Abstract
The generation of a new antifungal against Candida albicans biofilms has become a major priority, since biofilm formation by this opportunistic pathogenic fungus is usually associated with an increased resistance to azole antifungal drugs and treatment failures. Miltefosine is an alkyl phospholipid with promising antifungal activity. Here, we report that, when tested under planktonic conditions, miltefosine displays potent in vitro activity against multiple fluconazole-susceptible and -resistant C. albicans clinical isolates, including isolates overexpressing efflux pumps and/or with well-characterized Erg11 mutations. Moreover, miltefosine inhibits C. albicans biofilm formation and displays activity against preformed biofilms. Serial passage experiments confirmed that miltefosine has a reduced potential to elicit resistance, and screening of a library of C. albicans transcription factor mutants provided additional insight into the activity of miltefosine against C. albicans growing under planktonic and biofilm conditions. Finally, we demonstrate the in vivo efficacy of topical treatment with miltefosine in the murine model of oropharyngeal candidiasis. Overall, our results confirm the potential of miltefosine as a promising antifungal drug candidate, in particular for the treatment of azole-resistant and biofilm-associated superficial candidiasis.
INTRODUCTION
The recent elevated incidence of fungal infections is related to the indiscriminate use of broad-spectrum antibiotics and corticosteroids, the increase in the number of invasive medical procedures, and the AIDS epidemic. The persistence of these infections is usually associated with the fungal ability to form biofilms on implantable medical devices (1). Candida spp. can adhere and form biofilms on the surface of different medical devices (such as catheters, prostheses, pacemakers, and heart valves) and on the mucosal surface, leading to a superficial infection with a complex structure in which hyphae, pseudohyphae, and yeasts grow surrounded by a dense extracellular matrix, composed mainly of proteins, polysaccharides, and extracellular DNA (eDNA) (2). Hematogenous dissemination may occur due to the detachment of yeasts from the top layer of the biofilm, a phenomenon known as dispersion (3). Candida albicans is the third leading cause of infections related to the use of catheters (4, 5). Development of candidemia during hospitalization when central venous catheters are used can happen in 72% of cases in Latin America (6), and the worldwide mortality rate for catheter-related candidemia can reach 41% (7). Oropharyngeal candidiasis (OPC) is characterized by Candida growth as a biofilm over the tongue and oral mucosa. OPC has been described as the most frequent opportunistic fungal infection among HIV-positive patients, and it is estimated that more than 90% of these patients develop this infection at some time during the progression of their disease (8, 9).
The increased resistance to antifungal agents is the main clinical complication associated with biofilm formation. The list of resistance mechanisms proposed for fungal biofilms includes the association of antifungals with the extracellular matrix, high cellular density, the expression of drug efflux pumps (mainly CDR1, CDR2, and MDR, in Candida albicans), and the existence of a subpopulation of dormant cells (persister cells) (10). In vitro biofilm resistance was shown by several groups for biofilms of Candida spp. (11–13), Cryptococcus neoformans (14, 15), Aspergillus sp. (16), and Fusarium solani (17–19). Of the three classes of antifungal agents currently in clinical use (azoles, polyenes, and echinocandins), only lipid formulations of amphotericin B and the echinocandins (caspofungin) demonstrated a consistent in vitro activity against biofilms of C. albicans, Candida parapsilosis (12, 20, 21), and Candida tropicalis (22). However, despite the two options available, infections due to Candida spp. related to biofilm formation are extremely difficult to eradicate, requiring the removal of infected medical devices (23), which is not always possible (24). These findings illustrate the reduced number of drugs available for the treatment of fungal infections associated with biofilm formation and point to the urgent need to search for new molecules with antifungal activity not only against planktonic cells but also against biofilms.
Miltefosine (hexadecylphosphocholine) is an alkyl phospholipid, developed as an antitumor agent that currently constitutes an alternative chemotherapy for leishmaniasis in several countries (25). Recently, some reports have attributed the development of resistance to miltefosine during the treatment of visceral leishmaniasis to the overexpression of an ABC transporter (Leishmania tropica MDR1 [LtrMDR1]) and to changes in membrane sterol composition (25, 26). The broad-spectrum antifungal activity of miltefosine has been demonstrated in vitro against planktonic cells of several medically important fungi, including dermatophytes, Candida sp., Cryptococcus sp., Aspergillus sp., Fusarium sp., Scedosporium sp., Histoplasma capsulatum, Rhizopus sp., and Sporothrix schenckii (27–32), and no reports about resistance development have been made so far.
We have recently described the promising antibiofilm activity of miltefosine against C. albicans central venous catheter biofilms (33). In the present study, we have evaluated the activity of miltefosine against planktonic cells and biofilms formed by Candida albicans clinical isolates resistant to fluconazole (34, 35), demonstrated a low potential for the development of resistance to miltefosine, and reported susceptibility patterns for a series of C. albicans mutant strains mutated in different transcription factors (36). Finally, we investigated the effect of topical application of miltefosine using a murine model of oral candidiasis.
MATERIALS AND METHODS
Strains and culture conditions.
The clinical C. albicans wild-type strain SC5314 (a clinical isolate originally obtained from a patient with disseminated candidiasis [37]) was used in all experiments as a control standard. This strain was selected because it can form robust biofilms and is well characterized genetically (38). In addition, 12 C. albicans clinical isolates resistant to fluconazole by overexpression of drug efflux pumps and/or with mutations in the azole target gene ERG11 (34, 35) (Table 1) were used, together with C. albicans mutant strains with mutations in selected transcription factors developed by Homann and coworkers (36) (see Table S1 in the supplemental material). Cells from stocks stored at −80°C were propagated by streaking a loopful of culture onto yeast extract-peptone-dextrose (YPD) medium in an agarose gel (10 g yeast extract, 20 g Bacto peptone, 20 g dextrose, and 15 g of agar [Sigma] in 1 liter of sterile water) and incubated overnight at 30°C. A loopful of cells from YPD agar plates was inoculated into flasks (150 ml) containing 25 ml of YPD liquid medium and grown in an orbital shaker at 180 rpm for 14 to 16 h at 30°C. Under these conditions, C. albicans grows as budding yeasts. After 18 h, the cells were washed with phosphate-buffered saline (PBS) buffer and counted using a hemocytometer. The cells were adjusted to a final density of 1 × 106 cells/ml (for biofilm experiments) or 1 × 103 cells/ml (for planktonic cell experiments) in RPMI medium supplemented with l-glutamine (Cellgro; Corning, USA) and buffered with 165 mM morpholinepropanesulfonic acid (MOPS) (Thermo Fisher Scientific Inc., USA) at pH 7.0.
TABLE 1.
