION: The sonomorphological classification proposed in the present study facilitates the diagnosis, interpretation and comparison of hepatic alveolar echinococcosis in routine practice and in the context of scientific studies.
Keywords: Hepatic echinococcosis, Echinococcus multilocularis, Classification, Diagnosis, Ultrasonography, Alveolar echinococcosis
Core tip: Alveolar echinococcosis (AE) is a rare but potentially life-threatening parasitic disease. Despite the importance of ultrasonography as an imaging modality in the work-up of hepatic AE, there is no established sonomorphological classification of hepatic AE lesions analogous to the World Health Organization’s ultrasonographic classification for cystic echinococcosis. Objective of the present study was to establish an ultrasonographic classification based on a large sample of patients with confirmed hepatic AE. Assignment of hepatic AE lesions to one of the five sonomorphological patterns was successful in 95% of cases based on the ultrasonographic classification scheme proposed in the present study.
INTRODUCTION
Alveolar echinococcosis (AE) is a rare but potentially life-threatening parasitic disease caused by infection with the larval stage of the cestode tapeworm, Echinococcus alveolaris[1-3]. Worldwide, the distribution of the parasite is limited to the cool and temperate regions of the Northern Hemisphere[4]. A characteristic feature of AE is its tumor-like growth in the liver, which may infiltrate neighboring organs[1]. In a large majority of cases, the liver is the first organ to be infested by the larvae: in seven out of ten cases, hepatic lesions occur in the right hepatic lobe; in 40%, the liver hilus is also involved; while, in only two of ten cases, both hepatic lobes are affected[5].
In its initial phase, the infection is usually asymptomatic. First symptoms and signs may include upper abdominal pain or cholestatic jaundice. The incubation period ranges between five and fifteen years[6]. Complications, such as biliary obstruction, portal hypertension and bleeding esophageal varices, have been reported in advanced disease and are ascribed to the invasively growing mass of Echinococcus alveolaris in the liver[7]. Metastatic infiltration by Echinococcus alveolaris has been described for many organs[8,9] and is reflected in the PNM classification introduced by Kern et al[10].
Radical resection of echinococcal foci is the sole curative therapy for patients with AE. Curative therapy is followed by administration of benzimidazoles for two years; long-term administration of these agents is indicated for non-resectable lesions[9]. Left untreated, the disease is associated with a fatal outcome in more than 95% of cases within a period of ten years following diagnosis[11]. Only early diagnosis, based on diagnostic imaging and serological markers, can increase the rate of curative resections[12,13]. Early diagnostic imaging therefore takes on decisive importance[14].
Beside US, computed tomography (CT) represents the imaging method of choice among currently available diagnostic imaging modalities[15,16]. 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) is a sensitive and specific tool that uses 18F-fluorodesoxyglucose (18F-FDG) metabolism to estimate the metabolic activity of hepatic AE lesions[17-19]. The development of the US contrast enhanced SonoVue® (Bracco Medical Imaging Deutschland GmbH, Konstanz, Germany) has over the past few years facilitated US assessment of the vitality of AE lesions at follow-up monitoring. Assessment of the vascularization of hepatic AE lesions with contrast-enhanced ultrasound (CEUS) correlates with their metabolic activity at combined 18F-FDG-PET-CT[20,21] and can better delineate the spatial extent of hepatic alveolar echinococcosis lesions[22,23]. Lesions characterized by vesicles and small cysts show a high degree of correlation between 18F-FDG-PET and CEUS findings[24].
In 2003, Kodama et al[25] introduced a five-part classification for assessing hepatic AE with magnetic resonance imaging (MRI): Type 1: Multiple small round cysts without a solid component; Type 2: Multiple small round cysts with a solid component; Type 3: A solid component surrounding a large and/or irregular pseudo-cyst with multiple small round cysts; Type 4: A solid component without cysts; Type 5: A large cyst without a solid component.
No corresponding classification has yet been published for either CT or ultrasonography (US). Current studies suggest that the occurrence of alveolar echinococcosis is increasing worldwide and is spreading to previously unaffected regions. Especially in the Northern Hemisphere, there is a growing number of AE lesions occurring as coincidental findings at routine upper abdominal US[14,26,27]. Knowledge of the typical presentations of hepatic AE at diagnostic imaging may aid in making an early diagnosis[28]. Despite the importance of US as an image modality in the work-up of hepatic AE, there is no sonomorphological classification of hepatic AE lesions analogous to the World Health Organization (WHO)’s ultrasonographic classification for cystic echinococcosis, which has achieved worldwide acceptance for assessing the activity of that disease[14,15,28]. Objective of the present study was to establish an ultrasonographic classification based on a large sample of patients with confirmed hepatic AE as a way of facilitating the diagnosis, interpretation, classification and comparison of ultrasonographic findings of the rare disease entity.
