Abstract
AIM: To provide an overview of the clinical outcomes of self-expandable metal stent (SEMS) placement for malignant gastric outlet obstruction (MGOO).
METHODS: A systematic literature search was performed in PubMed of the literature published between January 2009 and March 2015. Only prospective studies that reported on the clinical success of stent placement for MGOO were included. The primary endpoint was clinical success, defined according to the definition used in the original article. Data were pooled and analyzed using descriptive statistics. Subgroup analyses were performed for partially covered SEMSs (PCSEMSs) and uncovered SEMSs (UCSEMSs) using Fisher’s exact test.
RESULTS: A total of 19 studies, including 1281 patients, were included in the final analysis. Gastric (42%) and pancreatic (37%) cancer were the main causes of MGOO. UCSEMSs were used in 76% of patients and PCSEMSs in 24%. The overall pooled technical success rate was 97.3% and the clinical success rate was 85.7%. Stent dysfunction occurred in 19.6% of patients, mainly caused by re-obstruction (12.6%) and stent migration (4.3%), and was comparable between PCSEMSs and UCSEMSs (21.2% vs 19.1%, respectively, P = 0.412). Re-obstruction was more common with UCSEMSs (14.9% vs 5.1%, P < 0.001) and stent migration was more frequent after PCSEMS placement (10.9% vs 2.2%, P < 0.001). The overall perforation rate was 1.2%. Bleeding was reported in 4.1% of patients, including major bleeding in 0.8%. The median stent patency ranged from 68 to 307 d in five studies. The median overall survival ranged from 49 to 183 d in 13 studies.
CONCLUSION: The clinical outcomes in this large population showed that enteral stent placement was feasible, effective and safe. Therefore, stent placement is a valid treatment option for the palliation of MGOO.
Keywords: Stents, Gastric outlet obstruction, Stomach neoplasms, Pancreatic neoplasms, Intestinal obstruction, palliative care, Systematic review
Core tip: In this pooled analysis of the prospective literature published since January 2009, we provide an extensive overview of the clinical outcomes of stent placement for malignant gastric outlet obstruction. We analyzed the technical and clinical success, stent dysfunction, stent patency, perforation, bleeding and overall survival in 1281 patients treated with enteral stent placement.
INTRODUCTION
Gastric outlet obstruction is a syndrome characterized by nausea (90%), vomiting (83%), regurgitation (69%) and abdominal pain (66%)[1]. The majority of patients (> 75%) presenting with malignant gastric outlet obstruction (MGOO) cannot tolerate solids, and approximately 40% of patients have no oral intake at all[1]. Pancreatic cancer is the most common cause of MGOO in Western countries[1-3], while gastric cancer is the leading cause of MGOO in Eastern Asian studies[4-6]. Gastric outlet obstruction is usually a late sign of a locally advanced or metastatic cancer, requiring palliative management. These patients have a poor prognosis with a mean survival of approximately 100 d (3.3 mo)[7], and an impaired quality of life[8,9]. The aim of palliative therapy is to relieve obstructive symptoms and to allow oral intake. Treatment options for MGOO are endoscopic stent placement (Figure 1), surgical bypass by means of a gastrojejunostomy, a percutaneous gastrostomy (PEG) serving for gastric decompressing with subsequent jejunal feeding tube placement, and pharmacological therapy aiming for improvement in gastric emptying, relief of symptoms and comfort[7,10-12]. Comparison of enteral stenting and gastrojejunostomy revealed sooner return to oral intake and shorter hospital stay after stent placement[7,13]. On the long term, however, patients with an enteral stent have more recurrent obstruction and require more re-interventions[9]. Therefore, one might argue that patients with a relatively short survival benefit the most from enteral stent placement.
Figure 1.
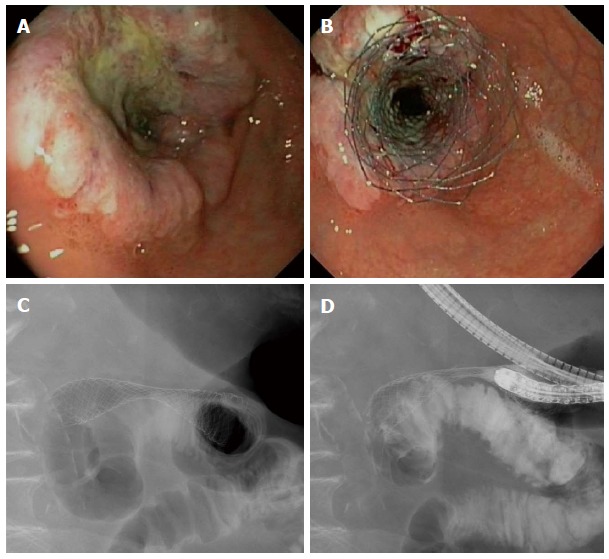
Endoscopic view of a gastric antrum adenocarcinoma involving the pylorus and causing obstructive symptoms (A) for which an uncovered WallFlex stent (Boston Scientific) was placed (B). Fluoroscopic view shows the fully deployed stent across the pylorus (C) with good passage of contrast to the duodenum (D).
The stents used for the endoscopic treatment of MGOO are self-expandable metal stents (SEMSs) (Figure 2). They consist of a flexible framework of wire mesh made of nitinol, a metal alloy of nickel and titanium, and are either uncovered or covered by a polytetrafluoroethylene, polyurethane or silicone membrane. Over the past years many studies have been published on the clinical outcomes of enteral stent placement for MGOO. With a pooled analysis of the recent literature we aim to provide an overview of the clinical outcomes of SEMS placement for MGOO, including subgroup analyses for covered and uncovered SEMSs.
Figure 2.
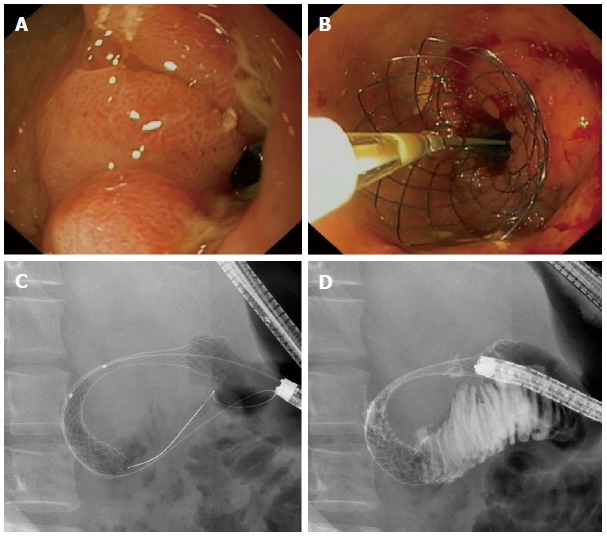
Endoscopic view of an adenocarcinoma of the distal stomach invading the duodenal bulb causing a gastric outlet obstruction (A) for which an uncovered WallFlex stent (Boston Scientific) was placed (B). Fluoroscopic view shows the fully deployed stent in the duodenal bulb (C) with good passage of contrast to the distal duodenum (D).
MATERIALS AND METHODS
The PubMed database was searched for relevant articles published between January 2009 and March 2015. This period was chosen because during the past years new stent designs have emerged and before 2009 the studies were usually small and retrospective. The search terms used were gastric outlet obstruction, duodenal obstruction, malignant and stents. A single reviewer (van Halsema EE) selected relevant articles by title and abstract. Only prospective studies that reported on the clinical success and safety of stent placement for MGOO were included. Studies with a sample size of less than 10 patients were excluded to avoid pilot studies with experimental stent designs and because the average series in this field usually contains a minimum of at least 30 patients. The search strategy and exclusion criteria are presented in Figure 3. The primary endpoint was clinical success of stent placement. Secondary endpoints were technical success of stent placement, stent dysfunction, stent patency, perforation, bleeding and survival. Clinical success was defined according to the definition used in the original article. These definitions all comprised the ability to tolerate oral intake, improvement in Gastric Outlet Obstruction Severity Score or relief of obstructive symptoms, up to 14 d after enteral stent placement. Stent dysfunction included re-obstruction by tumor in- or overgrowth, stent migration, stent compression by tumor pressure, insufficient expansion after deployment, stent fracture and food occlusion. Technical success was defined as successful stent placement across the obstructing tumor. Perforation and bleeding were analyzed when reported, regardless whether they were thought to be unrelated to enteral stent placement.
