Abstract
Janus kinases (JAK) are intracellular tyrosine kinases that transduce cytokine-mediated signals to the nucleus, promoting gene expression. Cytokines play a major role in microbial sepsis, which is often associated with uncontrolled inflammation leading to death. JAK inhibitors have been used for the treatment of several autoimmune diseases by modulating immune response, but they have never been tested against microbial sepsis. Ruxolitinib is a small-molecule inhibitor of JAK1/2 proteins, which are involved in the downstream signaling pathway of the vast majority of proinflammatory and anti-inflammatory cytokines. We therefore studied the effect of ruxolitinib in a mouse model of sepsis due to Candida albicans. When ruxolitinib therapy (50 mg/kg [of body weight]/day) was started 1 day before infection, the median survival time was reduced by 3 days, the fungal loads in all organs were higher, the inflammation was significantly less, and serum tumor necrosis factor alpha (TNF-α) and interleukin 10 (IL-10) levels and IL-10/TNF-α ratios were higher than in controls. When ruxolitinib therapy (50 to 1.5 mg/kg/day) was started 1 day after infection, an inverted-U relationship was found, with 6.25 mg/kg/day prolonging median survival time by 6 days, resulting in similar fungal loads, less inflammation, and similar cytokine levels but higher IL-10/TNF-α ratios than the controls. The optimal dose of ruxolitinib controlled infection and prolonged survival with less inflammation than in control animals. Administration of JAK inhibitors may be a promising therapeutic adjunct that needs further investigation.
INTRODUCTION
Sepsis is a leading cause of death in critically ill patients in developed countries (1). Candida species cause 10 to 15% of all bloodstream infections and 5% of all septic episodes (2, 3). Approximately one-third of patients with candidemia develop severe sepsis, with significant mortality (>60%) despite antifungal treatment (3). Alternative therapeutic approaches for severe sepsis are urgently needed.
Pathophysiologically, sepsis is considered the systemic inflammatory response syndrome resulting from the immune response of the host against the invading pathogen. Indeed, in many cases, death is directly caused by the uncontrolled and exaggerated inflammation rather than the pathogen itself (4). The effects of various agents, including corticosteroids, antiendotoxin, and anticytokine antibodies, on inflammatory cascade blockage have been investigated in several clinical trials (5). The results from these studies failed to show a clear therapeutic benefit, and in many circumstances inflammatory blockade was harmful. Indeed, a recent meta-analysis of clinical trials showed that in general, high-intensity blocking of inflammation has a negative impact on sepsis outcome. However, in many studies there was a small subgroup of patients who actually benefited from anti-inflammatory intervention (5).
Failure of anti-inflammatory blocking therapy might be attributed to several factors (6). First, slowing of the inflammatory process needs to occur at the right time. Inhibition might not be effective if it does not occur at an early phase of the septic process. In accordance with this hypothesis is the fact that patients admitted to an intensive care unit (ICU) are already in an established septic phase. Second, the intensity of the inhibition may be of crucial importance, since a certain degree of inflammation is required for the effective clearance of the pathogen (6). An absence of fine control and monitoring of the inhibition in a time-specific manner may contribute to the failure of anti-inflammatory trials. Finally, inhibition of a single cytokine may not be effective simply because the function of one cytokine is counterbalanced by one or more of the other mediators of the cytokine storm that occurs during sepsis (7).
The septic process is mediated through the orchestrated expression of various proinflammatory and anti-inflammatory cytokines acting upon binding to specific receptors of innate immune cells. The type I and type II classes are the two major kinds of cytokine receptors (8). The cytoplasmic domain of each receptor binds to members of the Janus family of tyrosine kinases (JAKs), consisting of JAK1, JAK2, JAK3, and Tyk2 protein. Different cytokine receptors bind with different combinations of JAKs, which are activated after cytokine binding. After binding of a cytokine to its cognate receptor, JAK homodimerization occurs and results in phosphorylation of the signal transducer and activator of transcription (STAT) molecules (9). The activated STATs form dimers before migrating to the nucleus, where they regulate gene expression through binding to specific DNA promoter elements. Examples of molecules that use the JAK/STAT signaling pathways are the colony-stimulating factor, prolactin, growth hormone, and a multitude of other cytokines. JAK inhibitors result in suppression of activation and expression of STATs and ultimately of downstream inflammatory genes (10).
