Abstract
Invasive aspergillosis (IA) is a severe disseminated fungal disease that occurs mostly in immunocompromised patients. However, central nervous system IA, combining meningitis and skull base involvement, does not occur only in groups with classic risk factors for IA; patients with chronic renal failure and diabetes mellitus are also at risk for more chronic forms. In both of our proven IA cases, voriconazole monotherapy was effective without surgery, and cerebrospinal fluid and serum 1,3-β-d-glucan test results were initially positive, in contrast to galactomannan antigen results.
TEXT
Invasive aspergillosis (IA) is a life-threatening disease, with a mortality rate of 45%, that occurs mostly in immunocompromised patients (1). Central nervous system (CNS) localization represents an independent risk factor for death in IA and can appear with or without pulmonary localization (1). Here, we report two proven cases of skull base aspergillosis with meningitis, involving patients who did not belong to groups at high risk for IA and who successfully responded to voriconazole treatment without surgery.
Case 1 involved a 71-year-old woman who was admitted with visual acuity loss in her right eye. Symptoms had been worsening for 1 year, leading to complete loss of sight in her right eye before the patient sought the health care system. Her medical history revealed high blood pressure, nephroangiosclerosis, and chronic renal failure leading to hemodialysis for 2 years.
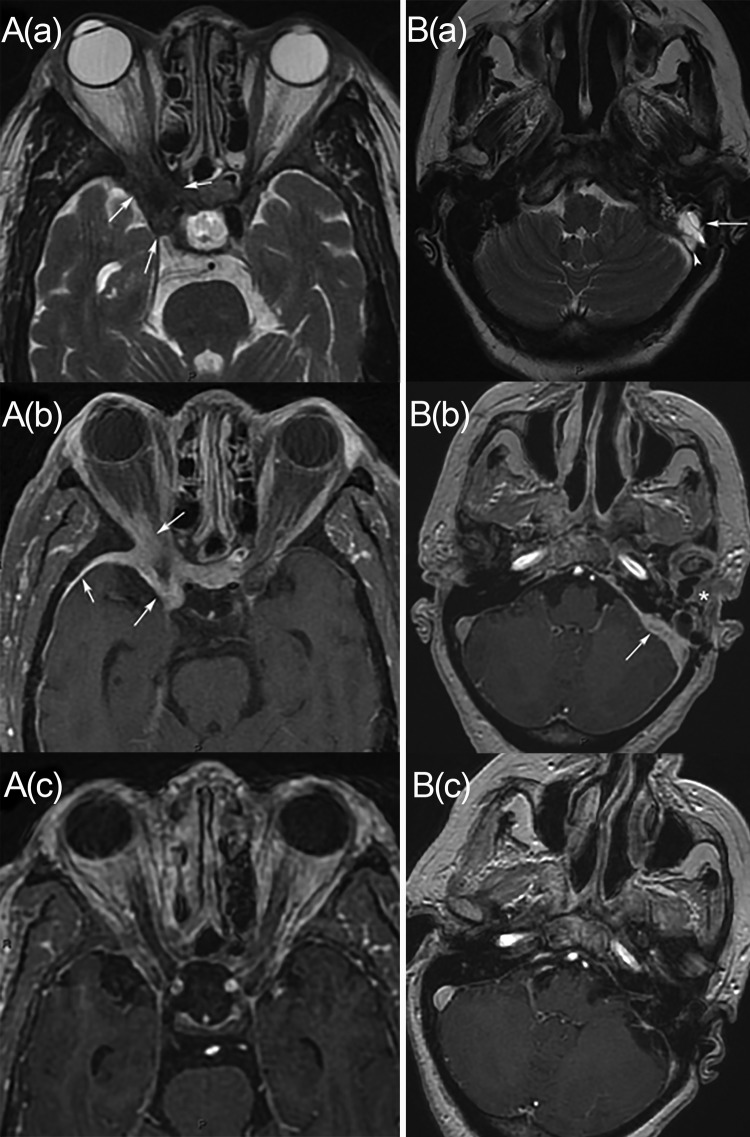
On admission, the patient was afebrile; in addition to the loss of sight in her right eye, she presented with intense hemicranial headache, stiff neck, and right exophthalmia. There were no other cranial nerve palsies. Orbitocerebral computed tomographic and magnetic resonance imaging (MRI) studies showed right orbital and optic canal infiltration, compressing the optic nerve and spreading to the right sphenoid and cavernous sinuses, associated with pachymeningitis (Fig. 1A). Retroorbital surgical biopsy samples, including ethmoidal and sphenoidal sinus samples, showed subacute inflammatory lesions with multifocal hemorrhage. Gomori-Grocott staining revealed hyaline septate hyphae (Fig. 2), and culture yielded