Figure 3.
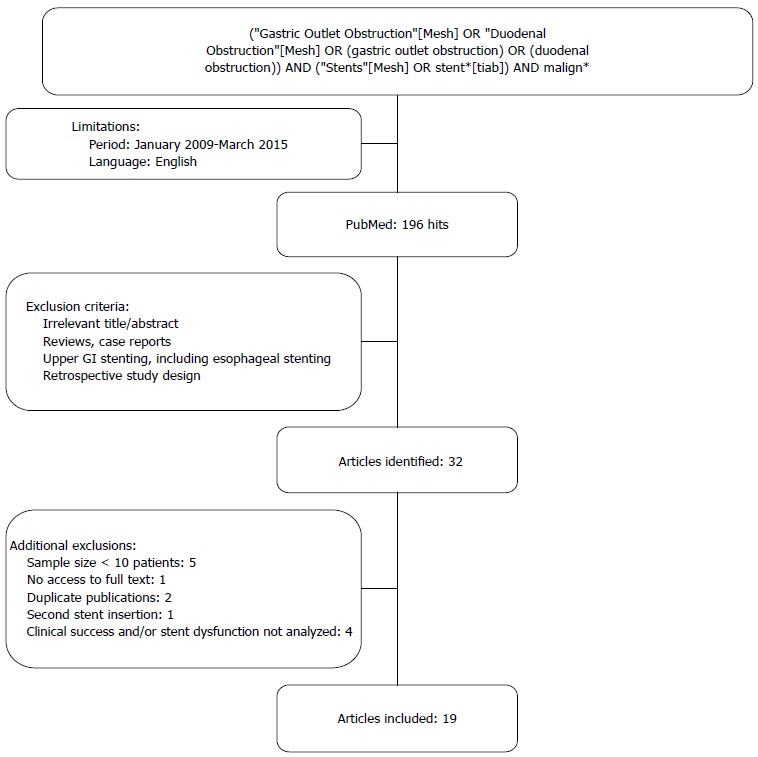
PubMed search. GI: Gastrointestinal.
Statistical analysis
Data were pooled and analyzed as an intention-to-treat analysis. Pooled data were presented as frequency and proportion. The median in days was used to report the stent patency and overall survival, because the median was reported most frequently in the original articles. Fisher’s Exact Test was used to compare two proportions using WinPepi, Version 11.26, freeware computer programs for epidemiologists[14]. Two-sided P values < 0.05 were considered statistically significant.
RESULTS
Thirty-two relevant prospective studies were identified. Figure 3 shows the results of the literature search. Thirteen articles were excluded because of the following reasons: the sample size was insufficient (n = 5)[30-34], the primary endpoint as defined before was not analyzed (n = 4)[8,35-37], second stent insertion was analyzed (n = 1)[38], the full text was not accessible (n = 1)[39] or because of duplicate publication (n = 2)[40,41]. Nineteen prospective studies, including four randomized controlled trials (RCTs), were included in the final analysis (Table 1[1,2,5,9,15-29]). A total of 1281 patients underwent enteral stent placement for MGOO. Gastric cancer (42%) was the most common indication for stent placement, followed by pancreatic cancer (37%). Uncovered SEMSs (UCSEMS) were used in 75.7% of patients and partially covered SEMSs (PCSEMS) in 24.3%. The majority of patients (93.5%, 692/740) received a single stent during the initial procedure and 6.5% (48/740) required two stents. The baseline characteristics are summarized in Table 2.
Table 1.
Literature table
| Ref. | Design, patients, indication | No. of stents1, biliary obstruction | Stent type | Technical success | Clinical Success, definition | Adverse events | Stent dysfunction | Stent patency, median (range) | Survival, follow-up, median (range) |
| Maetani et al[15] 2014 Japan | RCT, n = 62 GC: n = 27 PC: n = 26 BDC: n = 7 OT: n = 2 | No. of stents: 1: n = 58 2: n = 4 Biliary drainage: Yes: n = 24 No: n = 38 | UCSEMS: -Niti-S PCSEMS: -Niti-S Comvi | 100% (62/62) | 90.3% (56/62) -UCSEMS: 93.5% (29/31) -PCSEMS: 87.1% (27/31) ≥ 1 grade of improvement in GOOSS at any visit compared to baseline | Perforation: 1.6% (1/62) -PCSEMS: 3.2% (1/31) Major bleeding: 1.6% (1/62) -UCSEMS: 3.2% (1/31) | Overall: 22.6% (14/62) -UCSEMS: 29.0% (9/31) -PCSEMS: 16.1% (5/31) Re-obstruction: 9.7% (6/62); -UCSEMS: 19.4% (6/31) -PCSEMS: 0% (0/31) Migration: 4.8% (3/62) -UCSEMS: 3.2% (1/31) -PCSEMS: 6.5% (2/31) Fracture: 4.8% (3/62) -UCSEMS: 6.5% (2/31) -PCSEMS: 3.2% (1/31) Insufficient expansion: 3.2% (2/62) -UCSEMS: 0% (0/31) -PCSEMS: 6.5% (2/31) | PCSEMS: 68 d UCSEMS: 88 d (P = 0.70) | PCSEMS: 73 d UCSEMS: 93 d (P = 0.34) FU: 83.5 d; until death |
| Shi et al[16] 2014 China | RCT, n = 65 GC: n = 65 | No. of stents: 1: n = 65 Biliary obstruction: NR | UCSEMS: -Micro-Tech PCSEMS: -Micro-Tech (tailored cup- or funnel-shaped) | 96.9% (63/65) -UCSEMS: 96.9% (31/32) -PCSEMS: 97.0% (32/33) | 93.7% (59/63) -UCSEMS: 93.5% (29/31) -PCSEMS: 93.8% (30/32) Resolution of symptoms and the ability to restart a low residue diet after stent placement | Mild bleeding: 20% (13/65) -UCSEMS: 6.3% (2/32) -PCSEMS: 33.3% (11/33) Mild abdominal pain: 21.5% (14/65) -UCSEMS: 3.1% (1/32) -PCSEMS: 39.4% (13/33) | Overall: 18.5% (12/65) -UCSEMS: 25% (8/32) -PCSEMS: 12.1% (4/33) Re-obstruction: 12.3% (8/65) -UCSEMS: 21.9% (7/32) -PCSEMS: 3.0% (1/33) Migration: 3.1% (2/65) -UCSEMS: 0% -PCSEMS: 6.1% (2/33) Food impaction: 3.1% (2/65) -UCSEMS: 3.1% (1/32) -PCSEMS: 3.0% (1/33) | NR | Tailored PCSEMS: mean 231 (30-387) d Standard UCSEMS: mean 212 (43-267) d FU: until death |
| Tringali et al[1] 2014 Italy, Netherlands, Australia, Czech Republic, Canada, United States | Pros, n = 108 PC: n = 58 GC: n = 14 BDC: n = 7 GBC: n = 7 DC: n = 5 APC: n = 3 OT: n = 14 | No. of stents: 1: n = 106 2: n = 2 Biliary obstruction: Yes: n = 56 No: n = 52 | UCSEMS: -Evolution | 99.1% (107/108) | 84.5% (82/97) Relief of symptoms and/or improvement of oral intake at 14 d | Overall: 32.4% (35/108), including 19.4% (21/108) stent-related Perforation: 1.9% (2/108) Bleeding: 4.6% (5/108) No intervention required Abdominal pain: 1.9% (2/108) Other GI events: 15.7% (17/108) | Overall: 17.6% (19/108) Re-obstruction: 15.7% (17/108) Migration: 1.9% (2/108) | Estimated patency rates: -At 14 d: 94.6% (88/93) -At 60 d: 86.2% -At 180 d: 63.4% | Patients who completed 6 mo follow-up (11/108): 182 (178-195) d Patients who died before 6 mo follow-up: 52 (9-180) d FU: until 6 mo, death or re-intervention |
| Shi et al[17] 2013 China | Pros, n = 37 GC: n = 37 | No. of stents: 1: n = 35 2: n = 2 Biliary obstruction: NR | PCSEMS: -Micro-Tech (cup- or funnel-shaped) | 97.3% (36/37) | 94.4% (34/36) Relief of obstructive symptoms | Mild bleeding: 40.5% (15/37) Major hemorrhage: 2.7% (1/37) Abdominal pain: 37.8% (14/37) Perforation: 0% | Overall: 5.4% (2/37) Food impaction: 5.4% (2/37) Migration: 0% Re-obstruction: 0% | NR | Mean 232 (28-387) d FU: until death |
| van den Berg et al[18] 2013 Netherlands | Pros, n = 46 PC: n = 25 GC: n = 5 BDC: n = 7 DC: n = 3 GBC: n = 1 OT: n = 5 | No. of stents: 1: n = 43 2: n = 3 Biliary drainage: Yes: n = 34 No: n = 12 | UCSEMS: -Evolution | 89.1% (41/46) | 71.7% (33/46) Improvement of GOOSS of ≥ 1 point and/or relief of symptoms after 1 wk | Overall: 56.5% (26/46) Procedure-related: -Perforation: 2.2% (1/46) -Pancreatitis: 2.2% (1/46) -Pain: 2.2% (1/46) -Cholangitis: 2.2% (1/46) Non procedure-related: -Acute abdomen: 2.2% (1/46) -Cholangitis: 13.0% (6/46) -Jaundice: 4.3% (2/46) -Anemia: 8.7% (4/46) -Pneumonia: 2.2% (1/46) -CVA: 6.5% (3/46) -Ascites: 4.3% (2/46) -Motility disorder: 10.9% (5/46) | Overall: 30.4% (14/46) Re-obstruction: 19.6% (9/46) Stent compression: 4.3% (2/46) Migration: 4.3% (2/46) Food impaction: 2.2% (1/46) | 67% for up to 395 d, accounting for death unrelated to stent | 87 (IQR 35-237) d FU: until death |