Ruxolitinib is a JAK1 and JAK2 small-molecule inhibitor recently approved for the treatment of patients with primary and secondary myelofibrosis (11). Myelofibrosis is caused by the proliferation of an abnormal clone of hematopoietic stem cells which produces an excessive amount of cytokines, leading to bone marrow collagen fibrosis, splenomegaly, and severe anemia. Ruxolitinib offers an excellent opportunity for testing the effect of blocking inflammatory cytokines in the treatment of sepsis because (i) monitoring the extent of cytokine inhibition can be accomplished by administration of incremental doses of ruxolitinib and (ii) ruxolitinib provokes a more global cytokine effect, since JAK1 and JAK2 inactivation results in downstream signaling pathway inhibition of the vast majority of pro- and anti-inflammatory cytokines (12). Ruxolitinib was previously tested in animal models of myeloproliferative neoplasms (13), graft-versus-host disease (14), lymphoblastic leukemia (15), and arthritis (10) with promising results, posing this agent as important immunomodulator with many applications. Therefore, in the present study, we investigated the effect of ruxolitinib on the outcome of a mouse model of severe sepsis due to Candida albicans, measuring its impact on fungal load, cytokines, and inflammation.
(This study has been presented at the 24th European Conference for Clinical Microbiology and infectious Diseases in Barcelona, Spain, May 2014.)
MATERIALS AND METHODS
Isolate.
A clinical strain of Candida albicans ATCC UC820 isolated from the blood of a patient with disseminated candidiasis was used. The strain was stored at −70°C and was subcultured twice on Sabouraud dextrose agar (SDA) plates and incubated at 30°C for 24 h. Yeast suspensions were prepared in saline and adjusted using a Neubauer counting chamber in order to obtain a final suspension of 5 × 107 CFU/ml, which was verified by quantitative cultures.
Animal model of sepsis.
Female CD1 mice 4 to 6 weeks old and weighing 20 to 25 g (Hellenic Pasteur Institute, Athens, Greece) were housed and fed ad libitum. The animals were maintained in accordance with the 2010/66/ΕΕ guidelines of the animal welfare body of the European Commission. All animal studies were approved by the regional Animal Welfare Ethics Committee, Veterinary Department, Prefecture of Athens. After acclimatization for 1 week, mice were infected through the tail vein with 0.1 ml of yeast suspension, which corresponded to 5 × 106 CFU per mouse. In preliminary experiments, this inoculum was found to result in 90% mortality in 10 days and a median survival time of 5 days (16, 17). Previous studies showed that the murine model of hematogenously disseminated candidiasis accurately reflects the septic process observed in clinical cases, enabling the study of the pathophysiological aspect of this disease (16, 17). Sepsis syndrome was manifested by worsening hypotension, tachycardia, hypothermia, metabolic acidosis, profound acidemia, hypoglycemia, and severe renal insufficiency starting 1 day after infection and leading ultimately to death after day 3. Although one isolate was used, the host-microbe interactions are expected to be similar for different isolates. For all the following experiments, survival was monitored daily and moribund mice were sacrificed and considered deceased at the time of sacrifice.
Treatment.
The JAK inhibitor ruxolitinib phosphate (INCB018424, Jakavi; Novartis) was dissolved in 0.5% methylcellulose and serially diluted to obtain all doses. Ruxolitinib was administered daily by oral gavage. The control group consisted of mice treated with placebo consisting of 0.5% methylcellulose.
(i) Initiation time-finding studies.
In order to find the appropriate time for initiating treatment, groups of 10 mice were treated with daily doses of 50 mg/kg of ruxolitinib that started 1 day before (D−1 regimen) or 1 day after (D+1 regimen) the infection and continued for up to 14 days or the death of the mouse. This dose was chosen based on previous studies with mice (13) and for practical reasons related to the highest concentration of ruxolitinib that was dissolved in 0.5% methylcellulose. Because ruxolitinib therapy affects spleen size, spleens were removed after death and weighed for each group. It was previously found that ruxolitinib reduce the spleen size in a mouse model of malignant disease by selective decrease of neoplastic cell burden without loss of normal lymphoid tissue (13).
(ii) Optimal dose-finding studies.
