Abstract
A total of 421 methicillin-resistant Staphylococcus aureus (MRSA) clinical isolates were tested for ceftaroline susceptibility by Etest (bioMérieux). A multidrug resistant phenotype was found in 40.9%, and clonal complex 239 (CC239) was found in 33.5%. Ceftaroline nonsusceptibility (MIC, >1.0 μg/ml) was 16.9% overall. Nonsusceptibility was significantly higher in CC239 (41.1%, 58/141) and in isolates with a multidrug resistant phenotype (35.5%, 61/172) compared with comparators (P < 0.0001). Nonsusceptibility of common multidrug resistant MRSA clones limits the empirical use of ceftaroline for these infections.
TEXT
Ceftaroline, an oxyimino-cephalosporin active against methicillin-resistant Staphylococcus aureus (MRSA) due to enhanced affinity for penicillin binding protein (PBP) 2a, was approved for use in Australia in 2013 for the treatment of complicated skin and soft tissue infections (SSTIs) and community-acquired bacterial pneumonia (CABP), following approvals for the same indications in the United States in 2010 and Europe in 2012. S. aureus MIC breakpoints have been set by the Clinical and Laboratory Standards Institute (CLSI) (1) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (2). Both report a susceptibility MIC of ≤1.0 μg/ml, although they differ in the resistance designation (CLSI, resistant MIC of >4.0 μg/ml; EUCAST, resistant MIC of >1.0 μg/ml). Resistance appears to be associated with decreased PBP2a binding affinity (3–7) and heteroresistance (8). Reports have largely demonstrated minimal resistance, although geographical variation has been noted (4, 7, 9–33) (Table 1). More recently, in hospital-associated MRSA (HA-MRSA) isolates from China, ceftaroline nonsusceptibility was 33.5% (84/251); most (95.2%) belonged to clonal complex (CC) 8, and sequence type (ST) 239-III was the majority (9). Similarly, ceftaroline nonsusceptible MRSA isolates from Eastern Australia were all ST239-III clones (10). A German study also demonstrated increased ceftaroline MIC50 and MIC90 values for isolates of clonal lineages ST228 and ST239 (7).
TABLE 1.
Published reports of ceftaroline susceptibility in MRSA isolates
| Time period | Region | No. isolates | Source | MIC50 | MIC90 | % susceptiblea | Reference |
|---|---|---|---|---|---|---|---|
| 1997–2008 | Australia | 103 | Blood | 0.5 | 1.0 | 100 | 12 |
| 1.0 | 1.0 | 100b | |||||
| 2008 | United States | 2,254 | SSTIc | 1.0 | 1.0 | 94.8 | 13 |
| Europe | 734 | 1.0 | 2.0 | 82.6 | |||
| 2008–2009 | United States | 215 | RESPd | 1.0 | 1.0 | 94.4 | 14 |
| Europe | 60 | 1.0 | 2.0 | 81.7 | |||
| 2008–2010 | United States | 4,453 | Various | 1.0 | 1.0 | 96.1 | 15 |
| 2008–2011 | United States | 9,875 | Various | 0.5 | 1.0 | 97.1 | 16 |
| 2009 | United States | 2,247 | Various | 0.5 | 1.0 | 98.0 | 17 |
| 2009 | Canada | 74 CA-MRSAe | Various | 0.5 | 0.5 | 100 | 18 |
| 151 HA-MRSAf | 1.0 | 1.0 | 100 | ||||
| 2009–2011 | United States | 532 | RESP | 0.5 | 1.0 | 96.2 | 19 |
| 2009–2011 | United States | 1,492 | SSTI | 0.5 | 1.0 | 99.1 | 20 |
| 2009–2013 | United States | 12,514 | Various | 0.5 | 1.0 | 97.6 | 21 |
| 2009–2013 | United States | 2,013 | Blood | 0.5 | 1.0 | 95.4 | 22 |
| 2010 | United States | 1,072 | Various | 0.5 | 1.0 | 98.4 | 23 |
| 2010 | Europe | 331 | SSTI | 1.0 | 2.0 | 88.8 | 24 |
| 2010 | South Africa and Asia Pacific | 211 | SSTI and RESP | 1.0 | 2.0 | 80.6 | 25 |
| 2010–2011g | United States | 2,143 | Various | 0.5 | 1.0 | 96.8 | 26 |
| 2010–2012g | United States | 4,333 | SSTI | 0.5 | 1.0 | 98.9 | 27 |
| 2011 | Latin America | 409h | Various | 1.0 | 2.0 | 61.6 | 28 |
| 2011 | Australia | 713 | Various | 0.75 | 1.0 | 98.2i | 11 |
| 2011 | United States | 2,093 | Various | 0.5 | 1.0 | 98.9 | 29 |
