Abstract
AIM: To study the safety of epidural anesthesia (EA), its effect on pancreatic perfusion and the outcome of patients with acute pancreatitis (AP).
METHODS: From 2005 to August 2010, patients with predicted severe AP [Ranson score ≥ 2, C-reactive protein > 100 or necrosis on computed tomography (CT)] were prospectively randomized to either a group receiving EA or a control group treated by patient controlled intravenous analgesia. Pain management was evaluated in the two groups every eight hours using the visual analog pain scale (VAS). Parameters for clinical severity such as length of hospital stay, use of antibiotics, admission to the intensive care unit, radiological/clinical complications and the need for surgical necrosectomy including biochemical data were recorded. A CT scan using a perfusion protocol was performed on admission and at 72 h to evaluate pancreatic blood flow. A significant variation in blood flow was defined as a 20% difference in pancreatic perfusion between admission and 72 h and was measured in the head, body and tail of the pancreas.
RESULTS: We enrolled 35 patients. Thirteen were randomized to the EA group and 22 to the control group. There were no differences in demographic characteristics between the two groups. The Balthazar radiological severity score on admission was higher in the EA group than in the control group (mean score 4.15 ± 2.54 vs 3.38 ± 1.75, respectively, P = 0.347) and the median Ranson scores were 3.4 and 2.7 respectively (P = NS). The median duration of EA was 5.7 d, and no complications of the epidural procedure were reported. An improvement in perfusion of the pancreas was observed in 13/30 (43%) of measurements in the EA group vs 2/27 (7%) in the control group (P = 0.0025). Necrosectomy was performed in 1/13 patients in the EA group vs 4/22 patients in the control group (P = 0.63). The VAS improved during the first ten days in the EA group compared to the control group (0.2 vs 2.33, P = 0.034 at 10 d). Length of stay and mortality were not statistically different between the 2 groups (26 d vs 30 d, P = 0.65, and 0% for both respectively).
CONCLUSION: Our study demonstrates that EA increases arterial perfusion of the pancreas and improves the clinical outcome of patients with AP.
Keywords: Severe acute pancreatitis, Epidural anesthesia, Pancreatic necrosectomy, Pancreatic perfusion, Computed tomography
Core tip: We conducted this prospective randomized study to explore the safety of epidural anesthesia (EA), its effect on pancreatic perfusion and the outcome of patients with acute pancreatitis, as high mortality is linked with necrosis of the gland. We found an improvement in perfusion of the pancreas in the EA group. Necrosectomy was performed in 1/13 patients in the EA group vs 4/22 patients in the control group.
INTRODUCTION
Acute pancreatitis (AP) is a common disease whose incidence in the US reaches 35 per 100000 population annually[1]. The main causes of AP in adults are gallstone migration into the common bile duct and alcohol abuse. Approximately 80% of patients with AP will develop a mild disease for which the management is mainly conservative[2]. However 20% will develop a severe form that is associated with the development of local complications such as pancreatic and peripancreatic necrosis, pseudocysts, as well as systemic complications such as adult respiratory distress syndrome or renal failure. In the severe form of AP, the mortality rate can reach 17% and is primarily due to multiple organ failure and pancreatic necrosis. In particular, pancreatic necrosis is associated with a death rate of up to 40%[3].
The pathophysiology of necrotizing AP is not yet fully understood, though animal studies[4-6] suggest that an alteration in pancreatic microcirculatory blood flow as well as arterial vasoconstriction and ischemia-reperfusion injury are contributing factors. Microcirculatory dysfunction results in part from hypercoagulability and an increase in microvascular permeability that is mediated by the local and systemic inflammatory response (leukocyte activation as well as release of free radicals and cytokines). Kusterer et al[7] showed that, in animals, pancreatitis is associated with early arteriolar vasoconstriction and hypoperfusion of the pancreatic microcirculation. Thus, early in the course of AP, a decrease in pancreatic blood flow occurs that potentially plays a role in the development of necrotizing AP[4].
Epidural anesthesia (EA) is widely used to induce analgesia in the perioperative period and has also been used to decrease pain in patients with AP[8]. In addition, experimental studies have shown a specific beneficial effect of EA in AP attributed to a sympathetic nerve blockade that redistributes splanchnic blood flow to non-perfused pancreatic regions[9,10].
We previously performed an animal study showing that EA improves pancreatic hypoperfusion induced by AP and decreases the severity of metabolic acidosis and tissue injury, thus preventing progression of an edematous disease to necrotizing AP[4]. Clinically this can be measured with radiological perfusion studies of the pancreas[11].
We conducted a clinical randomized pilot study aimed at evaluating: (1) the safety of EA in predicted severe AP patients; (2) whether EA increases the perfusion of the pancreatic gland, which explains a decrease in severity of the disease; and (3) whether EA improves the clinical outcome of patients with predicted severe AP.
MATERIALS AND METHODS
Study design
We conducted a prospective randomized controlled study and included data from all adult patients admitted to the surgical unit of the University Hospital of Geneva for predicted severe AP.