MATERIALS AND METHODS
Study collective
Clinical data and US findings of 185 patients (n = 100 males; 85 females; mean age at diagnosis: 51.4 ± 17.6 years; mean age at time of US examination: 58.7 ± 18.2 years) followed at the Echinococcosis outpatient clinic of Ulm University Hospital (n = 385 patients) were reviewed with respect to the ultrasonographic morphology of hepatic AE lesions. Patients were originally examined between 1999 and 2014. A total of 200 patients were excluded from this analysis due to limitations in image quality impacting interpretation or incomplete data sets. The US findings of all patients (n = 185) with confirmed hepatic AE stored in the ViewPoint US documentation system (GE Healthcare Technologies, ViewPoint Bildverarbeitung GmbH, Weßling, Germany) were re-interpreted by a single reviewer (WK) with broad experience in the US of AE and grouped according to a novel five-part sonomorphological classification scheme (Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5).
Figure 1.
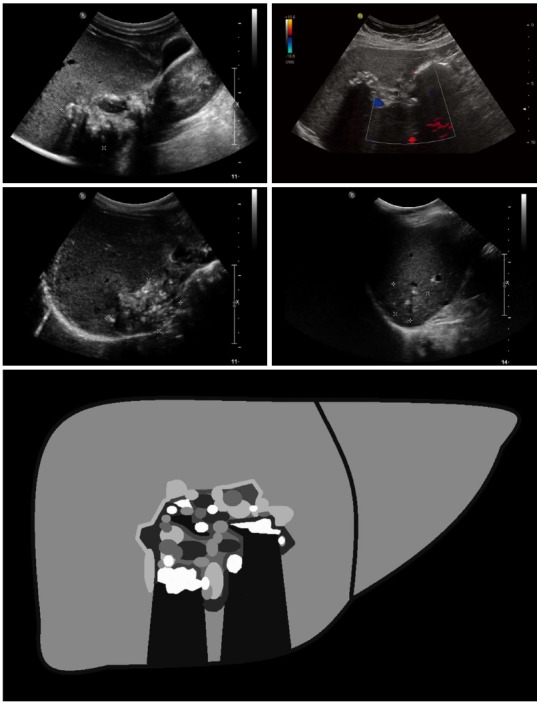
Hailstorm: The typical hailstorm appearance is characterized by indistinct, irregular boundaries, non-homogeneous pattern and hyperechoic formations, with or without dorsal acoustic shadow.
Figure 2.
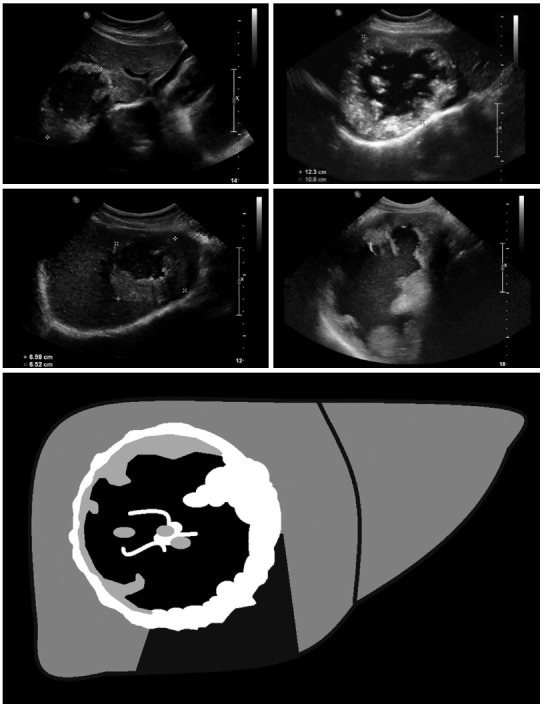
Pseudocystic: Pseudocystic alveolar echinococcosis lesions are primarily characterized by an hyperechoic, irregular and non-homogeneous rim that is non-vascularized at power Doppler and color-coded duplex ultrasonography. It may appear to be > 10 mm in thickness. There is a hypo- or anechoic, often non-homogeneous central zone that may contain hyperechoic material. Pseudocystic lesions may be already present at first diagnosis and involve an entire hepatic lobe, or may develop from primary hailstorm lesions following therapy with benzimidazoles.
Figure 3.
Hemangioma-like: These lesions are difficult to distinguish from atypical (e.g., partially thrombosed) hemangiomas, and often represent a significant diagnostic challenge. Sonomorphologically, the lesions present as a relatively clearly demarcated non-homogeneous tumor that appears hyperechoic in comparison with the surrounding hepatic parenchyma. Echogenicity ranges from slightly and non-homogeneously hyperechoic to strongly and homogeneous hyperechoic.
Figure 4.