| 2011 | China | 251 HA-MRSA | SSTI | 1.0 | 2.0 | 66.5 | 9 |
| 2012 | Global | 4,324 | Various | 1.0 | 2.0 | 85.2 | 4 |
| 2012 | Germany | 133 | Various | 1.0 | 1.5 | 63.9j | 7 |
| 0.5 | 0.75 | 96.2i | |||||
| 2012–2013 | U.K. | 531 | SSTI | 1.0 | 1.0 | 95.3k | 30 |
| 2012–2013 | France | 67 | SSTI | 0.5 | 1.0 | 97.0 | 31 |
| 2013 | Australia | 100 | Various | 0.5 | 2.0 | 85.0 | 10 |
| 1.0 | 2.0 | 85.0i | |||||
| 2013 | United States | 2,642 | SSTI | 1.0 | 1.0 | 98.8 | 32 |
| 2014 | India | 50 | Various | 0.5 | 1.0 | 96.0i | 33 |
Isolates were tested for susceptibility to ceftaroline by reference broth microdilution methods, as described by CLSI M07-A9, unless otherwise indicated.
Suceptibility testing by MIC evaluator (MICE) strips (Oxoid).
SSTI, skin and soft tissue infection.
RESP, respiratory.
CA-MRSA, community-associated MRSA.
HA-MRSA, health care-associated MRSA.
Study drug was ceftaroline-avibactam.
Includes isolates from Chile, of which 83.9% had a ceftaroline MIC of 2.0 μg/ml.
Suceptibility testing by Etest (bioMérieux).
Broth microdilution methods, as described by EUCAST.
Broth microdilution methods, as described by BSAC (British Society for Antimicrobial Chemotherapy).
Nonduplicate, consecutive clinical MRSA isolates were tested from patients treated at the Alfred Hospital, a tertiary-care metropolitan hospital in Melbourne, Australia, over two time periods: July to December 2010 and August to November 2013. Ceftaroline was not in use clinically before 2014. Isolates from 2010 and 2013 were identified using Vitek 2 and Vitek MS (bioMérieux), respectively. Susceptibility testing was performed using the Vitek 2 Gram-positive susceptibility card (AST-P612), applying EUCAST breakpoints. The multidrug resistant phenotype was defined as resistance to ≥3 non-β-lactam antibiotics, including ciprofloxacin, erythromycin/clindamycin, gentamicin, co-trimoxazole, and tetracycline. All isolates were assessed for ceftaroline and vancomycin susceptibility by Etest (bioMérieux). Isolates from 2010 that had been frozen were thawed and subcultured twice before testing. Isolates from 2013 were collected and tested in real time. Ceftaroline Etests were set up on Mueller-Hinton agar (Becton Dickinson BBL 211438), incubated, and read per manufacturer's guidelines. Ceftaroline nonsusceptibility was defined as a MIC of >1.0 μg/ml. Confirmatory broth microdilution (BMD) was not available. Typing was performed by a high-resolution melting-based method, giving inferred CCs (34).
A total of 421 nonduplicate MRSA isolates were identified for testing. Of the total, 270 isolates were from 2010, and 151 isolates were from 2013. SSTIs accounted for the majority (70.3%) of specimens. Strains with a multidrug-resistant phenotype made up 40.9% of the isolates, although this proportion declined over the two time periods, from 47.0% (127/270) in 2010 to 29.8% (24/151) in 2013. CC239, the dominant HA-MRSA strain found in Australia, and often multidrug resistant (11), was the most commonly identified clonal complex, accounting for 33.5% of all isolates. This proportion also decreased between the two time periods, from 41.5% (112/270) in 2010 to 19.2% (29/151) in 2013. CC22 and CC45 were the next most commonly identified clonal complexes, found in 28.5% and 11.4%, respectively. The proportion of CC22 isolates increased from 26.7% (72/270) in 2010 to 31.8% (48/151) in 2013. Among all MRSA isolates, the vancomycin MIC50 and MIC90 were 1.5 μg/ml. Reduced vancomycin susceptibility (i.e., vancomycin MIC, >1.0 μg/ml) was most common in isolates with a multidrug-resistant phenotype (84.9%, 146/172) and in CC239 isolates (83.7%, 118/141) compared with those that had a non-multidrug-resistant phenotype (32.9%, 82/249) or another CC type (39.3%, 110/280).