The study began in July 2005 and ended in August 2010. The Ethics Board of Geneva’s University Hospital approved the study in 2004 (HUG 02-0555). The trial began as a pilot study when registration was not mandatory. However, registration for randomized clinical trials was subsequently performed: NCT01607996.
Upon admission, the severity of pancreatitis was established according to the Ranson classification[12,13]. We included all patients presenting an AP with a Ranson score ≥ 2 and/or a C-reactive protein > 100 (mg/L) and/or necrosis on computed tomography (CT). After obtaining written consent from the patients who met the inclusion criteria, they were randomized into two study groups: (1) study group treated with EA; and (2) control group treated by patient controlled intravenous analgesia (PCA). Randomization was established with a closed envelope.
Exclusion criteria were absence of severe pancreatitis as defined previously, patients with contraindications to epidural anesthesia (skin infection of the vertebral region, coagulation disorders, iodine allergy), inability to obtain consent and concurrent participation in another clinical trial. According to our standardized protocol, all information on admission was gathered, including demographic characteristics such as age, sex and race. All medical information on past history, diagnostic, radiological and biochemical data and past and current medication was included.
For patients allocated to group 1 (treated with EA), EA was inserted immediately after the admission CT scan was obtained and used for up to 5 d following randomization. The standardized protocol for EA contained a mix of Carbostesine (Bupivacaine) at a concentration of 0.1% and Fentanyl at 2 μg/mL. The doses were established according to a pain score at rest and at mobilization and according to the sensitivo-motor bloc (ether test and Bromage scale). It was administered with a continuous infusion of the mix at a minimum of 6 mL to a maximum of 15 mL/h. A bolus between 3 to 5 mL every 30 to 60 min could be added upon request.
Patients allocated to group 2 received standardized intravenous analgesia using a PCA, which was started as soon as the patient was randomized. The medication contained Fentanyl at a concentration of 10 μg/mL. The continuous debit was used at a rate of 10 to 20 μg/h in association with pushes on demand of 1 to 2 mL every 5 to 10 min. The maximum dose was 400 μg every four hours. The duration of this therapy was between 3 to 5 d and was conducted using the same criteria as the EA.
We evaluated pain management in the two groups every eight hours using the visual analog pain scale (VAS)[14,15], scaled from zero to ten (zero meant no pain, and ten meant the worst pain tolerable). All modifications in pain management, blood pressure surveillance, cardiac and respiratory frequency and venous oxygen saturation were reported.
The same management was applied to the two groups, including a nothing by mouth regimen, a urinary catheter, prophylactic anticoagulation (Liquemine 2-3 × 5000 U/d sc according to weight), an antisecretory medication (Omeprazol 40 mg/d iv) and parenteral nutrition according to patient weight and local protocol.
CT scan protocol
Both groups were evaluated according the same protocol. A CT scan was obtained on admission and 72 h after admission along with a radiological perfusion study of the pancreas to evaluate pancreatic blood flow. A significant variation in blood flow was defined as a 20% difference in perfusion between admission and 72 h measured in the head, body and tail of the pancreas, which is higher than the maximal standard deviation associated with the perfusion measures (19.4%), as shown in a previous study at our center[11]. Perfusion series were anonymized and downloaded separately from the rest of the CT examination, onto a dedicated workstation. These perfusion series were analyzed in a delayed fashion, once all patients had been included in the series. For this reading the radiologists were blinded to the treatment allocation and especially to the presence of an epidural catheter because perfusion images were limited to the upper abdomen.
Perfusion studies were obtained on a 16 row CT-scanner (MX 8000, Philips Medical Systems, Best, the Netherland). Dynamic series (90 kV, 100 mAs) were performed using four slices with a beam collimation of 6 mm, targeted on the pancreas. Acquisition started simultaneously with an IV injection of a 40-mL bolus of iodinated contrast [Ultravist 300 (iopromide), Schering] at a flow-rate of 5 mL/s, performed during a single breath-hold. A total of 160 images (40 images for each slice level) were obtained during the dynamic examination.
Perfusion images were analyzed on a dedicated workstation (Advantage Windows, GE healthcare) using the positive enhancement integral (PEI) method, compatible with Contrast Enhanced Dynamic Acquisitions, based on rates of contrast uptake by the parenchyma.
Whenever possible, two different perfusion measurements by PEI were obtained on the head, body, and tail of the pancreas for both the admission and control CT. Perfusion measures were never performed in necrotic tissue. For each of these areas, the examination was considered relevant when the region of interest remained within the pancreas parenchyma during the entire acquisition. The perfusion of a given area was considered to be improved (respectively, to be impaired) between the first and the second CT when the PEI value measured on the second CT was at least 20% higher (respectively, lower) when compared to the admission CT. When the difference in perfusion was less than 20% between the two CT, the perfusion was considered unchanged. When one of the two measures was not feasible, no value was reported.
Definition of the “primary endpoint”
Our primary endpoint was determined by “Safety of EA in patients with AP”.