Planktonic susceptibility of Candida albicans clinical isolates obtained from HIV-positive patients from prospective clinical study of oropharyngeal candidiasis from University of Texas Health Science Center in San Antonio and South Texas Veterans Health Care System, San Antonio, Texas, USAe
| Strain | MIC (μg/ml) |
Erg11p substitution(s)a,b | Overexpressed gene(s)a,b | ||||
|---|---|---|---|---|---|---|---|
| Miltefosine |
Fluconazole |
||||||
| 24 h | 48 h | 24 h | 48 h | Previously publisheda,b | |||
| 3034a | 2 | 2 | >64 | >64 | >64 | NDd | MDR1, CDR, CDR1, CDR2 |
| 4617a | 2 | 2 | >64 | >64 | 64 | F449S, T229A | MDR1, CDR, CDR1 |
| 4639a | 1 | 1 | 64 | >64 | >64 | F449S, T229A | ND |
| 5106a | 2 | 2 | >64 | >64 | 8 | V437I | ND |
| 4380a | 0.25–1 | 1 | 0.5 | 1 | 64 | V437I | CDR, CDR1, CDR2 |
| 2440a | 2 | 2 | 32 | >64 | 64 | V437I | MDR1, ERG11, V437I |
| 2307a | 1 | 1–2 | 0.5 | 0.5 | >64 | K128T | ERG11, CDR, CDR1, CDR2 |
| 412a | 2 | 2 | 2 | >64 | 0.5 | K128T | ND |
| 1691a | 1–2 | 1–2 | 0.5 | >64 | 0.25 | K128T | ND |
| 3731a | 1–2 | 2 | >64 | >64 | >64 | F126L, K143R | MDR1 |
| 6482b | 1–2 | 1–2 | >64 | >64 | >32 | Point mutations | ND |
| 6191b | 1 | 1 | >64 | >64 | >32 | Point mutations | ND |
| SC5314c | 1 | 1 | 0.125–0.25 | 4 | |||
Antifungals.
Miltefosine (Cayman Chemical Company, USA) was diluted in sterile Milli-Q water and evaluated for in vitro and in vivo antifungal activity. Fluconazole (Pfizer Inc., New York, NY, USA), caspofungin (Sigma Chemical Co., St. Louis, MO, USA), and amphotericin B (Sigma Chemical Co., St. Louis, MO, USA) were used as reference antifungals. The final concentration of dimethyl sulfoxide (DMSO) after antifungal dilution was not higher than 0.14% in each test well. Stock solutions of the different antifungals were maintained at −80°C, and dilutions were made fresh for experiments.
MICs.
MICs of antifungal agents were determined for planktonic cells using the broth microdilution assay described in document M27-A3 published by the Clinical and Laboratory Standards Institute (39). Briefly, serial 2-fold dilutions of the compounds were prepared in RPMI 1640 medium, buffered with 165 mM MOPS, pH 7.0, in round-bottom 96-well microtiter trays (Corning Inc., Corning, NY, USA) to obtain concentration ranges from 0.03 to16 μg/ml for amphotericin B, caspofungin, and miltefosine and from 0.125 to 64 μg/ml for fluconazole. Yeasts were then added to each well at final concentrations of 0.5 × 103 to 1 × 103 cells/ml. Microtiter trays were incubated at 36°C for 48 h. Minimum concentrations that inhibited 50% and 90% of the fungal yeast growth in relation to control (IC50 and IC90, respectively) were determined by visual analysis and confirmed by spectrophotometry at 492 nm in a microtiter plate reader (Benchmark Microplate reader; Bio-Rad, CA). The percentage of inhibition was calculated with the equation % inhibition = [100 − (A × 100/C)], where A is the optical density (OD) of wells containing antifungal agent and C is the OD of control wells with fungi only. The document M27-A3 states that the IC50 should be considered the MIC for all azoles and the IC90 should be considered the MIC for all polyenes (39). Here, we also considered the IC90 value to be the MIC for miltefosine.
Resistance/tolerance induction assay.
Induction of resistance by serial passage in subinhibitory concentrations of antifungal drugs was based on a previous description (40) with some modifications. Briefly, a single colony of C. albicans strains SC5314 and 6482 (the latter is a clinical isolate obtained from an HIV-infected patient with recalcitrant oropharyngeal candidiasis with a high predisposition to develop resistance to multiple antifungal agents) was used to inoculate 25 ml of YPD broth which was incubated overnight in an orbital shaker (180 rpm) at 30°C. An aliquot (10 μl) of this culture containing 1 × 105 cells/ml was then transferred to 1 ml of RPMI medium (final inoculum of 103 cells/ml), containing subinhibitory concentrations (sub-IC) of miltefosine or fluconazole, and the cells were incubated in an orbital shaker (180 rpm) at 37°C for 24 h. After each cycle of 24-h growth in the presence of subinhibitory concentrations, 10 μl of the cell suspension was added to a fresh antifungal dilution and incubated under the same conditions for 24 h. Serial passage was performed for 35 days, and after each 7-day passage, drug concentration was doubled, until it reached the IC50 (fluconazole) or IC90 (miltefosine). At each passage, a 1-ml aliquot of the culture suspension was mixed with glycerol and frozen at −80°C for subsequent susceptibility testing.
Biofilm formation assay.
One hundred microliters of cell suspension (final density of 1 × 106 cells/ml) was placed in each well of a 96-well, flat-bottom microtiter plate (Corning Inc., NY, USA), and plates were sealed with Parafilm and incubated at 37°C for 24 h (41). Then, cells were gently washed with 200 μl of PBS buffer twice in order to remove the free-floating cells and leave the biofilms intact in the bottom of the well.
Biofilm MICs (BMICs).
The minimum antifungal concentration that inhibited 50% and 90% of both biofilm formation and preformed biofilm cell viability was defined as previously described (41). To evaluate the effect of the drugs in preventing biofilm formation, 50 μl of each drug serially diluted in RPMI medium was added to plates containing 50 μl of 2 × 106 cells/ml in a 96-well plate. To evaluate the efficacy of drugs against preformed biofilms, biofilms grown for 24 h were gently washed, and 100 μl of the serially diluted drug was added. After the addition of drugs, the plates were sealed with Parafilm and incubated at 37°C for 24 h. The plates were washed twice with PBS to remove nonadherent cells. The XTT viability test was performed to determine the efficacy of drugs. The dose-response experiments were performed in duplicate at each dose in two different plates.
XTT reduction assay.
To test the viability of cells within the biofilms, we used a colorimetric assay based on the reduction of the tetrazolium salt 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide (XTT; Sigma) by metabolically active cells to yield a formazan-colored product (41). Briefly, 100 μl of 50-μg/ml sterile XTT containing 1 μM menadione (Sigma) was added to each well of the microtiter plate and incubated for 2 h at 37°C. After incubation, 90 μl of XTT supernatant was removed and added to a fresh 96-well flat-bottom plate, and the plate was read in a microtiter plate reader (Benchmark Microplate reader; Bio-Rad, CA) at 490 nm. The percentage of biofilm inhibition was calculated as described for the MIC. The 50% and 90% inhibitory concentrations (BMIC50 and BMIC90) were defined as the concentrations causing 50% and 90% inhibition of either biofilm formation or preformed biofilms due to drug treatment, respectively.
Murine model of oropharyngeal candidiasis.