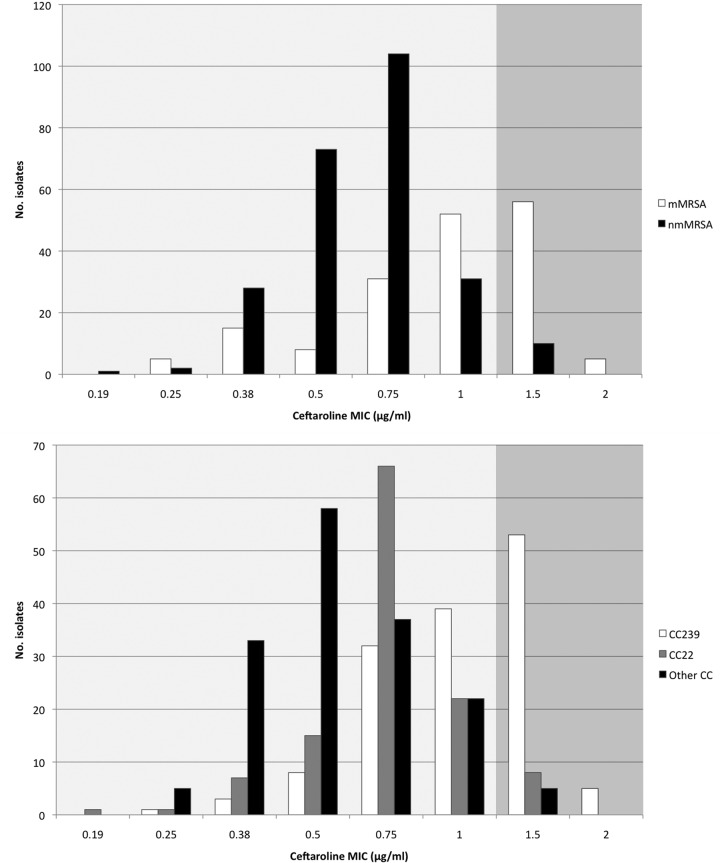
Overall, the ceftaroline MIC50 and MIC90 values were 0.75 μg/ml and 1.5 μg/ml, respectively, with a nonsusceptibility rate of 16.9% (71/421). The majority of ceftaroline-nonsusceptible isolates had a multidrug-resistant phenotype (85.9%, 61/71), were typed as CC239 (81.7%, 58/71) and had a vancomycin MIC of >1.0 μg/ml (76.1%, 54/71). All ceftaroline-nonsusceptible isolates had MICs that were either 1.5 μg/ml or 2.0 μg/ml, representing the upper tail of a unimodal distribution, and were characterized as resistant by EUCAST criteria and intermediate by CLSI (Fig. 1). A two-sample test of proportions demonstrated that ceftaroline nonsusceptibility was significantly higher in CC239 MRSA isolates than in non-CC239 isolates (58/141 [41.1%] versus 13/280 [4.6%]; P < 0.0001). Similarly, ceftaroline nonsusceptibility in multidrug-resistant MRSA isolates was significantly higher than in non-multidrug-resistant isolates (61/172 [35.5%] versus 10/249 [4.0%]; P < 0.0001).
FIG 1.
Ceftaroline MIC distributions in relation to antibiotic susceptibility phenotype and clonal complex (CC). Dark shaded area represents ceftaroline nonsusceptible MIC range. CC22, clonal complex 22 strain; CC239, clonal complex 239 strain; mMRSA, multidrug-resistant MRSA phenotype; nmMRSA, non-multidrug-resistant MRSA phenotype.