Definition of covariates
Including: (1) pancreatic blood perfusion determined by the CT analysis according to our perfusion protocol; (2) parameters for clinical severity: length of stay in the hospital, use of antibiotics, admission to the intensive care unit, the clinical systemic and loco-regional complication score established by Clavien[16], and requirements for surgical necrosectomy; (3) pain was evaluated using the VAS to measure the effect of EA on the severity of the pain due to AP; and (4) complications detected by imaging studies: cysts or fluid collections, necrotic collections and other infectious adverse events.
Statistical analysis
Fisher’s exact test and the Wilcoxon Mann-Whitney test were used to compare dichotomous and continuous variables between the two groups and results were expressed as means with standard deviation and proportions. Crude odds ratios (OR) and 95%CIs were calculated. All statistical analyses were performed using SPSS version 18.0.
We calculated that 50 patients were needed in each group to detect an OR of > 2.5 with a significance level of 0.05 and a power of 80% using power and sample size calculation software version 2.1.31.
RESULTS
Between July 2005 and August 2010, a total of 49 patients met the inclusion criteria, as shown in Figure 1. After obtaining a written consent, 15 patients were randomized to the EA group and 23 patients to the control group. Of these, we had to exclude 2 patients in the EA group because of contraindications to the insertion of an epidural catheter and 1 patient in the control group because of involvement in another study. In summary, 13 patients were randomized to the EA group and 22 patients to the control group. Follow-up was the duration of the hospital stay.
Figure 1.
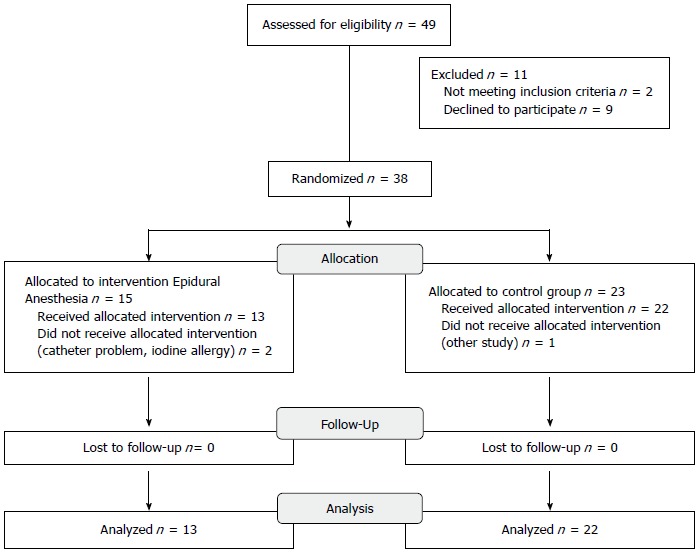
CONSORT diagram showing randomization and allocation of the study cohort.
Demographics of study population
Characteristics of each group and the results of univariate analyses are summarized in Table 1. Univariate analyses revealed that both groups were similar with respect to age, sex, BMI, and comorbid conditions.
Table 1.
Clinical characteristics of patients with severe acute pancreatitis
| Factor | Epidural anesthesia (n = 13) | Control group (n = 22) | P value1 |
| Demographics | |||
| Age (yr) | 66.08 (11.67) | 57.36 (17.97) | 0.092 |
| Male sex, n (%) | 7 (55.8) | 12 (54.5) | 0.968 |
| BMI (kg/m2) | 27.82 (6.13) | 29.15 (7.08) | 0.623 |
| Pain duration prior hospitalization (h) | 87.46 (198.85) | 90.86 (208.36) | 0.962 |
| Comorbid conditions, n (%) | 0.561 | ||
| Diabetes | 3 (23.1) | 3 (13.6) | |
| Hypertension | 2 (15.4) | 7 (31.8) | |
| High cholesterol | 2 (15.4) | 3 (13.6) | |
| Obesity | 1 (7.7) | 5 (22.7) | |
| Chronic alcoholism | 3 (23.1) | 3 (13.6) | |
| Cardiovascular condition | 2 (15.4) | 1 (4.5) | |
| Aetiology of pancreatitis, n (%) | 0.901 | ||
| Alcoholic | 3 (23.1) | 6 (27.27) | |
| Biliary | 7 (53.8) | 13 (59.1) | |
| Hyperlipidaemia | 1 (7.7) | 1 (4.5) | |
| Medication | 0 | 0 | |
| Unknown | 2 (15.4) | 2 (9.1) | |
| Ranson score at admission | 3.38 (1.12) | 2.68 (0.945) | 0.056 |
Groups were compared with a t-test for continuous variables (or Wilcoxon Mann-Whitney test) and a Pearson χ2 test (or Fisher exact test) for categorical variables. Values are mean (standard deviation) for continuous variables and n (%) for categorical variables.
The etiology of pancreatitis was not different between the two groups (alcoholic AP in 23% of the EA group vs in 27% of the control group, biliary AP 54% vs 59%, hyperlipidemic AP 7.7% vs 4.5%, unknown causes 15% vs 9.1% respectively, P = NS).
In the EA group, the mean Ranson score was higher than in the control group (Ranson score 3.38 ± 1.12 vs 2.68 ± 0.9, P = 0.056), although this did not reach statistical significance.