The effect of topical application of miltefosine was evaluated using a murine model of oropharyngeal candidiasis mostly as previously described (42). Cultures of C. albicans SC5314 for infection were grown overnight in YPD medium in a rocker table at 30°C. Under these conditions, the cells grew solely as yeast cells. Cells were harvested by centrifugation, washed three times in sterile saline solution, and counted using a hemocytometer. Animals were immunosuppressed with cortisone acetate (225 mg/kg of body weight) on alternate days during the entire experiment and anesthetized with chlorpromazine (2 mg/ml) before infection. Mice were infected with C. albicans suspensions (1 × 106 cells/ml in Hanks balanced salt solution [HBSS]) by placing a saturated calcium alginate swab impregnated sublingually for 75 min. Topical treatment started 1 day before infection and was carried out twice a day, using calcium alginate swabs impregnated with miltefosine (2 mg/ml) (treated group, n = 6) or saline (control group, n = 6). Animals were treated for 4 days, and at the end of the experiment (total of 6 days), remaining mice were euthanized. During the experiment, OPC progression was evaluated daily according to a previously established score, based on the oral mucosa and tongue surface area covered by Candida biofilm with 0 denoting a healthy tongue surface and 5 denoting the most severe stage (white patches covering 100% of the surface). Scoring data were compared using the nonparametric Mann-Whitney test. On days 3 and 4, fungal burden was determined on euthanized mice. Tongues and kidneys were removed and processed for colony counting and histology. Organs were homogenized, and fungal loads were determined by plating dilutions onto Sabouraud-chloramphenicol agar plates. For histology, tongues and kidneys retrieved from sacrificed mice were fixed in 10% buffered formalin and embedded in paraffin, and thin tissue slices were obtained and stained with Grocott-Gomori methenamine-silver (GMS) stain prior to microscopic evaluation. Mice were allowed a 1-week acclimatization period before experiments were started. All animal experimentation was conducted in an AAALAC-certified facility at The University of Texas at San Antonio (UTSA) according to the National Institutes of Health guidelines for housing and care of laboratory animals and performed in accordance with institutional regulations after pertinent review and approval by the Institutional Animal Care and Use Committee at The University of Texas at San Antonio.
Statistical analysis.
For quantitative assays, statistical analyses were performed with Dunnett's test (one-way analysis of variance) or Student's t test, and in vivo clinical scoring and CFU data were analyzed using the nonparametric Mann-Whitney test. Statistical significance was accepted at a P value of <0.05.
RESULTS
Miltefosine inhibits growth of planktonic fluconazole-resistant Candida albicans.
All the 12 C. albicans clinical isolates tested, including fluconazole-susceptible and -resistant (MIC, >64 μg/ml) isolates, irrespective of the underlying resistance mechanism (alterations in ERG11 and/or upregulation of genes encoding efflux pumps), were susceptible to miltefosine, presenting MICs between 1 and 2 μg/ml, similar to those of standard strain SC3514 (1 μg/ml) (Table 1). The resistance characteristics of these strains are compiled in Table 1. Selected clinical isolates have been previously shown to be susceptible to amphotericin B and to caspofungin, in planktonic forms (34, 35).
Miltefosine inhibits C. albicans biofilm formation and is active against preformed C. albicans biofilms in vitro.
The antibiofilm potential of miltefosine was evaluated using the same C. albicans clinical isolates previously characterized in regard to their fluconazole resistance, with two different approaches: (i) its ability to inhibit biofilm formation and (ii) its activity against preformed biofilms. For all isolates tested, and also for the control strain (SC5314), the presence of 2 to 4 μg/ml of miltefosine prevented 90% of biofilm formation (Table 2). A reduction of >90% of the metabolic activity of biofilm cells was observed in the case of preformed biofilms treated with concentrations ranging from 8 to 32 μg/ml of miltefosine (Table 3). Fluconazole showed no inhibitory activity against any stage of biofilm development, while amphotericin B and caspofungin were effective against both biofilm developmental phases (Tables 2 and 3). Amphotericin B concentrations ranging from 2 to 16 μg/ml inhibited >90% of C. albicans biofilm formation, whereas concentrations between 0.5 and 8 μg/ml reduced the metabolic activity of cells within preformed biofilms by >50%. For caspofungin, a >90% inhibition of biofilm formation was observed at concentrations between 0.12 and 16 μg/ml, whereas concentrations ranging from 0.25 to >16 μg/ml decreased the metabolic activity of cells within preformed biofilms by >50%. Again, biofilms from most fluconazole-resistant clinical isolates showed a paradoxical growth effect (i.e., reduced activity of the drug in concentrations higher than the BMIC) when treated with caspofungin (Tables 2 and 3).
TABLE 2.
Inhibitory effect of alkyl phospholipid miltefosine and standard antifungals amphotericin B, fluconazole, and caspofungin on biofilm formation of Candida albicans clinical isolatesb
| Strain | BMIC (μg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Miltefosine |
Amphotericin B |
Fluconazole |
Caspofungin |
|||||
| BMIC50 | BMIC90 | BMIC50 | BMIC90 | BMIC50 | BMIC90 | BMIC50 | BMIC90c | |
| 3034 | 2 | 4 | 2 | 8–16 | >1,000 | >1,000 | 0.25 | 0.2–0.5 |
| 4617 | 2 | 4 | 0.5 | 2 | 4 | 500 | 0.06–0.125 | 0.125 |
| 4639 | <2 | 4 | 0.25–0.5 | 2 | 8 | >1,000 | 0.03–0.06 | 0.06–0.12 |
| 5106 | 1–2 | 2 | 2 | 8 | >1,000 | >1,000 | 0.06–0.125 | 0.12–0.25 |
| 4380 | <2 | 4 | 2–4 | 8 | 2 | >1,000 | 0.125–0.25 | 0.25–16 |
| 2440 | <2 | 4 | 4 | 8 | >1,000 | >1,000 | 0.03 | 0.5 |
| 2307 | <2 | 2–4 | 0.5 | 4 | 2 | >1,000 | 0.125–0.25 | 0.25 |
| 412 | 2 | 4 | 1 | 8 | 2 | >1,000 | 0.06–0.125 | 0.125 |
| 1691 | 1–2 | 2 | 1 | 4 | 250 | >1,000 | 0.03–0.06 | 0.06–0.12 |
| 3731 | <2 | 2 | 0.5 | 4 | 125 | >1,000 | 0.06–0.125 | 0.12–0.25 |
| 6482 | 2 | 2–4 | 0.5–1 | 4 | 32 | >1,000 | 0.03–0.06 | 0.125 |
| 6191 | 2–4 | 4 | 1–2 | 2 | >1,000 | >1,000 | 0.25 | 0.5 |
| SC5314a | <2 | 4 | 0.5–1 | 2–4 | <2 | >1,000 | 0.06–0.125 | 0.125 |
Control strain.
Metabolic activity of biofilm cells was quantified using the XTT reduction assay after 24 h of incubation, and the inhibitory concentration was calculated in relation to control (not treated) biofilms.
All data in this column represent the paradoxical effect.
TABLE 3.
