Abstract
Extensively drug-resistant (XDR) Acinetobacter spp. have emerged as a cause of nosocomial infections, especially under conditions of intensive care. Unfortunately, resistance to colistin is increasing and there is a need for new therapeutic options. We aimed to study the effect of some novel combinations against XDR Acinetobacter baumannii in an in vitro pharmacokinetics-pharmacodynamics (PK/PD) model. Three nonrelated clinical strains of XDR A. baumannii were investigated. Antibiotic-simulated regimens were colistin at 3 MU every 8 h (q8h) (first dose, 6 MU), daptomycin at 10 mg/kg of body weight q24h, imipenem at 1 g q8h, and ertapenem at 1 g q24h. Combination regimens included colistin plus daptomycin, colistin plus imipenem, and imipenem plus ertapenem. Samples were obtained at 0, 1, 2, 4, 8, and 24 h. Among the single-agent regimens, only the colistin regimen resulted in significant reductions in log10 CFU per milliliter compared to the control for all the strains tested. Although colistin achieved bactericidal activity at 4 h, it was not able to reach the limit of detection (1 log10 CFU/ml). One strain had significant regrowth at 24 h without the emergence of resistance. Daptomycin-colistin combinations led to a significant reduction in levels of log10 CFU per milliliter that were better than those achieved with colistin as a single-agent regimen, reaching the limit of detection at 24 h against all the strains. The combination of imipenem plus ertapenem outperformed the colistin regimen, although the results did not reach the limit of detection, with significant regrowth at 24 h. Similarly, colistin-plus-imipenem combinations reduced the levels of log10 CFU per milliliter at 8 h, with significant regrowth at 24 h but with development of resistance to colistin. We have shown some potentially useful alternatives for the treatment of extensively drug-resistant A. baumannii. Among them, the daptomycin-colistin combination was the most effective and should be investigated in future studies.
INTRODUCTION
Many Gram-negative rods display significant antimicrobial resistance. Among them, Acinetobacter baumannii deserves special consideration. Currently, carbapenem-resistant A. baumannii is included in the Infectious Diseases Society of America (IDSA) list of nosocomial pathogens of particular concern (1).
Therapeutic options for this pathogen are extremely limited, a situation made worse by the drying up of the pharmaceutical development pipeline for anti-infective agents. This has forced clinicians to return to older, previously discarded drugs, such as the polymyxins and tigecycline (despite controversies regarding its efficacy), or to drug combinations. However, their efficacy is limited because of resistance or heteroresistance to colistin, drug-drug interactions, or severe side effects such as renal failure (2).
Some authors have reported the efficacy of novel combinations, including daptomycin combinations, against multidrug-resistant (MDR) A. baumannii strains (3, 4).
The exact mechanisms involved in this synergistic combination are not fully understood. Daptomycin-colistin combinations have shown an absence of activity against other multidrug-resistant Gram-negative rods but have displayed significant activity against A. baumannii (4).
MATERIALS AND METHODS
Bacterial isolates.
We used three nonrelated extensively drug-resistant (XDR) isolates of Acinetobacter baumannii (5). The strains were obtained from blood samples from two different hospitals.
All isolates were susceptible only to colistin and tigecycline.
Antibiotics and media.
Colistin sulfate (COL), daptomycin (DAP), ertapenem (ERT), and imipenem (IMI) were purchased from Sigma-Aldrich Co., Madrid, Spain (colistin), Novartis Pharmaceuticals, Basel, Switzerland (daptomycin), and MSD, Madrid, Spain (ertapenem and imipenem).
Mueller-Hinton broth supplemented with 25 mg/liter calcium and 12.5 mg/liter magnesium (SMHB; Difco Laboratories, Spain) was used for all susceptibility testing and in vitro pharmacokinetics-pharmacodynamics (PK/PD) experiments. For experiments using daptomycin, SMHB was supplemented with 50 mg/liter calcium and 25 mg/liter magnesium as recommended by the CLSI guidelines (6).
Antibiotic concentrations were measured by high-performance liquid chromatography following previously published methods (7–9).
In vitro PK/PD model.
A previously described in vitro PK/PD model consisting of a 250-ml one-compartment glass chamber with multiple ports for delivery and removal of medium, delivery of antibiotics, and collection of bacterial and antimicrobial samples was used (10, 11). The model was set with a targeted initial bacterial inoculum of ∼108 CFU/ml.
Antibiotics were administered as boluses into the central compartment via an injection port. Peristaltic pumps supplying fresh media were set to simulate antibiotic half-lives (t1/2).
