Abstract
Trimethoprim-sulfamethoxazole (SXT) is a possible alternative for the treatment of community- and hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) due to the susceptibility of most MRSA strains to the drug. However, after long-term treatment with SXT, thymidine-dependent (TD) SXT-resistant small-colony variants (SCVs) emerge. In TD-SCVs, mutations of thymidylate synthase ([TS] thyA) occur. Until now, it has never been systematically investigated that SXT is triggering the induction and/or selection of TD-SCVs. In our study, we performed induction, reversion, and competition experiments in vitro and in vivo using a chronic mouse pneumonia model to determine the impact of SXT on the emergence of TD-SCVs. SCVs were characterized by light and transmission electron microscopy (TEM) and auxotrophism testing. Short-term exposure of S. aureus to SXT induced the TD-SCV phenotype in S. aureus SH1000, while selection of TD-SCVs with thyA mutations occurred after long-term exposure. In reversion experiments with clinical and laboratory TD-SCVs, all revertants carried compensating mutations at the initially identified mutation site. Competition experiments in vitro and in vivo revealed a survival and growth advantage of the ΔthyA mutant under low-thymidine availability and SXT exposure although this advantage was less profound in vivo. Our results show that SXT induces the TD-SCV phenotype after short-term exposure, while long-term exposure selects for thyA mutations, which provide an advantage for TD-SCVs under specified conditions. Thus, our results further an understanding of the dynamic processes occurring during SXT exposure with induction and selection of S. aureus TD-SCVs.
INTRODUCTION
Staphylococcus aureus is an important human pathogen which causes a variety of infections in healthy and hospitalized patients (1). The increase of methicillin-resistant S. aureus (MRSA) isolates not only in hospitals but also in the community threatens the use of β-lactam antibiotics, which are most efficient for the treatment of S. aureus infections. Since more than 90% of community-acquired (CA-MRSA) and hospital acquired (HA-MRSA) strains are still susceptible to trimethoprim-sulfamethoxazole (TMP-SMX, or SXT) (2–5), new attention has been drawn to this old drug. For example, two recent randomized placebo-controlled studies evaluated the effect of SXT in skin abscesses after incision and drainage in children and adults mostly caused by CA-MRSA (6–8). Furthermore, De Angelis et al. suggested the use of SXT and clindamycin as first-line drugs as a therapeutic option in patients suffering from CA-MRSA infections (9). In contrast, the role of SXT in infections associated with tissue damage and extracellular available thymidine was questioned by Proctor (10) because S. aureus can bypass the inhibitory effect of SXT by external uptake of thymidine.
However, if patients were treated with SXT for extended periods, the emergence of thymidine-dependent (TD) small-colony variants (SCVs) of S. aureus has been reported (11–13). TD-SCVs were recovered from patients with chronic infections, such as soft tissue infection, bronchitis, peritonitis, endocarditis, and septicemia, and in particular with a high prevalence from the airways of cystic fibrosis (CF) patients, often in combination with an isogenic normal phenotype (11, 12, 14, 15). Just recently, Wolter et al. reported a high prevalence of SCVs in children with CF (24%), and most of these (95%) were TD-SCVs (16). A detailed characterization of clinical S. aureus TD-SCVs is given in Kahl et al. (17–19), where special features including gross morphological changes, impaired cell separation, and altered transcription patterns of important metabolism and virulence genes as well as of virulence regulators are described. TD-SCVs grow unaffected in the presence of SXT if TD-SCVs have access to extracellular thymidine such as that present in infected tissues and purulent airway secretions (13, 20).
The antibacterial effects of SXT are due to the interference of the drug with the bacterial folate pathway by competitive inhibition of dihydropteroate synthase and dihydrofolate reductase, two proteins involved in the synthesis and conversion of tetrahydrofolic acid (THF). THF acts as a cofactor for thymidylate synthase ([TS] thyA), which is essential for the de novo thymidylate biosynthesis (21, 22) required for DNA synthesis and bacterial replication. We along with others have shown that mutations in thyA are responsible for thymidine dependency of S. aureus TD-SCVs and that the emergence of these SCVs is associated with SXT treatment (21, 22).
However, until now the emergence of TD-SCVs has never been studied systematically, which we aimed to do in this study.
MATERIALS AND METHODS
Ethics statement.
Animal studies were conducted according to protocols approved by the San Raffaele Scientific Institute (Milan, Italy) Institutional Animal Care and Use Committee (IACUC) and adhered strictly to the Italian Ministry of Health guidelines for the use and care of experimental animals.
Bacterial strains and plasmids.
Strains and plasmids used in this study are listed in Table 1. S. aureus SH1000, a sigB-positive variant (rsbU+) of S. aureus 8325-4 (23), and its ΔthyA mutant (24) were used for competition experiments. Three clinical strain pairs of S. aureus, consisting of TD-SCVs and the respective isogenic normal phenotype, were isolated from airway secretions of CF patients who were treated long term with SXT (24).
TABLE 1.
Analysis of the thyA genes from revertant strains in comparison to genes of their parent isolates
| Parent strain and variant | TD phenotypea | Alteration(s) in thyAb | Predicted alteration or point mutation (position)c | Reference or source |
|---|---|---|---|---|
| Normal-5 | − | G564T | Trp → Tyr (188) | 1 |
| SCV-5 | + | T564G | Trp → stop (188) | 1 |
| Revertant-5 | − | T562C, T564G | Trp → Gln (188) | This study |
| Normal-6 | − | 1 | ||
| SCV-6 | + | C941A | Ala → Asp (314) | 1 |
| Revertant-6 | − | C941T | Ala → Phe (314) | This study |
| Normal-7 | − | 24 | ||
| SCV-7 | + | C705A | Ser → Arg (235) | 24 |
| Revertant-7 | − | This study | ||
| Newman WT | − | 24 | ||
| Newman SCV | + | G748T | Glu → stop (249) | 24 |
| Newman revertant | − | G748A | Glu → Lys (249) | This study |
TD, thymidine-dependent.