Inhibitory effect of alkyl phospholipid miltefosine and standard antifungals amphotericin B, fluconazole, and caspofungin on preformed biofilms of Candida albicans clinical isolatesb
| Strain | BMIC (μg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Miltefosine |
Amphotericin B |
Fluconazole |
Caspofungin |
|||||
| BMIC50 | BMIC90 | BMIC50 | BMIC90 | BMIC50 | BMIC90 | BMIC50 | BMIC90 | |
| 3034 | 4–8 | 8 | 4–8 | >16 | >1,000 | >1,000 | 2c | >16c |
| 4617 | 8 | 16 | 4 | 16 | 500 | >1,000 | 0.5–1 | 2c |
| 4639 | 8 | 16 | 2 | 16 | >1,000 | >1,000 | 0.5–1 | 1c |
| 5106 | 16–31.25 | 31.25 | 8 | >16 | >1,000 | >1,000 | >16 | >16 |
| 4380 | 8 | 8–16 | 0.5 | 4 | 2 | >1,000 | 0.25–0.5 | 1c |
| 2440 | 4–8 | 8 | 4 | >16 | >1,000 | >1,000 | 1–16 | >16 |
| 2307 | 4–8 | 16 | 4 | 16 | 4 | >1,000 | 0.5 | 1c |
| 412 | 8 | 31.25 | 2–4 | 16 | >1,000 | >1,000 | 0.5–1 | 2c |
| 1691 | 8 | 31.25 | 4 | 16 | >1,000 | >1,000 | 0.5–1 | 1c |
| 3731 | 4–8 | 8–16 | 4 | >16 | >1,000 | >1,000 | 1 | 4c |
| 6482 | 8 | 16 | 4 | 16 | >1,000 | >1,000 | 0.25 | 1c |
| 6191 | 4–8 | 8 | 2–4 | ≥16 | >1,000 | >1,000 | >16 | >16 |
| SC5314a | 8 | 16 | 2 | 8 | >1,000 | >1,000 | 0.5 | 1c |
Candida albicans control strain.
Metabolic activity of biofilm cells was quantified using the XTT reduction assay after 24 h of incubation with the drugs (except for control, which received only medium), and the inhibitory concentration was calculated in relation to control (not treated) biofilms.
Paradoxical effect.
Miltefosine activity is not affected by efflux pump transcription factor modulation.
In order to determine the role of drug efflux pump expression in the susceptibility of C. albicans to miltefosine, we used a group of C. albicans mutants in which transcription factors responsible for the expression of CDR, MDR, and other factors involved in drug resistance were deleted (for specific mutant information, see Table S1 in the supplemental material). Mutant susceptibility was evaluated in vitro, for both planktonic and biofilm growth conditions (in two stages of biofilm development—initial and preformed).
Mutants lacking TAC1 (Δtac1) and MRR1 (Δmrr1) have CDR (CDR1 and CDR2) and MDR transcription reduced, respectively, while mutants lacking CRZ1 (Δcrz1) are hypersensitive to fluconazole. Mutants with CAP1 (Δcap1) deleted lose their ability to become resistant to multiple drugs, and when NDT80 (Δndt80) is lost, mutants cease to be resistant by failing to enable production of Cdr1 in response to drugs. Moreover, FCR1 mutants (Δfcr1) become resistant to fluconazole. In our experiments, for all developmental stages tested—planktonic cells and initial and preformed biofilms—mutants associated with CDR (Δtac1 and Δndt80) or associated with clinical resistance (Δrme1) were susceptible to miltefosine to the same extent as was the parental strain (C. albicans SN152, used for mutant construction) (Table 4) and showed MICs and BMICs similar to those of C. albicans SC5314 (Tables 1 to 3). Interestingly, preformed biofilms of Δmrr1 mutants (associated with MDR pumps) and Δcrz1 mutants (hypersensitive to fluconazole, acting “downstream” of calcineurin) were slightly more sensitive to miltefosine than the wild-type strain (1- to 2-fold), despite the similar susceptibilities observed for planktonic cells (Table 4). Additionally, mutant transcription factors responsible for controlling the stress response, synthesis and cell wall remodeling, cell respiration, ergosterol biosynthesis, adhesion, biofilm formation, and virulence were also evaluated, but these processes do not seem to have a role in the antifungal activity of miltefosine (data not shown). Interestingly, knockout mutants for UPC2 (Δupc2), a transcriptional regulator of ergosterol biosynthetic genes and sterol uptake, formed biofilms with partially increased susceptibility to miltefosine (1- to 4-fold) (data not shown).
TABLE 4.
Inhibitory effect of alkyl phospholipid miltefosine on planktonic cells, biofilm formation, and preformed biofilms of Candida albicans transcription factor mutant library
| Target | Gene | IC90 for planktonic cells (μg/ml) | BMIC (μg/ml) |
|||
|---|---|---|---|---|---|---|
| Biofilm formation |
Preformed biofilm |
|||||
| BMIC50 | BMIC90 | BMIC50 | BMIC90 | |||
| Drug response | TAC1 | 1 | 2 | 4 | 8–16 | 32 |
| RME1 | 2 | 1–2 | 4 | 8 | 32 | |
| CRZ1 | 2 | 1–2 | 2 | 2 | 8 | |
| MRR1 | 1–2 | 2 | 4 | 2–4 | 8 | |
| NDT80 | 1 | 1–2 | 2 | 32–64 | 64 | |
| FCR1 | 1–2 | 2 | 4 | 8–16 | 32 | |
| CAP1 | 1 | 2 | 4 | 8 | 16 | |
| Stress response | WAR1 | 1 | 0.5 | 2 | 8 | 32 |
| MNL1 | 2 | 2 | 2 | 8–16 | 32 | |
| NRG1 | 1–2 | NDb | ND | ND | 16 | |
| Cell wall | RLM1 | 2 | 1–2 | 2 | 2–4 | 8 |
| CAS5 | 2 | 2 | 4 | 2–4 | 8 | |
| SKO1 | 1 | 1–2 | 2–4 | 8 | 32 | |
| Cell respiration/metabolism | HAP5 | 1 | 1–2 | 2 | 32–64 | 64 |
| HAP3 | 1 | 1 | 4 | 8–16 | 32 | |
| Ergosterol | UPC2 | 2 | 2–4 | 4–8 | 2–4 | 8 |
| Adherence, biofilm, and virulence | EFG1 | 0.5–1 | 1–2 | 2 | 4–8 | 8–16 |
| ACE2a | 1 | 4 | 8–16 | 2–4 | 4–8 | |
| BCR1a | 2 | 2–8 | 8 | 16–32 | 32 | |
| C. albicans SN152 | Parental strain | 1–2 | 2 | 4 | 8 | 16–32 |
Not a good biofilm-forming strain.
ND, not defined.
Repeated exposure of C. albicans to miltefosine in vitro does not induce resistance.