Six 2-fold doses of ruxolitinib (1.5, 3.1, 6.25, 12.5, 25, and 50 mg/kg/day) were studied starting the treatment 1 day after infection (D+1) for 14 days in groups of 10 mice. Survival curves were constructed, and the median survival time which corresponded to 50% survival was determined for each dose. The body weight of animals was also monitored during the experimental period. In order to exclude myelosuppression as a factor contributing to death, blood was drawn through cardiac puncture after euthanasia and white blood cells, platelets, and hemoglobin were measured in treated and control mice.
Fungal burden.
Groups of 5 animals were treated with 50-mg/kg D−1, 50-mg/kg D+1, and 6.25-mg/kg D+1 ruxolitinib dosing regimens or a placebo as in survival studies for 3 days when samples were taken for fungal burden, cytokines, and histology. These doses were chosen because they were associated with the largest reduction, no significant difference, and the largest increase in the median survival time (MST), respectively. Three days of treatment was chosen in order to have a sufficient amount of control animals because after this period control mice started to die; in addition, it is a critical period of treatment, since after that period the survival curves between control and treatment groups start to deviate. After 3 days of treatment, all mice were sacrificed by cervical dislocation after anesthesia and organs (kidneys, spleen, liver, lungs, and brain) were aseptically removed and placed separately in 2 ml of sterile saline. After mechanical homogenization, the homogenates were serially diluted in sterile saline and 100 μl of each dilution was plated on SDA plates, followed by incubation at 30οC for 24 h in order to determine the number of viable colonies of C. albicans (expressed as CFU/g of tissue). The lowest limit of detection was 20 CFU/g.
Cytokines.
After 3 days of treatment with 50 mg/kg (D−1 and D+1 groups) and 6.25 mg/kg (D+1) of ruxolitinib or placebo, cohorts of 5 mice were anesthetized 24 h after the last dose and blood was collected via cardiac puncture before euthanasia. Serum was collected after centrifugation at 4,000 × g for 10 min and stored at −70οC. Levels of gamma interferon (IFN-γ), TNF-α, interleukin 10 (IL-10), IL-4, IL-1β, and IL-6 in serum were measured by using Luminex xMAP technology and the Milliplex Map cytokine/chemokine bead panel (Millipore, Athens, Greece) in accordance with the manufacturer's protocol. The principle of this immunoassay is based on the capture of a specific cytokine to internally color-code microspheres with two fluorescent dyes which are coated with a specific capture antibody. After the capture, a biotinylated detection antibody and streptavidin-phycoerythrin (PE) conjugate (reporter molecule) are introduced, a laser excites the internal and the PE fluorescent dyes of the microspheres, and a digital-signal processor identifies each individual microsphere and quantifies the result of its bioassay based on the fluorescent reporter signals.
Histology.
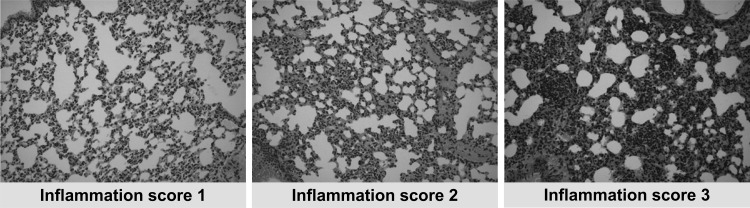
For histopathological examination, cohorts of 5 mice were treated with different ruxolitinib dosing regimens or placebo for 3 days, and after euthanasia, kidneys, lungs, liver, and brain were excised and fixed in 10% neutral buffered formaldehyde. Paraffin-embedded tissues were sectioned and stained with hematoxylin and eosin. The evaluation of inflammation for each individual mouse was based on a three-tiered grading system and was performed by two experienced pathologists in a blinded fashion. Scoring of inflammation was based on the extent of inflammatory cell (neutrophils, lymphocytes, and macrophages) infiltration, as previously described (18). Tissues with no or minimal inflammatory infiltration (less than 20% inflammatory cells) were graded as 1. Grade 3 was given to severely inflamed organs (more than 50% inflammatory cells), and those samples in between were evaluated as grade 2 (20 to 50% inflammatory cells) (Fig. 1). For each individual mouse, the total inflammation score was estimated as the sum of the scores of all examined organs.
FIG 1.
Typical photos from paraffin-embedded tissues with inflammation scores 1, 2, and 3, corresponding to progressive inflammation assessed by an experienced pathologist blinded to the treatments.