Only nonsynonymous mutations in thyA compared to the thyA gene of S. aureus 8325-4 are shown.
Amino acid position.
Media and growth conditions.
For cultivation of S. aureus, tryptic soy agar (BD, Heidelberg, Germany), Columbia blood agar (BD), Mueller-Hinton (MH) agar (Heipha Dr. Müller GmbH, Eppelheim, Germany), brain heart infusion (BHI) broth (Merck, Darmstadt, Germany), chemically defined medium to determine auxotrophisms of SCVs (25), and Luria-Bertani (LB) broth (BD) were used.
DNA extraction and sequencing.
S. aureus cells were lysed with lysostaphin (WAK Chemie Medical GmBH, Steinbach/Ts, Germany). Chromosomal DNA was prepared using a PrestoSpin D kit (Molzym GmbH and Co. KG, Bremen, Germany). All thyA sequences were cloned in the vector pQE30Xa (Qiagen, Hilden, German) using the primers thyA-fwd (TTG AAT TCA TTT GAT GCA GCA TAT CAC) and thyA-rev (TCA GGA TCC CTA CAC TGC TAT TGG AGC) as described before (25) and sequenced at Eurofins MWG Operon (Martinsried, Germany) using the primers pQE-for (GTA TCA CGA GGC CCT TTC GTC T) and pQE-rev (CAT TAC TGG ATC TAT CAA CAG GAG).
Induction of TD-SCVs by SXT.
To induce/select for TD-SCVs, the laboratory S. aureus strain Newman was cultured with SXT (240 μg/ml) in BHI broth (10 ml of medium in 100-ml baffled flasks at 160 rpm and 37°C) for several days. After each overnight (ON) culture, appropriate dilutions were streaked on Columbia blood agar. Small colonies were selected, subcultured, and tested for thymidine auxotrophism (11).
Reversion experiments.
First, the background frequency of mutation was determined by assessing the mutation rate of normal SCVs and TD-SCVs subjected to rifampin treatment (26). Briefly, one bacterial colony was resuspended in 20 ml of BHI broth and incubated overnight at 37°C at 160 rpm. Bacterial cells were collected by centrifugation at 3,000 rpm for 5 min and resuspended in 1 ml of BHI broth. A 100-μl sample of this suspension was further diluted and plated onto BHI agar plates with and without rifampin. The same dilutions were plated on MH agar plates, which are low in thymidine, to test for reversion of TD-SCVs. After 48 h, CFU were enumerated, and the mutation frequency was determined by dividing the number of CFU on the rifampin plates by the number of bacteria on BHI agar without rifampin. The reversion frequency was determined as the number of CFU on MH agar.
Competition experiments.
To investigate which conditions would select for TD-SCVs, we performed competition experiments with the wild type (WT) and a constructed stable ΔthyA mutant (24). After individual overnight cultures of the ΔthyA mutant, which is erythromycin resistant, and the WT in BHI broth under aerobic conditions (shaking at 160 rpm), both phenotypes were combined and inoculated to a final optical density at 578 nm (OD578) of 0.1, consisting of 0.05 OD units of each strain in fresh medium. The medium was BHI broth (i) without SXT or thymidine, (ii) with 240 μg/ml SXT, (iii) with 240 μg/ml SXT and 100 μg/ml thymidine, and (iv) with 100 μg/ml thymidine. After 24 h, cultures were transferred into fresh broth (OD of 0.1) with the respective substrates, and 100 μl of appropriate diluted aliquots were streaked on Columbia blood agar with (to select for the mutant) and without erythromycin and on MH agar for colony counting. This procedure was repeated for 5 days.
Transmission electron microscopy.
Samples were prepared for ultrastructure analysis as described previously (18). Ultrathin sections were visualized on a transmission electron microscope (Philips EM201) equipped with a digital imaging system (Ditabis, Pforzheim, Germany). Normal and SCV phenotypes of cocci were counted on the various images.
Chronic pneumonia mouse model.
The agar bead chronic pneumonia mouse model was used for competition experiments between the WT (S. aureus SH1000) and the ΔthyA mutant (27). A starting amount of 5 × 109 ΔthyA mutant cells/WT bacteria, mixed at a 1:1 ratio, was used for inclusion in the agar beads prepared according to a method described previously for P. aeruginosa, with modifications (28, 29). Briefly, S. aureus strains were cultured in BHI broth with erythromycin (2.5 μ/ml) for the mutant and without erythromycin for the WT overnight at 37°C, adjusted to a starting OD600 of 0.1, grown for an additional 3 h (SH1000) and 5 h (ΔthyA mutant) to allow agar bead preparation. Briefly, the bacteria were harvested by centrifugation and resuspended in 1 ml of phosphate-buffered saline (PBS; pH 7.4). Bacteria were added to 9 ml of BHI agar (BD), prewarmed to 45°C. This mixture was pipetted forcefully into 150 ml of heavy mineral oil at 45°C and stirred rapidly with a magnetic stirring bar for 6 min at room temperature, followed by cooling at 4°C with continuous slow stirring for 35 min. The oil-agar mixture was centrifuged at 4,000 rpm for 20 min to sediment the beads and washed six times in PBS. The size of the beads was verified microscopically, and only the preparations containing beads of 100 μm to 200 μm in diameter were used as inocula for animal experiments. The number of S. aureus CFU in the beads was determined by plating serial dilutions of the homogenized bacteria-bead suspension on Columbia blood agar plates with or without erythromycin (2.5 μl/ml). The inoculum was prepared by diluting the bead suspension with PBS to 1 × 107 CFU/ml.
