Abstract
Carbapenemase-producing, carbapenem-resistant Enterobacteriaceae, or CP-CRE, are an emerging threat to human and animal health, because they are resistant to many of the last-line antimicrobials available for disease treatment. Carbapenemase-producing Enterobacter cloacae harboring blaKPC-3 recently was reported in the upper midwestern United States and implicated in a hospital outbreak in Fargo, North Dakota (L. M. Kiedrowski, D. M. Guerrero, F. Perez, R. A. Viau, L. J. Rojas, M. F. Mojica, S. D. Rudin, A. M. Hujer, S. H. Marshall, and R. A. Bonomo, Emerg Infect Dis 20:1583–1585, 2014, http://dx.doi.org/10.3201/eid2009.140344). In early 2009, the Minnesota Department of Health began collecting and screening CP-CRE from patients throughout Minnesota. Here, we analyzed a retrospective group of CP-E. cloacae isolates (n = 34) collected between 2009 and 2013. Whole-genome sequencing and analysis revealed that 32 of the strains were clonal, belonging to the ST171 clonal complex and differing collectively by 211 single-nucleotide polymorphisms, and it revealed a dynamic clone under positive selection. The phylogeography of these strains suggests that this clone existed in eastern North Dakota and western Minnesota prior to 2009 and subsequently was identified in the Minneapolis and St. Paul metropolitan area. All strains harbored identical IncFIA-like plasmids conferring a CP-CRE phenotype and an additional IncX3 plasmid. In a single patient with multiple isolates submitted over several months, we found evidence that these plasmids had transferred from the E. cloacae clone to an Escherichia coli ST131 bacterium, rendering it as a CP-CRE. The spread of this clone throughout the upper midwestern United States is unprecedented for E. cloacae and highlights the importance of continued surveillance to identify such threats to human health.
INTRODUCTION
Enterobacter cloacae is ubiquitous in nature, existing as a saprophyte in the environment and a commensal organism in the human intestine (1). Historically, E. cloacae rarely causes disease in immunocompetent humans. E. cloacae infections most commonly occur as health care-associated infections in immunocompromised patients, including those with comorbidities and the elderly. In recent years, E. cloacae also has emerged as a significant nosocomial pathogen in neonatal intensive care units (2). E. cloacae has gained further attention for its association with antimicrobial resistance (1). Numerous mechanisms of resistance toward antibiotics that are critical to the treatment of human disease have been identified in this organism, both intrinsic and acquired, and selective pressures appear to be driving E. cloacae toward a multidrug-resistant phenotype (3–8).
The global emergence of carbapenemase-producing, carbapenem-resistant Enterobacteriaceae (CP-CRE) represents one of the greatest infectious disease threats to humans and has been described as requiring “urgent and aggressive action” by the Centers for Disease Control and Prevention (CDC) (9). Most cases of CP-CRE in North America and worldwide are attributed to the Klebsiella pneumoniae carbapenemase (KPC) enzyme and most commonly are associated with the clonal dissemination of K. pneumoniae ST258 (10–13). To date, the occurrence of carbapenemase-producing E. cloacae (CP-E. cloacae) remains relatively low (14). However, there have been recent reports of KPC- and New Delhi metallo-β-lactamase (NDM)-producing E. cloacae isolates circulating and persisting in health care systems in Pennsylvania (15), India (8), Greece (16), Spain (17), and Brazil (18).
Conjugative plasmids are the driving force behind the emergence of CP-CRE, including CP-E. cloacae. These plasmids enable not only the acquisition of genes such as blaKPC and subsequent clonal dissemination but also the conjugal transfer of such elements between unrelated bacteria. Whole-genome sequencing recently was performed on a number of CP-CRE isolates found within the National Institutes of Health (NIH) clinical center and hospital environment (3). This work demonstrated that plasmid transfer between unrelated strains and species of bacteria within a hospital might be further contributing to the dissemination of CP-CRE (3). Other reports of the apparent in vivo transfer of plasmids exist (19), with evidence of plasmid-mediated transfer of blaKPC-3 between E. cloacae isolates in a health care setting (20).
Clonal outbreaks of CP-E. cloacae are rarely reported but do occur; outbreaks have been reported involving Verona integron-encoded metallo-β-lactamase 1 (VIM-1)-producing E. cloacae in Croatia (7) and NDM-1-producing E. cloacae in Nepal (21). A recent outbreak involving a single clone of KPC-producing E. cloacae was reported in a hospital system in Fargo, North Dakota (22). Isolates identified within this system had uniform genetic backgrounds and possessed blaKPC-3 on a transmissible plasmid, suggesting clonal dissemination of an outbreak strain.
The Minnesota Department of Health (MDH) initiated surveillance for CRE in 2009 and has observed a constant influx of CP-E. cloacae isolates proportionate to CP-K. pneumoniae isolates, which is a phenomenon that has been reported only recently in other areas of the United States (personal correspondence). Therefore, the purpose of this study was to examine the genotypic characteristics, genetic relatedness, and phylogeography of a subset of CP-E. cloacae clinical isolates collected between 2009 and 2013 and the possible dissemination of KPC-encoding plasmids between E. cloacae and Escherichia coli within a single patient.