Samples (1 ml of media) were obtained at 0, 1, 2, 4, 8, and 24 h and applied to tryptic soy agar (TSA) plates using drop plating. Plates were read after 24 h of incubation at 35°C. For all samples, antimicrobial carryover was accounted for by serial dilution of the plated samples or by vacuum filtration if the drug level of the anticipated dilution was near the MIC value for the organism. The limit of detection of these methods of colony count determination was 2 log10 CFU/ml (extended to 1 log10 CFU/ml by vacuum filtration).
Bactericidal activity was defined as >3 log10 CFU/ml kill from the initial inoculum.
Emergence of resistance was evaluated by performing susceptibility testing of colonies recovered at 24 h according to CLSI guidelines (6).
Experiments were performed in duplicate to account for biological variability.
Simulated antibiotic regimens.
For colistin, the regimen consisted of a 6-MU loading dose (maximum concentration of free, unbound drug in serum [fCmax], 4.5 mg/liter; t1/2, 4 h; protein binding, 50%) followed by 3 MU administered every 8 h (q8h) (fCmax, 3 mg/liter) (12); for daptomycin, the regimen consisted of 10 mg/kg of body weight q24h (fCmax, 11.3 mg/liter; t1/2, 8 h; protein binding, 92%) (13); for ertapenem, the regimen consisted of 1 g q24h (fCmax, 7.75 mg/liter; t1/2, 4 h; protein binding, 95%) (14); and for imipenem, the regimen consisted of 1 g q8h (fCmax, 80 mg/liter; t1/2, 1 h; protein binding, 20%) (15).
Statistical analysis.
Changes in bacterial CFU counts per milliliter were compared by analysis of variance (ANOVA) and Turkey's post hoc test. A P value of <0.05 was considered significant.
RESULTS
Susceptibilities of the isolates are displayed in Table 1. Observed PK parameters were within 15% of the targeted values.
TABLE 1.
MIC of antimicrobial agents
| Antimicrobial agent | MIC (mg/liter) for strain: |
||
|---|---|---|---|
| AJ001 | AJ002 | AJ003 | |
| Colistin | 2 | 2 | 4 |
| Daptomycin | 256 | 256 | 256 |
| Imipenem | 32 | 64 | 32 |
| Ertapenem | 64 | 128 | 64 |
| Tigecycline | 1 | 1 | 1 |
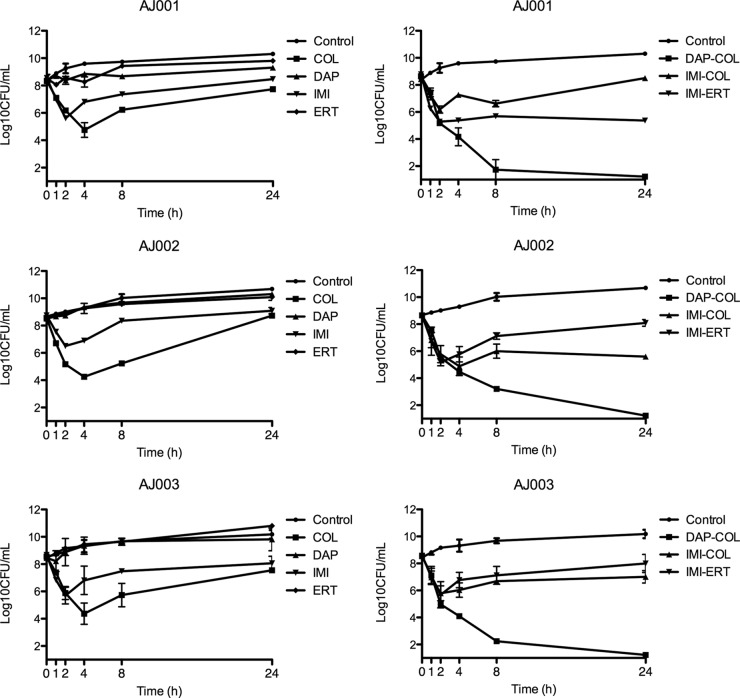
The daptomycin-colistin combination demonstrated bactericidal activity against all three clinical isolates of XDR A. baumannii evaluated (Fig. 1), reaching the limit of detection at 24 h. No other combination therapy reached the limit of detection.
FIG 1.
Activity of different single-agent regimens and combination regimens against three nonrelated strains of extensively drug-resistant A. baumannii. Symbols represent the mean numbers of viable colonies at each time interval. COL, colistin; DAP, daptomycin; IMI, imipenem, ERT, ertapenem.