| Costamagna et al[2] 2012 Italy, Czech Republic, South Africa, Canada, Sweden, Brazil, France, Germany, Finland, Spain | Pros, n = 202 PC: n = 104 GC: n = 37 DC: n = 18 BDC: n = 12 GBC: n = 12 APC: n = 2 OT: n = 17 | No. of stents: 1: n = 192 2: n = 10 Biliary drainage: Yes: n = 127 No: n = 75 | UCSEMS: -WallFlex | 98.0% (198/202) | 91% (177/195) Relief of obstruction as measured by oral intake | Overall: 20.3% (41/202) Transient periprocedural symptoms: 3.5% (7/202) Bleeding: 3.0% (6/202) -Major: 2.0% (4/202) -Self-limiting: 1.0% (2/202) Perforation: 0.5% (1/202) | Re-obstruction: 12.4% (25/202) Migration: 1.5% (3/202) Food impaction: 0.5% (1/202) | Maintaining GOOS score of 2-3 (n = 149): 91 d (95%CI: 87-182) | Survival rate at 9 mo: 28.2% FU: until 9 mo |
| Isayama et al[19] 2012 Japan | Pros, n = 50 PC: n = 26 GC: n = 14 BDC: n = 9 OT: n = 1 | No. of stents: NR Biliary obstruction: Yes: n = 30 No: n = 20 | PCSEMS: -ComVi Niti-S (modified) | 100% (50/50) | 90% (45/50) Relief of symptoms or improvement in GOOSS after 3 d | Cholangitis: 2% (1/50) Mild pancreatitis: 2% (1/50) Minor perforation: 2% (1/50) | Overall: 18% (9/50) Re-obstruction: 10% (5/50) Stent migration: 6% (3/50) Insufficient expansion: 2% (1/50) | Mean ± SD: 149.8 ± 8.9 d | 106 d FU: NR |
| Moura et al[20] 2012 Brazil | Pros, n = 15 PC: n = 9 GC: n = 3 BDC: n = 1 OT: n = 2 | No. of stents: NR Biliary drainage: Yes: 8 No: 7 | UCSEMS: -WallFlex | 100% (15/15) | 80% (12/15) Improvement of GOOSS at 15 d | Removal of foreign body: 7% (1/15) | Re-obstruction: 13% (2/15) Migration: 13% (2/15) | Mean time to first failure to maintain GOOS 2-3: 2.35 mo | NR FU: until 180 d |
| Dolz et al[21] 2011 Spain | Pros, n = 77 GC: n = 29 PC: n = 20 DC: n = 5 GBC: n = 4 BDC: n = 3 APC: n = 3 OT: n = 6 Unk: n = 7 | No. of stents: NR Biliary obstruction: NR | UCSEMS: -WallFlex -Wallstent -Ultraflex | 92.2% (71/77) | 81.7% (58/71) GOOSS 2-3 post-stenting | Pneumonia: 1.4% (1/71) Central catheter infection: 1.4% (1/71) Self-limiting bleeding: 7.0% (5/71) Late perforation: 2.8% (2/71) Intense pain: 2.8% (2/71) | Re-obstruction: 14.1% (10/71) Insufficient expansion: 4.2% (3/71) | NR | 91 (9-552) d FU: NR |
| Kim et al[22] 2011 South Korea | Pros, n = 50 GC: n = 31 PC: n = 11 BDC: n = 6 GBC: n = 2 | No. of stents: NR Biliary drainage: Yes: n = 17 No: n = 33 | PCSEMS: -Niti-S Comvi | 100% (50/50) | 88% (44/50) Ability to tolerate oral food intake without vomiting | Hyperamylasemia: 2% (1/50) Obstructive jaundice: 10% (5/50) | Re-intervention rate: 28% (14/50) Stent migration: 10% (5/50) Re-obstruction: 8% (4/50) Stent compression: 10% (5/50) | Mean 92 (4-238) d | Mean 110 (30-290) d FU: until death |
| van Hooft et al[23] 2011 Netherlands | Pros, n = 52 PC: n = 32 GC: n = 7 BDC: n = 10 APC: n = 1 DC: n = 1 OT: n = 1 | No. of stents: 1: n = 45 2: n = 7 Biliary obstruction: NR | UCSEMS: -Niti-S D-Weave | 96.2% (50/52) | 76.9% (40/52) Relief of symptoms or improvement of GOOSS after 1 wk | Overall complications: 23.1% (12/52) Procedure-related: -Pain: 7.7% (4/52) -Cholangitis: 1.9% (1/52) Non procedure-related: -Anemia: 3.8% (2/52) -Pneumonia: 1.9% (1/52) -Ascites: 1.9% (1/52) -Gastroenteritis: 1.9% (1/52) -Peritonitis carcinomatosis: 1.9% (1/52) -Bacteremia: 1.9% (1/52) | Overall: 25% (13/52) Re-obstruction: 21.2% (11/52) Migration: 3.8% (2/52) | 75% for up to 190 d, accounting for death unrelated to stent | 82 (IQR 31-135) d FU: until death |
| Jeurnink et al[9], 2010 Netherlands | RCT, n = 21 PC: n = 15 GC: n = 2 DC: n = 3 OT: n = 1 | No. of stents: 1: n = 17 2: n = 4 Biliary drainage: Yes: n = 12 No: n = 9 | UCSEMS: -WallFlex | 95.2% (20/21) | 85.7% (18/21); persistent obstruction within 4 wk in 3/21 | Bacterial infection: 4.8% (1/21) Delayed gastric emptying: 14.3% (3/21) Jaundice post stent: 19.0% (4/21) Cholangitis: 4.8% (1/21) | Overall: 19.0% (4/21) Re-obstruction: 9.5% (2/21) Migration: 4.8% (1/21) Food obstruction: 9.5% (2/21) Re-intervention rate: 33% (7/21) | NR | 56 d FU: until death |
| Kim et al[24] 2010 South Korea | RCT, n = 80 GC: n = 80 | No. of stents: NR Biliary obstruction: NR | PCSEMS: -Niti-S pyloric -Niti-S Comvi UCSEMS: -Wallstent -WallFlex | 100% (80/80) | 92.5% (74/80) -PCSEMS: 95% (38/40) -UCSEMS: 90% (36/40) Relief of symptoms or improvement of GOOSS at 3 d | Perforation by migrated stent: 1.5% (1/67) -PCSEMS: 3.2% (1/31) Intestinal obstruction by migrated stent fragment after fracture: 1.5% (1/67) -PCSEMS: 3.2% (1/31) | Re-obstruction: 25.4% (17/67) -PCSEMS: 3.2% (1/31) -UCSEMS: 44.4% (16/36) Migration: 19.4% (13/67) -PCSEMS: 32.3% (10/31) -UCSEMS: 8.3% (3/36) Fracture: 4.5% (3/67) -PCSEMS: 9.7% (3/31) Stent collapse: 1.5% (1/67) -PCSEMS: 3.2% (1/31) | PCSEMS: 14 wk (95%CI: 8.9-19.1) UCSEMS: 13 wk (95%CI: 9.5-16.5) | PCSEMS: 26 wk (95%CI: 11-41) UCSEMS: 19 wk (95%CI: 10-28) FU: until 8 wk |
| Maetani et al[25] 2010 Japan | Pros, n = 53 GC: n = 29 PC: n = 14 BDC: n = 5 OT: n = 5 | No. of stents: 1: n = 44 2: n = 9 Biliary drainage: Yes: n = 17 No: n = 36 | UCSEMS: -Niti-S | 98.1% (52/53) | 94.3% (50/53) Ability to tolerate oral intake without vomiting | Procedure-related perforation: 1.9% (1/53) Obstructive jaundice: 1.9% (1/53) Major bleeding: 1.9% (1/53) | Overall: 18.9% (10/53) Insufficient expansion: 3.8% (2/53) Re-obstruction: 13.2% (7/53) Food impaction: 1.9% (1/53) Fracture: 1.9% (1/53) Re-intervention rate: 20.8% (11/53) | NR | 88 d FU: until death |