Safety of epidural anesthesia in patients with acute pancreatitis
Our study showed no complications of the epidural procedure in patients with predicted severe AP. There were no catheter-related infections and no cases of hemodynamic complications during procedure. The median time of EA was 5.7 d.
Epidural anesthesia improves pancreatic perfusion
Altogether, 57 comparative perfusion measurements were obtained in the same pancreatic area in both groups on the first and on the second CT (114 measures in total). When comparing perfusion studies on admission to those obtained at 72 h (Figures 2 and 3), a significant improvement in arterial perfusion of the pancreas was observed in 13 (43%) of 30 measurements in the EA group, and in 2 (7%) of 27 measurements in the control group. No change in perfusion was observed in 10 (33%) and 9 (33%) perfusion measurements in the EA group and in the control group respectively. The difference between the perfusion improvement (n = 15) and all other cases (no change or decrease, n = 42) was statistically significant using a two-tailed Fisher’s exact test (P = 0.0025) (Figure 4).
Figure 2.
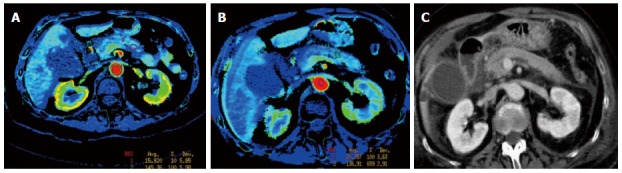
Perfusion computed tomography color map and standard computed tomography of the pancreas obtained in a 75-year-old woman admitted for acute severe pancreatitis, randomized to group 1 (epidural anesthesia). A: Axial image obtained at admission shows a positive enhancement integral (PEI) value of 15.9 in the pancreatic body; B: Axial image obtained with control computed tomography (CT), 72 h after admission, shows a 29% improvement of the perfusion in the pancreatic body (PEI of 20.5) when compared to admission values; C: Standard CT axial oblique image at the level of the pancreas obtained on admission, during portal phase.
Figure 3.
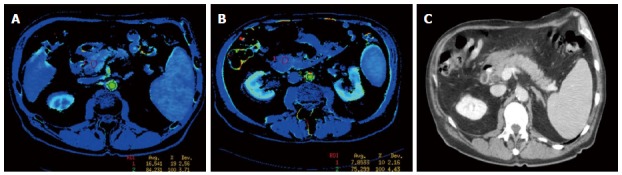
Perfusion computed tomography color map and standard computed tomography of the pancreas obtained in a 73-year-old man admitted for acute severe pancreatitis, randomized to group 2 (control group). A: Axial image obtained on admission shows a positive enhancement integral (PEI) value of 16.5 in the pancreatic head; B: Axial image obtained with control computed tomography (CT), 72 h after admission, shows a 53% impairment of the perfusion in the pancreatic head (PEI of 7.8) when compared to admission values; C: Standard CT axial oblique image, at the level of the pancreas obtained on admission, during portal phase.
Figure 4.
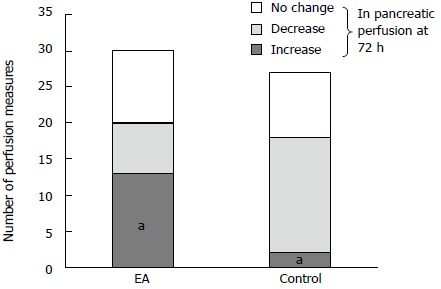
Changes in pancreatic perfusion measurements at 72 h compared to the measurements. On admission in the epidural anesthesia (EA) and control group (significant increase, decrease or no change in perfusion). aP = 0.0025 vs control.
Comparison of pancreatic necrosis on CT scan
On the CT scans, we compared the radiological severity score “Balthazar severity index”[17] between the two groups ranging from 1 to 10 and based on pancreatic morphology, pancreatic necrosis, and retroperitoneal complications. As shown in Table 2, the Balthazar score on admission was higher in the EA group than in the control group (mean score 4.15 ± 2.54 vs 3.38 ± 1.75, respectively, P = 0.347) and was higher as well at 48 h (mean score 4.69 ± 2.59 in the EA group and 4.17 ± 2.01 in the control group, P = 0.548).
Table 2.