Aspergillus fumigatus complex. A. fumigatus was identified morphologically; unfortunately, antifungal susceptibility testing was not performed with this isolate, because of the very low incidence of primary isolates resistant to triazoles in France (2). Cerebrospinal fluid (CSF) analyses revealed an isolated elevated protein level of 0.72 g/liter. CSF bacterial and fungal cultures were sterile. Baseline serum and CSF 1,3-β-d-glucan (BDG) measurements (Fungitell assay; threshold, 80 pg/ml) were positive at 216 and 152 pg/liter, respectively. Serum and CSF galactomannan (GM) antigen test results were negative in an A. fumigatus-specific PCR assay (3). Voriconazole (200 mg, twice a day) was administered orally. Voriconazole trough serum concentrations were monitored regularly and adjusted to ∼2.5 mg/liter. The patient experienced clinical improvement within 1 week, but the right eyesight loss was irreversible. Tolerance of antifungal therapy was good. A control MRI study performed after 7 months of treatment showed a significant decrease in the right cavernous sinus and pachymeningitis uptake (Fig. 1A). Antifungal treatment was continued for 10 months. No end-of-treatment radiological control study was performed, since the patient was lost to follow-up monitoring.
FIG 1.
Two cases of meningeal invasive aspergillosis. (A) Case 1, involving a 71-year-old woman. (a and b) Axial T2-weighted (a) and axial enhanced T1-weighted (b) MRI images show soft tissue thickening in the region of the right cavernous sinus and orbital apex (arrows). There is also meningeal enhancement adjacent to the thickening (arrowhead). (c) An axial enhanced T1-weighted MRI study performed after treatment shows important decreases in soft tissue thickening and meningitis. (B) Case 2, involving a 68-year-old woman. (a) An axial T2-weighted MRI image shows left filling of the mastoid air cells (arrow) and dural venous sinus thrombosis (arrowhead). (b) An axial enhanced T1-weighted MRI image shows soft tissue thickening involving the left temporal bone and the mandibular region (asterisk), with meningitis (arrow). (c) A follow-up axial enhanced T1-weighted MRI image shows an important decrease in the involvement and persistent dural venous sinus thrombosis.