Groups of 12 to 13 C57BL/6 male mice (20 to 22 g; Charles River Laboratories) were infected with 5 ×105 CFU of S. aureus as described previously (27, 30). After anesthesia and exposure of the trachea, mice were inoculated with 50 μl of agar bead suspension into the lung. After inoculation, all incisions were closed by suture. All mice were maintained under specific-pathogen-free conditions in sterile cages, which were put into a ventilated isolator. Twenty-four hours after infection, mice were treated with SXT (Cotrim-ratiopharm Ampullen SF; Ratiopharm) (20 mg of the TMP component/kg of body weight intraperitoneally [i.p.]) or with saline as a control by intraperitoneal injection once a day. Antibiotic treatment and dose were established according to previous papers (31, 32). Twelve days after infection and repeated antibiotic treatments, the murine lungs were excised, homogenized, and plated onto BHI agar plates in the presence and absence of erythromycin (2.5 μ/ml).
The mean of the competition index (CI) was calculated as the ratio between the number of ΔthyA mutant and WT CFU recovered from the murine lungs at 12 days postinfection, adjusted by the input ratio of the inoculum of each animal (in vivo CI) (29, 33).
Statistical analysis.
Statistical analyses for the in vivo experiments were performed by Mann-Whitney U tests for unpaired samples, using GraphPad software. Tests were considered statistically significant at a significance level of ≤0.05.
RESULTS
SXT-induced S. aureus TD-SCVs.
To verify the impact of SXT on the emergence of TD-SCVs, we set up induction/selection experiments in vitro. We tried to select TD-SCVs by cultivating the laboratory S. aureus strain Newman, SH1000, and USA300 with SXT (240 μg/ml) in BHI broth for several days. (SXT MICs for the strains were 0.38 mg/liter, 0.047 mg/liter, and 0.064 mg/ml). After three overnight cultures with SXT, appropriate dilutions were streaked on Columbia blood agar. Small colonies were selected and subcultured. Only after several repetitions of these experiments were we able to isolate one particular SCV in strain Newman but not in S. aureus strain SH1000 or USA300. The strain Newman SCV failed to grow on MH agar plates and was determined to be thymidine dependent by auxotrophism testing. To confirm thymidine dependency of this isolate also on the molecular level, we sequenced the thyA gene of this strain and identified a point mutation at position 748 leading to a premature stop codon (Table 1). It was not possible to retrieve TD-SCVs directly after one ON culture with SXT on agar plates. Therefore, it was not possible to determine the exact thyA mutation frequency. Since the background mutation rate of strain Newman was 9.44 × 10−10 cells per generation, as determined in three independent experiments using the rifampin mutation frequency assay, we could only estimate the induction frequency as being approximately less than 1 × 10−10 cells per generation.
Primary mutational events in thyA of TD-SCVs were compensated in revertants.
It is known that clinical SCVs are often not stable and revert back to the normal phenotype (34, 35). Therefore, we aimed to study reversion of TD-SCVs systematically. Three consecutive overnight cultures in BHI broth of clinical TD-SCVs and an in vitro-induced TD-SCV of strain Newman (Table 1) were performed. Serial dilutions of each passage were streaked on Columbia blood agar. We were able to recover revertants for SCVs with point mutations (Table 1) but not for TD-SCVs with deletions in thyA (data not shown). We tried to estimate the reversion frequency of SCVs by plating the samples after overnight culture in addition to BHI with and without rifampin also on MH agar to assess reversion frequency. While TD-SCVs are not able to grow on MH agar, which contains small amounts of thymidine, revertants can grow on this agar. Since SCVs are impaired in their replication, not all of the SCVs yield high numbers of bacteria after overnight culture. Therefore, it was not possible to determine the mutation frequency for all TD-SCVs. We were able to get a high enough density for one of the clinical TD-SCVs with 6.45 × 108 CFU/ml. For this TD-SCV we determined a background mutation rate of 1.55 × 10−9 with no growth on MH agar. From these results we estimated the reversion frequency of this TD-SCV as being lower than 1 × 10−9. Sequence analysis of the thyA genes of all revertants revealed second point mutations at the initially identified mutation site leading back to thyA of the WT strain or to a novel sequence leading to another amino acid exchange (Table 1).
SXT exposure induced the SCV phenotype morphologically in normal S. aureus cells.
To analyze the effects of SXT on S. aureus, we exposed S. aureus WT (SH1000) and the ΔthyA mutant (24) strains to SXT (240 μg/ml). We analyzed the effects on S. aureus morphology by light microscopy (see Fig. S1 in the supplemental material) and by transmission electron microscopy (TEM). In BHI broth, the ΔthyA mutant showed significantly enlarged cocci with multiple and partially incomplete cross walls in contrast to the homogenous morphology of the WT (Fig. 1A). If thymidine was added to BHI broth, the sizes of the cocci decreased, with a mixture of normal and enlarged cells in the mutant, while the phenotype of the WT did not change (Fig. 1B). Challenge with SXT did not affect the morphology of the TD-SCV (Fig. 1C) but caused enlarged cocci with impaired cell division in the WT strain comparable to the phenotype of the mutant (Fig. 1C). Further addition of thymidine to the medium containing SXT caused changes in both strains almost leading back to the normal morphology (Fig. 1D). The prevalences of normal and SCV phenotypes were determined for the respective images (Table 2). During SXT exposure, 46% of cocci of the WT exhibited the SCV phenotype, while 69% of the cocci of the ΔthyA mutant showed this phenotype. Addition of thymidine under SXT challenge caused reversion to the normal phenotype in 94% of cocci of the WT and in 79% of the cocci of the mutant. Using light microscopy analysis (37) of Gram stainings (see Fig. S1 in the supplemental material), the sizes of 100 cocci of three biological replicates of each condition were measured and showed the same effects: the sizes of the TD-SCVs were significantly smaller with additional thymidine in the medium, with or without SXT, while the sizes of the WT cells were significantly larger under SXT challenge, a characteristic which was reverted by the addition of thymidine (Fig. 2).