MATERIALS AND METHODS
Bacterial isolates.
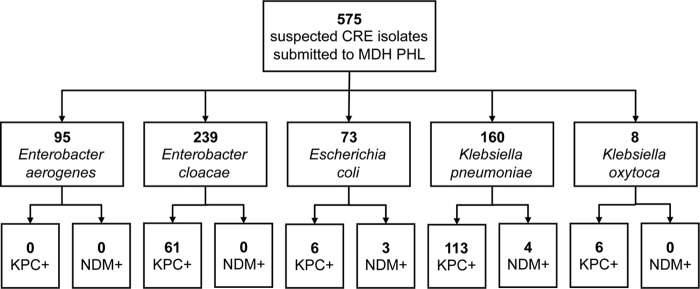
A total of 575 suspected CRE clinical isolates (Enterobacter species, E. coli, and Klebsiella species) were submitted by clinical laboratories to the MDH-Public Health Laboratory (PHL) between 2009 and 2013 as part of a statewide surveillance program, including border facilities in North Dakota and South Dakota (Fig. 1); active surveillance for CRE was initiated in June 2011 in Hennepin and Ramsey counties as part of the CDC Multi-Site Gram-Negative Surveillance Initiative Program. Starting in 2009, clinical laboratories were asked to submit isolates according to the following definition: reduced susceptibility to at least one carbapenem antibiotic (imipenem, meropenem, doripenem, or ertapenem [MIC ≥ 2 μg/ml]) or suspected carbapenemase producer based on a phenotypic (e.g., the modified Hodge test) or genotypic (e.g., PCR for carbapenemase genes) test. Criteria were modified in 2011 to exclude ertapenem and also require resistance to all third-generation cephalosporins tested (23). Isolates in this study did not result from screening for fecal carriage of the organism. The identification of each isolate was confirmed phenotypically through conventional biochemical testing.
FIG 1.
Carbapenem-resistant Enterobacteriaceae (CRE) isolates submitted to the MDH-PHL for further characterization according to the MDH CRE definition. Isolates were collected between February 2009 and December 2013. Other species of CRE (e.g., Citrobacter spp.) submitted for additional testing were excluded. Whole-genome sequencing (WGS) was performed subsequently on a subset of CRE isolates (40 E. cloacae and 4 E. coli isolates) for this study.
Of the 575 suspected CRE isolates submitted, 61 CP-E. cloacae isolates were identified by real-time PCR targeting blaKPC and blaNDM carbapenemase genes with an additional CP isolate (blaIMI-3) identified by whole-genome sequencing; 49 isolates were from unique patients. Thirty-four CP-E. cloacae isolates representing 32 patients were initially examined in this study; the isolation of a second E. cloacae isolate was observed for two patients. Additionally, 6 non-CP clinical E. cloacae isolates were included as comparison strains. Four E. coli isolates cultured from a single patient coinfected with CP-E. cloacae also were analyzed (Table 1).
TABLE 1.
Isolates examined in this study
| ID | Collection date | Source | blaKPC PCR status | ECL subtypea | NCBI accession no. |
|---|---|---|---|---|---|
| E. cloacae | |||||
| MNCRE03 | September 2009 | Urine | − | 2 | JZCX00000000 |
| MNCRE04 | November 2009 | Urine | − | 6 | JZDF00000000 |
| MNCRE09 | December 2009 | Respiratory | + | 8 | JZDE00000000 |
| MNCRE08 | January 2010 | Blood | + | 1 | JYLY00000000 |
| MNCRE07 | January 2010 | Blood | + | 1 | JYLX00000000 |
| MNCRE06 | January 2010 | Urine | + | 1 | JYLW00000000 |
| MNCRE12 | May 2010 | Urine | − | 10 | JYME00000000 |
| MNCRE14b | July 2010 | Respiratory | − | 11 | JYMF00000000 |
| MNCRE15 | August 2010 | Urine | − | 12 | JYMG00000000 |
| MNCRE16 | September 2010 | Urine | − | 13 | JYMH00000000 |
| MNCRE17 | October 2010 | Fluid | + | 14 | JYMI00000000 |
| MNCRE18 | October 2010 | Respiratory | + | 1 | JYLZ00000000 |
| MNCRE19 | June 2011 | Fluid | + | 19 | JYMJ00000000 |
| MNCRE20 | July 2011 | Respiratory | − | 16 | JYMM00000000 |
| MNCRE22 | August 2011 | Blood | + | 32 | LKAY00000000 |
| MNCRE23 | August 2011 | Respiratory | + | 17 | JYMN00000000 |
| MNCRE25 | September 2011 | Urine | + | 17 | JYMO00000000 |
| MNCRE28 | September 2011 | Urine | + | 32 | JYMA00000000 |
| MNCRE29c | October 2011 | Urine | + | 15 | JYMK00000000 |
| MNCRE30 | October 2011 | Urine | + | 32 | JYMB00000000 |
| MNCRE35 | December 2011 | Respiratory | + | 20 | JZCV00000000 |
| MNCRE36 | December 2011 | Fluid | + | 21 | JZCY00000000 |
| MNCRE37 | December 2011 | Urine | + | 17 | JYMP00000000 |