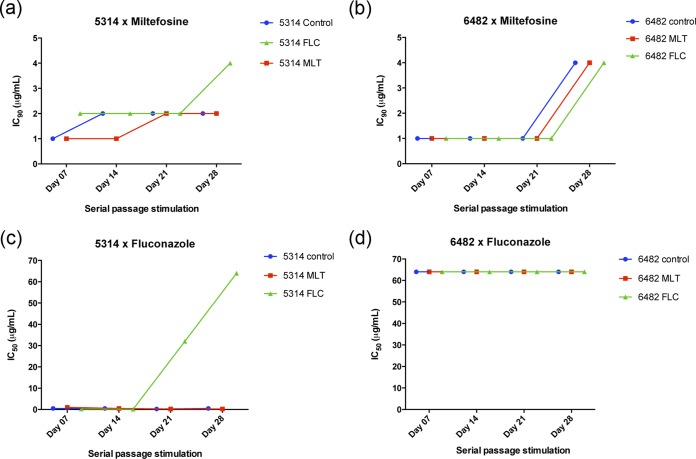
We performed a series of serial passage experiments to evaluate the potential to induce resistance upon repeated exposure to miltefosine. Planktonic cells of the control strain (SC5314) and of isolate 6482 (with a high predisposition to develop resistance to multiple antifungals) were exposed to increasing subinhibitory concentrations of miltefosine or fluconazole, for 35 days. Changes in the susceptibility to the drugs were monitored by microdilution assays during the experiments. Continuous stimulation with subinhibitory concentrations of miltefosine did not alter the susceptibility profile of the standard strain (SC5314), and the MIC remained similar to that of the control (exposed to culture medium only during the length of experiments) (Fig. 1a). Interestingly, the clinical isolate 6482 showed a slight reduction in its susceptibility to miltefosine, with MIC increasing from 1 to 4 μg/ml (2 dilutions) between days 21 and 28. However, this susceptibility change was observed simultaneously in three experimental variables: miltefosine exposure, fluconazole exposure, and fresh medium only (control). Therefore, we consider that this change is more likely an adaptation of the strain during the continuous batch culture in RPMI medium and not a direct consequence of the antifungal drug pressure (Fig. 1b). Additionally, exposure to miltefosine did not induce the development of cross-resistance to fluconazole, and the MIC for fluconazole in the miltefosine-exposed group remained between 0.25 and 1 μg/ml throughout the experiment, similar to the control group (0.25 to 0.5 μg/ml) (Fig. 1c). Continuous growth in the presence of fluconazole for 14 days induced the development of tolerance to this drug, increasing the MIC to 32 and >64 μg/ml on days 21 and 28, respectively (Fig. 1c), while C. albicans strain 6482 remained resistant to fluconazole (MIC of >64 μg/ml) throughout the experiment, in all groups (Fig. 1d). Yet, populations of C. albicans SC5314 grown in the presence of fluconazole yielded no cross-resistance to miltefosine, and the MIC values remained similar to those of the control and the miltefosine-exposed group, during the initial 21 days (Fig. 1c). Between days 21 and 28, a small increase was observed in the MIC of miltefosine (1-fold). The increase of only 1 dilution is not significant, but a longer test should be conducted to clarify the cross-tolerance development potential after stimulation with fluconazole for periods longer than 30 days.
FIG 1.
Induction of in vitro development of resistance to drugs by continuous exposure. Both strains of Candida albicans, SC5314 and 6482, were challenged daily with fresh culture medium (control; blue line), increasing subinhibitory concentrations of miltefosine (MLT; red line), and increasing subinhibitory concentrations of fluconazole (FLC; green line), for 30 days. (a and b) Susceptibility of the three groups to miltefosine over time; (c and d) susceptibility of the three groups to fluconazole over time.
In vivo topical treatment with miltefosine impaired the progression of oral candidiasis.
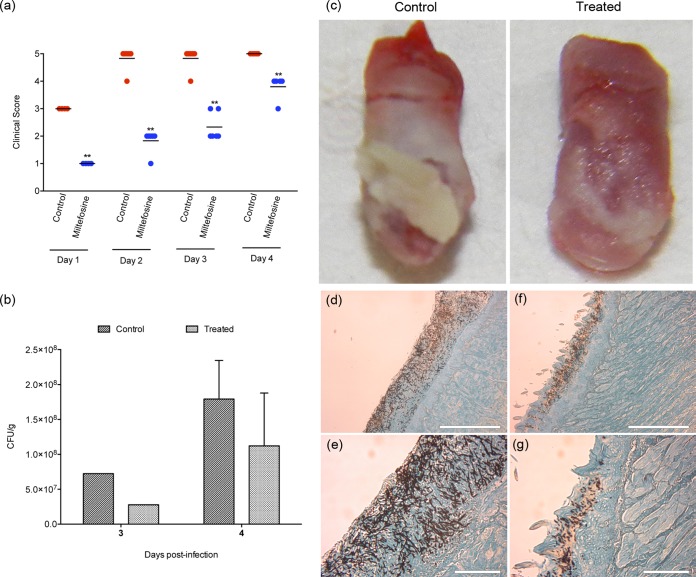
The potential protective effect of miltefosine as a topical treatment for OPC was evaluated using a mouse model of oral candidiasis previously described (42). The effectiveness of the treatment was assessed using three different parameters: (i) clinical (visual) analysis of disease progression, using predefined parameters (see Materials and Methods); (ii) assessment of the extent of colonization of the animal tongues through histological sections; and (iii) determination of fungal burden in tongues. Topical treatment with 50 mg/kg (2 mg/ml) of miltefosine administered twice a day was started 1 day before the infection, and the drug prevented the establishment of infection on days 1 and 2 (Fig. 2a). Animals from the treated group remained with reduced clinical signs compared to the control group treated with saline until the end of the experiment (P < 0.01) (Fig. 2a). Visual analysis of the tongues from control and treated animals, after 3 and 4 days of treatment, confirmed that topical miltefosine significantly reduced the colonization and infection of mouse tongues (Fig. 2c). At the end of the 4th day, the tongues of the animals from the control group were completely covered by a thick fungal biofilm layer, which was not observed so intensely in treated animals (Fig. 2c). In addition, mice from the treated group also showed reduced fungal burdens as determined by lower CFU values than those for control animals treated with saline (Fig. 2b). We note that filamentous forms that predominate in the tongues from the untreated group (Fig. 2c) may have a lower plating efficiency than yeast cells, which are mostly seen in the miltefosine-treated group, and therefore, fungal burdens in organs from control mice may be underestimated. Dissemination to the kidneys was not observed in any of the groups tested, as CFU plates of kidney homogenates (obtained at time of necropsy) had no colonies after 48 h of incubation (data not shown). Histological sections from control group tongues revealed that the superficial tissue of the tongue was covered by C. albicans, with many hyphae actively penetrating the tissue, characterizing the invasive behavior during an active infection (Fig. 2d and e). On the other hand, histological sections of tongues from the group treated with miltefosine showed a reduced number of yeasts and hyphae colonizing only the superficial layers of tissue, confirming a less invasive behavior (Fig. 2f and g).
FIG 2.
In vivo evaluation of the protective effect of miltefosine against oral candidiasis. Treated groups received 50 mg/kg of miltefosine, twice a day, by topical administration. Miltefosine treatment was initiated the day before infection (day −1). The control group received saline twice a day, also by topical administration. (a) Clinical score of candidiasis progression, showing that miltefosine has a protective role, preventing the development of disease. **, P < 0.01; statistical analysis by nonparametric Mann-Whitney test. (b) Extent of C. albicans colonization on tongues from control mice and mice treated with miltefosine. Only one animal from each group was sacrificed at the end of day 3. Bars indicate the standard deviations. The graph shows the reduction in C. albicans colonizing the tongues of animals treated with miltefosine in comparison to the untreated control group. Statistical analysis by nonparametric Mann-Whitney test (4th day), P = 0.0952, and Mann-Whitney U test, 4.000. (c) Visual analysis of the extension of tongue colonization by C. albicans in mice from the control group (left) and the group treated with miltefosine (right) at the end of day 4. Control tongues showed a dense biofilm covering the entire tongue surface on day 4 (left), and miltefosine treatment showed a protective role by reducing the tongue colonization and reducing the biofilm formation (right). (d to g) Histological sections of tongues from control mice (d and e) and mice treated with miltefosine (f and g) sacrificed on the 4th day and stained with Grocott-Gomori stain and silver methanamine. The administration of miltefosine reduced tissue colonization, inhibited hypha formation, and reduced invasive behavior at the infection site (f and g).