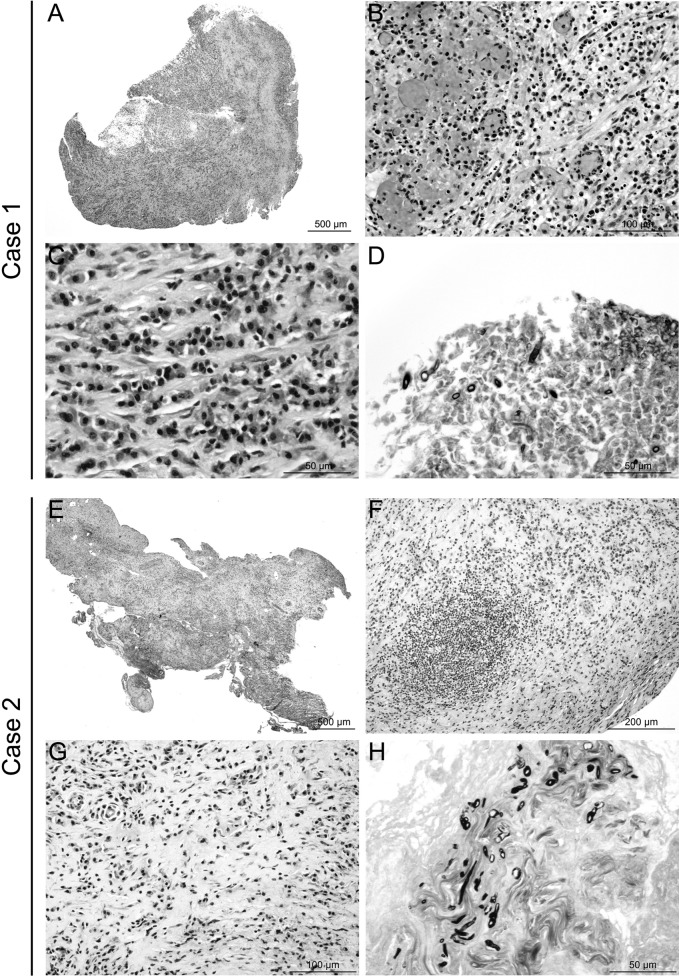
FIG 2.
Histological lesion profiles and fungus identification. (A to D) Case 1, retroorbital biopsy samples. A diffuse subacute inflammatory lesion was observed (A), characterized by infiltration of neutrophils, macrophages (B), lymphocytes, and plasma cells (C), sometimes associated with vascular and ischemic necrosis, with hemorrhage (B). Rare fragmented hyphae were detected (size, <50 μm) (D). These hyaline hyphae were septate but no branching was identified, probably because of the small sizes of the samples. (E to H) Case 2, meningeal biopsy samples. A diffuse chronic inflammatory lesion was observed (E), characterized by infiltration of macrophages, lymphocytes, and plasma cells, sometimes organized in pseudolymphoid follicles (F), included in dense mature connective tissue (G). Small colonies of irregularly dispersed hyaline hyphae were identified (H). These hyphae were septate and branching, with the branches forming acute angles. Both lesion presentations were consistent with aspergillosis. Hematoxylin-eosin staining (A, B, C, E, F, and G) or Gomori-Grocott staining (D and H).
Case 2 involved a 68-year-old woman who was admitted with left hearing loss and hemicranial headache. Her medical history revealed uncontrolled type 2 diabetes mellitus and high blood pressure. Nine months before her admission, she had experienced microbiologically undocumented left invasive otitis externa, invading the mastoid process, which was treated by mastoidectomy and ciprofloxacin administration for 2 months. Despite treatment, otalgia reappeared, with progressive left middle ear, temporal, and mandibular osteomyelitis and pachymeningitis, as assessed by MRI. A first surgical cavum biopsy, without extensive surgical debridement, did not identify any infectious agent, but a second biopsy, performed 1 month later, showed a lymphocytic inflammatory infiltrate and culture yielded Candida albicans, which was identified on selective medium (BBL CHROMagar Candida; BD, Sparks, MD) and by the rapid latex coagglutination slide test (Bichro-Latex Albicans; Fumouze Diagnostics, Levallois-Perret, France). Candida albicans was susceptible in vitro to fluconazole and voriconazole, with MICs of 0.25 μg/ml and ≤0.01 μg/ml, respectively, determined by the EUCAST microdilution method. Although fluconazole was administered for 2 months, symptoms worsened, with the onset of recurrent left laryngeal paralysis. A new MRI study revealed growth of the former lesions and left lateral venous and sigmoid sinus thrombosis, without any sign of intracranial hypertension (Fig. 1B). The patient was then referred to our ward. In addition to the former symptoms, tinnitus and paralysis of the left soft palate completed the clinical presentation. CSF analysis showed an isolated elevated protein level of 0.84 g/liter, and the CSF mycological culture results remained negative. CSF and serum BDG levels were initially slightly elevated at 93 and 118 pg/ml, respectively. Serum and CSF GM test results remained negative. The tissue culture from an osteomeningeal biopsy sample grew Aspergillus flavus, which was identified on the basis of morphological characteristics and sequencing of the β-tubulin gene. The strain was susceptible to itraconazole, with a MIC of 0.125 μg/ml determined by the EUCAST microdilution method; the MICs for amphotericin B and voriconazole were 0.25 μg/ml, that for posaconazole was 0.125 μg/ml, that for caspofungin was 0.5 μg/ml, and that