| MNCRE38 | January 2012 | Urine | + | 17 | JYMQ00000000 |
| MNCRE39 | January 2012 | Respiratory | + | 17 | JZDG00000000 |
| MNCRE42 | March 2012 | Urine | + | 17 | JYLQ00000000 |
| MNCRE41d | March 2012 | Respiratory | + | 17 | JYLR00000000 |
| MNCRE48 | April 2012 | Bone | + | 1 | JYMC00000000 |
| MNCRE51d | May 2012 | Respiratory | + | 21 | JYLU00000000 |
| MNCRE52 | May 2012 | Urine | + | 30 | JZDD00000000 |
| MNCRE55 | July 2012 | Urine | + | 24 | JZDA00000000 |
| MNCRE56 | August 2012 | Urine | + | 22 | JZCZ00000000 |
| MNCRE57c | August 2012 | Respiratory | + | 15 | JYML00000000 |
| MNCRE71 | November 2012 | Wound | + | 28 | JZDB00000000 |
| MNCRE79 | February 2013 | Urine | + | 17 | LDEN00000000 |
| MNCRE81 | March 2013 | Fluid | + | 17 | LDEO00000000 |
| MNCRE83 | April 2013 | Urine | + | 29 | JZDC00000000 |
| MNCRE86 | April 2013 | Urine | + | 18 | JZCW00000000 |
| MNCRE84 | April 2013 | Urine | + | 32 | JYMD00000000 |
| MNCRE85 | May 2013 | Urine | + | 17 | LBMV00000000 |
| E. coli | |||||
| MNCRE46 | April 2012 | Urine | + | NA | JYLS00000000 |
| MNCRE47 | April 2012 | Respiratory | − | NA | JYLT00000000 |
| MNCRE45 | April 2012 | Urine | + | NA | JYLV00000000 |
| MNCRE44 | April 2012 | Respiratory | + | NA | NZ_CP010876.1 |
ECL subtypes were determined using pulsed-field gel electrophoresis; ECL numbers are PFGE subtype designations determined by the MDH-PHL. Subtypes were not determined for E. coli isolates examined in this study.
blaIMI-3 carbapenemase positive.
Isolates were cultured from a single patient, termed patient A.
Isolates were cultured from a single patient, termed patient B.
PFGE.
E. cloacae isolates were characterized by pulsed-field gel electrophoresis (PFGE) according to the previously published protocol by Ribot et al., with modifications (24). Electrophoresis conditions were changed to have an initial switch time of 4.0 s and a final switch time of 40.0 s, and gels were run for 18 h. The resulting PFGE patterns were analyzed in BioNumerics software (Applied Maths, Austin, TX) with dendrograms based on the Dice coefficient with a band position tolerance of 1%. Patterns with no discernible differences were considered indistinguishable and given the same PFGE pattern designation.
Real-time PCR.
Singleplex (submission dates, July 2009 to December 2011) or multiplex (submission dates, January 2012 to present) real-time PCR targeting blaKPC or blaKPC and blaNDM carbapenemase genes, respectively, was performed on all analyzed isolates based on a modified CDC protocol (25). Primers and dually labeled fluorescence resonance energy transfer (FRET) probes were ordered from Biosearch Technologies (Petaluma, CA) using sequences described previously (25); probes were labeled with equivalent fluorophores and quenchers recommended by the manufacturer.
DNA templates were prepared via boiling lysis of bacterial cells (26). Reaction mixtures containing bacterial lysate, primers, probes, and PerfeCTa quantitative PCR ToughMix (Quanta Biosciences, Gaithersburg, MD) were mixed and amplified on a Bio-Rad CFX96 real-time PCR detection system instrument (Bio-Rad Laboratories, Inc., Hercules, CA) under the following conditions: denaturing step of 95°C for 3 min, followed by 40 cycles of 95°C for 30 s and 60°C for 1 min. Collected amplification curve data were visualized and analyzed using Bio-Rad CFX Manager 3.1 software (Bio-Rad Laboratories, Inc., Hercules, CA).
Antimicrobial susceptibilities.
Antimicrobial susceptibility profiles from automated or manual test methods were generated by the clinical laboratories and submitted with most isolates. Additional broth microdilution susceptibility testing was performed at MDH-PHL for 34 CP-E. cloacae isolates using GNX2F Sensititre plates (TREK Diagnostic Systems, Oakwood Village, OH) with subsequent analysis on a Sensititre Vizion system (Trek Diagnostic Systems).
DNA sequencing.