Clinical outcome of patients with severe acute pancreatitis
| Factor | Epidural anesthesia (n = 13) | Control group (n = 22) | P value1 |
| Biochemical data | |||
| Blood pH at admission | 7.4 (0.05) | 7.38 (0.07) | 0.270 |
| Blood pH day 2 | 7.43 (0.04) | 7.4 (0.04) | 0.180 |
| CRP at admission (mg/L) | 58 (102) | 87 (118) | 0.470 |
| CRP day 2 | 274 (108) | 245 (145) | 0.550 |
| Glucose at admission (mmol/L) | 10.66 (4.29) | 9.5 (4.76) | 0.480 |
| Glucose day 2 | 8.36 (2.1) | 7.9 (3.07) | 0.630 |
| Amylase at admission (U/L) | 1479 (1183) | 1700 (1374) | 0.640 |
| Amylase day 2 | 239 (188) | 306 (262) | 0.440 |
| Lipase at admission (U/L) | 1844 (1568) | 1804 (1675) | 0.940 |
| Lipase day 2 | 108 (68) | 211 (261) | 0.190 |
| CT at admission Balthazar score | 4.15 (2.54) | 3.38 (1.75) | 0.347 |
| CT day 2 Balthazar score | 4.69 (2.59) | 4.17 (2.01) | 0.548 |
| CT guided puncture | 5 (38.5%) | 10 (45.5%) | 0.686 |
| Infected | 1 (20%) | 6 (54.5%) | 0.308 |
| Surgical treatments | |||
| Cholecystectomy | 5 (38.5%) | 13 (59.1%) | 0.240 |
| Necrosectomy | 1 (7.7%) | 4 (18.2%) | 0.630 |
| Caudal pancreatectomy | 1 | ||
| Clinical severity | |||
| ICU | 4 (33.3%) | 10 (45.5%) | 0.493 |
| Sepsis | 0 (0%) | 2 (10%) | 0.508 |
| Intubation | 1 (7.7%) | 6 (27.3%) | 0.220 |
| Medical treatments | |||
| Antibiotics | 8 (61.5%) | 15 (68.2%) | 0.689 |
| Nb of days | 19.7 (13.2) | 16.3 (12.75) | 0.580 |
| Systemic complications | 10 (76.9%) | 13 (59.1%) | 0.283 |
| Grading 1-4 | 1.77 (1.64) | 1.73 (1.75) | 0.945 |
| Loco regional complications | 9 (69.2%) | 12 (54.5%) | 0.392 |
| Grading 1-4 | 1.54 (1.45) | 1.55 (1.65) | 0.990 |
| Length of stay (d) | 26.15 (21.94) | 30.05 (25.06) | 0.646 |
| Death | 0 | 0 | 1 |
| Pain score VAS | |||
| Before randomization | 6.55 (3.39) | 7.31 (3.44) | 0.572 |
| VAS day0, EA implementation | 1.6 (1.838) | 3.5 (2.2) | 0.020 |
| VAS day 1 | 0.57 (1.51) | 2.0 (2.89) | 0.066 |
| VAS day 2 | 1.63 (3.46) | 1.67 (2.693) | 0.637 |
| VAS day 5 | 1.86 (3.485) | 1.38 (1.768) | 0.694 |
| VAS day 7 | 3 (2.38) | 2 (2.39) | 0.346 |
| VAS day 10 | 0.2 (0.447) | 2.33 (2.309) | 0.034 |
| VAS day 12 | 2.8 (2.28) | 0 (0) | 0.071 |
Groups were compared with a t-test for continuous variables (or Wilcoxon Mann-Whitney test) and a Pearson χ2 test (or Fisher exact test) for categorical variables. Values are mean +/- SD for continuous variables and n (%) for categorical variables.
Analysis of radiological interventions showed that five patients in the EA group and ten patients in the control group had a CT guided puncture of peri-pancreatic fluid or of necrotic collections (38.5% vs 45.5%, P = 0.68). Of these punctures, one in the EA group and six in the control group were infected (20% vs 54.5%, P = 0.3). This was not a statistically significant difference.
Epidural anesthesia and requirements for necrosectomy
Surgical pancreatic necrosectomy was performed in one patient in the EA group compared to four patients in the control group (7.7% vs 18.2%, P = 0.63) (Table 2). There was a trend towards EA reduction in the risk of necrosectomy that did not reach statistical significance.
Clinical outcome and mortality
To evaluate the clinical severity of AP, we looked at sepsis, organ failure and the need for an ICU stay. As shown in Table 2, the biochemical data on admission and on day 2 (48 h) did not differ between the two groups. Further, four patients in the EA group and ten in the control group had to be admitted to the intensive care unit (33% vs 45.5%, P = 0.493). In the EA group, none of the patients developed clinical sepsis and only one needed intubation, whereas in the control group, six patients needed intubation for acute respiratory distress (7.7% vs 27.3% had an intubation, respectively, P = 0.22). Furthermore, the use of antibiotics was not different between the two groups (61.5% of patients of the EA group and 68.2% of the control group, P = 0.689), nor was the duration of therapy.
During hospitalization, the EA group developed 9 cases of loco-regional complications and 10 cases of systemic complications compared to the control with 12 cases of loco-regional complications and 13 systemic complications. According to Claviens’ grading system from one to four, there was no significant difference between the two groups in the mean score for loco-regional and for systemic complications (loco-regional: 1.54 ± 1.45 vs 1.55 ± 1.65, P = 0.99 and systemic: 1.77 ± 1.64 vs 1.73 ± 1.75, P = 0.95) (Table 2).
Also, length of stay and mortality were not statistically different between the two groups (26 d in the EA group vs 30 d in the control group, P = 0.65, and 0 mortality in both groups).
Epidural anesthesia improves pain management
The VAS showed an improvement in subjective pain during the first twelve days in the EA group compared to the control group, with a significant difference on the day of EA implementation and at ten days. The results for the mean pain score on a scale from one to ten were before randomization 6.55 vs 7.31, P = 0.57; after EA implementation 1.6 vs 3.5, P = 0.02; at day one 0.57 vs 2, P = 0.06; at day five 1.86 vs 1.38, P = 0.69; at day ten 0.2 vs 2.33, P = 0.034; at day twelve 0 vs 2.8, P = 0.071 (Table 2).