FIG 1.
WT S. aureus SH1000 and the ΔthyA mutant under SXT challenge with and without thymidine. Strains were cultured in BHI broth under SXT exposure with and without additional thymidine. TEM was performed to analyze the phenotypic changes of the wild-type (SH1000) and the mutant (ΔthyA strain) under different conditions, as follows: BHI, showing the typical phenotypes of WT S. aureus and the ΔthyA mutant, which is characterized by enlarged cocci with several division planes (A); BHI broth plus 100 μg/ml thymidine, showing that the WT phenotype did not change but that the mutant was partially complemented with smaller cocci than in culture with BHI broth without additional thymidine (B); BHI broth plus 240 μg/ml SXT, which demonstrates that the WT resembles the mutant under these conditions, with enlarged cocci, while the phenotype of the mutant did not change (C); BHI plus 100 μg/ml thymidine plus 240 μg/ml SXT, where adding thymidine to SXT-challenged bacteria almost reverts both SCV phenotypes back to the normal phenotypes in the WT and in the mutant (D).
TABLE 2.
Frequency of normal and SCV phenotypes of the WT and the ΔthyA mutant strains under different conditions
| Strain and phenotype | No. of cocci (%) for the indicated Fig. 1 panel and treatment |
|||
|---|---|---|---|---|
| A (none) | B (+thymidine) | C (+SXT) | D (+thymidine, +SXT) | |
| WT | ||||
| Normal | 100 (100) | 92 (100) | 18 (54) | 50 (94) |
| SCV | 0 (0) | 0 (0) | 15 (46) | 3 (6) |
| ΔthyA strain | ||||
| Normal | 3 (18) | 19 (58) | 8 (31) | 31 (79) |
| SCV | 14 (82) | 14 (52) | 18 (69) | 8 (21) |
FIG 2.
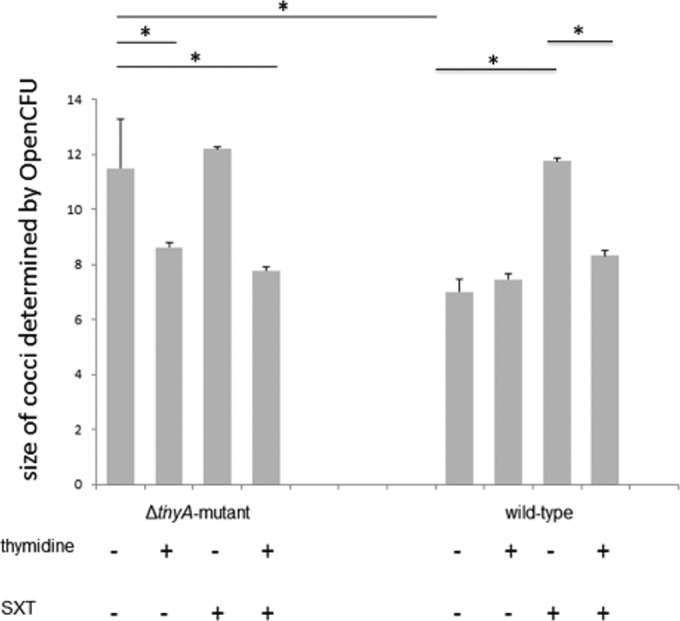
Size analysis of SH1000 and the ΔthyA mutant cocci under SXT challenge with and without thymidine by light microscopy. The WT and the ΔthyA mutant were cultured under the same conditions as described in the legend of Fig. 1. The overnight culture was centrifuged, resuspended in 1 ml of BHI broth, and subjected to Gram staining and size analysis of each 100 cocci using OpenCFU. Data are shown for three biological replicates with means and standard deviations. The significance of differences in sizes of cocci was calculated by a t test (*, P < 0.05).
Advantage of the ΔthyA mutant under SXT exposure in vitro.
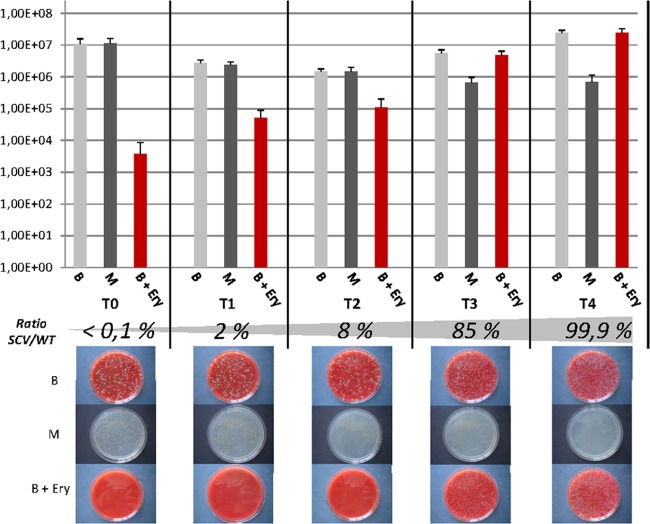
To assess both the relative fitness of the ΔthyA mutant compared to that of its parent strain and the conditions which would select for TD-SCVs, we performed competition experiments exposing the ΔthyA mutant and the WT strain to SXT with and without additional thymidine in BHI broth (Fig. 3). By adding thymidine to BHI broth, we expected a growth advantage for the WT under SXT challenge. The WT showed a considerably higher growth rate than the ΔthyA mutant in BHI broth without SXT (data not shown) and comparable growth to that of the ΔthyA mutant under SXT challenge. As expected, the WT was the predominant phenotype under three of the four tested conditions (BHI, BHI plus SXT plus thymidine, and BHI plus thymidine) after 5 days of serial subcultures (data not shown). Only under low-thymidine conditions, as with BHI broth and SXT exposure (BHI plus SXT), the ΔthyA mutant outcompeted the WT and almost entirely displaced the WT within 5 days of serial subcultures (Fig. 3).