for micafungin was ≤0.008 μg/ml. No extended surgical debridement was performed. Histopathological analysis revealed chronic meningitis and osteomyelitis characterized by diffuse infiltration of macrophages, lymphocytes, and plasma cells. Gomori-Grocott staining revealed hyaline septate hyphae with acute-angle branching (Fig. 2). Voriconazole oral therapy and anticoagulation were started. The voriconazole trough plasma level initially was 3.05 mg/liter and then was adjusted to ∼2 mg/liter in the context of cholestasis. Symptoms improved within 2 weeks. However, left hearing loss, retroauricular hyperesthesia, and occasional tinnitus persisted. After 6 months, the lesions showed improvement on MRI scans. Soft tissue lesions decreased and meningeal uptake disappeared, but signs of left temporal bone osteomyelitis and left sinus thrombosis remained (Fig. 1B). Antifungal treatment was discontinued after 18 months. A MRI study performed 6 months after the discontinuation of treatment showed residual lesions.
These two cases emphasize the diagnostic difficulties regarding meningeal and skull base aspergillosis in the absence of classic risk factors. Both cases had a chronic presentation and a long delay between the onset of clinical signs and diagnosis. IA frequently occurs with hematological malignancies, solid organ transplantation, or long-term corticosteroid use (1). Although chronic renal failure requiring hemodialysis was associated with IA-related death for a patient with cancer (4, 5), it is not a common risk factor. However, chronic renal failure is known to lead to an immunocompromised state combining malnutrition and immune system impairment (5, 6). Diabetes mellitus is not recognized as an IA risk factor per se, although innate immunity and adaptive immunity are altered (1, 5, 7, 8). However, uncontrolled diabetes is a risk factor for fungal invasive otitis (9–12) and was the main underlying disease in three reported cases of CNS IA (13–15).
Voriconazole is the first-line treatment for IA, particularly with CNS localizations (16–18). It has good penetration across the blood-brain barrier, and its CNS concentrations exceed inhibitory concentrations for Aspergillus spp. (19, 20). However, the Infectious Diseases Society of America recommends surgery in combination with antifungal therapy for osteomyelitis and cerebral lesions. Despite the chronic courses in our cases, voriconazole was effective without surgical debridement, at least in a nonneutropenic setting.
CSF GM testing has a reported sensitivity of 80% for CNS IA (21, 22). However, the sensitivity varies widely according to sample type and patient condition, giving poor performance among nonneutropenic patients (1). While serum and CSF GM test results remained negative in both cases, BDG was detected in baseline serum and CSF samples. It should be noted that at least one serum sample positive for BDG is a microbiological criterion per se for the diagnosis of probable invasive fungal disease (IFD) (23). A previous preliminary study showed that mean CSF BDG concentrations of 383 pg/ml were found in three patients with CNS aspergillosis (24, 25). More recently, during a large devastating outbreak of iatrogenic fungal meningitis, CSF BDG concentrations ranged from 39 to 2,396 pg/ml, guiding the diagnosis of probable fungal meningitis (26). Thus, BDG might be a valuable biomarker in CSF for the diagnosis of Aspergillus meningitis and/or brain lesions, especially among nonneutropenic patients.