DISCUSSION
The potent antifungal activity of miltefosine has been demonstrated in recent years against different fungal species (27–32). Our previous report showed that this drug also has an important in vitro activity against C. albicans biofilms formed in the lumen of central venous catheters (33). Here, we expanded these analyses to the examination of the effects of miltefosine on a panel of C. albicans clinical isolates, many of them exhibiting frank fluconazole resistance, and the examination of its potential to elicit resistance in C. albicans. Our in vivo results also indicate its promising topical activity for the treatment of superficial candidiasis.
Under planktonic conditions, miltefosine was highly active against all clinical isolates tested, with MIC values between 0.25 and 2 μg/ml. Interestingly, similar concentrations of miltefosine were able to inhibit 90% of biofilm formation (BMIC90 ranging from 2 to 4 μg/ml). This may happen because fungal cells promptly incorporate miltefosine and mitochondrial damage begins even before biofilm formation starts, impairing biofilm formation with concentrations similar to those that kill planktonic cells. The quick incorporation of miltefosine by yeast cells was demonstrated by Zuo and coworkers (43) using 14C-labeled miltefosine, and in Saccharomyces cerevisiae, mitochondrial damage and disruption of its membrane potential were observed after 1 h of treatment with 2 μg/ml of miltefosine (43). Additionally, our data show that even when a highly dense preformed biofilm is challenged, miltefosine effectively inhibits 90% of cell metabolic activity with concentrations only 4 to 16 times higher than the mean planktonic MIC (2 μg/ml) (Table 3). Existing literature shows that, in vitro, all azoles, including the reference fluconazole and the newer molecule voriconazole, have a BMIC90 for preformed biofilms higher than 1,000 and 256 μg/ml, respectively. Also, the amphotericin B inhibitory concentration of 50% for biofilms is 8 to 32 times higher than its planktonic MIC (12). In this scenario, miltefosine demonstrated a promising activity against C. albicans biofilms.
For this report, a set of 12 C. albicans fluconazole-resistant strains, obtained from HIV-positive patients with OPC (35), was selected. The development of “acquired resistance” to fluconazole by C. albicans is a fairly common process and most often is the result of alterations in the target enzyme (C14-α-lanosterol demethylase) or the increase in drug efflux pumps on the cell surface. The two main changes observed in the target enzyme are overexpression of the enzyme itself and point mutations in the gene that encodes the same (ERG11). Activation of drug efflux pumps in the fungal cell membrane reduces azole accumulation inside the cells, preventing their operation. This mechanism is mediated by two types of multidrug efflux transporters, belonging to the major facilitator superfamily (MFS), encoded by multiple drug resistance genes (such as C. albicans MDR1 [CaMDR1]), and those belonging to the superfamily of ABC transporters, encoded by genes CDR1 and CDR2. The CDR overexpression can confer resistance to multiple azoles, while upregulation of CaMDR1 alone leads to exclusive resistance to fluconazole (35). Overexpression of CDR1, CDR2, and MDR1 has been observed since the early stages of development of C. albicans biofilms (44, 45) and is one of the factors that contribute to the intrinsic resistance of biofilms to fluconazole. Here, it was shown that clinical isolates resistant to fluconazole by overexpression of CDR and/or MDR pumps or by point mutations in the gene ERG11 are susceptible to miltefosine. Also, combinatory assays of miltefosine and the efflux pump inhibitor FK506 on both planktonic and biofilm forms did not lead to increases in the antifungal activity of this drug (data nor shown). These results corroborate the hypothesis of independence between the miltefosine mechanism of action and the presence of efflux pumps and highlight the inhibitory activity of miltefosine against isolates with reduced susceptibility to standard drugs. Additionally, the susceptibility of strains with Erg11p mutations places miltefosine as a potential option against resistant strains exhibiting this resistance mechanism.
Our serial passage experiments clearly indicate that repeated exposure to miltefosine is unlikely to elicit the emergence of resistance. Recently, Biswas and coworkers showed that S. cerevisiae mutants overexpressing the gene HXT13 developed resistance to miltefosine through Hxt13p the protein, a hexose transporter which also appears to function as an efflux pump belonging to the MFS family (same as CaMDR1) (46). Miltefosine resistance development has been observed during treatment of visceral leishmaniasis, which has been attributed to the reduction of drug accumulation in the parasite resulting from overexpression of ABC transporter LtrMDR1 and to changes in membrane sterol composition, affecting the initial interaction and internalization of the drug (25, 26). In order to gain further insight into the in vitro activity of miltefosine under both planktonic and biofilm growth conditions, including development of resistance, we evaluated the susceptibility to miltefosine of a series of C. albicans transcription factor mutants (36). Our results indicate that mutant strains resulting in inhibition of expression of CDR1 and/or CDR2 genes remained susceptible to miltefosine under both planktonic and biofilm conditions (Table 4). Moreover, C. albicans mutants lacking the MDR1 regulator MRR1 (Δmrr1) displayed planktonic and biofilm susceptibility against miltefosine similar to that of the parental strain (Table 4).
In humans, miltefosine has an extended half-life (∼150 h) and a low therapeutic index. Clinically, this means that, even after the completion of the treatment, patients are exposed to subtherapeutic concentrations of the drug for a few weeks, a characteristic that could stimulate resistance (26). Seifert et al. (47) described the in vitro development of resistance in Leishmania donovani strains by serial passages in the presence of miltefosine. Using the in vitro model of tolerance induction by serial passage (40), we demonstrated that, in C. albicans, continuous in vitro stimulation with subtherapeutic concentrations of miltefosine does not induce the development of in vitro resistance. Using the same serial passage stimulation, we also showed that exposure to miltefosine does not affect the susceptibility of C. albicans to fluconazole and, fortunately, that previous exposure to fluconazole does not alter the susceptibility of C. albicans to miltefosine.
The major concerns in the use of miltefosine are related to its effect on the mucosa of the gastrointestinal tract and its teratogenicity, as shown in preclinical animal studies (25). These gastrointestinal side effects are probably related to oral ingestion of the drug and the detergent properties of miltefosine, which irritates the gastrointestinal mucosa directly, causing vomiting and diarrhea. In general, miltefosine has a good oral bioavailability (82 to 95%) and is distributed to all tissues with accumulation mainly in liver, lungs, kidneys, and spleen (25), and its cytotoxicity is not greater than that of the current gold standard drug, amphotericin B (29). The in vivo efficacy of oral treatment with miltefosine was initially demonstrated by Widmer et al. (28) using models of disseminated cryptococcosis in mice, where doses of 3.6 and 7.2 mg/kg/day increased survival of animals and reduced the fungal load in the brain and lungs. However, Wiederhold et al. found that, for cryptococcal meningitis and disseminated cryptococcosis models, doses up to 45 mg/kg/day of miltefosine did not improve the survival or reduce the infectious process (48). Similarly, intraperitoneal treatment with doses up to 10 mg/kg/day of miltefosine was not effective in a murine model of disseminated candidiasis (49). These negative results may be associated with the high binding of miltefosine to plasma proteins (up to 95%) (25), reducing their systemic activity and its penetration across the blood-brain barrier.