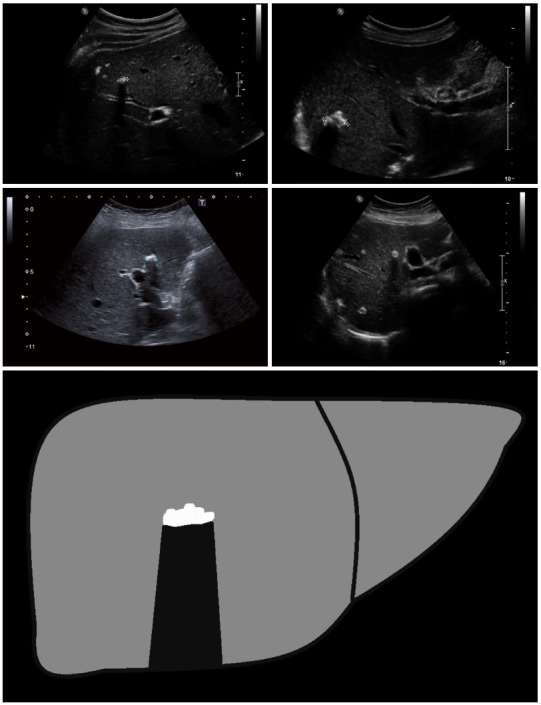
Ossification: The ossification pattern presents with solitary or grouped, mostly sharply delineated lesions with dorsal acoustic shadow. In terms of their differential diagnosis, these alveolar echinococcosis (AE) lesions are often difficult to distinguish from inflammatory or hyperechoic metastases of various carcinomas. Very large ossification-type AE lesions represent a rarity. Both uni- and multifocal involvement is possible.
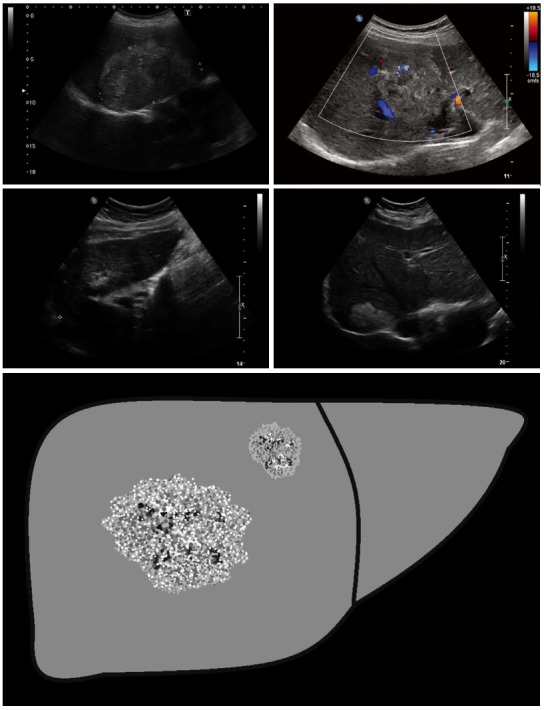
Figure 5.
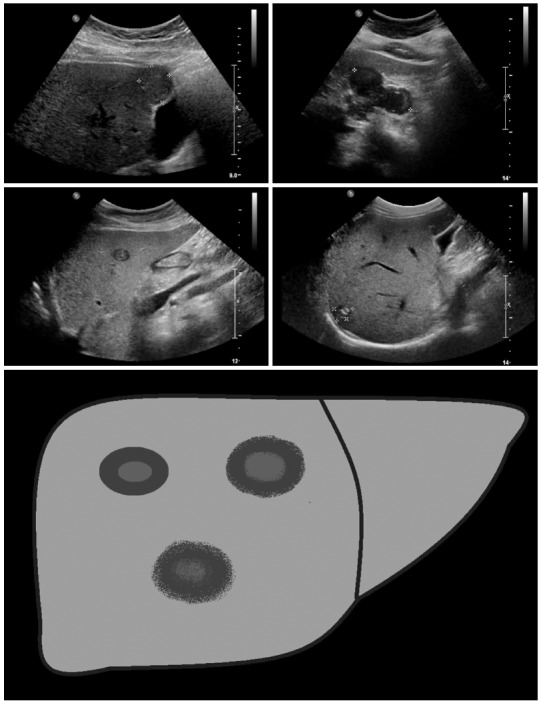
Metastasis-like: Beside the hemangioma-like lesions, the metastasis-like lesions of alveolar echinococcosis represent the greatest diagnostic challenge. Mostly hypoechoic, these lesions exhibit as a typical characteristic-compared to typical hepatic metastases (e.g., of colorectal cancer)-the absence of the halo phenomenon. Instead, there is a central, hyperechoic, non-homogeneous scar.
The study design complies with the requirements of the Helsinki Declaration and was approved by the Ethics Commission of Ulm University.