REFERENCES
- 1.Lortholary O, Gangneux J-P, Sitbon K, Lebeau B, de Monbrison F, Le Strat Y, Coignard B, Dromer F, Bretagne S. 2011. Epidemiological trends in invasive aspergillosis in France: the SAIF network (2005–2007). Clin Microbiol Infect 17:1882–1889. doi: 10.1111/j.1469-0691.2011.03548.x. [DOI] [PubMed] [Google Scholar]
- 2.Alanio A, Sitterlé E, Liance M, Farrugia C, Foulet F, Botterel F, Hicheri Y, Cordonnier C, Costa J-M, Bretagne S. 2011. Low prevalence of resistance to azoles in Aspergillus fumigatus in a French cohort of patients treated for haematological malignancies. J Antimicrob Chemother 66:371–374. doi: 10.1093/jac/dkq450. [DOI] [PubMed] [Google Scholar]
- 3.Suarez F, Lortholary O, Buland S, Rubio MT, Ghez D, Mahé V, Quesne G, Poirée S, Buzyn A, Varet B, Berche P, Bougnoux ME. 2008. Detection of circulating Aspergillus fumigatus DNA by real-time PCR assay of large serum volumes improves early diagnosis of invasive aspergillosis in high-risk adult patients under hematologic surveillance. J Clin Microbiol 46:3772–3777. doi: 10.1128/JCM.01086-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nivoix Y, Velten M, Letscher-Bru V, Moghaddam A, Natarajan-Amé S, Fohrer C, Lioure B, Bilger K, Lutun P, Marcellin L, Launoy A, Freys G, Bergerat J-P, Herbrecht R. 2008. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis 47:1176–1184. doi: 10.1086/592255. [DOI] [PubMed] [Google Scholar]
- 5.Herbrecht R, Bories P, Moulin J-C, Ledoux M-P, Letscher-Bru V. 2012. Risk stratification for invasive aspergillosis in immunocompromised patients. Ann N Y Acad Sci 1272:23–30. doi: 10.1111/j.1749-6632.2012.06829.x. [DOI] [PubMed] [Google Scholar]
- 6.Vaziri ND, Pahl MV, Crum A, Norris K. 2012. Effect of uremia on structure and function of immune system. J Ren Nutr 22:149–156. doi: 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao Y, Zhong M-K, Xu H-B, Li L. 2013. Development and validation of a risk score for predicting invasive fungal infectious in an intensive care unit. Pharmazie 68:459–464. [PubMed] [Google Scholar]
- 8.Rammaert B, Lanternier F, Poirée S, Kania R, Lortholary O. 2012. Diabetes and mucormycosis: a complex interplay. Diabetes Metab 38:193–204. doi: 10.1016/j.diabet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Koirala J, Khardori R, Khardori N. 2007. Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am 21:617–638. doi: 10.1016/j.idc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Parize P, Chandesris M-O, Lanternier F, Poirée S, Viard J-P, Bienvenu B, Mimoun M, Méchai F, Mamzer M-F, Herman P, Bougnoux M-E, Lecuit M, Lortholary O. 2009. Antifungal therapy of Aspergillus invasive otitis externa: efficacy of voriconazole and review. Antimicrob Agents Chemother 53:1048–1053. doi: 10.1128/AAC.01220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ling SS, Sader C. 2008. Fungal malignant otitis externa treated with hyperbaric oxygen. Int J Infect Dis 12:550–552. doi: 10.1016/j.ijid.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Rubin Grandis J, Branstetter BF IV, Yu VL. 2004. The changing face of malignant (necrotising) external otitis: clinical, radiological, and anatomic correlations. Lancet Infect Dis 4:34–39. doi: 10.1016/S1473-3099(03)00858-2. [DOI] [PubMed] [Google Scholar]
- 13.Pongbhaesaj P, Dejthevaporn C, Tunlayadechanont S, Witoonpanich R, Sungkanuparph S, Vibhagool A. 2004. Aspergillosis of the central nervous system: a catastrophic opportunistic infection. Southeast Asian J Trop Med Public Health 35:119–125. [PubMed] [Google Scholar]