MIC gradient strip testing and disc diffusion remain the most common ways for clinical laboratories to test ceftaroline susceptibility in the absence of automated methods and with the impracticalities of BMD (35). Ceftaroline gradient strip tests have been reported to underestimate (7), overestimate (12), and demonstrate reasonably good MIC correlation with BMD (10). Livermore et al. reported that discrimination between MICs of 1.0 and 2.0 μg/ml by Etest, compared with agar dilution, was poor (36). Despite this, and despite differences in clinical breakpoints set by CLSI (1) and EUCAST (2), an Etest ceftaroline MIC of 1.0 μg/ml remains an important and conservative breakpoint for the laboratory to report susceptibility.
The pharmacokinetic and pharmacodynamic index that best correlates with ceftaroline efficacy is the percentage of time during the dosing interval that free-drug concentrations remain above the MIC of the infecting organism (f%T>MIC). In a Staphylococcus aureus neutropenic murine thigh model, the mean ± standard deviation f%T>MIC required for bacterial stasis, 1-log10 kill, and 2-log10 kill was 26 ± 8, 33 ± 9, and 45 ± 13, respectively. Less killing was observed with more widely spaced dosing intervals (12 h and 24 h) (37). Suggestions that ceftaroline susceptibility test interpretive criteria could be as high as a MIC of ≤2.0 μg/ml (4, 38) are based on achieving an f%T>MIC target of 26%, reflecting bacterial stasis, and would be considered the minimum value required when uncomplicated infections in patients with an intact immune system are being treated (39). At a MIC of 2.0 μg/ml, standard dosing of ceftaroline with 600 mg twice daily demonstrated lower target attainments, aiming for 1- or 2-log10 kill (74.5% and 28.8%, respectively, in simulated patients with normal renal function, applying f%T>MIC of 36% and 51% for a 1- and 2-log10 kill, respectively) (38). Monte Carlo simulation with ceftaroline administered 600 mg every 8 hours as a 2-h infusion in patients with normal renal function demonstrated a higher probability of treatment success (from 72% in CABP and 79% in SSTI to ≥99% in both), and the every-8-hour schedule may represent a better option than standard dosing, especially when targeting MRSA (40). In practice, off-label dosing has been commonly reported for serious infections (e.g., bacteremia, endocarditis, MRSA pneumonia) with good clinical outcomes, although such dosing risks higher toxicity rates (41).
Ceftaroline resistance among MRSA isolates from Melbourne, Australia, especially the isolates with a multidrug-resistant phenotype and the CC239 strain, is significant and would preclude its empirical use prior to dedicated susceptibility testing. Empirical usage in other settings should be determined at an individual institutional level. Ceftaroline nonsusceptibility has been identified prior to the clinical use of the drug and, therefore, does not represent the emergence of resistance. Despite the limitations of this study, which might be biased toward hospital-specific clones, or which might represent the known laboratory issues of the gradient strip susceptibility test with the lack of confirmatory BMD testing, our findings linking ceftaroline resistance to a multidrug resistance phenotype and to CC239 are supported by other recently published studies (4, 9, 10). Our results may also represent changes in resistance over time, highlighting the importance of monitoring MRSA molecular epidemiology in order to understand the underlying genetic background to nonsusceptibility and the dynamic relationship that is seen between the pathogen and the drug. In regions where CC239 contributes to a large proportion of MRSA, caution should be exercised in using ceftaroline for suspected or known MRSA infections. Although the association of CC239 and the multidrug-resistant phenotype are inherently linked, the clinical appreciation at the time of culture result, that a multidrug-resistant MRSA isolate has a higher background rate of ceftaroline nonsusceptibility, is an important one. Further studies are required on the clinical efficacy and safety of using more intensive ceftaroline dosing regimens in such settings.
ACKNOWLEDGMENTS
We thank the Microbiology Department, Alfred Hospital, for laboratory assistance.
Funding was received from Alfred Research Trusts Small Projects Grants.
S.Y.C.T. is an Australian National Health and Medical Research Council Career Development Fellow (1065736).
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 2.European Committee On Antimicrobial Susceptibility Testing. 2015. Breakpoint tables for interpretation of MICs and zone diameters, version 5.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_5.0_Breakpoint_Table_01.pdf.