FIG 3.
In vitro competition assay of the SH1000 WT and the ΔthyA mutant under low-thymidine conditions (in BHI broth). After individual overnight cultures in BHI broth (37°C and 160 rpm), the strains were combined and inoculated to a final OD of 0.1 consisting of 0.05 OD units of each strain in fresh medium (BHI plus 240 μg/ml SXT). After 24 h of coincubation, an aliquot of the culture (corresponding to an OD of 0.1) was transferred into fresh medium. One hundred microliters of appropriate dilutions (10−1 to 10−6) were streaked on Columbia blood agar (B), on which all bacteria grow, on Columbia blood agar with erythromycin (B+Ery), on which only the mutant grows, and on Mueller-Hinton agar (M), which allows growth of only the WT and not of the mutant, which needs additional thymidine. CFU/ml was determined by colony counting (see graphs). The ratio of the number of the SH1000 ΔthyA mutant CFU to the number of the SH1000 WT CFU was calculated. Images of the agar plates show the growth of the WT and mutant. At the beginning only low numbers of the mutant were detected on cultures plated on Columbia blood agar with erythromycin compared to numbers of the WT plated on Mueller-Hinton agar (5.0 × 103 CFU versus 1.0 × 107 CFU), while after 5 days of SXT challenge more CFU of the mutant than of the WT were cultured (1.3 × 107 CFU versus 8.0 × 105 CFU), indicating that the mutant outcompeted the WT under these conditions. T0 to T4, time period day 1 to day 5 of coincubation.
Advantage of the ΔthyA mutant in a chronic murine pneumonia model under SXT treatment.
To further investigate the relative fitness of the ΔthyA mutant in comparison with that of the WT under in vivo conditions, we performed competition experiments in a chronic murine pneumonia model with or without SXT treatment (Fig. 4). First, the WT has a significantly better fitness in the murine lung than the ΔthyA mutant. After antibiotic treatment for 12 days, the total number of bacteria present in lung samples was lower (Fig. 4, top), indicating the therapeutic benefit of SXT to reduce bacterial infection. However, SXT treatment was more efficient in reducing bacterial counts of the WT than of the ΔthyA mutant strain. As already observed for other pathogens, the antibiotic treatment failed to completely eradicate chronic infection under these experimental conditions (33). Thus, significant reduction of the WT population was observed under treated conditions compared to the level of the untreated condition (P < 0.05), while bacterial counts of the ΔthyA mutant were unchanged. In terms of the competition index (CI), the nontreated group showed a CI value of 0.95 × 10−1, indicating an advantage of the WT over the ΔthyA mutant strain, whereas the treated group increased the CI to 0.78, resulting in almost comparable fitness levels of the mutant and the WT under this condition (Fig. 4, bottom). These results show that during SXT challenge the fitness of the mutant, but not of the WT, increased (CI, P < 0.05).
FIG 4.
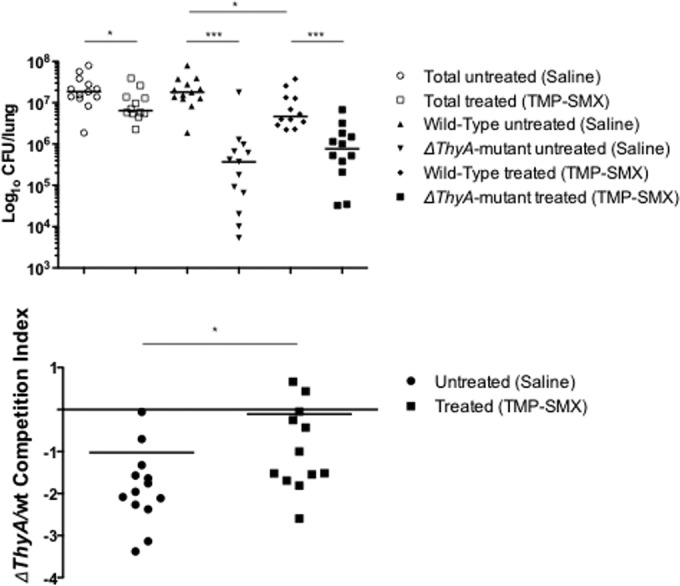
In vivo competition between the ΔthyA mutant and the WT S. aureus strain in C57BL/6 mice. The ΔthyA mutant and the WT S. aureus strain SH1000 were embedded in agar beads and used to infect 12 to 13 C57BL/6 mice/group. Chronic infection was sustained for 12 days with and without treatment by SXT, and the numbers of CFU per lung were evaluated on erythromycin-selective plates to distinguish the two competitors (top). CI was calculated by dividing the ratio of the number of CFU of the ΔthyA mutant/number of CFU of the WT strain recovered from the lungs by the ratio of the number of the CFU of the ΔthyA mutant/number of the CFU of the WT strain of the inoculum for each animal (bottom). Dots represent individual measurements, and horizontal lines represent the geometric means. A CI of <1 indicated a disadvantage for the ΔthyA mutant in being maintained in vivo compared with the WT strain SH1000. Statistical analysis was calculated for each in vivo competition between the two treated groups with and without SXT (*, P < 0.05; *** P < 0.01, Mann-Whitney two-tailed test). The results show that during SXT challenge, the fitness of the mutant but not of the WT increased. TMP-SMX, trimethoprim-sulfamethoxazole (SXT).