Diagnosis and classification
The diagnosis of AE was made in cases with unequivocal seropositivity, positive histological findings following diagnostic puncture or partial resection of the liver, as well as findings typical for AE in either US, CT, MRI or PET-CT[15]. According to the modified WHO criteria of Brunetti et al[15] 79 cases were confirmed by positive histopathology and proven specific enzyme linked immunosorbent assay from tissue samples. An additional 85 patients were considered probable cases with positive serology in two different procedures and positive imaging for AE in two imaging techniques, while 21 patients were considered possible cases with a positive medical history and a positive result for imaging and serology in one test each. Based on the reviewer’s many years’ experience together with reports of sonomorphological findings in patients with hepatic AE in the literature, individual US findings were grouped into one of the following patterns: hailstorm, pseudocystic, hemangioma-like, ossification, and metastasis-like[14,16,17] (Figures 1-5). As an acronym, we propose EMUC-US (Echinococcosis Multilocularis Ulm Classification - Utrasound). In addition to patient-specific parameters, the number, maximum diameter and localization of the largest echinococcus-specific lesion were documented and interpreted. US examinations were performed exclusively using convex transducer heads (1-6 MHz) with different US units (Philips HDI 3000, HDI 5000, IU 22, Toshiba Aplio 500, Siemens S3000, Hitachi Ascendus).
Statistical analysis
Statistical analyses were performed using the SAS statistical software package (version 9.2; SAS Institute Inc., Cary, NC, United States). Data were analyzed descriptively with regard to absolute and relative frequencies, means and standard deviation. The AE lesions were divided into five morphological patterns. One-way analysis of variance was applied to analyze differences between the patterns.
RESULTS
The most frequently encountered sonomorphological pattern among the 185 patients was the hailstorm pattern (54.1%, n = 100), followed, in 13.5% (n = 25) by the pseudocystic appearance and in 13% (n = 24) by the ossification appearance. Much less frequently observed were the hemangioma-like appearance (8.1%, n = 15) and the metastasis-like appearance (6.5%, n = 12). In terms of their mean diameters, the hailstorm lesions measured 59.6 ± 27.9 mm; the pseudocystic lesions, 120.0 ± 47.3 mm; the hemangioma-like lesions, 68.1 ± 37.3 mm; the ossification lesions, 28.0 ± 19.4 mm; and metastasis-like lesions, 35.3 ± 33.1 mm (Figure 6). The diameters of lesions exhibiting pseudocystic sonomorphology were significantly larger than any of the other four lesion types (P < 0.05). In terms of their mean diameters, lesions of both the hailstorm and hemangioma-like types differed significantly from those of the ossification type (P < 0.05).
Figure 6.
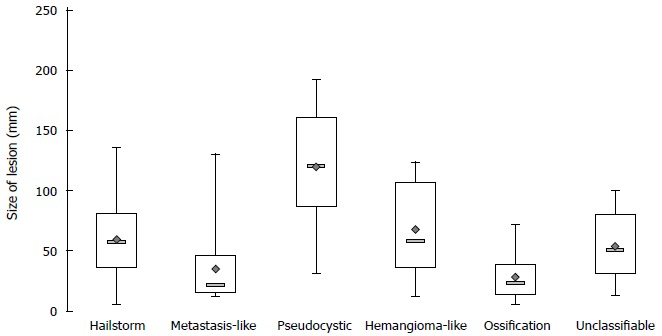
Lesion size depending on the sonomorphological pattern.
In nine cases (4.9%), the complexity of the sonomorphological appearance or the simultaneous occurrence of characteristics typical for more than one sonomorphological pattern precluded assignment of sonomorphological findings to any one of the defined sonomorphological types in the new classification (Table 1).
Table 1.
Patient characteristics n (%)
| Characteristics | mean ± SD |
| Number of patients | 185 |
| Gender | |
| Female | 100 (54.1) |
| Male | 85 (45.9) |
| Age at diagnosis | 51.4 ± 17.6 |
| Age at ultrasonographic examination | 58.7 ± 18.2 |
| Sonomorphological classification | |
| Hailstorm | 100 (54.1) |
| Pseudocystic | 25 (13.5) |
| Ossification | 24 (13.0) |
| Hemangioma-like | 15 (8.1) |
| Metastasis-like | 12 (6.5) |
| Unclassifiable | 9 (4.9) |
| Number of lesions | |
| 1 | 116 |
| 2 | 24 |
| 3 | 16 |
| 4 | 5 |
| 5 | 7 |
| 6-10 | 4 |
| > 10 | 13 |
| Mean diameter of the largest lesion | 62.5 ± 40.4 |
| Mean lesion diameter according to sonomorphological classification | |
| Hailstorm | 59.6 ± 27.9 |
| Pseudocystic | 120.0 ± 47.3 |
| Ossification | 28.0 ± 19.4 |
| Hemangioma-like | 68.1 ± 37.3 |
| Metastasis-like | 35.3 ± 33.1 |
| Unclassifiable | 53.9 ± 30.6 |
| Localization of the largest lesion (hepatic lobe) | |
| Right | 113 (61.1) |
| Left | 58 (31.4) |
| Both | 14 (7.6) |
| Calcification | |
| No | 47 (25.4) |
| Yes | 138 (74.6) |
| Affected liver segments (multiple segments possible) | |
| I | 6 (3.2) |
| II | 19 (10.3) |
| III | 22 (11.9) |
| IVa | 30 (16.2) |
| IVb | 28 (15.1) |
| V | 44 (23.8) |
| VI | 41 (22.2) |
| VII | 44 (23.8) |
| VIII | 41 (22.2) |
| Steatosis hepatis | |
| No | 147 (79.5) |
| Yes | 38 (20.5) |
| Liver size in mid-clavicular line (mm) | 146.1 ± 25.2 |
Solitary echinococcus foci were by far the most frequent, being observed in 62.7% of cases. Only 13 patients (7%) exhibited more than ten identifiable foci (Table 1). Typical calcifications with dorsal acoustic shadow were visualized ultrasonographically in nearly three-fourths of cases (74.6%). A majority of lesions (61.1%) were localized in the right hepatic lobe compared with only 31.4% in the left hepatic lobe. Echinococcal lesions affecting both hepatic lobes were identified in only 7.6%. Further characteristics and findings are summarized in Table 1.