Selected isolates in this study were sequenced (n = 44) using Illumina MiSeq (Table 1). Isolates were grown overnight from several colonies in 3 ml lysogeny broth (LB) with shaking. DNA was extracted from each isolate using a Qiagen DNeasy kit (Valencia, CA) according to the manufacturer's instructions. Nextera libraries were created using genomic DNA. Sequencing was performed at the University of Minnesota Genomics Center (Minneapolis, MN) using a single 250-bp dual-index run on an Illumina MiSeq to generate approximately 50- to 100-fold coverage per genome.
Two isolates, MNCRE41 and MNCRE44, also were sequenced using PacBio technology at the Rochester Mayo Medical Genome Facility. SMRTbell template libraries were generated from previously isolated unsheared raw genomic DNA using Pacific Biosciences SMRTbell template preparation kit 1.0 (Pacific Biosciences, Menlo Park, CA). Finished DNA libraries subsequently were subjected to DNA size selection using the BluePippin DNA size selection system (Sage Science Inc.) with a 7-kb cutoff to select DNA fragments greater than 7 kb. Sequencing was performed on the PacBio RSII (Pacific Biosciences, Menlo Park, California) using P6 polymerase binding and C4 sequencing kits with magnetic bead loading and 240-min acquisition.
Analysis and annotation.
Genome assembly of MiSeq reads was performed using CLC Genomics Workbench, version 7.5 (CLC Bio, Boston, MA). Assembly parameters were the following: automatic word size, automatic bubble size, minimum contig size of 500 bp, length fraction of 0.5, and similarity fraction of 0.8. Genome annotation was performed using the National Center for Biotechnology Information (NCBI) Prokaryotic Genomes Automatic Annotation Pipeline (PGAAP; http://www.ncbi.nlm.nih.gov/genome/annotation_prok/). XPlasMap was used to construct plasmid maps (http://www.iayork.com/Widgets.shtml#XPlasMap). Resistance genes were identified using the Comprehensive Antibiotic Resistance database (27). Plasmid types were identified using PlasmidFinder (28). E. cloacae multilocus sequence types (MLST) were identified in silico using MLST 1.7 (29). PacBio genome assemblies were performed using HGAP 3 as part of SMRTAnalysis, version 2.2. Assemblies were subjected to three rounds of polishing with Quiver. SMRT-based assemblies were further validated and error corrected using MiSeq read data by reassembling the MiSeq reads to the finished PacBio sequences and resolving remaining ambiguities through manual inspection.
SNP identification and analysis.
As an initial approach to compare all sequenced E. cloacae isolates, the draft assemblies of each genome were aligned in MAUVE and their single-nucleotide polymorphisms (SNPs) were extracted. MAUVE was used for this analysis because of the sequence divergence between CP and non-CP strains and the lack of raw sequencing reads for NCBI database reference isolates to assemble to a common reference strain. For all subsequent SNP-based comparisons using closely related strains, sequencing reads from each isolate were mapped to the assembled and annotated chromosome of strain MNCRE09 as an index isolate. Mapping was performed in CLC Genomics Workbench with the following parameters: length fraction of 0.5, similarity fraction of 0.9, and random mapping of nonspecific matches. Following mapping, SNP identification was performed for each isolate using basic variant detection with the following parameters: minimum coverage of 10, maximum coverage of 1,000, minimum frequency of 90%, and ignoring broken pairs. SNPs were exported and used to infer phylogenetic relatedness using MEGA6 (30). Prior to inferring phylogenetic relatedness, recombinant regions were identified in the genomes using BratNextGen and subsequently were removed from the analysis (31). The estimation of recombination was performed with default settings with 5-kb blocks using 20 iterations of the estimation algorithm. The significance of a recombinant region was determined with 100 permutations, and a threshold of 5% was used to conclude significance for each region. SNPs were further analyzed for effect on coding sequences using SnpEff (32).
Phylogenetic analyses.
To estimate the phylogeny for the alignment of all SNPs, Bayesian framework implemented in BEAST software package version 1.5.4 (33) under the general time-reversible nucleotide substitution model was used. Three replications were run under this model. The molecular clock was calibrated under a relaxed clock (which allows different rates on different branches, drawn from a specified distribution) (34). The Markov chain Monte Carlo (MCMC) analysis was run until evidence of proper mixing was obtained (up to 107 generations and sampled every 10,000th generation). Results were visualized in Tracer v.1.5, and proper mixing of the MCMC analysis was assessed by calculating the effective sampling size (ESS) for each parameter (33). The maximum clade credibility (MCC) tree, which is the tree with the largest product of posterior clade probabilities, was selected from the posterior tree distribution using TreeAnnotator version 1.5.4 (part of the BEAST package). Final trees were annotated with FigTree version 1.3 (http://tree.bio.ed.ac.uk/software/figtree/).
Global scan for genes under positive selection.