| Shaw et al[26], 2010 South Africa | Pros, n = 70 GC: n = 19 PC: n = 34 GBC: n = 5 DC: n = 2 BDC: n = 3 OT: n = 7 | No. of stents: NR Biliary drainage: Yes: n = 35 No: n = 35 | UCSEMS: -WallFlex | 92.9% (65/70) | 88.6% (62/70) Resumption of intake that enabled the patient to return home independent of nutritional support | Minor bleeding: 2.9% (2/70) Perforation: 0% | Overall: 7.1% (5/70) Re-obstruction: 4.3% (3/70) Insufficient expansion: 1.4% (1/70) Stent migration: 1.4% (1/70) | NR | 1.8 (0.1-19) mo FU: 54 (range 1-570) d; until death |
| Havemann et al[27] 2009 Denmark | Pros, n = 45 PC: n = 30 GC: n = 5 OT: n = 10 | No. of stents: NR Biliary drainage: Yes: n = 11 No: n = 34 | UCSEMS: -Hanaro | 91.1% (41/45) | 63.4% (26/41) Improvement in GOOSS by ≥ 1 point | Procedure-related perforation: 4.4% (2/45) Biliary obstruction: 17.8% (8/45) | Re-obstruction: 8.9% (4/45) Migration: 6.7% (3/45) Re-intervention rate: 13.3% (6/45) | NR | Mean 121 (95%CI: 62-181) d FU: NR |
| Lee et al[5] 2009 South Korea | Pros, n = 154 GC: n = 122 PC: n = 19 GBC: n = 3 BDC: n = 3 APC: n = 4 DC: n = 2 OT: n = 1 | No. of stents: NR Biliary obstruction: NR | UCSEMS: -Niti-S PCSEMS: -Niti-S | 100% (154/154) | 97.4% (150/154) -PCSEMS: 98.6% (69/70) -UCSEMS: 96.4% (81/84) Relief of vomiting and resumption of diet | No procedure-related complications | Migration: 7.8% (12/154) -PCSEMS: 17.1% (12/70) -UCSEMS: 0% Re-obstruction: 13.6% (21/154) -PCSEMS: 7.1% (5/70) -UCSEMS: 19.0% (16/84) Re-intervention rate: 17.5% (27/154) -PCSEMS: 21.4% (15/70) -UCSEMS: 14.3% (12/84) | UCSEMS: 73 (95%CI: 44-102) d PCSEMS: 75 (95%CI: 47-134) d | UCSEMS: 108 (95%CI: 60-151) d PCSEMS: 115 (95%CI: 80-156) d FU: until death |
| Piesman et al[28] 2009 United States | Pros, n = 43 PC: n = 21 GC: n = 8 BDC: n = 3 GBC: n = 1 OT: n = 9 Unk: n = 1 | No. of stents: 1: n = 39 2: n = 4 Biliary drainage: Yes: n = 23 No: n = 20 | UCSEMS: -WallFlex | 95.3% (41/43) | 81.4% (35/43) GOOSS increase of ≥ 1 point | Duodenal perforation: 4.7% (2/43) Vomiting: 9.3% (4/43) Cholangitis: 2.3% (1/43) Hemorrhage: 2.3% (1/43) -Endoscopy performed Nausea: 2.3% (1/43) Sepsis: 2.3% (1/43) | Overall: 18.6% (8/43) Re-obstruction: 9.3% (4/43) Malposition: 2.3% (1/43) Stent collapse: 2.3% (1/43) Incomplete expansion: 4.7% (2/43) Occlusion by jejunal wall: 2.3% (1/43) Migration: 0% | GOOS score increase of ≥ 1 until death or end of follow-up: 45% (95%CI: 27-74) | 49 d; At 24 wk: 44.1% (19/43) FU: until 24 wk |
| Van Hooft et al[29] 2009 Netherlands | Pros, n = 51 PC: n = 35 GC: n = 2 BDC: n = 3 DC: n = 3 GBC: n = 2 APC: n = 1 OT: n = 5 | No. of stents: 1: n = 48 2: n = 3 Biliary drainage: Yes: n = 38 No: n = 13 | UCSEMS: -WallFlex | 98.0% (50/51) | 84.3% (43/51) Relief of symptoms or improvement of GOOSS after 1 wk | Motility dysfunction: 3.9% (2/51) Intermittent pain: 3.9% (2/51) Cholangitis: 5.9% (3/51) Major bleeding: 3.9% (2/51) | Re-obstruction: 11.8% (6/51) Migration: 2.0% (1/51) | 307 d; 75% functional at 135 d, 25% functional at 470 d | 62 d; 75% alive at 35 d, 25% alive at 156 d FU: until death |
Number of enteral stents inserted at the initial procedure. Pros: Prospective; Retro: Retrospective; RCT: Randomized controlled trial; GC: Gastric cancer; PC: Pancreatic cancer; BDC: Bile duct cancer; GBC: Gallbladder cancer; APC: Ampullary cancer; DC: Duodenal cancer; OT: Other malignancies; UCSEMS: Uncovered self-expandable metal stent; PCSEMS: Partially covered self-expandable metal stent; FU: Follow-up; GOOSS: Gastric outlet obstruction severity score; NR: Not reported; IQR: Interquartile range; GI: Gastrointestinal; CVA: Cerebrovascular accident.
Table 2.
Baseline characteristics n (%)
| Patients with MGOO | 1281 (100) |
| Cause of MGOO | |
| Gastric cancer | 536 (41.8) |
| Pancreatic cancer | 479 (37.4) |
| Bile duct cancer | 79 (6.2) |
| Duodenal cancer | 42 (3.3) |
| Gallbladder cancer | 37 (2.9) |
| Ampullary cancer | 14 (1.1) |
| Other malignancies | 86 (6.7) |
| Unknown | 8 (0.6) |
| Biliary obstruction1 | |
| Yes | 432 (52.9) |
| No | 384 (47.1) |
| Stent type | |
| Uncovered SEMS | 970 (75.7) |
| Partially covered SEMS | 311 (24.3) |
| No. of enteral stents inserted at initial procedure2 | |
| Single stent | 692 (93.5) |
| Two stents | 48 (6.5) |
Total group: n = 816, no data of 465 patients;
Total group: n = 740, no data of 541 patients. MGOO: Malignant gastric outlet obstruction; SEMS: Self-expandable metal stent.
Technical and clinical success
Technical success was achieved in 97.3% (range 89.1%-100%) of patients and was significantly higher for PCSEMSs in comparison with UCSEMSs: 99.4% vs 96.6% (P = 0.008). The main reasons for technical failure were the inability to pass the guidewire across the stenosis (1.0%), stent migration during deployment (0.3%) and insufficient deployment (0.3%). Technical failure due to a procedure-related perforation was reported in one case (0.1%)[25]. The overall clinical success rate was 85.7% (range, 57.8%-97.4%). PCSEMSs had a significantly higher clinical success rate than UCSEMSs: 92.3% vs 83.6% (P < 0.001). Four studies compared the clinical outcomes of PCSEMSs and UCSEMSs[5,15,16,24]. In those comparative studies, the pooled clinical success rates of PCSEMSs and UCSEMSs were 94.3% (164/174) and 93.6% (175/187), respectively (P = 0.829). Further details are summarized in Table 3.