- 3.Mendes RE, Tsakris A, Sader HS, Jones RN, Biek D, McGhee P, Appelbaum PC, Kosowska-Shick K. 2012. Characterization of methicillin-resistant Staphylococcus aureus displaying increased MICs of ceftaroline. J Antimicrob Chemother 67:1321–1324. doi: 10.1093/jac/dks069. [DOI] [PubMed] [Google Scholar]
- 4.Lahiri SD, McLaughlin RE, Whiteaker JD, Ambler JE, Alm RA. 2015. Molecular characterization of MRSA isolates bracketing the current EUCAST ceftaroline-susceptible breakpoint for Staphylococcus aureus: the role of PBP2a in the activity of ceftaroline. J Antimicrob Chemother 70: 2488–2498. doi: 10.1093/jac/dkv131. [DOI] [PubMed] [Google Scholar]
- 5.Kelley WL, Jousselin A, Barras C, Lelong E, Renzoni A. 2015. Missense mutations in PBP2A affecting ceftaroline susceptibility detected in epidemic hospital-acquired methicillin-resistant Staphylococcus aureus clonotypes ST228 and ST247 in Western Switzerland archived since 1998. Antimicrob Agents Chemother 59:1922–1930. doi: 10.1128/AAC.04068-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan LC, Basuino L, Diep B, Hamilton S, Chatterjee SS, Chambers HF. 2015. Ceftobiprole- and ceftaroline-resistant methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 59:2960–2963. doi: 10.1128/AAC.05004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strommenger B, Layer F, Klare I, Werner G. 2015. Pre-use susceptibility to ceftaroline in clinical Staphylococcus aureus isolates from Germany: is there a non-susceptible pool to be selected? PLoS One 10:e0125864. doi: 10.1371/journal.pone.0125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saravolatz SN, Martin H, Pawlak J, Johnson LB, Saravolatz LD. 2014. Ceftaroline-heteroresistant Staphylococcus aureus. Antimicrob Agents Chemother 58:3133–3136. doi: 10.1128/AAC.02685-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Xiao M, Kong F, O'Sullivan MV, Mao LL, Zhao HR, Zhao Y, Wang H, Xu YC. 2015. A multicentre study of meticillin-resistant Staphylococcus aureus in acute bacterial skin and skin-structure infections in China: susceptibility to ceftaroline and molecular epidemiology. Int J Antimicrob Agents 45:347–350. doi: 10.1016/j.ijantimicag.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Mubarak N, Sandaradura I, Isaia L, O'Sullivan M, Zhou F, Marriott D, Iredell JR, Harkness J, Andresen D. 2015. Nonsusceptibility to ceftaroline in healthcare-associated multiresistant MRSA in eastern Australia. J Antimicrob Chemother 70:2413–2414. doi: 10.1093/jac/dkv124. [DOI] [PubMed] [Google Scholar]
- 11.Coombs G, Pearson J, Nimmo G, Collignon P, Bell J, McLaws ML, Christiansen K, Turnidge J, Australian Group on Antimicrobial Resistance . 2013. Antimicrobial susceptibility of Staphylococcus aureus and molecular epidemiology of methicillin-resistant S. aureus isolated from Australian hospital inpatients: report from the Australian Group on Antimicrobial Resistance 2011 Staphylococcus aureus Surveillance Programme. J Glob Antimicrob Resist 1:149–156. doi: 10.1016/j.jgar.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Espedido BA, Jensen SO, van Hal SJ. 2015. Ceftaroline fosamil salvage therapy: an option for reduced-vancomycin-susceptible MRSA bacteraemia. J Antimicrob Chemother 70:797–801. doi: 10.1093/jac/dku455. [DOI] [PubMed] [Google Scholar]
- 13.Jones RN, Mendes RE, Sader HS. 2010. Ceftaroline activity against pathogens associated with complicated skin and skin structure infections: results from an international surveillance study. J Antimicrob Chemother 65(Suppl 4):iv17–iv31. doi: 10.1093/jac/dkq252. [DOI] [PubMed] [Google Scholar]
- 14.Jones RN, Farrell DJ, Mendes RE, Sader HS. 2011. Comparative ceftaroline activity tested against pathogens associated with community-acquired pneumonia: results from an international surveillance study. J Antimicrob Chemother 66(Suppl 3):iii69–iii80. doi: 10.1093/jac/dkr101. [DOI] [PubMed] [Google Scholar]
- 15.Farrell DJ, Castanheira M, Mendes RE, Sader HS, Jones RN. 2012. In vitro activity of ceftaroline against multidrug-resistant Staphylococcus aureus and Streptococcus pneumoniae: a review of published studies and the AWARE Surveillance Program (2008 to 2010). Clin Infect Dis 55(Suppl 3):S206–S214. doi: 10.1093/cid/cis563. [DOI] [PubMed] [Google Scholar]