DISCUSSION
For more than a decade, it has been shown that the emergence of S. aureus TD-SCVs is associated with prolonged SXT treatment (11). However, the reasons why TD-SCVs emerge and why they are especially associated with chronic S. aureus infections are not known. This study showed for the first time that SXT exposure induced, selected, and conferred a survival and growth advantage to TD-SCVs in low-thymidine-containing environments and that mutational inactivation of thyA is the molecular mechanism leading to the clinical TD-SCV phenotype.
Having shown earlier that mutations in thyA caused inactivity of the protein (24), we aimed to induce TD-SCVs with thyA mutations in vitro by SXT challenge. This was only possible after prolonged exposure of S. aureus to SXT in BHI broth as shown here for the widely used laboratory S. aureus strain Newman but not for strain SH1000 or USA300. After several attempts of induction, we isolated a typical TD-SCV which did not grow on MH agar and which was thymidine dependent. Such a low mutation frequency might be due to the high fitness costs that thymidine dependency causes for the bacteria, indicating that selection of TD-SCVs requires long-term exposure. Such results are in line with earlier studies in CF patients, which showed that TD-SCVs were isolated after approximately 18 months of SXT treatment (11). The induced TD-SCV in the S. aureus strain Newman carried a point mutation in thyA, leading to a premature stop codon. Similar mutations were already seen in clinical TD-SCVs (21, 22, 24).
The fact that TD-SCVs were isolated from patients in vivo who were treated with SXT for long periods (11–13, 16, 24, 36) indicates that particular conditions seem to favor the emergence of TD-SCVs. To simulate such clinical conditions, we performed competition experiments comparing the WT and the ΔthyA mutant during SXT exposure in vitro and in vivo. In the in vitro experiments, the ΔthyA mutant had a clear growth and survival advantage under SXT exposure compared to that of the WT in one out of four tested conditions. Only in BHI broth plus SXT, which contains low but sufficient amounts of thymidine, did the mutant out-compete the WT. Low concentrations of thymidine, such as those present in BHI broth, are supposed to be available in chronically infected tissues in contrast to conditions in acute infections, with large amounts of thymidine present due to cell detritus, pus, and DNA degradation (20). Only these conditions favored the selection of TD-SCVs. In addition, the in vitro results were supported by our in vivo competition experiments.
In the in vivo competition experiments using a chronic pneumonia model, which compared SXT-treated to nontreated mice, we show that the WT was the dominant phenotype in the nontreated group. However, SXT treatment reduced the number of the WT cells significantly but not the numbers of the ΔthyA mutant cells, indicating an advantage of the mutant in this model under antibiotic treatment. Although we established for the first time a 2-week mouse pneumonia infection model for S. aureus, this extended period still seems to be too short to reflect real long-term infections in humans with CF, which can go on for months or even years.
However, the in vitro and in vivo results of our competition experiments indicate that our results can be directly transferred to the in vivo situation, in which treatment with SXT causes selection of TD-SCVs at sites where sufficient amounts of thymidine are available. Zander et al. demonstrated that thymidine is available in various human specimens (20), showing that thymidine is provided by pus and cell detritus and thereby allows growth and survival of TD-SCVs.
Our results allow us to propose a three-step model for the dynamics of TD-SCV formation and reversion (Fig. 5). If thymidine availability is low, as at some infection sites, short-term challenge by SXT induces the formation of SCV phenotypes in the whole S. aureus population, as shown by TEM (Fig. 1), due to blocking de novo thymidylate synthesis. If SXT is present for extended periods, such conditions will favor a thymidine-dependent phenotype based on random mutations within thyA, leading to inactivity of the TS protein. Two types of functional inactivation of thyA are possible: (i) point mutations, leading to single amino acid substitutions, and (ii) deletions, leading to frameshift or in-frame mutations. If SXT exposure is halted (e.g., end of antibiotic treatment), TD-SCVs with point mutations may revert, while TD-SCVs with deletions are very unlikely to revert, thereby representing a stable TD-SCV population. Once the population is no longer exposed to SXT, phenotypically induced SCVs revert back to the WT phenotype while stable TD-SCVs (with mutations in thyA) will remain. However, treatment with SXT favors TD-SCVs in vivo and in vitro, and one could speculate that several episodes of treatment will lead to a diversification of the whole S. aureus population. This population then consists of WT phenotypes including revertants with and without point mutations in thyA and TD-SCVs, which can revert or are stable, depending on the underlying mutational event.
FIG 5.

A model for the dynamics of TD-SCVs. A three-step model explains the dynamics of TD-SCV formation and reversion. In a low-thymidine environment, treatment with SXT will induce the formation of SCV phenotypes in the whole S. aureus population by blocking thymidylate de novo synthesis (step 1). Two types of functional inactivation of thyA are possible: (i) point mutations, leading to single amino acid substitutions, and (ii) deletions, leading to frameshift or in-frame mutations (step 2). TD-SCVs with point mutations can revert back to the WT phenotype representing unstable SCVs, while TD-SCVs with deletions are very unlikely to revert, therefore representing a stable TD-SCV population (step 3). Once the strains are no longer exposed to SXT, induced SCVs and TD-SCVs with point mutations revert back to the WT phenotype.
In summary, our results provide for the first time clear evidence that short-term SXT exposure induces the TD-SCV phenotype in WT S. aureus and that long-term exposure drives the selection of mutations in thyA, resulting in TD-SCVs which have a survival advantage in a specific environment with low thymidine availability. Thus, our results help further an understanding of the dynamic processes of S. aureus phenotypic adaptation and selection during SXT challenge.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Deiwick, D. Kuhn, and E. Leidig (Institute of Medical Microbiology, University Hospital of Muenster, Muenster, Germany) for excellent technical assistance. We thank Wolfgang Völker (Leibniz Institute of Atherosclerosis Research, University Hospital of Muenster, Muenster, Germany) for excellent transmission electron microscopy. We thank Melisa Zengin, a summer student from Hacettepe University, Ankara, Turkey, for performing Gram stainings and size analysis of cocci.