DISCUSSION
Alveolar echinococcosis is a rare disease[14,15,29]. AE is characterized by destructive growth and exhibits all the characteristics of a malignant disease with infiltration of adjacent organs and formation of distance metastases[10,15]. Hence, rapid and definitive diagnosis is essential. Due to the rarity of the disease, however, especially in non-endemic areas, AE presents a significant diagnostic challenge in routine clinical practice[30]. US is the imaging method of choice in the work-up of symptomatic patients and especially as a screening tool[14,31]. The widespread use of imaging modalities, such as US, CT and MRI, has led to an increase in the detection of previously unsuspected liver masses in asymptomatic patients[32]. These hepatic incidentalomas in asymptomatic patients are mostly benign and, in most cases, US (including CEUS) will suffice to definitively distinguish them from malignant lesions[32,33]. Certain hepatic incidentalomas, such as regenerative nodules, angiomyolipomas of the liver or hepatic AE, however, remain a diagnostic challenge for all imaging modalities[14,30,34]. Not infrequently, a final diagnosis is made only upon histopathological examination of material obtained at puncture or resection[35,36].
In the present study population, over 80% of cases corresponded sonomorphologically to the hailstorm, pseudocystic or ossification patterns. These morphologically very characteristic appearances have already been described by many authors, though not in the context of an ultrasonographic classification[16,37]. The so-called “hailstorm” and “pseudocystic” patterns were described as early as 1984 by Didier et al[37]. In fact, in their small series of 24 patients, the distribution of the hailstorm and pseudocystic pattern in 62.5% and 12%, respectively, was quite similar to that observed in the present study with 54.1% and 13.5% for the hailstorm and pseudocystic patterns, respectively[37]. Bresson-Hadni et al[16] also describe patterns that correspond to our hailstorm and pseudocystic patterns. Taken together, these two forms comprise about 70% of “typical” AE lesions among the lesions studied. The French research group also reported an hemangioma-like pattern as well as a usually small, calcified form of AE lesion (ossification pattern)[16]. AE lesions exhibiting an ossification appearance may present a diagnostic challenge. The differential diagnosis encompasses other hyperechoic, calcified lesions occurring in a wide range of benign, infectious or vascular disorders; with hepatic metastases of colorectal or breast cancer; or metastases of malignant melanomas[38]. A metastasis-like appearance for hepatic AE has not previously been described. Unlike typical liver metastases, which exhibit an hypoechoic halo, lesions characterized by a metastasis-like appearance may be visualized as a hypoechoic growth without the halo sign or often with a central, hyperechoic scar[39].
In cases with pseudocystic manifestation, especially when the lesion is very large, the differential diagnosis includes liver abscess, cystadenoma or cystic echinococcosis[14]. In our series, the pseudocystic lesions were significantly larger than lesions of other sonomorphological types (68.1 ± 37.3 mm, P < 0.05).
Since AE is a very rare disease conducting an inter-rater reliability is difficult. The lack of inter-rater reliability remains a limitation of the proposed classification. In the present series, very few hepatic lesions (4.9%) could not be assigned to one of the five sonomorphological patterns. In routine clinical practice, only histopathological confirmation can clarify these unclear hepatic findings[36]. Depending on the experience of the pathologist, even the histopathological diagnosis of AE may be difficult. Immunohistochemical examination using Em-specific monoclonal antibodies facilitates a definitive diagnosis even in archived formalin-fixed or paraffin-embedded tissue[40].