DISCUSSION
This study is the first to randomize the use of EA for the treatment of AP. Previous studies have shown a beneficial effect of EA on pain management in patients with predicted severe AP[8,18]. Furthermore, the safety of EA has been widely documented in the literature[19,20] and its benefit on postoperative morbidity and mortality are well known[21-23].
We chose to present the results as a pilot study because one primary and one secondary endpoint showed statistical significance and several others showed a trend towards better outcome after EA. Our data indicated a better outcome for the EA group, though this should be confirmed by a multi-center phase II clinical trial using a larger sample size.
More than one factor seems to activate the pathological process leading to AP. The activation of pancreatic enzymes leading to edema and necrosis[24], vasoconstriction and pancreatic ischemia may convert mild disease to predicted severe AP with parenchymal necrosis[13,25,26]. This has been demonstrated by Klar et al[6] who have shown experimentally that pancreatic blood flow decreases with severe pancreatitis.
Previous studies have shown that EA increases blood flow and delays metabolic acidosis, though this has been largely investigated in the gut[9,27]. These effects have been attributed to a sympathetic nerve blockade that redistributes blood flow to non-perfused regions[9,10].
At our center, using an animal model reproducing AP, Demirag et al[4] showed that EA has a beneficial effect on the severity of AP, suggesting that EA leads to an improvement in pancreatic blood flow with a concomitant decrease in the severity of metabolic acidosis and diminished tissue injury.
Development of pancreatic necrosis is a critical event in AP that determines patient prognosis because it is often accompanied by infection and multiple organ dysfunction syndromes and, thus, is associated with a high mortality[3,28-30]. Therefore, early detection of necrosis is important for the appropriate treatment of predicted severe AP. The literature supports the use of CT scan perfusion studies to measure blood flow and diagnose necrosis in the pancreas[11,31,32]. Our measures of pancreatic perfusion showed significant improvement of the parenchymal blood flow within the pancreatic gland in the group treated with EA when compared to the control group on admission and at 72 h. This observation substantiates the theory that the severity of AP may be related to a vasoconstriction phenomenon, which can be attenuated by EA. It also suggests that the use of EA decreases progression from edematous to severe necrotizing pancreatitis caused by early ischemia of the gland and thus could reduce the severity of the disease.
In our study, there was a significant drop in subjective pain feeling at ten days as well as fewer organ failures and admissions to the ICU in the group treated with EA. Furthermore, the number of patients with infected necrosis and subsequent sepsis requiring necrosectomy was lower in the EA group, although this was not statistically significant. In general, infection occurs in about 40%-70% of patients with necrotizing pancreatitis requiring surgical debridement with necrosectomy[33]. However, these procedures have a high complication and mortality rate, ranging from 14%-26%[34,35].
There is one main limitation to our study. The study was interrupted and enrollment was closed after 49 patients because of the extreme difficulty encountered in recruiting patients from the emergency setting with severe acute pancreatitis. In addition, patients randomized for EA had a higher dropout rate compared to the standard therapy group. This resulted in uneven study groups, which could bias our results. Further trials are needed to increase the study population.
We propose that EA is beneficial for preventing early tissue damage during AP by enhancing pancreatic blood flow, as shown by our previous animal study[4] and by the CT perfusion images in this study. In conclusion, our study showed that EA significantly increased arterial perfusion of the pancreatic gland and suggested a trend towards improvement of the clinical outcome for patients with predicted severe AP. To confirm this statement, we plan to initiate a multi-center phase II study.
ACKNOWLEDGMENTS
The authors thank Mrs. Eicher Nicole and Caroline Gilbert de Vautibault for conducting follow-up and database management.
COMMENTS
Background
Severe acute pancreatitis is linked to necrosis of the pancreatic gland, which is worsened by local vasoconstriction. This can lead to high mortality for patients. Animal studies have shown that epidural anesthesia restores pancreatic microcirculation and decreases the severity of acute pancreatitis by inducing a blockade of sympathetic nerves.
Research frontiers
Epidural anesthesia is widely used to induce analgesia in the perioperative period and has also been used to decrease pain in patients with acute pancreatitis. In addition, experimental studies have shown a specific beneficial effect of epidural anesthesia in acute pancreatitis, attributed to a sympathetic nerve blockade that redistributes splanchnic blood flow to non-perfused pancreatic regions. The hotspot in current research is focusing on how to prevent or reverse pancreatic necrosis (and the risk of infection and sepsis), which in its severe form has a high mortality rate primarily due to multiple organ failure.
Innovations and breakthroughs
The innovation in this study is the use of epidural anesthesia because of its effect on splanchnic blood-flow rather than its widely approved use for analgesia. Furthermore, we present the clinical application of a computed tomography (CT) scan perfusion measuring protocol for better assessment of the perfusion status of the pancreatic gland. Different perfusion measurements using the Positive Enhancement Integral method were performed in the head, body, and tail of the pancreas, for both admission and follow-up CT.