- 16.Sader HS, Flamm RK, Jones RN. 2013. Antimicrobial activity of ceftaroline tested against staphylococci with reduced susceptibility to linezolid, daptomycin, or vancomycin from U.S. hospitals, 2008 to 2011. Antimicrob Agents Chemother 57:3178–3181. doi: 10.1128/AAC.00484-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Costello AJ, Kroeger JS, Biek D, Critchley IA, Diekema DJ, Doern GV. 2011. Activity of ceftaroline and epidemiologic trends in Staphylococcus aureus isolates collected from 43 medical centers in the United States in 2009. Antimicrob Agents Chemother 55:4154–4160. doi: 10.1128/AAC.00315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlowsky JA, Adam HJ, Decorby MR, Lagace-Wiens PR, Hoban DJ, Zhanel GG. 2011. In vitro activity of ceftaroline against gram-positive and gram-negative pathogens isolated from patients in Canadian hospitals in 2009. Antimicrob Agents Chemother 55:2837–2846. doi: 10.1128/AAC.01787-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flamm RK, Sader HS, Jones RN. 2014. Ceftaroline activity against organisms isolated from respiratory tract infections in USA hospitals: results from the AWARE Program, 2009 to 2011. Diagn Microbiol Infect Dis 78:437–442. doi: 10.1016/j.diagmicrobio.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller MA, Flamm RK, Sader HS, Jones RN. 2014. Ceftaroline activity against bacterial organisms isolated from acute bacterial skin and skin structure infections in United States medical centers (2009 to 2011). Diagn Microbiol Infect Dis 78:422–428. doi: 10.1016/j.diagmicrobio.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Sader HS, Flamm RK, Streit JM, Farrell DJ, Jones RN. 2015. Ceftaroline activity against bacterial pathogens frequently isolated in U.S. medical centers: results from five years of the AWARE surveillance program. Antimicrob Agents Chemother 59:2458–2461. doi: 10.1128/AAC.04614-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sader HS, Farrell DJ, Flamm RK, Jones RN. 2015. Activity of ceftaroline and comparator agents tested against Staphylococcus aureus from patients with bloodstream infections in US medical centers (2009 to 2013). J Antimicrob Chemother 70:2053–2056. doi: 10.1093/jac/dkv076. [DOI] [PubMed] [Google Scholar]
- 23.Flamm RK, Sader HS, Farrell DJ, Jones RN. 2012. Summary of ceftaroline activity against pathogens in the United States, 2010: report from the Assessing Worldwide Antimicrobial Resistance Evaluation (AWARE) surveillance program. Antimicrob Agents Chemother 56:2933–2940. doi: 10.1128/AAC.00330-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrell DJ, Flamm RK, Sader HS, Jones RN. 2013. Spectrum and potency of ceftaroline tested against leading pathogens causing skin and soft-tissue infections in Europe (2010). Int J Antimicrob Agents 41:337–342. doi: 10.1016/j.ijantimicag.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Sader HS, Flamm RK, Jones RN. 2013. Antimicrobial activity of ceftaroline and comparator agents tested against bacterial isolates causing skin and soft tissue infections and community-acquired respiratory tract infections isolated from the Asia-Pacific region and South Africa (2010). Diagn Microbiol Infect Dis 76:61–68. doi: 10.1016/j.diagmicrobio.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Sader HS, Flamm RK, Jones RN. 2013. Antimicrobial activity of ceftaroline-avibactam tested against clinical isolates collected from U.S. medical centers in 2010 to 2011. Antimicrob Agents Chemother 57:1982–1988. doi: 10.1128/AAC.02436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flamm RK, Farrell DJ, Sader HS, Jones RN. 2014. Antimicrobial activity of ceftaroline combined with avibactam tested against bacterial organisms isolated from acute bacterial skin and skin structure infections in United States medical centers (2010 to 2012). Diagn Microbiol Infect Dis 78:449–456. doi: 10.1016/j.diagmicrobio.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Flamm RK, Sader HS, Jones RN. 2014. Ceftaroline activity tested against contemporary Latin American bacterial pathogens (2011). Braz J Infect Dis 18:187–195. doi: 10.1016/j.bjid.