In conclusion, ninety-five per cent of cases of hepatic alveolar echinococcosis could be successfully assigned to one of the sonomorphological patterns based on the ultrasonographic classification scheme proposed in the present study. The hailstorm pattern represented the most frequent form, being observed in over 50%. The sonomorphological classification proposed in the present study can facilitate the diagnosis, interpretation, classification and comparison of ultrasonographic findings in patients with alveolar echinococcosis of the liver, both in routine clinical practice and in the context of scientific studies. The evaluation of different clinical courses (PNM classification) with inclusion of biological markers and other imaging modalities should be investigated in further studies.
ACKNOWLEDGMENTS
Thanks to the members of the Echinococcus Multilocularis Study Group who not list as authors, in alphabetical order: Max G Bachem, Ambros J Beer, Meinrad Beer, Bernhard O Boehm, Franziska Ehing, Martina Furitsch, Martin Gottstein, Doris Henne-Bruns, Max Kurlbaum, Thomas Seufferlein.
COMMENTS
Background
Human alveolar echinococcosis (AE) is the most Iethal human helminthic infection and is one of the 17 neglected tropical diseases prioritized by the World Health Organization (WHO). Its incidence is low in endemic regions of Central and Western Europe (0.03-0.05/100000) and high in central Asia. Current studies suggest that the occurrence of alveolar echinococcosis is increasing worldwide and is spreading to previously unaffected regions. Morbidity and treatment costs of the disease are high.
Research frontiers
Despite the importance of ultrasonography as an image modality in the work-up of hepatic AE, there is no sonomorphological classification of hepatic AE lesions analogous to the WHO’s ultrasonographic classification for cystic echinococcosis, which has achieved worldwide acceptance for assessing the activity of that disease.
Innovations and breakthroughs
Objective of the present study was to establish an ultrasonographic classification based on a large sample of patients with confirmed hepatic AE as a way of facilitating the diagnosis of the disease entity.
Applications
The sonomorphological classification proposed in the present study can facilitate the diagnosis, interpretation, classification and comparison of ultrasonographic findings in patients with alveolar echinococcosis of the liver, both in routine clinical practice and in the context of scientific studies.
Peer-review
This is a good study in which the authors introduce a new ultrasound classification for alveolar echinococcosis. The results are interesting and 95% of cases of hepatic alveolar echinococcosis could be successfully assigned to one of the five sonomorphological patterns based on the ultrasonographic classification scheme proposed in the present study. The results of the present study will be important for further research in this field.
Footnotes
Institutional review board statement: The study was reviewed and approved by the local Ethics Committee of University of Ulm.
Informed consent statement: Because of retrospective and anonymous character of this study the need for informed consent was waived by the Institutional Review Board.
Conflict-of-interest statement: The authors declare that there are no conflicts of interest.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 15, 2015
First decision: June 2, 2015
Article in press: September 14, 2015
P- Reviewer: Bottcher D, Martakis K, Tamarozzi F S- Editor: Yu J L- Editor: A E- Editor: Liu XM
References
- 1.Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miguet JP, Bresson-Hadni S. Alveolar echinococcosis of the liver. J Hepatol. 1989;8:373–379. doi: 10.1016/0168-8278(89)90037-8. [DOI] [PubMed] [Google Scholar]
- 3.Nunnari G, Pinzone MR, Gruttadauria S, Celesia BM, Madeddu G, Malaguarnera G, Pavone P, Cappellani A, Cacopardo B. Hepatic echinococcosis: clinical and therapeutic aspects. World J Gastroenterol. 2012;18:1448–1458. doi: 10.3748/wjg.v18.i13.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romig T. Epidemiology of echinococcosis. Langenbecks Arch Surg. 2003;388:209–217. doi: 10.1007/s00423-003-0413-3. [DOI] [PubMed] [Google Scholar]
- 5.Heyd B, Weise L, Bettschart V, Gillet M. [Surgical treatment of hepatic alveolar echinococcosis] Chirurg. 2000;71:16–20. doi: 10.1007/s001040051007. [DOI] [PubMed] [Google Scholar]