Colistin was the only single-agent regimen that was able to produce a significant reduction in the bacterial count, reaching a bactericidal effect against all isolates at 4 h; however, there was regrowth in all three isolates at 24 h, without emergence of resistance. Despite regrowth at 24 h, colistin produced a significant reduction of the bacterial count compared to controls against all three strains (P = 0.012).
The imipenem regimen led to a small reduction of the bacterial count at 2 h, followed by bacterial regrowth. At 24 h, there were no statistically significant differences between the results seen with imipenem, ertapenem, and daptomycin used as single-agent regimens and control results.
Imipenem-colistin and imipenem-ertapenem combinations outperformed colistin as a single-agent regimen and demonstrated a bactericidal effect at 4 h, although there was significant regrowth at 24 h. The imipenem-colistin combination led to the emergence of resistance to colistin.
DISCUSSION
Using this in vitro pharmacokinetic/pharmacodynamic model of bacteremia, we have shown the efficacy of the daptomycin-colistin combination against three nonrelated strains of extensively drug-resistant Acinetobacter baumannii. Daptomycin-colistin combinations outperformed colistin as a single-agent regimen and colistin-imipenem combinations.
Extensively drug-resistant A. baumannii has become a serious problem worldwide. Limited antibiotic options and high severity of disease in patients suffering from XDR A. baumannii infections account for the high rates of mortality described.
Although other authors have reported the efficacy of colistin-daptomycin combinations against MDR A. baumannii strains, they used different methodologies, mainly time-kill curves and checkerboard assays (3) or the Etest agar dilution method (4). We used an in vitro PK/PD model that resembles human pharmacokinetics and mimics A. baumannii bacteremia.
Other combinations of anti-Gram-positive agents with colistin against A. baumannii have been studied. Significantly enhanced in vitro killing activity has been described with vancomycin (16), teicoplanin (17), and telavancin (18). Similarly, glycopeptide-colistin combinations have been studied in vivo with promising results (19). Very recently, Petrosillo et al. reported the efficacy of glycopeptide-colistin combinations in intensive care unit (ICU) patients infected with multidrug-resistant Gram-negative microorganisms (60% A. baumannii). Patients who received glycopeptide-colistin combinations had an increased chance of survival (20). However, renal failure was more frequent among patients who received combined therapy with glycopeptides, limiting its utility.
Daptomycin has no renal toxicity (21), and it can attenuate the nephrotoxicity of other antimicrobials (22), so the daptomycin-colistin combination is not expected to increase renal toxicity as described with glycopeptide-colistin combinations, making this combination an attractive option in cases of XDR A. baumannii infection.
Colistin is thought to increase the permeability of the Gram-negative outer membrane, rendering Gram-negative bacteria susceptible to antimicrobials which otherwise would have no effect against them. Although this mechanism of action could explain the enhanced activity of vancomycin, teicoplanin, or rifampin combinations (16, 23), it does not fully explain the effect of the daptomycin-colistin combination against A. baumannii.
Phee et al. (4) reported that the daptomycin-colistin combination had no effect against colistin-susceptible strains of Escherichia coli, Klebsiella pneumonia, Enterobacter cloacae, or Pseudomonas aeruginosa but demonstrated high efficacy against all colistin-susceptible strains of A. baumannii. These results are consistent with data published previously where a mutant strain of E. coli that lacked the outer membrane (E. coli imp) was fully susceptible to vancomycin (24) whereas the strain remained fully resistant to daptomycin and its derivatives (25). It has been suggested that daptomycin, after binding into cell membrane, induces alterations in the operon encoding YycFG, a two-component system present only in Gram-positive organisms and involved in controlling the cytoplasmic membrane integrity, leading to rapid cell death without lysis (26, 27). This proposed mechanism of action might explain daptomycin's absence of activity against Gram-negative bacteria. However, the high activity displayed against A. baumannii but not against many other Gram-negative bacterial species suggests the presence of striking differences between A. baumannii and other Gram-negative bacteria that should be further studied.
We have also explored other combinations, such as imipenem-colistin or double-carbapenem combinations, that have proven to be synergistic against other MDR Gram-negative organisms (28, 29). However, we did not find any activity with the ertapenem-imipenem combination and found limited efficacy of imipenem-colistin combinations. Interestingly, the imipenem-colistin combination led to the emergence of colistin resistance, but administration of colistin as a single-agent regimen did not result in colistin resistance. We do not have an explanation for this, but our results suggest that there might be some kind of interaction with imipenem and colistin.