Statistics.
Survival analysis was performed by using the Kaplan-Meier test, and comparison between survival curves was performed by using the log rank (Mantel-Cox) test. In addition, the mean and 95% confidence interval of survival curves were reported at the end of treatment. Comparison of fungal burden and cytokine levels among groups of mice was performed with analysis of variance (ANOVA) after log transformation to approximate normal distribution, followed by Bonferonni's multiple-comparison test. Differences among the inflammation scores of the groups were assessed using Kruskal-Wallis test, followed by Dunn's multiple-comparison test. A P value of <0.05 was considered statistically significant.
RESULTS
Survival and treatment initiation time-finding studies.
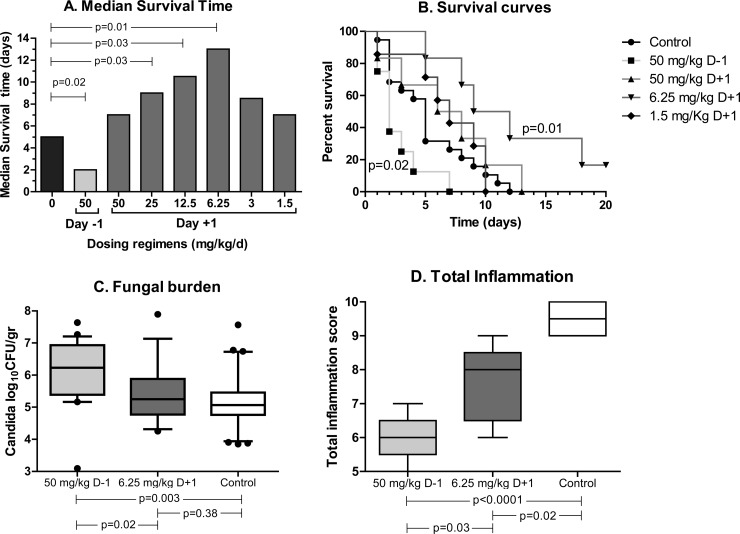
The median survival time (MST) of control mice was 5 days. Ruxolitinib administered at 50 mg/kg D−1 resulted in decreased survival of infected animals compared with that of control animals (P = 0.02) (Fig. 2Α, light gray bar). In contrast, the MST of mice treated with the same dose of ruxolitinib 1 day after infection was not different from that of control animals but was significantly increased compared with that of the mice treated with ruxolitinib in the D−1 regimen (P = 0.03) (Fig. 2Α, first dark gray bar). Based on these results, all subsequent experiments were performed with ruxolitinib administered 1 day after infection. The mean (95% confidence interval) at the end of treatment was 0% for all dosing regimens except for 6.25 mg/kg (33%[28 to 34%]) and 3 mg/kg (16% [16 to 35%]). The weights of spleens were significantly (P < 0.05) lower than in the placebo group only in the mice receiving the 50-mg/kg D+1 regimen. Ruxolitinib administered at doses of ≤50 mg/kg was not associated with any significant myelosuppressive effect. In order to ensure reproducibility, the test with a group of control animals and a group treated with the lowest dose of ruxolitinib was repeated; the difference in median survival times was ±1 day. White blood cells, platelets, and hemoglobin in mice treated with the highest dose were not different from those in control mice (data not shown).
FIG 2.
Median survival times (A), survival curves (B), fungal burdens (C), and total inflammation scores (D) of mice treated with increasing doses of ruxolitinib or placebo started 1 day before (D−1) or after (D+1) infection.
Survival and optimal dose-finding studies.