- 14.Chan HS, Yuen HY, Ng WK, Vlantis AC, Ahuja AT, Tong CFM, van Hasselt CA. 2011. Aspergillus pachymeningitis mimicking nasopharyngeal carcinoma. J Laryngol Otol 125:103–107. doi: 10.1017/S0022215110001623. [DOI] [PubMed] [Google Scholar]
- 15.Lee G-J, Jung T-Y, Choi S-M, Jung M-Y. 2013. Cerebral aspergillosis with multiple enhancing nodules in the right cerebral hemisphere in the immune-competent patient. J Korean Neurosurg Soc 53:312–315. doi: 10.3340/jkns.2013.53.5.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik J-A, Wingard JR, Patterson TF. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz S, Ruhnke M, Ribaud P, Corey L, Driscoll T, Cornely OA, Schuler U, Lutsar I, Troke P, Thiel E. 2005. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood 106:2641–2645. doi: 10.1182/blood-2005-02-0733. [DOI] [PubMed] [Google Scholar]
- 18.Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann J-W, Kern WV, Marr KA, Ribaud P, Lortholary O, Sylvester R, Rubin RH, Wingard JR, Stark P, Durand C, Caillot D, Thiel E, Chandrasekar PH, Hodges MR, Schlamm HT, Troke PF, de Pauw B. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz S, Thiel E. 2009. Cerebral aspergillosis: tissue penetration is the key. Med Mycol 47(Suppl 1):S387–S393. doi: 10.1080/13693780802537953. [DOI] [PubMed] [Google Scholar]
- 20.Clemons KV, Schwartz JA, Stevens DA. 2012. Experimental central nervous system aspergillosis therapy: efficacy, drug levels and localization, immunohistopathology, and toxicity. Antimicrob Agents Chemother 56:4439–4449. doi: 10.1128/AAC.06015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kami M, Ogawa S, Kanda Y, Tanaka Y, Machida U, Matsumura T, Sakamaki H, Hirai H. 1999. Early diagnosis of central nervous system aspergillosis using polymerase chain reaction, latex agglutination test, and enzyme-linked immunosorbent assay. Br J Haematol 106:536–537. doi: 10.1046/j.1365-2141.1999.01542.x. [DOI] [PubMed] [Google Scholar]
- 22.Viscoli C, Machetti M, Gazzola P, De Maria A, Paola D, Van Lint MT, Gualandi F, Truini M, Bacigalupo A. 2002. Aspergillus galactomannan antigen in the cerebrospinal fluid of bone marrow transplant recipients with probable cerebral aspergillosis. J Clin Microbiol 40:1496–1499. doi: 10.1128/JCM.40.4.1496-1499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petraitiene R, Petraitis V, Hope WW, Mickiene D, Kelaher AM, Murray HA, Mya-San C, Hughes JE, Cotton MP, Bacher J, Walsh TJ. 2008. Cerebrospinal fluid and plasma (1→3)-β-d-glucan as surrogate markers for detection and monitoring of therapeutic response in experimental hematogenous Candida meningoencephalitis. Antimicrob Agents Chemother 52:4121–4129. doi: 10.1128/AAC.00674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikulska M, Furfaro E, Bono VD, Raiola AM, Grazia CD, Bacigalupo A, Viscoli C. 2013. (1-3)-β-d-Glucan in cerebrospinal fluid is useful for the diagnosis of central nervous system fungal infections. Clin Infect Dis 56:1511–1512. doi: 10.1093/cid/cit073. [DOI] [PubMed] [Google Scholar]
- 26.Lyons JL, Roos KL, Marr KA, Neumann H, Trivedi JB, Kimbrough DJ, Steiner L, Thakur KT, Harrison DM, Zhang SX. 2013. Cerebrospinal fluid (1,3)-β-d-glucan detection as an aid for diagnosis of iatrogenic fungal meningitis. J Clin Microbiol 51:1285–1287. doi: 10.1128/JCM.00061-13. [DOI] [PMC free article] [PubMed] [Google Scholar]