Our work has some limitations. First, we tested only three strains of A. baumannii, so our results may not be generalizable to all strains; however, the consistency of the results against all three strains, and the fact that we used three unrelated strains, makes us believe that our results can be generalized. Second, our model ran for only 24 h and thus would not detect antimicrobial activity that might appear after 24 h or mechanisms of resistance. We also have to admit that the high inoculum we used might have impaired the activity of imipenem. However, recent data from studies using the colistin-carbapenem combination did not show differences at low (<∼108 CFU/ml) or high (∼1011 CFU/ml) bacterial density (30).
Finally, we acknowledge that in vitro studies do not always translate into similar results in clinical practice, so caution has to be advised prior to use. However, similar results have been reported with three other methodologies (3, 4), giving robustness to our results.
In the present situation of almost no therapeutic option against some strains of XDR A. baumannii, we believe that our results represent a novel strategy to add to the limited options against XDR A. baumannii.
ACKNOWLEDGMENT
We declare that we have no conflicts of interest.
REFERENCES
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Bergen PJ, Li J, Nation RL. 2011. Dosing of colistin-back to basic PK/PD. Curr Opin Pharmacol 11:464–469. doi: 10.1016/j.coph.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galani I, Orlandou K, Moraitou H, Petrikkos G, Souli M. 2014. Colistin/daptomycin: an unconventional antimicrobial combination synergistic in vitro against multidrug-resistant Acinetobacter baumannii. Int J Antimicrob Agents 43:370–374. doi: 10.1016/j.ijantimicag.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Phee L, Hornsey M, Wareham DW. 2013. In vitro activity of daptomycin in combination with low-dose colistin against a diverse collection of Gram-negative bacterial pathogens. Eur J Clin Microbiol Infect Dis 32:1291–1294. doi: 10.1007/s10096-013-1875-z. [DOI] [PubMed] [Google Scholar]
- 5.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2008. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed CLSI, Wayne, PA. [Google Scholar]
- 7.He H, Li JC, Nation RL, Jacob J, Chen G, Lee HJ, Tsuji BT, Thompson PE, Roberts K, Velkov T, Li J. 2013. Pharmacokinetics of four different brands of colistimethate and formed colistin in rats. J Antimicrob Chemother 68:2311–2317. doi: 10.1093/jac/dkt207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polillo M, Tascini C, Lastella M, Malacarne P, Ciofi L, Viaggi B, Bocci G, Menichetti F, Danesi R, Del Tacca M, Di Paolo A. 2010. A rapid high-performance liquid chromatography method to measure linezolid and daptomycin concentrations in human plasma. Ther Drug Monit 32:200–205. doi: 10.1097/FTD.0b013e3181d3f5cb. [DOI] [PubMed] [Google Scholar]
- 9.Dailly E, Bouquie R, Deslandes G, Jolliet P, Le Floch R. 2011. A liquid chromatography assay for a quantification of doripenem, ertapenem, imipenem, meropenem concentrations in human plasma: application to a clinical pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci 879:1137–1142. doi: 10.1016/j.jchromb.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 10.Steed ME, Vidaillac C, Rose WE, Winterfield P, Kaatz GW, Rybak MJ. 2011. Characterizing vancomycin-resistant Enterococcus strains with various mechanisms of daptomycin resistance developed in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 55:4748–4754. doi: 10.1128/AAC.00084-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidaillac C, Parra-Ruiz J, Winterfield P, Rybak MJ. 2011. In vitro pharmacokinetic/pharmacodynamic activity of NXL103 versus clindamycin and linezolid against clinical Staphylococcus aureus and Streptococcus pyogenes isolates. Int J Antimicrob Agents 38:301–306. doi: 10.1016/j.ijantimicag.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW. 2008. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J Antimicrob Chemother 61:636–642. doi: 10.1093/jac/dkm511. [DOI] [PubMed] [Google Scholar]
- 13.Parra-Ruiz J, Bravo-Molina A, Pena-Monje A, Hernandez-Quero J. 2012. Activity of linezolid and high-dose daptomycin, alone or in combination, in an in vitro model of Staphylococcus aureus biofilm. J Antimicrob Chemother 67:2682–2685. doi: 10.1093/jac/dks272. [DOI] [PubMed] [Google Scholar]
- 14.Congeni BL. 2010. Ertapenem. Expert Opin Pharmacother 11:669–672. doi: 10.1517/14656561003631397. [DOI] [PubMed] [Google Scholar]