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richter SS, Diekema DJ, Heilmann KP, Dohrn CL, Crispell EK, Riahi F, McDanel JS, Satola SW, Doern GV. 2014. Activities of vancomycin, ceftaroline, and mupirocin against Staphylococcus aureus isolates collected in a 2011 national surveillance study in the United States. Antimicrob Agents Chemother 58:740–745. doi: 10.1128/AAC.01915-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livermore DM, Mushtaq S, Warner M, James D, Kearns A, Woodford N. 2015. Pathogens of skin and skin-structure infections in the United Kingdom and their susceptibility to antibiotics, including ceftaroline. J Antimicrob Chemother 70:2844–2853. doi: 10.1093/jac/dkv179. [DOI] [PubMed] [Google Scholar]
- 31.Leprince C, Desroches M, Emirian A, Coutureau C, Anais L, Fihman V, Soussy CJ, Decousser JW, Premium Study Group . 2015. Distribution and antimicrobial susceptibility of bacteria from adults with community-acquired pneumonia or complicated skin and soft tissue infections in France: the nationwide French PREMIUM study. Diagn Microbiol Infect Dis 83:175–182. doi: 10.1016/j.diagmicrobio.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Sader HS, Farrell DJ, Mendes RE, Flamm RK, Castanheira M, Jones RN. 2015. Antimicrobial activity of ceftaroline tested against bacterial isolates causing respiratory tract and skin and skin structure infections in US medical centers in 2013. Diagn Microbiol Infect Dis 82:78–84. doi: 10.1016/j.diagmicrobio.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Basireddy S, Singh M, Ali S, Kabra V. 2015. In vitro activity of ceftaroline against methicillin-resistant Staphylococcus aureus isolates. Indian J Med Microbiol 33:464–465. doi: 10.4103/0255-0857.158612. [DOI] [PubMed] [Google Scholar]
- 34.Lilliebridge RA, Tong SY, Giffard PM, Holt DC. 2011. The utility of high-resolution melting analysis of SNP nucleated PCR amplicons: an MLST-based Staphylococcus aureus typing scheme. PLoS One 6:e19749. doi: 10.1371/journal.pone.0019749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones RN, Holliday NM, Critchley IA. 2015. Accuracy of the Thermo Fisher Scientific (Sensititre) dry-form broth microdilution MIC product when testing ceftaroline. Diagn Microbiol Infect Dis 81:280–282. doi: 10.1016/j.diagmicrobio.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Livermore DM, Mushtaq S, Warner M, James D, Woodford N. 27 August 2015. Susceptibility testing challenges with ceftaroline, MRSA and a 1 mg/liter breakpoint. J Antimicrob Chemother doi: 10.1093/jac/dkv265. [DOI] [PubMed] [Google Scholar]
- 37.Andes D, Craig WA. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob Agents Chemother 50:1376–1383. doi: 10.1128/AAC.50.4.1376-1383.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Wart SA, Ambrose PG, Rubino CM, Khariton T, Riccobene TA, Friedland HD, Critchley IA, Bhavnani SM. 2014. Pharmacokinetic-pharmacodynamic target attainment analyses to evaluate in vitro susceptibility test interpretive criteria for ceftaroline against Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother 58:885–891. doi: 10.1128/AAC.01680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mouton JW, Brown DF, Apfalter P, Canton R, Giske CG, Ivanova M, MacGowan AP, Rodloff A, Soussy CJ, Steinbakk M, Kahlmeter G. 2012. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin Microbiol Infect 18:E37–E45. doi: 10.1111/j.1469-0691.2011.03752.x. [DOI] [PubMed] [Google Scholar]
- 40.Canut A, Isla A, Rodriguez-Gascon A. 2015. Pharmacokinetic/pharmacodynamic analysis to evaluate ceftaroline fosamil dosing regimens for the treatment of community-acquired bacterial pneumonia and complicated skin and skin-structure infections in patients with normal and impaired renal function. Int J Antimicrob Agents 45:399–405. doi: 10.1016/j.ijantimicag.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Stryjewski ME, Jones RN, Corey GR. 2015. Ceftaroline: clinical and microbiology experience with focus on methicillin-resistant Staphylococcus aureus after regulatory approval in the USA. Diagn Microbiol Infect Dis 81:183–188. doi: 10.1016/j.diagmicrobio.2014.11.016. [DOI] [PubMed] [Google Scholar]