A scan for genes under positive selection within the circulating lineage was performed by selecting representative genomes from each major clade from the circulating lineage (MNCRE19 and MNCRE25) and comparing it with reference genomes from the following strains: 34399 (NZ_CP010384), 34977 (NZ_CP010376.2), 34983 (NZ_CP010377), ATCC 13047 (NC_014121.1), ECNIH5 (NZ_CP009854), and NDA (NZ_JWRO01000001). A set of all protein and coding sequence fasta files were extracted from GenBank annotation files for each organism using biopython. All-versus-all blastp searches (NCBI BLAST+, v. 2.2.28) were performed with an E value cutoff of 1e−5 (35). The resulting tabular (-outfmt 7) blastp output was filtered to retain only the reciprocal best hits and then clustered into orthologous groups using MCL (v. 14-137). Multifasta coding DNA sequences were collected for sequences within each orthologous group and then aligned using MUSCLE v 3.8.31 (36). Phylogenetic trees were generated for each alignment with MrBayes v. 3.2.1 (nucmodel = codon; nst = 6; rates = gamma; 2 runs, 20,000 generations) (37). Finally, PAML v. 4.6 was used to scan for positive selection along each branch in the phylogenetic tree (specified within the tree file as a free parameter for the codeml module). PAML output was parsed to retain instances where the dN/dS (the ratio of nonsynonymous to synonymous evolutionary changes) along any branch was >1.0, provided that appreciable observations of nonsynonymous substitutions were actually observed (i.e., dN > 0.01) (38). Cases of nonconvergence (dN/dS in PAML of 999) were discarded.
Genome comparisons.
For the analysis of gene content, the assembled genomes were uploaded to the RAST Server for automated annotation and comparisons between strains (39). Using MNCRE09 as a reference, the genomes of all strains in this study, along with all available reference genomes of E. cloacae, were compared using protein BLAST with a cutoff of 90% amino acid similarity across 90% of the predicted protein. Genes encoding proteins unique to E. cloacae lineages of interest were further analyzed using protein BLAST. For nucleotide similarities between genomes, nucleotide BLAST and circular comparison maps were generated using the CGView comparison tool (40). The following reference strain genomes were included for comparison purposes: ATCC 13047 (CP001918), ECNIH5 (CP009854), 34983 (CP010377), 34977 (CP010376), and 34399 (CP010384).
Plasmid profiling.
S1 nuclease PFGE was performed on selected isolates for plasmid visualization, as previously described (41). PCR also was performed to confirm the apparent junctions of cointegrate plasmid formation in E. coli isolates. Primers are listed in Table S1 in the supplemental material.
Nucleotide sequence accession numbers.
Whole-genome draft sequence assemblies have been deposited at NCBI GenBank and are listed in Table 1. The complete sequences of the chromosome of E. coli MNCRE44 and its six plasmids, pMNCRE44_1 through pMNCRE44_6, have been deposited in GenBank under the accession numbers CP010876, CP010877, CP010878, CP010879, CP010880, CP010881, and CP010882, respectively (42).
RESULTS
Occurrence of carbapenemase-producing E. cloacae in Minnesota, 2009 to 2013.
Between 2009 and 2013, a total of 575 suspected CRE isolates (334 Enterobacter species, 73 E. coli isolates, and 168 Klebsiella species; Fig. 1) were submitted to the MDH-PHL by clinical laboratories. Roughly one-third of submitted CRE isolates (193/575; 34%) possessed a blaKPC or blaNDM carbapenemase gene, as determined by real-time PCR. E. cloacae formed the largest proportion of submitted isolates (239/575; 42%).
Among the 239 E. cloacae isolates submitted, 62 CP-E. cloacae isolates were detected, representing 49 unique patients. CP-E. cloacae isolates were submitted by clinical laboratories affiliated with 17 different hospitals, four long-term-care facilities, three long-term acute-care hospitals, and one clinic throughout Minnesota and Fargo, North Dakota. Initial isolates were submitted primarily from west central Minnesota and Fargo, North Dakota; after active surveillance for CRE was initiated in Hennepin and Ramsey counties in June 2011, most isolates were submitted from clinical laboratories serving the Minneapolis and St. Paul metropolitan area health care facilities. Urine was the most common source of isolation for CP-E. cloacae (n = 30; 48%), followed by respiratory (n = 10; 16%), blood (n = 4; 7%), and other sources (n = 18; 30%).
PCR analysis revealed that 61/62 CP-E. cloacae isolates were blaKPC positive and 0/62 were blaNDM positive; an additional CP-E. cloacae isolate was later identified as blaIMI-3 positive by whole-genome sequencing analysis. Forty-two of 62 isolates were further characterized at the MDH-PHL using PFGE. A total of 18 different pulsotypes were identified, with 13 pulsotype patterns demonstrating 85% or greater pattern similarity, suggesting clonality.
MIC broth microdilution testing was performed on a subset of the 62 CP-E. cloacae isolates (n = 34). MIC profiles for 33/34 isolates met the MDH CRE case definition. One isolate possessing blaIMI-3 was resistant to carbapenems but susceptible to all third-generation cephalosporins tested. These 34 isolates were further characterized by whole-genome sequencing.