Thus, we decided to determine the effectiveness of a topical treatment with miltefosine in a murine model of OPC, which is associated with a biofilm etiology. Usually, the clinical response to fluconazole in patients with OPC is satisfactory, but relapses or reinfections frequently occur, probably because of an incomplete eradication of yeast cells in treated patients that may result from biofilm persister cells. Consequently, many AIDS patients receive fluconazole for long periods of time, for several cycles, or even as prophylaxis, favoring the development of resistance (50). A preliminary in vivo study was conducted to evaluate if the topical treatment with miltefosine could reduce the development of oral candidiasis. Our results confirmed that topical application of 50 mg/kg (2 mg/ml) of miltefosine twice a day reduced the development of oral candidiasis in immunocompromised mice (Fig. 2). Miltefosine treatment reduced the colonized area, biofilm formation in the tongue, and tissue damage. These results are relevant from a clinical point of view as miltefosine topical formulations are commercially available and may represent a new therapeutic option, especially for those recurrent cases recalcitrant to fluconazole treatment. The development of a new drug is a long-lasting, laborious, and expensive process. Defining a new application for a commercially available product cuts out much time in this process.
In conclusion, this work confirmed that low concentrations of miltefosine are efficient to inhibit C. albicans biofilm formation in vitro and that preformed biofilms are also susceptible to this drug. Its efficacy against azole-resistant clinical isolates and its reduced potential to foster development of resistance highlight the importance of expanding studies upon its antifungal activities. Finally, the demonstration of miltefosine topical activity in vivo in the murine model of oropharyngeal candidiasis corroborates the potential of this molecule as a promising antifungal candidate for the treatment of superficial Candida infections.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—Brazil) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ—Brazil). T.V.M.V. was a fellow of the PDSE program supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes/Brasil) (Prot. no. 14165/13-9). Biofilm-related work in the J.L.L.-R. laboratory is supported by PHS grants numbered 1R01DE023510 and 1R01AI119554 from the National Institute of Dental and Craniofacial Research and the National Institute of Allergy and Infectious Diseases, respectively. Additional support was provided by the Army Research Office of the Department of Defense under contract no. W911NF-11-1-0136 (to J.L.L.-R.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, and the content is solely the responsibility of the authors.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01890-15.
REFERENCES
- 1.Kojic EM, Darouiche RO. 2004. Candida infections of medical devices. Clin Microbiol Rev 17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarnowski R, Westler WM, Lacmbouh GA, Marita JM, Bothe JR, Bernhardt J, Lounes-Hadj AS, Fontaine J, Sanchez H, Hatfield RD, Ntambi JM, Nett JE, Mitchell AP, Andes DR. 2014. Novel entries in a fungal biofilm matrix encyclopedia. mBio 5:e01333-14. doi: 10.1128/mBio.01333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Köhler JR, Kadosh D, Lopez-Ribot JL. 2010. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog 6:e1000828. doi: 10.1371/journal.ppat.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crump J, Collignon P. 2000. Intravascular catheter-associated infections. Eur J Clin Microbiol Infect Dis 19:1–8. doi: 10.1007/s100960050001. [DOI] [PubMed] [Google Scholar]
- 5.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 6.Nucci M, Queiroz-Telles F, Alvarado-Matute T, Tiraboschi IN, Cortes J, Zurita J, Guzman-Blanco M, Santolaya ME, Thompson L, Sifuentes-Osornio J, Echevarria JI, Colombo AL. 2013. Epidemiology of candidemia in Latin America: a laboratory-based survey. PLoS One 8:e59373. doi: 10.1371/journal.pone.0059373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen MH, Peacock JEJ, Tanner DC, Morris AJ, Nguyen ML, Snydman DR, Wagener MM, Yu VL. 1995. Therapeutic approaches in patients with candidemia: evaluation in a multicenter, prospective, observational study. Arch Intern Med 155:2429–2435. [PubMed] [Google Scholar]
- 8.Samaranayake LP. 1992. Oral mycoses in HIV infection. Oral Surg Oral Med Oral Pathol 73:171–180. doi: 10.1016/0030-4220(92)90191-R. [DOI] [PubMed] [Google Scholar]
- 9.Samaranayake L, Holmstrup P. 1989. Oral candidiasis and human immunodeficiency virus infection. J Oral Pathol Med 18:554–564. doi: 10.1111/j.1600-0714.1989.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 10.Ramage G, Rajendran R, Sherry L, Williams C. 2012. Fungal biofilm resistance. Int J Microbiol 2012:1–14. doi: 10.1155/2012/528521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, Ghannoum MA. 2001. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res 80:903–908. doi: 10.1177/00220345010800031101. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 46:1773–1780. doi: 10.1128/AAC.46.6.1773-1780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiori B, Posteraro B, Torelli R, Tumbarello M, Perlin DS, Fadda G, Sanguinetti M. 2011. In vitro activities of anidulafungin and other antifungal agents against biofilms formed by clinical isolates of different Candida and Aspergillus species. Antimicrob Agents Chemother 55:3031–3035. doi: 10.1128/AAC.01569-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez LR, Casadevall A. 2006. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother 50:1021–1033. doi: 10.1128/AAC.50.3.1021-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez LR, Casadevall A. 2006. Cryptococcus neoformans cells in biofilms are less susceptible than planktonic cells to antimicrobial molecules produced by the innate immune system. Infect Immun 74:6118–6123. doi: 10.1128/IAI.00995-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mowat E, Lang S, Williams C, McCulloch E, Jones B, Ramage G. 2008. Phase-dependent antifungal activity against Aspergillus fumigatus developing multicellular filamentous biofilms. J Antimicrob Chemother 62:1281–1284. doi: 10.1093/jac/dkn402. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee PK, Chandra J, Yu C, Sun Y, Pearlman E, Ghannoum MA. 2012. Characterization of fusarium keratitis outbreak isolates: contribution of biofilms to antimicrobial resistance and pathogenesis. Invest Ophthalmol Vis Sci 53:4450–4457. doi: 10.1167/iovs.12-9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imamura Y, Chandra J, Mukherjee PK, Lattif AA, Szczotka-Flynn LB, Pearlman E, Lass JH, O'Donnell K, Ghannoum MA. 2008. Fusarium and Candida albicans biofilms on soft contact lenses: model development, influence of lens type, and susceptibility to lens care solutions. Antimicrob Agents Chemother 52:171–182. doi: 10.1128/AAC.00387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Sun X, Wang Z, Zhang Y, Hou W. 2012. Keratitis-associated fungi form biofilms with reduced antifungal drug susceptibility. Invest Ophthalmol Vis Sci 53:7774–7778. doi: 10.1167/iovs.12-10810. [DOI] [PubMed] [Google Scholar]