Incremental dosing of ruxolitinib resulted in significant differences in the MST of infected mice (log rank test chi square = 29.5; degrees of freedom = 8; P = 0.0003), with a significant trend between MST and ruxolitinib doses (log rank test for trend chi square = 9.3; df = 1; P = 0.0022). An inverted-U correlation between ruxolitinib dosing and MST was observed when ruxolitinib was given at 1 day after infection (Fig. 2Α, dark gray bars). Progressive de-escalation of ruxolitinib dosing resulted in increased MST. Intermediate doses of ruxolitinib (25 to 12.5 mg/kg; D+1) resulted in increased MST compared with that of the cohort of mice treated with the highest dose (50 mg/kg; D+1) and placebo (P = 0.03). A further decrease in ruxolitinib dosing was associated with an even further increase of MST, with the largest increase in MST compared to placebo group observed with the dose of 6.25 mg/kg (P = 0.009) (Fig. 2Α). The therapeutic benefit of ruxolitinib was lost after further reduction of the dose. Mice treated with very low doses of ruxolitinib (1.5 to 3 mg/kg) had a decreased survival rate compared with that of mice treated with the dose of 6.25 mg/kg and a survival rate similar to that for control mice (P > 0.16). Among the different dosing regimens, statistically significant differences were found between the 50-mg/kg D−1 and each of the other regimens (P = 0.03 to 0.0001) and between 12.5 and 1.5 mg/kg (P = 0.03). Thus, the 6.25 mg/kg dose of ruxolitinib started 1 day after infection was considered the optimal dose for treatment of septic mice. In contrast, the most detrimental effect on survival was observed with high-dose ruxolitinib (50 mg/kg) administered 1 day before infection. Figure 2B shows the survival curves with the 50 mg/kg dose of ruxolitinib started 1 day before or after infection and the 6.25 and 1.5 mg/kg doses of ruxolitinib started 1 day after infection. The body weight of animals after an increase on day 1 declined on day 3 to 23 (22.5 to 24.5), 22 (22.5 to 24.5), and 20 (20 to 22) g in animals treated with placebo, 6.25 mg/kg D+1 regimen of ruxolitinib, and 50 mg/kg D−1 regimen of ruxolitinib, respectively (data not shown).
Fungal load.
Mice treated with the 50 mg/kg D−1 regimen of ruxolitinib had a significantly higher fungal burden in all organs tested than did control animals (P = 0.0003) and mice treated with the 6.25 mg/kg D+1 regimen of ruxolitinib (P = 0.001) (Fig. 2C). In contrast, the mice treated with the 6.25 mg/kg D+1 regimen of ruxolitinib had a fungal load similar to that of the control mice in all organs examined, including kidneys, liver, spleen, brain, and lungs. Figure 2C shows the differences between the sums of the Candida loads of all organs in each group.
Inflammation.
Among the three groups of animals, the highest total inflammation score was observed in the control group and the lowest in animals treated with the 50 mg/kg D−1 regimen of ruxolitinib, whereas the total inflammation score in organs of animals treated with the 6.25 mg/kg D+1 regimen was between those of the former two groups. The median (range among the animals of each group) total inflammation scores were 9.5 (9 to 10) in control animals, 6 (5 to 7) in mice treated with the 50 mg/kg D−1 regimen of ruxolitinib, and 8 (6 to 9) in mice treated with the 6.25 mg/kg D+1 regimen of ruxolitinib (Fig. 2D). The median inflammation score in the low- and high-dose groups were 16% and 38% lower than in the control group (P < 0.05), respectively.
Serum cytokine levels.
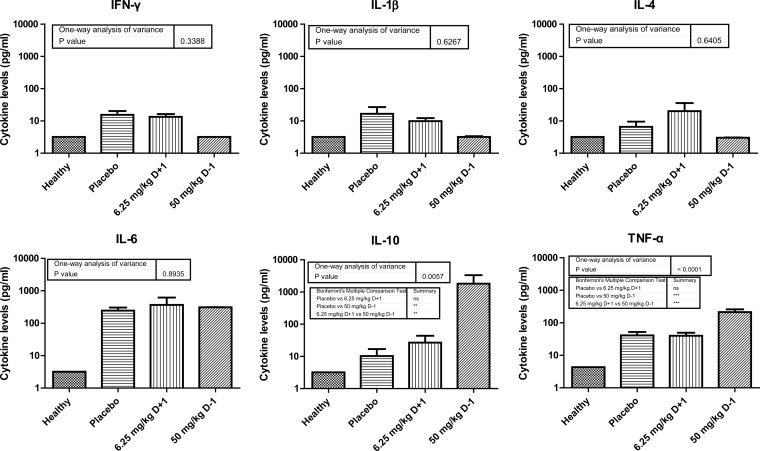
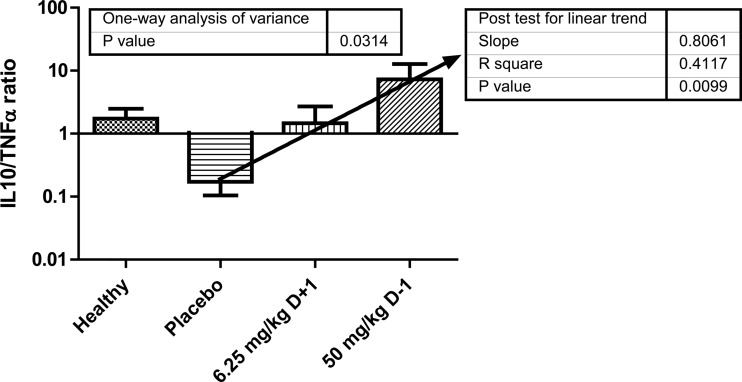