- 15.Breilh D, Texier-Maugein J, Allaouchiche B, Saux MC, Boselli E. 2013. Carbapenems. J Chemother 25:1–17. doi: 10.1179/1973947812Y.0000000032. [DOI] [PubMed] [Google Scholar]
- 16.Gordon NC, Png K, Wareham DW. 2010. Potent synergy and sustained bactericidal activity of a vancomycin-colistin combination versus multidrug-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother 54:5316–5322. doi: 10.1128/AAC.00922-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wareham DW, Gordon NC, Hornsey M. 2011. In vitro activity of teicoplanin combined with colistin versus multidrug-resistant strains of Acinetobacter baumannii. J Antimicrob Chemother 66:1047–1051. doi: 10.1093/jac/dkr069. [DOI] [PubMed] [Google Scholar]
- 18.Hornsey M, Phee L, Longshaw C, Wareham DW. 2013. In vivo efficacy of telavancin/colistin combination therapy in a Galleria mellonella model of Acinetobacter baumannii infection. Int J Antimicrob Agents 41:285–287. doi: 10.1016/j.ijantimicag.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Hornsey M, Wareham DW. 2011. In vivo efficacy of glycopeptide-colistin combination therapies in a Galleria mellonella model of Acinetobacter baumannii infection. Antimicrob Agents Chemother 55:3534–3537. doi: 10.1128/AAC.00230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrosillo N, Giannella M, Antonelli M, Antonini M, Barsic B, Belancic L, Inkaya AC, De Pascale G, Grilli E, Tumbarello M, Akova M. 2014. Clinical experience of colistin-glycopeptide combination in critically ill patients infected with Gram-negative bacteria. Antimicrob Agents Chemother 58:851–858. doi: 10.1128/AAC.00871-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kullar R, McClellan I, Geriak M, Sakoulas G. 2014. Efficacy and safety of daptomycin in patients with renal impairment: a multicenter retrospective analysis. Pharmacotherapy 34:582–589. doi: 10.1002/phar.1413. [DOI] [PubMed] [Google Scholar]
- 22.Thibault N, Grenier L, Simard M, Bergeron MG, Beauchamp D. 1994. Attenuation by daptomycin of gentamicin-induced experimental nephrotoxicity. Antimicrob Agents Chemother 38:1027–1035. doi: 10.1128/AAC.38.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timurkaynak F, Can F, Azap OK, Demirbilek M, Arslan H, Karaman SO. 2006. In vitro activities of non-traditional antimicrobials alone or in combination against multidrug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii isolated from intensive care units. Int J Antimicrob Agents 27:224–228. doi: 10.1016/j.ijantimicag.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Eggert US, Ruiz N, Falcone BV, Branstrom AA, Goldman RC, Silhavy TJ, Kahne D. 2001. Genetic basis for activity differences between vancomycin and glycolipid derivatives of vancomycin. Science 294:361–364. doi: 10.1126/science.1063611. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen KT, Ritz D, Gu JQ, Alexander D, Chu M, Miao V, Brian P, Baltz RH. 2006. Combinatorial biosynthesis of novel antibiotics related to daptomycin. Proc Natl Acad Sci U S A 103:17462–17467. doi: 10.1073/pnas.0608589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baltz RH. 2009. Daptomycin: mechanisms of action and resistance, and biosynthetic engineering. Curr Opin Chem Biol 13:144–151. doi: 10.1016/j.cbpa.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 27.Bayer AS, Schneider T, Sahl HG. 2013. Mechanisms of daptomycin resistance in Staphylococcus aureus: role of the cell membrane and cell wall. Ann N Y Acad Sci 1277:139–158. doi: 10.1111/j.1749-6632.2012.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergen PJ, Forrest A, Bulitta JB, Tsuji BT, Sidjabat HE, Paterson DL, Li J, Nation RL. 2011. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrob Agents Chemother 55:5134–5142. doi: 10.1128/AAC.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliva A, D'Abramo A, D'Agostino C, Iannetta M, Mascellino MT, Gallinelli C, Mastroianni CM, Vullo V. 2014. Synergistic activity and effectiveness of a double-carbapenem regimen in pandrug-resistant Klebsiella pneumoniae bloodstream infections. J Antimicrob Chemother 69:1718–1720. doi: 10.1093/jac/dku027. [DOI] [PubMed] [Google Scholar]
- 30.Ly NS, Bulitta JB, Rao GG, Landersdorfer CB, Holden PN, Forrest A, Bergen PJ, Nation RL, Li J, Tsuji BT. 2015. Colistin and doripenem combinations against Pseudomonas aeruginosa: profiling the time course of synergistic killing and prevention of resistance. J Antimicrob Chemother 70:1434–1442. doi: 10.1093/jac/dku567. [DOI] [PMC free article] [PubMed] [Google Scholar]