- 6.Ammann RW, Eckert J. Cestodes. Echinococcus. Gastroenterol Clin North Am. 1996;25:655–689. doi: 10.1016/s0889-8553(05)70268-5. [DOI] [PubMed] [Google Scholar]
- 7.Craig P. Echinococcus multilocularis. Curr Opin Infect Dis. 2003;16:437–444. doi: 10.1097/00001432-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis. 2009;13:125–133. doi: 10.1016/j.ijid.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 9.McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ. 2012;344:e3866. doi: 10.1136/bmj.e3866. [DOI] [PubMed] [Google Scholar]
- 10.Kern P, Wen H, Sato N, Vuitton DA, Gruener B, Shao Y, Delabrousse E, Kratzer W, Bresson-Hadni S. WHO classification of alveolar echinococcosis: principles and application. Parasitol Int. 2006;55 Suppl:S283–S287. doi: 10.1016/j.parint.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Buttenschoen K, Carli Buttenschoen D, Gruener B, Kern P, Beger HG, Henne-Bruns D, Reuter S. Long-term experience on surgical treatment of alveolar echinococcosis. Langenbecks Arch Surg. 2009;394:689–698. doi: 10.1007/s00423-008-0392-5. [DOI] [PubMed] [Google Scholar]
- 12.Kern P, Kratzer W, Reuter S. [Alveolar echinococcosis: diagnosis] Dtsch Med Wochenschr. 2000;125:59–62. doi: 10.1055/s-2007-1023907. [DOI] [PubMed] [Google Scholar]
- 13.Sezgin O, Altintaş E, Saritaş U, Sahin B. Hepatic alveolar echinococcosis: clinical and radiologic features and endoscopic management. J Clin Gastroenterol. 2005;39:160–167. [PubMed] [Google Scholar]
- 14.Liu W, Delabrousse É, Blagosklonov O, Wang J, Zeng H, Jiang Y, Wang J, Qin Y, Vuitton DA, Wen H. Innovation in hepatic alveolar echinococcosis imaging: best use of old tools, and necessary evaluation of new ones. Parasite. 2014;21:74. doi: 10.1051/parasite/2014072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Bresson-Hadni S, Delabrousse E, Blagosklonov O, Bartholomot B, Koch S, Miguet JP, André Mantion G, Angèle Vuitton D. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol Int. 2006;55 Suppl:S267–S272. doi: 10.1016/j.parint.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 17.Reuter S, Schirrmeister H, Kratzer W, Dreweck C, Reske SN, Kern P. Pericystic metabolic activity in alveolar echinococcosis: assessment and follow-up by positron emission tomography. Clin Infect Dis. 1999;29:1157–1163. doi: 10.1086/313438. [DOI] [PubMed] [Google Scholar]
- 18.Reuter S, Buck A, Manfras B, Kratzer W, Seitz HM, Darge K, Reske SN, Kern P. Structured treatment interruption in patients with alveolar echinococcosis. Hepatology. 2004;39:509–517. doi: 10.1002/hep.20078. [DOI] [PubMed] [Google Scholar]
- 19.Reuter S, Grüner B, Buck AK, Blumstein N, Kern P, Reske SN. Long-term follow-up of metabolic activity in human alveolar echinococcosis using FDG-PET. Nuklearmedizin. 2008;47:147–152. [PubMed] [Google Scholar]
- 20.Kaltenbach TE, Graeter T, Mason RA, Kratzer W, Oeztuerk S, Haenle MM, Gruener B, Gottstein M. Determination of vitality of liver lesions by alveolar echinococcosis. Comparison of parametric contrast enhanced ultrasound (SonoVue®) with quantified 18F-FDG-PET-CT. Nuklearmedizin. 2014;26:54. doi: 10.3413/Nukmed-0670-14-05. [DOI] [PubMed] [Google Scholar]
- 21.Ehrhardt AR, Reuter S, Buck AK, Haenle MM, Mason RA, Gabelmann A, Kern P, Kratzer W. Assessment of disease activity in alveolar echinococcosis: a comparison of contrast enhanced ultrasound, three-phase helical CT and [(18)F] fluorodeoxyglucose positron emission tomography. Abdom Imaging. 2007;32:730–736. doi: 10.1007/s00261-006-9173-1. [DOI] [PubMed] [Google Scholar]
- 22.Tao S, Qin Z, Hao W, Yongquan L, Lanhui Y, Lei Y. Usefulness of gray-scale contrast-enhanced ultrasonography (SonoVue®) in diagnosing hepatic alveolar echinococcosis. Ultrasound Med Biol. 2011;37:1024–1028. doi: 10.1016/j.ultrasmedbio.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Kratzer W, Reuter S, Hirschbuehl K, Ehrhardt AR, Mason RA, Haenle MM, Kern P, Gabelmann A. Comparison of contrast-enhanced power Doppler ultrasound (Levovist) and computed tomography in alveolar echinococcosis. Abdom Imaging. 2005;30:286–290. doi: 10.1007/s00261-004-0263-7. [DOI] [PubMed] [Google Scholar]
- 24.Azizi A, Blagosklonov O, Lounis A, Berthet L, Vuitton DA, Bresson-Hadni S, Delabrousse E. Alveolar echinococcosis: correlation between hepatic MRI findings and FDG-PET/CT metabolic activity. Abdom Imaging. 2015;40:56–63. doi: 10.1007/s00261-014-0183-0. [DOI] [PubMed] [Google Scholar]