This project was funded partly by grants of the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG]; KA 2249/1-3, DFG Spp 1316, and BE 2546/1-2), the Interdisciplinary Center for Clinical Research (IZKF Münster; Kah2/024/09), the Transregional Collaborative Research Center 34 (C7), and the Bundesministerium für Bildung und Forschung (BMBF Medizinische Infektionsgenomik; 0315829B).
There are no conflicts of interest for any of the authors.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00742-15.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Chua K, Laurent F, Coombs G, Grayson ML, Howden BP. 2011. Antimicrobial resistance: not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician's guide to community MRSA—its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis 52:99–114. doi: 10.1093/cid/ciq067. [DOI] [PubMed] [Google Scholar]
- 3.Talan DA, Krishnadasan A, Gorwitz RJ, Fosheim GE, Limbago B, Albrecht V, Moran GJ. 2011. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis 53:144–149. doi: 10.1093/cid/cir308. [DOI] [PubMed] [Google Scholar]
- 4.Hanaki H, Cui L, Ikeda-Dantsuji Y, Nakae T, Honda J, Yanagihara K, Takesue Y, Matsumoto T, Sunakawa K, Kaku M, Tomono K, Fukuchi K, Kusachi S, Mikamo H, Takata T, Otsuka Y, Nagura O, Fujitani S, Aoki Y, Yamaguchi Y, Tateda K, Kadota J, Kohno S, Niki Y. 2014. Antibiotic susceptibility survey of blood-borne MRSA isolates in Japan from 2008 through 2011. J Infect Chemother 20:527–534. doi: 10.1016/j.jiac.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Levesque S, Bourgault AM, Galarneau LA, Moisan D, Doualla-Bell F, Tremblay C. 2015. Molecular epidemiology and antimicrobial susceptibility profiles of methicillin-resistant Staphylococcus aureus blood culture isolates: results of the Quebec Provincial Surveillance Programme. Epidemiol Infect 143:1511–1518. doi: 10.1017/S095026881400209X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz GR, Bruner D, Pitotti R, Olderog C, Livengood T, Williams J, Huebner K, Lightfoot J, Ritz B, Bates C, Schmitz M, Mete M, Deye G. 2010. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med 56:283–287. doi: 10.1016/j.annemergmed.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Duong M, Markwell S, Peter J, Barenkamp S. 2010. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med 55:401–407. doi: 10.1016/j.annemergmed.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Pallin DJ, Binder WD, Allen MB, Lederman M, Parmar S, Filbin MR, Hooper DC, Camargo CA Jr. 2013. Clinical trial: comparative effectiveness of cephalexin plus trimethoprim-sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Dis 56:1754–1762. doi: 10.1093/cid/cit122. [DOI] [PubMed] [Google Scholar]
- 9.De Angelis G, Cipriani M, Cauda R, Tacconelli E. 2011. Treatment of skin and soft tissue infections due to community-associated methicillin-resistant Staphylococcus aureus in Europe: the role of trimethoprim-sulfamethoxazole. Clin Infect Dis 52:1471–1472. doi: 10.1093/cid/cir247. [DOI] [PubMed] [Google Scholar]
- 10.Proctor RA. 2008. Role of folate antagonists in the treatment of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46:584–593. doi: 10.1086/525536. [DOI] [PubMed] [Google Scholar]
- 11.Kahl B, Herrmann M, Everding As, Koch HG, Becker K, Harms E, Proctor RA, Peters G. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J Infect Dis 177:1023–1029. doi: 10.1086/515238. [DOI] [PubMed] [Google Scholar]
- 12.Besier S, Smaczny C, von Mallinckrodt C, Krahl A, Ackermann H, Brade V, Wichelhaus TA. 2007. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J Clin Microbiol 45:168–172. doi: 10.1128/JCM.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besier S, Zander J, Siegel E, Saum SH, Hunfeld KP, Ehrhart A, Brade V, Wichelhaus TA. 2008. Thymidine-dependent Staphylococcus aureus small-colony variants: human pathogens that are relevant not only in cases of cystic fibrosis lung disease. J Clin Microbiol 46:3829–3832. doi: 10.1128/JCM.01440-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yagci S, Hascelik G, Dogru D, Ozcelik U, Sener B. 2013. Prevalence and genetic diversity of Staphylococcus aureus small-colony variants in cystic fibrosis patients. Clin Microbiol Infect 19:77–84. doi: 10.1111/j.1469-0691.2011.03742.x. [DOI] [PubMed] [Google Scholar]
- 15.Gilligan PH, Gage PA, Welch DF, Muszynski MJ, Wait KR. 1987. Prevalence of thymidine-dependent Staphylococcus aureus in patients with cystic fibrosis. J Clin Microbiol 25:1258–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolter DJ, Emerson JC, McNamara S, Buccat AM, Qin X, Cochrane E, Houston LS, Rogers GB, Marsh P, Prehar K, Pope CE, Blackledge M, Deziel E, Bruce KD, Ramsey BW, Gibson RL, Burns JL, Hoffman LR. 2013. Staphylococcus aureus small-colony variants are independently associated with worse lung disease in children with cystic fibrosis. Clin Infect Dis 57:384–391. doi: 10.1093/cid/cit270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahl BC, Belling G, Becker P, Chatterjee I, Wardecki K, Hilgert K, Cheung AL, Peters G, Herrmann M. 2005. Thymidine-dependent Staphylococcus aureus small colony variants are associated with extensive changes in regulator and virulence gene expression profiles. Infect Immun 73:4119–4126. doi: 10.1128/IAI.73.7.4119-4126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahl BC, Belling G, Reichelt R, Herrmann M, Proctor RA, Peters G. 2003. Thymidine-dependent small-colony variants of Staphylococcus aureus exhibit gross morphological and ultrastructural changes consistent with impaired