Carbapenemase-producing E. cloacae isolates spanning 2009 to 2013 in Minnesota are clonal.
Forty clinical E. cloacae isolates (34 CP E. cloacae and 6 non-CP E. cloacae; Table 1) were sequenced using the Illumina MiSeq platform, and draft assemblies were generated for each strain. Multilocus sequence types were assessed in silico, and all CP-E. cloacae strains were typed as ST171 with two exceptions, strains MNCRE14 and MNCRE55. None of the six non-CP isolates belonged to the ST171 sequence type. Antibiotic resistance-associated genes were assessed among these isolates. All CP isolates except MNCRE14 and MNCRE55 contained the following resistance-associated genes: sul2, blaTEM-1, blaOXA-9, blaKPC-3, aadA, aph(3″)-Ib or strA, aph(6)-Id or strB, aac(6′)-Ib or aacA4, and cat. In contrast, strain MNCRE14 only possessed blaIMI-3 and strain MNCRE55 carried blaKPC-3 on a plasmid unrelated to and not found in the other CP isolates.
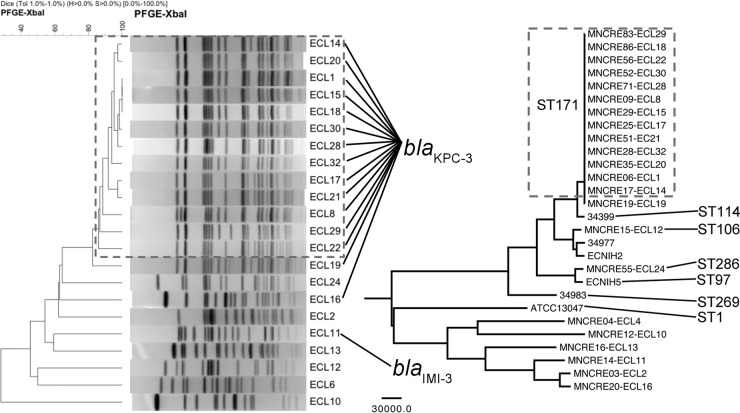
Whole-genome SNPs were used to infer overall evolutionary relatedness using maximum parsimony methods. This approach was compared to PFGE performed on the same isolates (Fig. 2) and confirmed that clinical non-CP E. cloacae isolates were distinct from blaKPC-3-positive isolates. PFGE subtypes and whole-genome SNP analysis agreed, and both indicated that all blaKPC-3-positive isolates except for MNCRE19 and MNCRE55 appeared clonal in nature (Fig. 2). This lineage subsequently is referred to as the circulating lineage (n = 31).
FIG 2.
Genetic relationships between E. cloacae isolates from Minnesota and North Dakota. (Left) Dendrogram of the pulsed-field gel electrophoresis XbaI banding patterns. The dendrogram was generated using the Dice coefficient with a band position tolerance of 1%. Patterns within the hashed box are ≥85% similar and are representative PFGE subtypes of the isolates belonging to the KPC-3-positive circulating lineage. ECL numbers are PFGE subtype designations determined by the MDH-PHL. Strains corresponding to ECL numbers are the following: ECL1, MNCRE06; ECL2, MNCRE03; ECL6, MNCRE04; ECL8, MNCRE09; ECL10, MNCRE12; ECL11, MNCRE14; ECL12, MNCRE15; ECL13, MNCRE16; ECL14, MNCRE17; ECL15, MNCRE29; ECL16, MNCRE20; ECL17, MNCRE25; ECL18, MNCRE86; ECL19, MNCRE19; ECL20, MNCRE35; ECL21, MNCRE51; ECL22, MNCRE56; ECL24, MNCRE55; ECL28, MNCRE71; ECL29, MNCRE83; ECL30, MNCRE52; and ECL32, MNCRE28. Isolates harboring blaKPC-3 or blaIMI-3 are noted. (Right) Phylogenetic relationships between the same isolates based upon whole-genome sequencing, also including existing genome sequences form the NCBI database (see Materials and Methods for a description). Evolutionary history was inferred using the maximum parsimony method on SNPs identified by multiple-genome alignment in MAUVE. Branches corresponding to partitions reproduced in fewer than 50% of bootstrap replicates are collapsed following 1,000 bootstrap replications. All branches were supported with >90% bootstrap confidence. Branches are displayed to scale, and the scale bar represents the number of substitutions. A total of 818,547 SNPs were included in the final data set, and analyses were conducted in MEGA6. The boxed isolates represent KPC-3-positive isolates belonging to the circulating lineage.
Evolutionary history of the circulating lineage of E. cloacae is traced to western Minnesota and eastern North Dakota.