Table 3.
Technical and clinical success of enteral stent placement n (%)
| Overall (n = 1281) | UCSEMS (n = 970) | PCSEMS (n = 311) | P value1 | |
| Technical success | 1246 (97.3) | 937 (96.6) | 309 (99.4) | 0.008 |
| Reasons for technical failure | ||||
| Inability to pass guidewire | 13 (1.0) | 13 (1.3) | 0 | |
| Looping/buckling of delivery system | 2 (0.2) | 0 | 2 (0.6) | |
| Stent malposition | 1 (0.1) | 1 (0.1) | 0 | |
| Stent migration during deployment | 4 (0.3) | 4 (0.4) | 0 | |
| Insufficient deployment | 4 (0.3) | 4 (0.4) | 0 | |
| Colonic stent inserted | 1 (0.1) | 1 (0.1) | 0 | |
| No stenosis at endoscopy | 1 (0.1) | 1 (0.1) | 0 | |
| Procedural perforation | 1 (0.1) | 1 (0.1) | 0 | |
| Not specified | 8 (0.6) | 8 (0.8) | 0 | |
| Clinical success | 1098 (85.7) | 811 (83.6) | 287 (92.3) | < 0.001 |
Comparison of UCSEMS and PCSEMS using Fisher’s exact test. UCSEMS: Uncovered self-expandable metal stents; PCSEMS: Partially covered self-expandable metal stents.
Stent dysfunction
Stent dysfunction occurred in 19.6% (range, 5.4%-42.5%) of patients. There was no difference between the stent dysfunction rate of PCSEMSs and UCSEMSs: 21.2% vs 19.1%, respectively (P = 0.412). The main reasons for stent failure were re-obstruction by tumor in- or overgrowth (12.6%) and stent migration (4.3%). Re-obstruction was more common with the use of UCSEMSs compared with PCSEMSs: 14.9% vs 5.1% (P < 0.001). The stent migration rate was significantly higher after PCSEMS placement: 10.9% vs 2.2% (P < 0.001). Stent compression or collapse by tumor pressure occurred in 0.7% of patients, and was significantly higher for PCSEMSs: 1.9% vs 0.3% (P = 0.008). Other reasons for stent dysfunction were insufficient expansion (0.9%), food occlusion (0.7%), stent fracture (0.5%) and other (0.2%) (Table 4).
Table 4.
Adverse events n (%)
| Overall (n = 1281) | UCSEMS (n = 970) | PCSEMS (n = 311) | P value1 | |
| Stent dysfunction | 251 (19.6) | 185 (19.1) | 66 (21.2) | 0.412 |
| Re-obstruction by tumor growth | 161 (12.6) | 145 (14.9) | 16 (5.1) | < 0.001 |
| Stent migration | 55 (4.3) | 21 (2.2) | 34 (10.9) | < 0.001 |
| Stent compression by tumor pressure | 9 (0.7) | 3 (0.3) | 6 (1.9) | 0.008 |
| Stent fracture | 7 (0.5) | 3 (0.3) | 4 (1.3) | 0.064 |
| Insufficient expansion | 11 (0.9) | 8 (0.8) | 3 (1.0) | 0.734 |
| Food occlusion | 9 (0.7) | 6 (0.6) | 3 (1.0) | 0.460 |
| Other | 2 (0.2) | 2 (0.2) | 0 | - |
| Perforation | 15 (1.2) | 12 (1.2) | 3 (1.0) | 1.000 |
| Bleeding | 52 (4.1) | 25 (2.6) | 27 (8.7) | < 0.001 |
| Major bleeding requiring intervention | 10 (0.8) | 9 (0.9) | 1 (0.3) | 0.466 |
Comparison of UCSEMS and PCSEMS using Fisher’s exact test. UCSEMS: Uncovered self-expandable metal stents; PCSEMS: Partially covered self-expandable metal stents.
Perforation and bleeding
The overall perforation rate was 1.2% and was comparable for PCSEMSs and UCSEMSs (Table 4). Perforation within 30 d was reported in 0.7% and late perforations in 0.5% of patients. Six (0.5%) perforations occurred during or immediately after the initial stent placement procedure. A description of the perforation cases is provided in Table 5.
Table 5.
Details on the perforation cases
| No. | Description | Day of onset | Treatment |
| 1 | Jejunal perforation at distal end of the stent[15] | 173 | Surgical closure |
| 2 | Intraprocedural perforation while the stricture was crossed with the catheter and guidewire[1] | 0 | Successfully treated with covered SEMS |
| 3 | Duodenal perforation after biliary stent placement[1] | 82 | Laparotomy, abdominal drainage and duodenal covered SEMS |
| 4 | Acute abdomen[18] | 42 | Refused treatment |
| 5 | Guidewire perforation[18] | 0 | Conservative treatment with antibiotics |
| 6 | Perforation likely due to stent-induced ischemia[2] | 15 | Surgical suture and gastrojejunostomy |
| 7 | Minor perforation after balloon dilation because of insufficient stent expansion[19] | 7 | Recovered without surgery |
| 8 | Late perforation, not related to dilatation[21] | NR | NR |
| 9 | Late perforation, not related to dilatation[21] | NR | NR |
| 10 | Late intestinal perforation by migrated stent[24] | NR | Surgical intervention |
| 11 | Perforation while pushing the delivery system across the initially placed stent[25] | 0 | Surgical closure and gastrojejunostomy |
| 12 | Perforation by the guidewire and/or ERCP catheter with subsequent misplacement of the stent[27] | 0 | Surgical suture, bowel patch and gastroenteric bypass |
| 13 | Perforation by the guidewire and/or ERCP catheter with subsequent misplacement of the stent[27] | 0 | Surgical suture, bowel patch and gastroenteric bypass |
| 14 | Abdominal pain and pneumoperitoneum immediately after stent placement[28] | 0 | Loop gastrojejunostomy and combined gastrostomy-jejunostomy tube placement |
| 15 | Abdominal pain, distension, vomiting, and free air on x-ray 6 d after second stent placement[28] | 12 | Nasogastric tube placement and hospitalized; died two days later of sepsis |
SEMS: Self-expandable metal stent; NR: Not reported; ERCP: Endoscopic retrograde cholangiopancreatography.
Bleeding was reported in 4.1% of patients and was more frequent in patients treated with PCSEMSs: 8.7% vs 2.6% (P < 0.001) (Table 4). Major bleeding, requiring an intervention, occurred in 10 (0.8%) cases.
Stent patency and overall survival
The median stent patency was reported in five studies[2,5,15,24,29], including 549 patients, and ranged from 68 d to 98 d, with exception of one study that reported a median stent patency of 307 d[29].
The median overall survival ranged from 49 d to 183 d in thirteen studies, including 867 patients[1,5,9,15,18,19,21,23-26,28,29]. When the majority (≥ 50%) of the study sample included patients with pancreatic cancer, the median overall survival ranged from 49 d to 106 d[1,9,18,19,23,26,28,29]. When the majority of the study sample included patients with gastric cancer, the median overall survival ranged from 88 d to 183 d[5,24,25].
DISCUSSION
This pooled analysis of 1281 patients identified from the prospective literature, showed that palliative SEMS placement for MGOO is feasible, effective and safe. Stent placement can therefore be regarded as a good alternative for surgery in the palliative setting. The clinical success rate was high (85.7%) and although stent dysfunction was frequently encountered (19.6%), it could usually be managed endoscopically by additional stent placement. Large, recently published, retrospective studies, each including more than 125 patients, reported comparable results[4,42,43].
In subgroup analysis, the technical and clinical success rates of PCSEMS placement were significantly higher than those of UCSEMSs. The reasons for technical failure (Table 3) were rather procedure-related than stent-related. The higher technical success rate of PCSEMSs can therefore not be easily explained. The higher clinical success rate of PCSEMSs is a notable finding, suggesting that these stent models have more capacity in relieving MGOO, for instance by a higher radial force than UCSEMSs. However, the validity of this finding may be questioned because of heterogeneity, such as the difference in definitions of clinical success between the included studies. To exclude this heterogeneity, a subgroup analysis was performed of the four studies that compared the outcomes of PCSEMSs and UCSEMSs, showing similar pooled clinical success rates for PCSEMSs and UCSEMSs. In addition, a meta-analysis of comparative studies found no difference in technical and clinical success between covered and uncovered SEMSs[44]. The data were insufficient and the samples would be too small to analyze the outcomes of the eleven different stent models, including modified and patient-tailored stents, used in the 19 included studies.