- 25.Kodama Y, Fujita N, Shimizu T, Endo H, Nambu T, Sato N, Todo S, Miyasaka K. Alveolar echinococcosis: MR findings in the liver. Radiology. 2003;228:172–177. doi: 10.1148/radiol.2281020323. [DOI] [PubMed] [Google Scholar]
- 26.Tennert U, Schubert S, Tröltzsch M, Ivanova Tchavdarova L, Mössner J, Schoppmeyer K. Pitfall alveolar echinococcosis in non-endemic areas. Alveolar echinococcosis migrating northward. Ann Hepatol. 2010;9:99–103. [PubMed] [Google Scholar]
- 27.Antolova D, Miterpakova M, Radoňak J, Hudačkova D, Szilagyiova M, Začek M. Alveolar echinococcosis in a highly endemic area of Northern Slovakia between 2000 and 2013. Euro Surveill. 2014;19:pii: 20882. [PubMed] [Google Scholar]
- 28.Madhusudhan KS, Srivastava DN, Dash NR, Venuthurimilli A, Sharma R, Gamanagatti S, Gupta AK. Alveolar echinococcosis of liver: a diagnostic problem in a nonendemic area. Curr Probl Diagn Radiol. 2015;44:221–226. doi: 10.1067/j.cpradiol.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Piarroux M, Piarroux R, Giorgi R, Knapp J, Bardonnet K, Sudre B, Watelet J, Dumortier J, Gérard A, Beytout J, et al. Clinical features and evolution of alveolar echinococcosis in France from 1982 to 2007: results of a survey in 387 patients. J Hepatol. 2011;55:1025–1033. doi: 10.1016/j.jhep.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Kantarci M, Bayraktutan U, Karabulut N, Aydinli B, Ogul H, Yuce I, Calik M, Eren S, Atamanalp SS, Oto A. Alveolar echinococcosis: spectrum of findings at cross-sectional imaging. Radiographics. 2012;32:2053–2070. doi: 10.1148/rg.327125708. [DOI] [PubMed] [Google Scholar]
- 31.Romig T, Kratzer W, Kimmig P, Frosch M, Gaus W, Flegel WA, Gottstein B, Lucius R, Beckh K, Kern P. An epidemiologic survey of human alveolar echinococcosis in southwestern Germany. Römerstein Study Group. Am J Trop Med Hyg. 1999;61:566–573. doi: 10.4269/ajtmh.1999.61.566. [DOI] [PubMed] [Google Scholar]
- 32.Koea JB. Hepatic incidentaloma: the rule of tens. HPB (Oxford) 2013;15:379–383. doi: 10.1111/j.1477-2574.2012.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dietrich CF, Sharma M, Gibson RN, Schreiber-Dietrich D, Jenssen C. Fortuitously discovered liver lesions. World J Gastroenterol. 2013;19:3173–3188. doi: 10.3748/wjg.v19.i21.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galanski M, Jördens S, Weidemann J. [Diagnosis and differential diagnosis of benign liver tumors and tumor-like lesions] Chirurg. 2008;79:707–721. doi: 10.1007/s00104-008-1522-x. [DOI] [PubMed] [Google Scholar]
- 35.Atanasov G, Benckert C, Thelen A, Tappe D, Frosch M, Teichmann D, Barth TF, Wittekind C, Schubert S, Jonas S. Alveolar echinococcosis-spreading disease challenging clinicians: a case report and literature review. World J Gastroenterol. 2013;19:4257–4261. doi: 10.3748/wjg.v19.i26.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller M, Kratzer W, Oeztuerk S, Wilhelm M, Mason RA, Mao R, Haenle MM. Percutaneous ultrasonographically guided liver punctures: an analysis of 1961 patients over a period of ten years. BMC Gastroenterol. 2012;12:173. doi: 10.1186/1471-230X-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Didier D, Weiler S, Rohmer P, Lassegue A, Deschamps JP, Vuitton D, Miguet JP, Weill F. Hepatic alveolar echinococcosis: correlative US and CT study. Radiology. 1985;154:179–186. doi: 10.1148/radiology.154.1.3880602. [DOI] [PubMed] [Google Scholar]
- 38.Stoupis C, Taylor HM, Paley MR, Buetow PC, Marre S, Baer HU, Vock P, Ros PR. The Rocky liver: radiologic-pathologic correlation of calcified hepatic masses. Radiographics. 1998;18:675–685; quiz 726. doi: 10.1148/radiographics.18.3.9599391. [DOI] [PubMed] [Google Scholar]
- 39.Wernecke K, Vassallo P, Bick U, Diederich S, Peters PE. The distinction between benign and malignant liver tumors on sonography: value of a hypoechoic halo. AJR Am J Roentgenol. 1992;159:1005–1009. doi: 10.2214/ajr.159.5.1329454. [DOI] [PubMed] [Google Scholar]
- 40.Barth TF, Herrmann TS, Tappe D, Stark L, Grüner B, Buttenschoen K, Hillenbrand A, Juchems M, Henne-Bruns D, Kern P, et al. Sensitive and specific immunohistochemical diagnosis of human alveolar echinococcosis with the monoclonal antibody Em2G11. PLoS Negl Trop Dis. 2012;6:e1877. doi: 10.1371/journal.pntd.0001877. [DOI] [PMC free article] [PubMed] [Google Scholar]