- 20.Bachmann SP, VandeWalle K, Ramage G, Patterson TF, Wickes BL, Graybill JR, Lopez-Ribot J. 2002. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob Agents Chemother 46:3591–3596. doi: 10.1128/AAC.46.11.3591-3596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cocuaud C, Rodier M-H, Daniault G, Imbert C. 2005. Anti-metabolic activity of caspofungin against Candida albicans and Candida parapsilosis biofilms. J Antimicrob Chemother 56:507–512. doi: 10.1093/jac/dki269. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira JAG, Carr JH, Starling CEF, de Resende MA, Donlan RM. 2009. Biofilm formation and effect of caspofungin on biofilm structure of Candida species bloodstream isolates. Antimicrob Agents Chemother 53:4377–4384. doi: 10.1128/AAC.00316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montejo M. 2011. Epidemiology of invasive fungal infection in solid organ transplant. Rev Iberoam Micol 28:120–123. doi: 10.1016/j.riam.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Sardi JDCO, Pitangui NDS, Rodríguez-Arellanes G, Taylor ML, Fusco-Almeida AM, Mendes-Giannini MJS. 2014. Highlights in pathogenic fungal biofilms. Rev Iberoam Micol 31:22–29. doi: 10.1016/j.riam.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Dorlo TPC, Balasegaram M, Beijnen JH, De Vries PJ. 2012. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother 67:2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- 26.Maltezou HC. 2010. Drug resistance in visceral leishmaniasis. J Biomed Biotechnol 2010:617521. doi: 10.1155/2010/617521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong Z, Widmer F, Sorrell TC, Guse Z, Jolliffe KA, Halliday C, Lee OC, Kong F, Wright LC, Chen SC. 2007. In vitro activities of miltefosine and two novel antifungal biscationic salts against a panel of 77 dermatophytes. Antimicrob Agents Chemother 51:2219–2222. doi: 10.1128/AAC.01382-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widmer F, Wright LC, Obando D, Handke R, Ganendren R, Ellis DH, Sorrell TC. 2006. Hexadecylphosphocholine (miltefosine) has broad-spectrum fungicidal activity and is efficacious in a mouse model of cryptococcosis. Antimicrob Agents Chemother 50:414–421. doi: 10.1128/AAC.50.2.414-421.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borba-Santos LP, Gagini T, Ishida K, de Souza W, Rozental S. 2015. Miltefosine is active against Sporothrix brasiliensis isolates with in vitro low susceptibility to amphotericin B or itraconazole. J Med Microbiol 64:415–422. doi: 10.1099/jmm.0.000041. [DOI] [PubMed] [Google Scholar]
- 30.Biswas C, Sorrell TC, Djordjevic JT, Zuo X, Jolliffe KA, Chen SC. 2013. In vitro activity of miltefosine as a single agent and in combination with voriconazole or posaconazole against uncommon filamentous fungal pathogens. J Antimicrob Chemother 68:2842–2846. doi: 10.1093/jac/dkt282. [DOI] [PubMed] [Google Scholar]
- 31.Imbert S, Palous M, Meyer I, Dannaoui E, Mazier D, Datry A, Fekkar A. 2014. In vitro combination of voriconazole and miltefosine against clinically relevant molds. Antimicrob Agents Chemother 58:6996–6998. doi: 10.1128/AAC.03212-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brilhante RSN, Malaquias ADM, Caetano EP, Castelo-Branco DDSCM, Lima RACD, Marques FJDF, Silva NF, Alencar LPD, Monteiro AJ, Camargo ZPD, Bandeira TDJPG, Rodrigues AM, Cordeiro RDA, Moreira JLB, Sidrim JJC, Rocha MFG. 2014. In vitro inhibitory effect of miltefosine against strains of Histoplasma capsulatum var. capsulatum and Sporothrix spp. Med Mycol 52:320–325. doi: 10.1093/mmy/myt027. [DOI] [PubMed] [Google Scholar]
- 33.Vila TVM, Ishida K, de Souza W, Prousis K, Calogeropoulou T, Rozental S. 2013. Effect of alkylphospholipids on Candida albicans biofilm formation and maturation. J Antimicrob Chemother 68:113–125. doi: 10.1093/jac/dks353. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Brown N, Chau AS, López-Ribot JL, Ruesga MT, Quindos G, Mendrick CA, Hare RS, Loebenberg D, DiDomenico B, McNicholas PM. 2004. Changes in susceptibility to posaconazole in clinical isolates of Candida albicans. J Antimicrob Chemother 53:74–80. [DOI] [PubMed] [Google Scholar]
- 35.Perea S, López-Ribot JL, Kirkpatrick WR, McAtee RK, Santillán RA, Martínez M, Calabrese D, Sanglard D, Patterson TF. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 45:2676–2684. doi: 10.1128/AAC.45.10.2676-2684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homann OR, Dea J, Noble SM, Johnson AD. 2009. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet 5:e1000783. doi: 10.1371/journal.pgen.1000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillum AM, Tsay EY, Kirsch DR. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 38.Swindell K, Lattif AA, Chandra J, Mukherjee PK, Ghannoum MA. 2009. Parenteral lipid emulsion induces germination of Candida albicans and increases biofilm formation on medical catheter surfaces. J Infect Dis 200:473–480. doi: 10.1086/600106. [DOI] [PubMed] [Google Scholar]
- 39.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A3. CLSI, Wayne, PA. [Google Scholar]
- 40.Calvet HM, Yeaman MR. 1997. Reversible fluconazole resistance in Candida albicans: a potential in vitro model. Antimicrob Agents Chemother 41:535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierce CG, Uppuluri P, Tristan AR, Wormley FL, Mowat E, Ramage G, Lopez-Ribot JL. 2008. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3:1494–1500. doi: 10.1038/nprot.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solis NV, Filler SG. 2012. Mouse model of oropharyngeal candidiasis. Nat Protoc 7:637–642. doi: 10.1038/nprot.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo X, Djordjevic JT, Bijosono Oei J, Desmarini D, Schibeci SD, Jolliffe KA, Sorrell TC. 2011. Miltefosine induces apoptosis-like cell death in yeast via Cox9p in cytochrome c oxidase. Mol Pharmacol 80:476–485. doi: 10.1124/mol.111.072322. [DOI] [PubMed] [Google Scholar]
- 44.Ramage G, Bachmann S, Patterson TF, Wickes BL, López-Ribot JL. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother 49:973–980. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee PK, Chandra J. 2004. Candida biofilm resistance. Drug Resist Updat 7:301–309. doi: 10.1016/j.drup.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Biswas C, Djordjevic JT, Zuo X, Boles E, Jolliffe KA, Sorrell TC, Chen SC. 2013. Functional characterization of the hexose transporter Hxt13p: an efflux pump that mediates resistance to miltefosine in yeast. Fungal Genet Biol 61:23–32. doi: 10.1016/j.fgb.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Seifert K, Matu S, Pérez-Victoria FJ, Castanys S, Gamarro F, Croft SL. 2003. Characterisation of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int J Antimicrob Agents 22:380–387. doi: 10.1016/S0924-8579(03)00125-0. [DOI] [PubMed] [Google Scholar]
- 48.Wiederhold NP, Najvar LK, Bocanegra R, Kirkpatrick WR, Sorrell TC, Patterson TF. 2013. Limited activity of miltefosine in murine models of cryptococcal meningoencephalitis and disseminated cryptococcosis. Antimicrob Agents Chemother 57:745–750. doi: 10.1128/AAC.01624-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ravu RR, Chen YL, Jacob MR, Pan X, Agarwal AK, Khan SI, Heitman J, Clark AM, Li XC. 2013. Synthesis and antifungal activities of miltefosine analogs. Bioorganic Med Chem Lett 23:4828–4831. doi: 10.1016/j.bmcl.2013.06.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanglard D, Kuchler K, Ischer F, Pagani JL, Monod M, Bille J. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother 39:2378–2386. doi: 10.1128/AAC.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.