Several factors have been identified as predictors for the outcomes of stent placement for MGOO. One prospective cohort study, including 71 patients, found a significantly lower clinical success rate for stents placed in the gastric antrum (29%) compared with success rates of stent placement in the duodenum (70%) or at the gastrojejunal anastomosis (87%)[21]. The authors speculated that antral tumors have to be larger to cause obstruction, resulting in more antral rigidity[21]. The two main indications for enteral stent placement in our pooled analysis were obstruction by gastric (42%) and pancreatic (37%) cancer. Unfortunately, the data were insufficient to analyze the clinical outcomes according to cause and site of obstruction. However, other retrospective and prospective studies never identified type of cancer and site of obstruction as predictors for success of enteral stent placement[4,42,45-47]. The main factors associated with a poor stent outcome in the literature are a poor performance status and peritoneal dissemination with ascites[4,36,37,43,48].
One fifth of the patients experienced stent dysfunction, mainly because of re-obstruction by tumor in- or overgrowth and stent migration. PCSEMSs were associated with the occurrence of stent migration, while re-obstruction was more frequently seen with the use of UCSEMSs. The overall stent dysfunction rates were comparable between both stent types, which is consistent with a recently published meta-analysis[44]. The fact that stent covering precludes tumor ingrowth, but provokes stent migration, has already been demonstrated[44]. A large retrospective analysis, including 583 patients with MGOO mainly caused by gastric cancer (57%), found that duodenal lesions, a shorter stricture length and longer survival time were associated with the occurrence of re-obstruction by tumor overgrowth[49]. Also short time to progression has been identified as a predictor for re-obstruction, while administration of first line chemotherapy was protective against re-stenosis[50]. Regarding the occurrence of stent migration, chemotherapy after stent placement was associated with migration in two studies, although only in univariate analysis[50,51]. In a prospective pilot study of 25 patients with MGOO, covered SEMSs were anchored into the mucosa by three endoscopic clips at the proximal end of the stent to prevent stent migration[41]. No cases of stent migration occurred, suggesting that endoscopic clipping may prevent stent migration[41]. Regarding the stent patency, one of the included studies estimated with a Kaplan-Meier analysis that 63% of the stents were patent at six months[1]. Another prospective cohort reported that the GOOS score increase persisted until death or end of follow-up in 45% (95%CI: 27%-74%) of patients[28].
Perforation and major bleeds were rare, both occurring in approximately 1% of patients. Seven of the 15 perforations were procedure- or balloon dilatation-related. A recently published, retrospective study reported perforation in 3.4% (10/292) of patients treated with SEMSs for MGOO[42]. The perforation rate according to the cause of obstruction was 4.6% (9/196) for pancreatic cancer and 1.0% (1/96) for nonpancreatic cancer[42], suggesting that the cause of obstructing may be associated with the occurrence of perforation after enteral stent placement. However, data are lacking to support this assumption. Minor bleeding was more frequently seen in patients treated with PCSEMSs, mainly contributed by two studies from the same institution that reported 56% (29/52) of bleedings using tailored, funnel- and cup-shaped, PCSEMSs[16,17]. Therefore, these tailored PCSEMSs may not be directly comparable with the other PCSEMS designs used in the literature. Nevertheless, the overall major bleeding rate in our pooled analysis was only 0.8%.
This analysis of the prospective literature has several limitations. Heterogeneity between the included studies is the main limitation. As mentioned before, the causes of MGOO, the definitions used for clinical success and the stent designs differed between the included studies. Furthermore, the included prospective studies are prone to selection-by-indication, since only one RCT was included that compared surgical gastrojejunostomy with enteral stent placement[9]. The patients included in the remaining articles therefore represent a selected population, because it was decided upfront that stent placement was indicated. This may overestimate the outcomes of enteral stent placement. Another issue is the clinically relevant question whether duodenal stent placement should be preceded by biliary stenting to maintain biliary drainage (Figure 4). However, that question was beyond our literature search.
Figure 4.
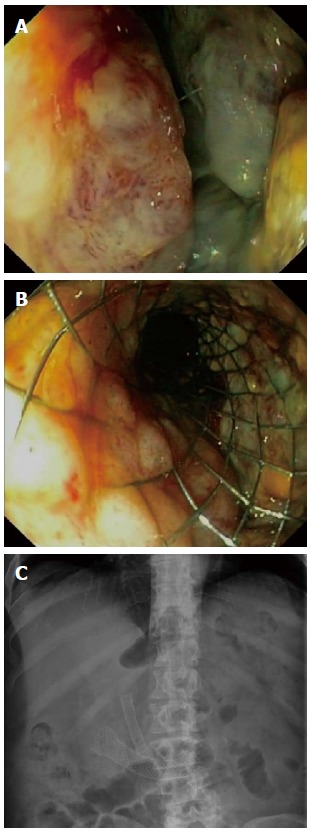
Endoscopic view of an ulcerative, obstructing gastrointestinal stromal tumor of the peri-ampullary region of the duodenum (A) for which an uncovered WallFlex stent (Boston Scientific) was placed (B). Fluoroscopic view shows the fully deployed duodenal stent overlapping the previously placed biliary SEMS (C).
In conclusion, this pooled analysis of the recently published, prospective literature provides an extensive overview of the clinical outcomes of stent placement for MGOO. In this large population enteral stent placement was feasible, effective and safe. Therefore, stent placement is a valid option for the palliation of MGOO.
COMMENTS
Background
Gastric outlet obstruction is usually a late sign of a locally advanced or metastatic cancer, requiring palliative management. Endoscopic self-expandable metal stent placement to relieve obstructive symptoms and allow oral intake, is a well-established treatment option in patients with malignant gastric outlet obstruction.
Research frontiers
Comparison of enteral stenting and gastrojejunostomy revealed sooner return to oral intake and shorter hospital stay after stent placement. On the long term, however, patients with an enteral stent have more recurrent obstruction and require more re-interventions.
Innovations and breakthroughs
To improve the long term patency of self-expandable metal stents, many different stent designs have been developed to reduce the risk of stent migration and re-obstruction by tumor ingrowth. In this systematic review, the authors provide an extensive overview of the prospective literature published since January 2009 on the clinical outcomes of stent placement for malignant gastric outlet obstruction.
Applications
This pooled analysis may be helpful for the endoscopist in the decision-making on the indication for duodenal stent placement and also to inform the patient on the risks and benefits of stent therapy.
Terminology
Gastric outlet obstruction is an obstruction at the level of the pylorus (gastric antrum, pylorus, duodenal bulb) causing problems with the passage of food into the small intestine. Self-expandable metal stents consist of a flexible framework of wire mesh made of nitinol, a metal alloy of nickel and titanium, and are either uncovered or covered by a polytetrafluoroethylene polyurethane or silicone membrane.
Peer-review
Interesting study, well written and deeply described.
Footnotes
Conflict-of-interest statement: Emo E van Halsema and Erik AJ Rauws no conflict of interest; Paul Fockens doing consultancy work for Boston Scientific, Covidien and Olympus Medical Systems; Jeanin van Hooft doing consultancy work for Cook Medical, Boston Scientific, Covidien and Abbott.