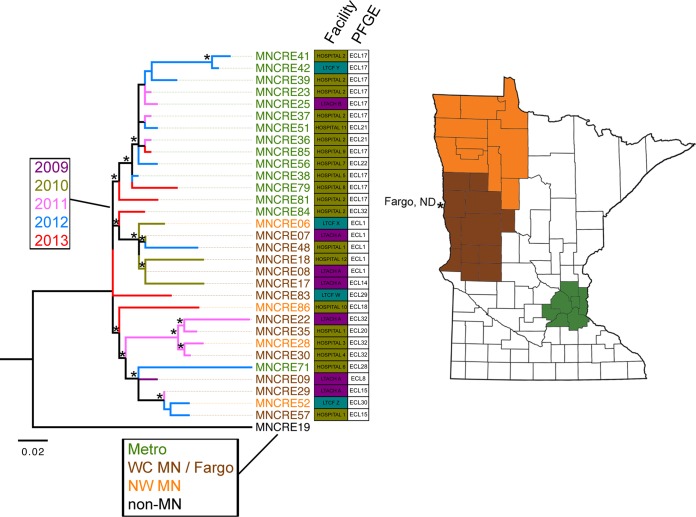
The raw sequencing reads of each blaKPC-3-positive isolate were mapped back to the assembled sequence of strain MNCRE09 to refine the comparison of SNPs within the circulating lineage. Strain MNCRE09 was used as a reference for mapping, because it was isolated in 2009 from a patient in a long-term acute-care hospital serving the western Minnesota and eastern North Dakota region and had the earliest isolation date of CP-E. cloacae in the collection. Prior to reconstructing the evolutionary history, four recombinant genomic regions were identified totaling 359,834 bp. SNPs within these regions were removed from the analysis. Outgroups also were removed in an effort to improve resolution, and the phylogenetic tree then was reconstructed using maximum likelihood methods (Fig. 3). Of note, strain MNCRE19 was closely related to but distinct from the circulating lineage, and epidemiological data suggest that the patient harboring this isolate likely acquired it outside Minnesota and North Dakota, so it was used as an outgroup in Fig. 3. Using this approach, isolates clustered into three main sublineages with strong bootstrap support. These included a sublineage containing 2011-2013 isolates which all originated from the Minneapolis and St. Paul metropolitan area and two other sublineages containing isolates originating from eastern North Dakota and western Minnesota that were mostly collected at earlier time points. While there was geographical clustering based upon inferred phylogenetic relationships, a temporal pattern indicative of singular stepwise clonal evolution was not evident. When examining health care facilities across sublineages, long-term acute-care hospital A and hospital 2 were the only facilities that harbored patients with CP-E. cloacae from multiple sublineages, suggesting microevolutionary lineages leading to multiple-case clusters.
FIG 3.
Phylogenetic relationships between CP-E. cloacae isolates within the circulating lineage. (Left) Evolutionary history was inferred using the maximum likelihood method based on the general time-reversible model, using SNPs identified by mapping to strain MNCRE09 as a reference. Branches corresponding to partitions reproduced in fewer than 50% of bootstrap replicates are collapsed following 10,000 bootstrap replications. Branches are displayed to scale and represent nucleotide changes over the entire sequence assessed. A total of 234 SNPs were included in the final data set, and analyses were conducted in MEGA6. Branches with >60% bootstrap confidence are noted with an asterisk. Branches are colored by year of isolation, and isolate labels are colored by the geographical location of the facility (WC MN, west central MN; NW MN, northwest MN). Facility type (LTACH, long-term acute-care hospital; LTCF, long-term-care facility) and isolate PFGE subtype are included. (Right) Isolate label colors correspond to colored locations on the map of Minnesota (Fargo, ND, isolates were included with WC MN isolates because the city borders WC MN).
Since an outbreak caused by KPC-producing CP-E. cloacae occurred in 2011 to 2012 at the Sanford Health System in Fargo, North Dakota (22), which is in eastern North Dakota bordering west central Minnesota, 14 NCBI whole-genome sequence database isolates from North Dakota were included as a part of the analysis (BioProject PRJNA259658, KPC Enterobacter species). When these isolates were included in the analysis, it was evident that they also belonged to the circulating lineage. Most isolates clustered within their own sublineage, while two isolates clustered with other isolates from west central MN (Fig. 4). This further suggests microevolutionary events related to geography and isolate mixing between health care facilities.
FIG 4.