The cytokine levels in healthy and in infected mice treated with placebo or the 6.25 mg/kg D+1 or 50 mg/kg D−1 regimens of ruxolitinib were estimated. Results of one-way analysis of variance for each cytokine are shown in Fig. 3. Significant differences were found for TNF-α (P < 0.0001) and IL-10 (P = 0.0057), with cytokine levels higher in mice treated with the 50 mg/kg D−1 regimen of ruxolitinib than in the control and the 6.25 mg/kg D+1 group. As a measure of proinflammatory and anti-inflammatory cytokine balance, the IL-10/TNF-α ratio was estimated separately for each infected mouse treated with placebo and the 6.25 mg/kg D+1 or 50 mg/kg D−1 regimens of ruxolitinib. The median ratios in these groups were 0.1, 1, and 10, respectively. This difference in IL-10/TNF-α ratio was significant (ANOVA, P = 0.034; posttest for linear trend, P = 0.013), indicating that increased or reduced IL-10 compared to TNF-α levels are detrimental for survival, whereas equal amounts are optimal (Fig. 4).
FIG 3.
Cytokine levels in healthy, infected mice treated with placebo (control group) and infected mice treated with 6.25 mg/kg of ruxolitinib started 1 day after infection or 50 mg/kg of ruxolitinib started 1 day before infection. Results of one-way analysis of variance are shown for each cytokine.
FIG 4.
IL-10/TNF-α ratios in sera of healthy, infected mice treated with placebo (control group) and infected mice treated with 6.25 mg/kg of ruxolitinib started 1 day after infection (D+1) and 50 mg/kg of ruxolitinib started 1 day before infection (D−1). Results of one-way analysis of variance are shown together with the posttest for linear trend.
DISCUSSION
In the present study, we investigated whether a drug that blocks the cytokine signal cascade could work as an inflammation controller during sepsis in an experimental Candida infection, and we found that administration of a high dose of ruxolitinib (50 mg/kg) 1 day before infection resulted in decreased median survival time (MST) compared with that in control mice, associated with an inappropriate downregulation of the inflammatory response and some kind of immunosuppression leading to death due to Candida overgrowth. In contrast, administration of a high dose of ruxolitinib 1 day after infection, which is a more clinically relevant scenario, resulted in an MST comparable to that of the control group. Furthermore, progressively decreasing doses of ruxolitinib resulted in a gradual increase in MST, with an optimal dose of 6.25 mg/kg corresponding to the highest MST. Further decrease in dosing, at less than the optimal, was associated with a decrease in MST to one comparable to that in control mice. Interestingly, mice treated with a high dose of ruxolitinib had significantly less inflammation and higher fungal loads than control animals. On the other hand, mice treated with the optimal dose had less inflammation than controls but similar fungal loads. Ιnflammation helps to control infection, but at the same time, it can have a detrimental effect due to excessive tissue injury. Intense inhibition of the inflammatory process, especially when it occurs before infection, might prevent organ damage but lead to death due to Candida overgrowth, as observed in the present study for the high-dose group treated with the D−1 regimen. Thus, only an appropriate degree of inflammation is necessary for controlling the infection. Indeed, at the optimal dose, ruxolitinib controlled the infection and prolonged survival with less inflammation than in control animals but more inflammation than with high-dose ruxolitinib.
Mice treated with high-dose ruxolitinib had significantly higher serum TNF-α and IL-10 levels than did control animals. The IL-10/TNF-α ratio was estimated in mice from different groups as a measure of proinflammatory and anti-inflammatory cytokine balance. In human studies, the IL-10/TNF-α ratio has shown a good correlation with survival of patients with sepsis (19). High ruxolitinib doses resulted in a 2-log increase of the ratio compared to that in controls. Optimal ruxolitinib dosing produced an IL-10/TNF-α ratio of 1, between the corresponding ratios in controls and in mice treated with the high dose of ruxolitinib, further proof of the concept that a balance between proinflammatory and anti-inflammatory signaling is required for a successful outcome. In accordance with our studies, human studies showed that a IL-10/TNF-α ratio close to 1 at 48 h after admission to ICU was associated with increased survival (19).