Data sharing statement: All data in this systematic review were extracted from the original articles and are presented in the literature Table 1. No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 6, 2015
First decision: July 14, 2015
Article in press: October 13, 2015
P- Reviewer: Attar A S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Tringali A, Didden P, Repici A, Spaander M, Bourke MJ, Williams SJ, Spicak J, Drastich P, Mutignani M, Perri V, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc. 2014;79:66–75. doi: 10.1016/j.gie.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 2.Costamagna G, Tringali A, Spicak J, Mutignani M, Shaw J, Roy A, Johnsson E, De Moura EG, Cheng S, Ponchon T, et al. Treatment of malignant gastroduodenal obstruction with a nitinol self-expanding metal stent: an international prospective multicentre registry. Dig Liver Dis. 2012;44:37–43. doi: 10.1016/j.dld.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Khashab M, Alawad AS, Shin EJ, Kim K, Bourdel N, Singh VK, Lennon AM, Hutfless S, Sharaiha RZ, Amateau S, et al. Enteral stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Surg Endosc. 2013;27:2068–2075. doi: 10.1007/s00464-012-2712-7. [DOI] [PubMed] [Google Scholar]
- 4.Hori Y, Naitoh I, Ban T, Narita K, Nakazawa T, Hayashi K, Miyabe K, Shimizu S, Kondo H, Nishi Y, et al. Stent under-expansion on the procedure day, a predictive factor for poor oral intake after metallic stenting for gastric outlet obstruction. J Gastroenterol Hepatol. 2015;30:1246–1251. doi: 10.1111/jgh.12933. [DOI] [PubMed] [Google Scholar]
- 5.Lee KM, Choi SJ, Shin SJ, Hwang JC, Lim SG, Jung JY, Yoo BM, Cho SW, Kim JH. Palliative treatment of malignant gastroduodenal obstruction with metallic stent: prospective comparison of covered and uncovered stents. Scand J Gastroenterol. 2009;44:846–852. doi: 10.1080/00365520902929849. [DOI] [PubMed] [Google Scholar]
- 6.Park CH, Park JC, Kim EH, Chung H, An JY, Kim HI, Shin SK, Lee SK, Cheong JH, Hyung WJ, et al. Impact of carcinomatosis and ascites status on long-term outcomes of palliative treatment for patients with gastric outlet obstruction caused by unresectable gastric cancer: stent placement versus palliative gastrojejunostomy. Gastrointest Endosc. 2015;81:321–332. doi: 10.1016/j.gie.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Nagaraja V, Eslick GD, Cox MR. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction-a systematic review and meta-analysis of randomized and non-randomized trials. J Gastrointest Oncol. 2014;5:92–98. doi: 10.3978/j.issn.2078-6891.2014.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt C, Gerdes H, Hawkins W, Zucker E, Zhou Q, Riedel E, Jaques D, Markowitz A, Coit D, Schattner M. A prospective observational study examining quality of life in patients with malignant gastric outlet obstruction. Am J Surg. 2009;198:92–99. doi: 10.1016/j.amjsurg.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 9.Jeurnink SM, Steyerberg EW, van Hooft JE, van Eijck CH, Schwartz MP, Vleggaar FP, Kuipers EJ, Siersema PD. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490–499. doi: 10.1016/j.gie.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Strand DS, Thlick JE, Patrie JT, Gaidhane MR, Kahaleh M, Wang AY. Gastroduodenal stents are associated with more durable patency as compared to percutaneous endoscopic gastrojejunostomy in the palliation of malignant gastric outlet obstruction. J Interv Gastroenterol. 2012;2:150–154. doi: 10.4161/jig.23749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CL, Perng CL, Chao Y, Li CP, Hou MC, Tseng HS, Lin HC, Lee KC. Application of stent placement or nasojejunal feeding tube placement in patients with malignant gastric outlet obstruction: a retrospective series of 38 cases. J Chin Med Assoc. 2012;75:624–629. doi: 10.1016/j.jcma.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res. 2012;4:159–169. doi: 10.2147/CMAR.S29297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng B, Wang X, Ma B, Tian J, Jiang L, Yang K. Endoscopic stenting versus gastrojejunostomy for palliation of malignant gastric outlet obstruction. Dig Endosc. 2012;24:71–78. doi: 10.1111/j.1443-1661.2011.01186.x. [DOI] [PubMed] [Google Scholar]
- 14.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011;8:1. doi: 10.1186/1742-5573-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maetani I, Mizumoto Y, Shigoka H, Omuta S, Saito M, Tokuhisa J, Morizane T. Placement of a triple-layered covered versus uncovered metallic stent for palliation of malignant gastric outlet obstruction: a multicenter randomized trial. Dig Endosc. 2014;26:192–199. doi: 10.1111/den.12117. [DOI] [PubMed] [Google Scholar]
- 16.Shi D, Ji F, Bao YS, Liu YP. A Multicenter Randomized Controlled Trial of Malignant Gastric Outlet Obstruction: Tailored Partially Covered Stents (Placed Fluoroscopically) versus Standard Uncovered Stents (Placed Endoscopically) Gastroenterol Res Pract. 2014;2014:309797. doi: 10.1155/2014/309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi D, Bao YS, Liu YP. Individualization of metal stents for management of gastric outlet obstruction caused by distal stomach cancer: a prospective study. Gastrointest Endosc. 2013;78:277–284. doi: 10.1016/j.gie.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 18.van den Berg MW, Haijtink S, Fockens P, Vleggaar FP, Dijkgraaf MG, Siersema PD, van Hooft JE. First data on the Evolution duodenal stent for palliation of malignant gastric outlet obstruction (DUOLUTION study): a prospective multicenter study. Endoscopy. 2013;45:174–181. doi: 10.1055/s-0032-1326077. [DOI] [PubMed] [Google Scholar]
- 19.Isayama H, Sasaki T, Nakai Y, Togawa O, Kogure H, Sasahira N, Yashima Y, Kawakubo K, Ito Y, Hirano K, et al. Management of malignant gastric outlet obstruction with a modified triple-layer covered metal stent. Gastrointest Endosc. 2012;75:757–763. doi: 10.1016/j.gie.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 20.Moura EG, Ferreira FC, Cheng S, Moura DT, Sakai P, Zilberstain B. Duodenal stenting for malignant gastric outlet obstruction: prospective study. World J Gastroenterol. 2012;18:938–943. doi: 10.3748/wjg.v18.i9.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolz C, Vilella À, González Carro P, González Huix F, Espinós JC, Santolaria S, Pérez Roldán F, Figa M, Loras C, Andreu H. Antral localization worsens the efficacy of enteral stents in malignant digestive tumors. Gastroenterol Hepatol. 2011;34:63–68. doi: 10.1016/j.gastrohep.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Kim YW, Choi CW, Kang DH, Kim HW, Chung CU, Kim DU, Park SB, Park KT, Kim S, Jeung EJ, et al. A double-layered (comvi) self-expandable metal stent for malignant gastroduodenal obstruction: a prospective multicenter study. Dig Dis Sci. 2011;56:2030–2036. doi: 10.1007/s10620-011-1566-5. [DOI] [PubMed] [Google Scholar]
- 23.van Hooft JE, van Montfoort ML, Jeurnink SM, Bruno MJ, Dijkgraaf MG, Siersema PD, Fockens P. Safety and efficacy of a new non-foreshortening nitinol stent in malignant gastric outlet obstruction (DUONITI study): a prospective, multicenter study. Endoscopy. 2011;43:671–675. doi: 10.1055/s-0030-1256383. [DOI] [PubMed] [Google Scholar]
- 24.Kim CG, Choi IJ, Lee JY, Cho SJ, Park SR, Lee JH, Ryu KW, Kim YW, Park YI. Covered versus uncovered self-expandable metallic stents for palliation of malignant pyloric obstruction in gastric cancer patients: a randomized, prospective study. Gastrointest Endosc. 2010;72:25–32. doi: 10.1016/j.gie.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 25.Maetani I, Ukita T, Nambu T, Shigoka H, Omuta S, Endo T, Takahashi K. Comparison of ultraflex and niti-s stents for palliation of unresectable malignant gastroduodenal obstruction. Dig Endosc. 2010;22:83–89. doi: 10.1111/j.1443-1661.2010.00942.x. [DOI] [PubMed] [Google Scholar]