cell separation. J Clin Microbiol 41:410–413. doi: 10.1128/JCM.41.1.410-413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee I, Herrmann M, Proctor RA, Peters G, Kahl BC. 2007. Enhanced post-stationary-phase survival of a clinical thymidine-dependent small-colony variant of Staphylococcus aureus results from lack of a functional tricarboxylic acid cycle. J Bacteriol 189:2936–2940. doi: 10.1128/JB.01444-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zander J, Besier S, Saum SH, Dehghani F, Loitsch S, Brade V, Wichelhaus TA. 2008. Influence of dTMP on the phenotypic appearance and intracellular persistence of Staphylococcus aureus. Infect Immun 76:1333–1339. doi: 10.1128/IAI.01075-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besier S, Ludwig A, Ohlsen K, Brade V, Wichelhaus TA. 2007. Molecular analysis of the thymidine-auxotrophic small colony variant phenotype of Staphylococcus aureus. Int J Med Microbiol 297:217–225. doi: 10.1016/j.ijmm.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee I, Kriegeskorte A, Fischer A, Deiwick S, Theimann N, Proctor RA, Peters G, Herrmann M, Kahl BC. 2008. In vivo mutations of thymidylate synthase (thyA) are responsible for thymidine-dependency in clinical small colony variants (TD-SCVs) of Staphylococcus aureus. J Bacteriol 190:834–842. doi: 10.1128/JB.00912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol 184:5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kriegeskorte A, Block D, Drescher M, Windmuller N, Mellmann A, Baum C, Neumann C, Lore NI, Bragonzi A, Liebau E, Hertel P, Seggewiss J, Becker K, Proctor RA, Peters G, Kahl BC. 2014. Inactivation of thyA in Staphylococcus aureus attenuates virulence and has a strong impact on metabolism and virulence gene expression. mBio 5:e01447–14. doi: 10.1128/mBio.01447-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Rijn I, Kessler RE. 1980. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun 27:444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besier S, Zander J, Kahl BC, Kraiczy P, Brade V, Wichelhaus TA. 2008. The thymidine-dependent small-colony-variant phenotype is associated with hypermutability and antibiotic resistance in clinical Staphylococcus aureus isolates. Antimicrob Agents Chemother 52:2183–2189. doi: 10.1128/AAC.01395-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bragonzi A, Worlitzsch D, Pier GB, Timpert P, Ulrich M, Hentzer M, Andersen JB, Givskov M, Conese M, Doring G. 2005. Nonmucoid Pseudomonas aeruginosa expresses alginate in the lungs of patients with cystic fibrosis and in a mouse model. J Infect Dis 192:410–419. doi: 10.1086/431516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bragonzi A, Farulla I, Paroni M, Twomey KB, Pirone L, Lore NI, Bianconi I, Dalmastri C, Ryan RP, Bevivino A. 2012. Modelling co-infection of the cystic fibrosis lung by Pseudomonas aeruginosa and Burkholderia cenocepacia reveals influences on biofilm formation and host response. PLoS One 7:e52330. doi: 10.1371/journal.pone.0052330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montanari S, Oliver A, Salerno P, Mena A, Bertoni G, Tummler B, Cariani L, Conese M, Doring G, Bragonzi A. 2007. Biological cost of hypermutation in Pseudomonas aeruginosa strains from patients with cystic fibrosis. Microbiology 153:1445–1454. doi: 10.1099/mic.0.2006/003400-0. [DOI] [PubMed] [Google Scholar]
- 30.Bragonzi A, Paroni M, Nonis A, Cramer N, Montanari S, Rejman J, Di SC, Doring G, Tummler B. 2009. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. Am J Respir Crit Care Med 180:138–145. doi: 10.1164/rccm.200812-1943OC. [DOI] [PubMed] [Google Scholar]
- 31.Bhagwat SP, Wright TW, Gigliotti F. 2010. Anti-CD3 antibody decreases inflammation and improves outcome in a murine model of Pneumocystis pneumonia. J Immunol 184:497–502. doi: 10.4049/jimmunol.0901864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Disney MD, Stephenson R, Wright TW, Haidaris CG, Turner DH, Gigliotti F. 2005. Activity of Hoechst 33258 against Pneumocystis carinii f. sp. muris, Candida albicans, and Candida dubliniensis. Antimicrob Agents Chemother 49:1326–1330. doi: 10.1128/AAC.49.4.1326-1330.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcala-Franco B, Montanari S, Cigana C, Bertoni G, Oliver A, Bragonzi A. 2012. Antibiotic pressure compensates the biological cost associated with Pseudomonas aeruginosa hypermutable phenotypes in vitro and in a murine model of chronic airways infection. J Antimicrob Chemother 67:962–969. doi: 10.1093/jac/dkr587. [DOI] [PubMed] [Google Scholar]
- 34.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 35.Tuchscherr L, Medina E, Hussain M, Volker W, Heitmann V, Niemann S, Holzinger D, Roth J, Proctor RA, Becker K, Peters G, Loffler B. 2011. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med 3:129–141. doi: 10.1002/emmm.201000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vergison A, Denis O, Deplano A, Casimir G, Claeys G, DeBaets F, DeBoeck K, Douat N, Franckx H, Gigi J, Ieven M, Knoop C, Lebeque P, Lebrun F, Malfroot A, Paucquay F, Pierard D, Van Eldere J, Struelens MJ. 2007. National survey of molecular epidemiology of Staphylococcus aureus colonization in Belgian cystic fibrosis patients. J Antimicrob Chemother 59:893–899. doi: 10.1093/jac/dkm037. [DOI] [PubMed] [Google Scholar]
- 37.Geissmann Q. 2013. OpenCFU, a new free and open-source software to count cell colonies and other circular objects. PLoS One 8:e54072. doi: 10.1371/journal.pone.0054072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.