In vitro gene expression studies have shown that TNF-α is highly increased at an early phase when human monocytes encounter C. albicans (20). Indeed, inhibition of endogenous TNF-α through anticytokine antibodies is associated with decreased survival in an animal model of Candida sepsis (21). In contrast, a posttranscriptional late modulation of Toll-like receptor 2-derived signals to C. albicans appears to mediate increased production of IL-10 (22). Hypersecretion of an anti-inflammatory cytokine, such as IL-10, might result in severe immunosuppression, which is a major cause of death for patients with sepsis. The late immunosuppressive phase of the septic process should be taken into consideration in any immune-modulating intervention. These data are in agreement with observations from ex vivo studies showing the deleterious effect of increased IL-10 activity on human monocyte function against serum-opsonized Candida albicans (23). Data from mouse models showed that exogenous administration of IL-10 resulted in decreased survival, while the opposite was observed after neutralization of endogenous IL-10 (24). IL-10 inhibits production of proinflammatory cytokines through the JAK/STAT3-signaling pathway. Inhibition of JAKs increases secretion of proinflammatory cytokines due to its ability to block IL-10-mediated feedback inhibition on cytokine transcription. Data from animal studies showed that the initial wave of IL-10 secretion was independent of JAK signaling. However, prolonged inhibition of JAKs resulted in reduced IL-10 secretion at later time points, thus preventing the excessive immunosuppression observed during the late phase of sepsis (25).
Based on these data, we assume that any immune-modulating intervention in sepsis should take into consideration the immunosuppressive phase that may be exacerbated by an uncontrolled inhibition of the early hyperinflammatory phase. Ruxolitinib fulfills the above requirements, since it produces a global modulation in the action of the vast majority of both proinflammatory and anti-inflammatory cytokines through the JAK pathway. Although we used a Candida sepsis model, we believe that sepsis due to other organisms may have the same response to ruxolitinib, as the JAK pathway is universal in the response to various organisms and the creation of septic shock. This, however, has also to be demonstrated in animal models with other pathogens. The optimal ruxolitinib dose may differ for different Candida strains, species, and infection models given the pharmacokinetic variability and severity of infection.
In conclusion, the results of our study suggest that low-dose ruxolitinib administered early after initiation of Candida sepsis is a promising option that needs to be further investigation for different Candida species and animal models in combination with antifungals drugs. In future trials with patients with severe sepsis or septic shock, the effect of immune-modulating agents could be investigated in a time- and dose-specific manner with the aim of finding an optimal way of administration in order to avoid the excessive inflammatory reaction and the resulting organ damage but without compromising pathogen clearance.
ACKNOWLEDGMENT
We thank the Laboratory Animal Facility of Attikon University Hospital.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Vincent J-L, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K. 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 3.Delaloye J, Calandra T. 2014. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 5:161–169. doi: 10.4161/viru.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deitch EA. 1998. Animal models of sepsis and shock: a review and lessons learned. Shock 9:1–11. [DOI] [PubMed] [Google Scholar]
- 5.Zeni F, Freeman B, Natanson C. 1997. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med 25:1095–1100. doi: 10.1097/00003246-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Boomer JS, Green JM, Hotchkiss RS. 2014. The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence 5:45–56. doi: 10.4161/viru.26516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulte W, Bernhagen J, Bucala R. 2013. Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediators Inflamm 2013:165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott MJ, Godshall CJ, Cheadle WG. 2002. Jaks, STATs, cytokines, and sepsis. Clin Diagn Lab Immunol 9:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivashkiv LB, Donlin LT. 2014. Regulation of type I interferon responses. Nat Rev Immunol 14:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarilina A, Xu K, Chan C, Ivashkiv LB. 2012. Regulation of inflammatory responses in tumor necrosis factor-activated and rheumatoid arthritis synovial macrophages by JAK inhibitors. Arthritis Rheum 64:3856–3866. doi: 10.1002/art.37691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesa RA, Gotlib J, Gupta V, Catalano JV, Deininger MW, Shields AL, Miller CB, Silver RT, Talpaz M, Winton EF, Harvey JH, Hare T, Erickson-Viitanen S, Sun W, Sandor V, Levy RS, Kantarjian HM, Verstovsek S. 2013. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 31:1285–1292. doi: 10.1200/JCO.2012.44.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiu H, Nicholson SE. 2012. Biology and significance of the JAK/STAT signalling pathways. Growth Factors 30:88–106. doi: 10.3109/08977194.2012.660936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quintás-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA, Caulder E, Wen X, Li Y, Waeltz P, Rupar M, Burn T, Lo Y, Kelley J, Covington M, Shepard S, Rodgers JD, Haley P, Kantarjian H, Fridman JS, Verstovsek S. 2010. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 115:3109–3117. doi: 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, Verbeek M, Fischer J, Otten V, Schmickl M, Maas-Bauer K, Finke J, Peschel C, Duyster J, Poeck H, Zeiser R, von Bubnoff N. 2014. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood 123:3832–3842. doi: 10.1182/blood-2013-12-543736. [DOI] [PubMed] [Google Scholar]
- 15.Appelmann I, Rillahan CD, de Stanchina E, Carbonetti G, Chen C, Lowe SW, Sherr CJ. 2015. Janus kinase inhibition by ruxolitinib extends dasatinib- and dexamethasone-induced remissions in a mouse model of Ph+ ALL. Blood 125:1444–1451. doi: 10.1182/blood-2014-09-601062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spellberg B, Ibrahim AS, Edwards JE Jr, Filler SG. 2005. Mice with disseminated candidiasis die of progressive sepsis. J Infect Dis 192:336–343. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- 17.Matuschak GM, Lechner AJ. 1997. The yeast to hyphal transition following hematogenous candidiasis induces shock and organ injury independent of circulating tumor necrosis factor-alpha. Crit Care Med 25:111–120. doi: 10.1097/00003246-199701000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Cantley MD, Haynes DR, Marino V, Bartold PM. 2011. Pre-existing periodontitis exacerbates experimental arthritis in a mouse model. J Clin Periodontol 38:532–541. doi: 10.1111/j.1600-051X.2011.01714.x. [DOI] [PubMed] [Google Scholar]
- 19.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. 2000. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 181:176–180. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, Choi EH, Khan J, Roilides E, Francesconi A, Kasai M, Sein T, Schaufele RL, Sakurai K, Son CG, Greer BT, Chanock S, Lyman CA, Walsh TJ. 2005. Expression of genes encoding innate host defense molecules in normal human monocytes in response to Candida albicans. Infect Immun 73:3714–3724. doi: 10.1128/IAI.73.6.3714-3724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louie A, Baltch AL, Smith RP, Franke MA, Ritz WJ, Singh JK, Gordon MA. 1994. Tumor necrosis factor alpha has a protective role in a murine model of systemic candidiasis. Infect Immun 62:2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Netea MG, Sutmuller R, Hermann C, Van der Graaf CAA, Van der Meer JWM, van Krieken JH, Hartung T, Adema G, Kullberg BJ. 2004. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol 172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 23.Roilides E, Anastasiou-Katsiardani A, Dimitriadou-Georgiadou A, Kadiltsoglou I, Tsaparidou S, Panteliadis C, Walsh TJ. 1998. Suppressive effects of interleukin-10 on human mononuclear phagocyte function against Candida albicans and Staphylococcus aureus. J Infect Dis 178:1734–1742. doi: 10.1086/314479. [DOI] [PubMed] [Google Scholar]
- 24.Del Sero G, Mencacci A, Cenci E, d'Ostiani CF, Montagnoli C, Bacci A, Mosci P, Kopf M, Romani L. 1999. Antifungal type 1 responses are upregulated in IL-10-deficient mice. Microbes Infect 1:1169–1180. doi: 10.1016/S1286-4579(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 25.Pattison MJ, Mackenzie KF, Arthur JSC. 2012. Inhibition of JAKs in macrophages increases lipopolysaccharide-induced cytokine production by blocking IL-10-mediated feedback. J Immunol 189:2784–2792. doi: 10.4049/jimmunol.1200310. [DOI] [PMC free article] [PubMed] [Google Scholar]