Applications
The study results suggest that epidural anesthesia increases arterial perfusion of the pancreatic gland and shows a trend towards an improved clinical outcome for patients with predicted severe acute pancreatitis. Therefore, the authors propose that epidural anesthesia could be beneficial for preventing early tissue damage during acute pancreatitis by enhancing its blood flow.
Terminology
Acute pancreatitis is a process caused by inflammation of the pancreatic gland that is mainly due to migration of gallstones into the common bile duct or to alcohol abuse. It can lead to the development of local complications, such as pancreatic and peri-pancreatic necrosis and pseudocysts, as well as systemic complications such as adult respiratory distress syndrome or renal failure, with a risk of death in its most severe form. Epidural anesthesia is a technique that involves injection of drugs through a catheter placed into the epidural space. When injecting anesthetic drugs, it leads to loss of sensation (like pain), by blocking the transmission of signals through nerve fibers in or near the spinal cord.
Peer-review
This is a randomized prospective study evaluating the use of epidural anesthesia in patients with acute pancreatitis. Although the study did not reach its targeted accrual, the results are new and show that epidural anesthesia is safe in patients with acute pancreatitis. Further, the study demonstrates that there could be the benefit of preventing early tissue damage that could potentially improve the patient’s clinical outcome.
Footnotes
Supported by a research grant of the University Hospitals of Geneva (to Bühler L).
Institutional review board statement: The Ethics Board of Geneva’s University Hospital approved the study in 2004 (HUG 02-0555).
Clinical trial registration statement: This study is registered at http://www.clinicaltrials.gov. The registration identification number is NCT01607996.
Informed consent statement: All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement: This study was funded by a research grant from the University Hospitals of Geneva to Bühler Leo. JL Frossard received payment from the University Hospitals of Geneva for lectures. The other authors have nothing to disclose.
Data sharing statement: Consent was not obtained, but the presented data are anonymized and the risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 8, 2015
First decision: June 2, 2015
Article in press: September 15, 2015
P- Reviewer: Kim HK, Machado MCC S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Yang AL, Vadhavkar S, Singh G, Omary MB. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168:649–656. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- 2.Beger HG, Rau B, Isenmann R. Prevention of severe change in acute pancreatitis: prediction and prevention. J Hepatobiliary Pancreat Surg. 2001;8:140–147. doi: 10.1007/s005340170037. [DOI] [PubMed] [Google Scholar]
- 3.Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 4.Demirag A, Pastor CM, Morel P, Jean-Christophe C, Sielenkämper AW, Güvener N, Mai G, Berney T, Frossard JL, Bühler LH. Epidural anaesthesia restores pancreatic microcirculation and decreases the severity of acute pancreatitis. World J Gastroenterol. 2006;12:915–920. doi: 10.3748/wjg.v12.i6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schilling MK, Redaelli C, Reber PU, Friess H, Signer C, Stoupis C, Büchler MW. Microcirculation in chronic alcoholic pancreatitis: a laser Doppler flow study. Pancreas. 1999;19:21–25. [PubMed] [Google Scholar]
- 6.Klar E, Schratt W, Foitzik T, Buhr H, Herfarth C, Messmer K. Impact of microcirculatory flow pattern changes on the development of acute edematous and necrotizing pancreatitis in rabbit pancreas. Dig Dis Sci. 1994;39:2639–2644. doi: 10.1007/BF02087702. [DOI] [PubMed] [Google Scholar]
- 7.Kusterer K, Poschmann T, Friedemann A, Enghofer M, Zendler S, Usadel KH. Arterial constriction, ischemia-reperfusion, and leukocyte adherence in acute pancreatitis. Am J Physiol. 1993;265:G165–G171. doi: 10.1152/ajpgi.1993.265.1.G165. [DOI] [PubMed] [Google Scholar]
- 8.Bernhardt A, Kortgen A, Niesel HCh, Goertz A. [Using epidural anesthesia in patients with acute pancreatitis--prospective study of 121 patients] Anaesthesiol Reanim. 2002;27:16–22. [PubMed] [Google Scholar]
- 9.Sielenkämper AW, Eicker K, Van Aken H. Thoracic epidural anesthesia increases mucosal perfusion in ileum of rats. Anesthesiology. 2000;93:844–851. doi: 10.1097/00000542-200009000-00036. [DOI] [PubMed] [Google Scholar]
- 10.Freise H, Anthonsen S, Fischer LG, Van Aken HK, Sielenkämper AW. Continuous thoracic epidural anesthesia induces segmental sympathetic block in the awake rat. Anesth Analg. 2005;100:255–262. doi: 10.1213/01.ANE.0000140253.65577.1C. [DOI] [PubMed] [Google Scholar]