- 26.Shaw JM, Bornman PC, Krige JE, Stupart DA, Panieri E. Self-expanding metal stents as an alternative to surgical bypass for malignant gastric outlet obstruction. Br J Surg. 2010;97:872–876. doi: 10.1002/bjs.6968. [DOI] [PubMed] [Google Scholar]
- 27.Havemann MC, Adamsen S, Wøjdemann M. Malignant gastric outlet obstruction managed by endoscopic stenting: a prospective single-centre study. Scand J Gastroenterol. 2009;44:248–251. doi: 10.1080/00365520802530820. [DOI] [PubMed] [Google Scholar]
- 28.Piesman M, Kozarek RA, Brandabur JJ, Pleskow DK, Chuttani R, Eysselein VE, Silverman WB, Vargo JJ, Waxman I, Catalano MF, et al. Improved oral intake after palliative duodenal stenting for malignant obstruction: a prospective multicenter clinical trial. Am J Gastroenterol. 2009;104:2404–2411. doi: 10.1038/ajg.2009.409. [DOI] [PubMed] [Google Scholar]
- 29.van Hooft JE, Uitdehaag MJ, Bruno MJ, Timmer R, Siersema PD, Dijkgraaf MG, Fockens P. Efficacy and safety of the new WallFlex enteral stent in palliative treatment of malignant gastric outlet obstruction (DUOFLEX study): a prospective multicenter study. Gastrointest Endosc. 2009;69:1059–1066. doi: 10.1016/j.gie.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Zhou WZ, Yang ZQ, Liu S, Zhou CG, Xia JG, Zhao LB, Shi HB. A newly designed stent for management of malignant distal duodenal stenosis. Cardiovasc Intervent Radiol. 2015;38:177–181. doi: 10.1007/s00270-014-0899-9. [DOI] [PubMed] [Google Scholar]
- 31.van den Berg MW, Walter D, Vleggaar FP, Siersema PD, Fockens P, van Hooft JE. High proximal migration rate of a partially covered “big cup” duodenal stent in patients with malignant gastric outlet obstruction. Endoscopy. 2014;46:158–161. doi: 10.1055/s-0033-1359023. [DOI] [PubMed] [Google Scholar]
- 32.Didden P, Spaander MC, de Ridder R, Berk L, van Tilburg AJ, Leeuwenburgh I, Kuipers EJ, Bruno MJ. Efficacy and safety of a partially covered stent in malignant gastric outlet obstruction: a prospective Western series. Gastrointest Endosc. 2013;77:664–668. doi: 10.1016/j.gie.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Song HY, Hu HT, Kang YK, Jung HY, Yook JH, Kim BS. Palliative treatment of malignant gastric outlet obstructions with a large-diameter metallic stent: prospective preliminary study. J Vasc Interv Radiol. 2010;21:1125–1128. doi: 10.1016/j.jvir.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Moon JH, Choi HJ, Ko BM, Koo HC, Hong SJ, Cheon YK, Cho YD, Lee MS, Shim CS. Combined endoscopic stent-in-stent placement for malignant biliary and duodenal obstruction by using a new duodenal metal stent (with videos) Gastrointest Endosc. 2009;70:772–777. doi: 10.1016/j.gie.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Larssen L, Hauge T, Medhus AW. Stent treatment of malignant gastric outlet obstruction: the effect on rate of gastric emptying, symptoms, and survival. Surg Endosc. 2012;26:2955–2960. doi: 10.1007/s00464-012-2291-7. [DOI] [PubMed] [Google Scholar]
- 36.Jeurnink SM, Steyerberg EW, Vleggaar FP, van Eijck CH, van Hooft JE, Schwartz MP, Kuipers EJ, Siersema PD. Predictors of survival in patients with malignant gastric outlet obstruction: a patient-oriented decision approach for palliative treatment. Dig Liver Dis. 2011;43:548–552. doi: 10.1016/j.dld.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 37.van Hooft JE, Dijkgraaf MG, Timmer R, Siersema PD, Fockens P. Independent predictors of survival in patients with incurable malignant gastric outlet obstruction: a multicenter prospective observational study. Scand J Gastroenterol. 2010;45:1217–1222. doi: 10.3109/00365521.2010.487916. [DOI] [PubMed] [Google Scholar]
- 38.Kim CG, Choi IJ, Lee JY, Cho SJ, Kim SJ, Kim MJ, Park SR, Park YL. Outcomes of second self-expandable metallic stent insertion for malignant gastric outlet obstruction. Surg Endosc. 2014;28:281–288. doi: 10.1007/s00464-013-3185-z. [DOI] [PubMed] [Google Scholar]
- 39.Qureshi S, Ghazanfar S, Hafeez AB, Taj MA, Niaz SK, Quraishy MS. Malignant pyloro-duodenal obstruction: role of self expandable metallic stents. J Pak Med Assoc. 2014;64:16–19. [PubMed] [Google Scholar]
- 40.Jeurnink SM, Polinder S, Steyerberg EW, Kuipers EJ, Siersema PD. Cost comparison of gastrojejunostomy versus duodenal stent placement for malignant gastric outlet obstruction. J Gastroenterol. 2010;45:537–543. doi: 10.1007/s00535-009-0181-0. [DOI] [PubMed] [Google Scholar]
- 41.Kim ID, Kang DH, Choi CW, Kim HW, Jung WJ, Lee DH, Chung CW, Yoo JJ, Ryu JH. Prevention of covered enteral stent migration in patients with malignant gastric outlet obstruction: a pilot study of anchoring with endoscopic clips. Scand J Gastroenterol. 2010;45:100–105. doi: 10.3109/00365520903410554. [DOI] [PubMed] [Google Scholar]
- 42.Oh SY, Edwards A, Mandelson M, Ross A, Irani S, Larsen M, Gan SI, Gluck M, Picozzi V, Helton S, et al. Survival and clinical outcome after endoscopic duodenal stent placement for malignant gastric outlet obstruction: comparison of pancreatic cancer and nonpancreatic cancer. Gastrointest Endosc. 2015;82:460–8.e2. doi: 10.1016/j.gie.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 43.Jeon HH, Park CH, Park JC, Shim CN, Kim S, Lee HJ, Lee H, Shin SK, Lee SK, Lee YC. Carcinomatosis matters: clinical outcomes and prognostic factors for clinical success of stent placement in malignant gastric outlet obstruction. Surg Endosc. 2014;28:988–995. doi: 10.1007/s00464-013-3268-x. [DOI] [PubMed] [Google Scholar]
- 44.Pan YM, Pan J, Guo LK, Qiu M, Zhang JJ. Covered versus uncovered self-expandable metallic stents for palliation of malignant gastric outlet obstruction: a systematic review and meta-analysis. BMC Gastroenterol. 2014;14:170. doi: 10.1186/1471-230X-14-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng HT, Lee CS, Lin CH, Cheng CL, Tang JH, Tsou YK, Chang JM, Lee MH, Sung KF, Liu NJ. Treatment of malignant gastric outlet obstruction with metallic stents: assessment of whether gastrointestinal position alters efficacy. J Investig Med. 2012;60:1027–1032. doi: 10.2310/JIM.0b013e31826509c8. [DOI] [PubMed] [Google Scholar]
- 46.Kim JH, Song HY, Shin JH, Hu HT, Lee SK, Jung HY, Yook JH. Metallic stent placement in the palliative treatment of malignant gastric outlet obstructions: primary gastric carcinoma versus pancreatic carcinoma. AJR Am J Roentgenol. 2009;193:241–247. doi: 10.2214/AJR.08.1760. [DOI] [PubMed] [Google Scholar]
- 47.Kim JH, Song HY, Shin JH, Choi E, Kim TW, Jung HY, Lee GH, Lee SK, Kim MH, Ryu MH, et al. Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: prospective evaluation of results and factors influencing outcome in 213 patients. Gastrointest Endosc. 2007;66:256–264. doi: 10.1016/j.gie.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki T, Isayama H, Nakai Y, Togawa O, Kogure H, Kawakubo K, Mizuno S, Yashima Y, Ito Y, Yamamoto N, et al. Predictive factors of solid food intake in patients with malignant gastric outlet obstruction receiving self-expandable metallic stents for palliation. Dig Endosc. 2012;24:226–230. doi: 10.1111/j.1443-1661.2011.01208.x. [DOI] [PubMed] [Google Scholar]
- 49.Jang JK, Song HY, Kim JH, Song M, Park JH, Kim EY. Tumor overgrowth after expandable metallic stent placement: experience in 583 patients with malignant gastroduodenal obstruction. AJR Am J Roentgenol. 2011;196:W831–W836. doi: 10.2214/AJR.10.5861. [DOI] [PubMed] [Google Scholar]
- 50.Kim CG, Park SR, Choi IJ, Lee JY, Cho SJ, Park YI, Nam BH, Kim YW. Effect of chemotherapy on the outcome of self-expandable metallic stents in gastric cancer patients with malignant outlet obstruction. Endoscopy. 2012;44:807–812. doi: 10.1055/s-0032-1309893. [DOI] [PubMed] [Google Scholar]
- 51.Miyabe K, Hayashi K, Nakazawa T, Sano H, Yamada T, Takada H, Naitoh I, Shimizu S, Kondo H, Nishi Y, et al. Safety and benefits of self-expandable metallic stents with chemotherapy for malignant gastric outlet obstruction. Dig Endosc. 2015;27:572–581. doi: 10.1111/den.12424. [DOI] [PubMed] [Google Scholar]