- 11.Bize PE, Platon A, Becker CD, Poletti PA. Perfusion measurement in acute pancreatitis using dynamic perfusion MDCT. AJR Am J Roentgenol. 2006;186:114–118. doi: 10.2214/AJR.04.1416. [DOI] [PubMed] [Google Scholar]
- 12.Ranson JH. Acute pancreatitis--where are we? Surg Clin North Am. 1981;61:55–70. doi: 10.1016/s0039-6109(16)42332-7. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198–1210. doi: 10.1056/NEJM199404283301706. [DOI] [PubMed] [Google Scholar]
- 14.Hayes S, Patterson D. Experimental development of the graphic rating method. Psychological Bulletin. 1921;18:98–99. [Google Scholar]
- 15.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balthazar EJ. Staging of acute pancreatitis. Radiol Clin North Am. 2002;40:1199–1209. doi: 10.1016/s0033-8389(02)00047-7. [DOI] [PubMed] [Google Scholar]
- 18.Niesel HC, Klimpel L, Kaiser H, Bernhardt A, al-Rafai S, Lang U. [Epidural blockade for analgesia and treatment of acute pancreatitis] Reg Anaesth. 1991;14:97–100. [PubMed] [Google Scholar]
- 19.Freise H, Van Aken HK. Risks and benefits of thoracic epidural anaesthesia. Br J Anaesth. 2011;107:859–868. doi: 10.1093/bja/aer339. [DOI] [PubMed] [Google Scholar]
- 20.Auroy Y, Narchi P, Messiah A, Litt L, Rouvier B, Samii K. Serious complications related to regional anesthesia: results of a prospective survey in France. Anesthesiology. 1997;87:479–486. doi: 10.1097/00000542-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Tziavrangos E, Schug SA. Regional anaesthesia and perioperative outcome. Curr Opin Anaesthesiol. 2006;19:521–525. doi: 10.1097/01.aco.0000245278.22658.1e. [DOI] [PubMed] [Google Scholar]
- 22.Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, Sage D, Futter M, Saville G, Clark T, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321:1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuman KJ, McCarthy RJ, March RJ, DeLaria GA, Patel RV, Ivankovich AD. Effects of epidural anesthesia and analgesia on coagulation and outcome after major vascular surgery. Anesth Analg. 1991;73:696–704. doi: 10.1213/00000539-199112000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 25.Takeda K, Mikami Y, Fukuyama S, Egawa S, Sunamura M, Ishibashi T, Sato A, Masamune A, Matsuno S. Pancreatic ischemia associated with vasospasm in the early phase of human acute necrotizing pancreatitis. Pancreas. 2005;30:40–49. [PubMed] [Google Scholar]
- 26.Büchler M, Friess H, Uhl W, Beger HG. Clinical relevance of experimental acute pancreatitis. Eur Surg Res. 1992;24 Suppl 1:85–88. doi: 10.1159/000129243. [DOI] [PubMed] [Google Scholar]
- 27.Freise H, Lauer S, Anthonsen S, Hlouschek V, Minin E, Fischer LG, Lerch MM, Van Aken HK, Sielenkämper AW. Thoracic epidural analgesia augments ileal mucosal capillary perfusion and improves survival in severe acute pancreatitis in rats. Anesthesiology. 2006;105:354–359. doi: 10.1097/00000542-200608000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Isenmann R, Rau B, Zoellner U, Beger HG. Management of patients with extended pancreatic necrosis. Pancreatology. 2001;1:63–68. doi: 10.1159/000055794. [DOI] [PubMed] [Google Scholar]
- 29.Gloor B, Müller CA, Worni M, Martignoni ME, Uhl W, Büchler MW. Late mortality in patients with severe acute pancreatitis. Br J Surg. 2001;88:975–979. doi: 10.1046/j.0007-1323.2001.01813.x. [DOI] [PubMed] [Google Scholar]
- 30.Tenner S, Sica G, Hughes M, Noordhoek E, Feng S, Zinner M, Banks PA. Relationship of necrosis to organ failure in severe acute pancreatitis. Gastroenterology. 1997;113:899–903. doi: 10.1016/s0016-5085(97)70185-9. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji Y, Hamaguchi K, Watanabe Y, Okumura A, Isoda H, Yamamoto N, Kikuchi O, Yamamoto H, Matsueda K, Ueno K, et al. Perfusion CT is superior to angiography in predicting pancreatic necrosis in patients with severe acute pancreatitis. J Gastroenterol. 2010;45:1155–1162. doi: 10.1007/s00535-010-0267-8. [DOI] [PubMed] [Google Scholar]
- 32.Tsuji Y, Yamamoto H, Yazumi S, Watanabe Y, Matsueda K, Yamamoto H, Chiba T. Perfusion computerized tomography can predict pancreatic necrosis in early stages of severe acute pancreatitis. Clin Gastroenterol Hepatol. 2007;5:1484–1492. doi: 10.1016/j.cgh.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Beger HG, Rau B, Mayer J, Pralle U. Natural course of acute pancreatitis. World J Surg. 1997;21:130–135. doi: 10.1007/s002689900204. [DOI] [PubMed] [Google Scholar]
- 34.Connor S, Alexakis N, Raraty MG, Ghaneh P, Evans J, Hughes M, Garvey CJ, Sutton R, Neoptolemos JP. Early and late complications after pancreatic necrosectomy. Surgery. 2005;137:499–505. doi: 10.1016/j.surg.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Büchler MW, Gloor B, Müller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619–626. doi: 10.1097/00000658-200011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]