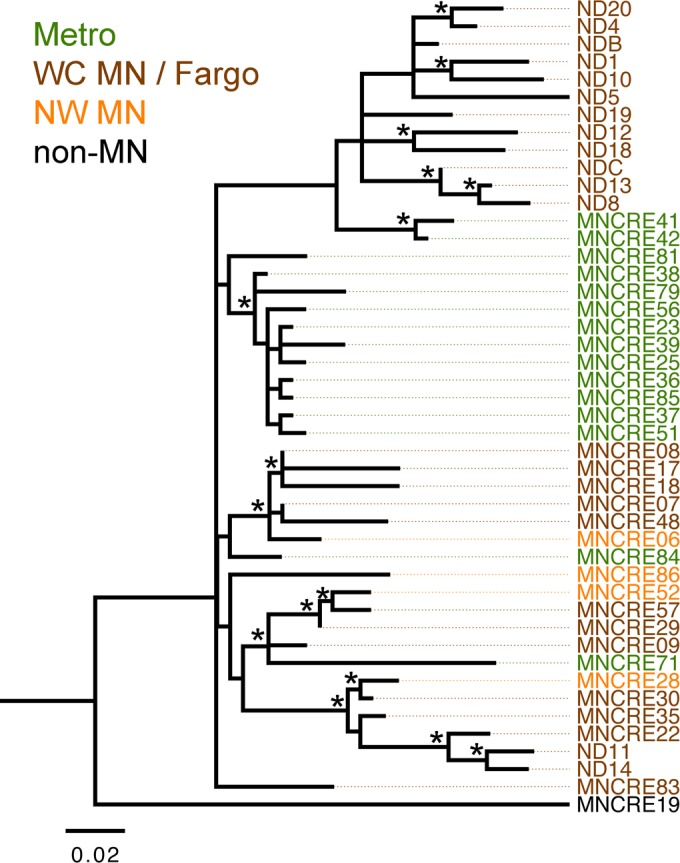
Phylogenetic relationships between CP-E. cloacae isolates in the upper midwestern United States. MNCRE isolates from the Minnesota Department of Health were compared with database isolates from an apparent outbreak in a hospital system in North Dakota (ND) during 2010 to 2012 (BioProject PRJNA259658). Evolutionary history was inferred using the maximum likelihood method based on the general time-reversible model, using SNPs identified by mapping to strain MNCRE09 as a reference. A total of 234 SNPs were included in the final data set, and analyses were conducted in MEGA6. Branches with >60% bootstrap confidence are noted with an asterisk. Branches are displayed to scale and represent nucleotide changes over the entire sequence. A total of 234 SNPs were included in the final data set, and analyses were conducted in MEGA6. Isolate label colors correspond to those in Fig. 3.
Nonsynonymous changes occurring in the circulating E. cloacae lineage.
The circulating clade was examined for evidence of positive selection by comparing it with representative strains across E. cloacae lineages. Beginning with 8 species, clustering of the sequence similarity results yielded 3,915 orthologous gene family groups. Tests of positive selection across every branch in the tree for each of these groups resulted in 22 groups with evidence of positive selection within the circulating lineage (see Table S2 in the supplemental material). Two of these groups had very high dN/dS values: phospholipid-lipid A palmitoyltransferase (dN/dS = 307) and Hcp1 family type VI secretion system effector (dN/dS = 188).
SNPs accumulated in the circulating lineage were analyzed further by comparing them to those of strain MNCRE09, the earliest strain in the collection (see Table S3 in the supplemental material), and indicated a dominance of nonsynonymous changes in these genomes. We further analyzed the coding regions impacted by nonsynonymous changes, and it was clear that many of the impacted proteins had predicted functions related to the regulation of antimicrobial resistance, transport of heavy metals, adhesion, or fitness/virulence (Table 2).
TABLE 2.
Nonsynonymous SNPs present in circulating E. cloacae sublineages compared to sequence of index isolate MNCRE09
| MNCRE09 gene locus | Change in amino acid | Protein description |
|---|---|---|
| 1145 | T234A | Hydroperoxidase |
| 1825 | A289T | Type 1 secretion-associated adhesin |
| 2230 | S56F | LysR DNA binding protein, xenobiotic response family |
| 3185 | G30E | Restriction endonuclease |
| 7060 | E389K | Trimethylamine N-oxide reductase |
| 7190 | H145R | DNA binding protein, xenobiotic response family |
| 8305 | P56T | Serine endoprotease |
| 8680 | A389S | DEAD/DEAH box helicase |
| 9255 | C47Y | Hypothetical protein upstream of a transcriptional regulator |
| 12115 | R654S | TonB receptor protein |
| 13980 | N281I | Spermidine/putrescine ABC transporter permease |
| 14960 | V7L | Hypothetical protein |
| 16825 | D125V | Acyl-coenzyme A esterase |
| 17550 | Y13H | Hypothetical protein upstream of lipid A biosynthesis palmitoleoyl acyltransferase |
| 19685 | T18A | Hypothetical protein |
| 20570 | L735R | Heavy-metal efflux pump protein |
| 21075 | K28Q | Transcriptional activator |
Bayesian molecular clock analysis also reveals recent emergence and spread of CP-E. cloacae.
The results of Bayesian evolutionary analysis sampling tree (BEAST) analysis indicated that the CP-E. cloacae data set is suitable for Bayesian phylogenetic analysis (43). Using strain MNCRE19 as a known closely related outlier, a phylogenetic tree was rooted and used for calculation of the most recent common ancestor (MRCA). The recent evolutionary history as simulated under BEAST estimated the time to most recent common ancestor (TMRCA) for the recent outbreak strains between 16 and 45 years ago (see Fig. S1 in the supplemental material).
Core genome of the circulating lineage.
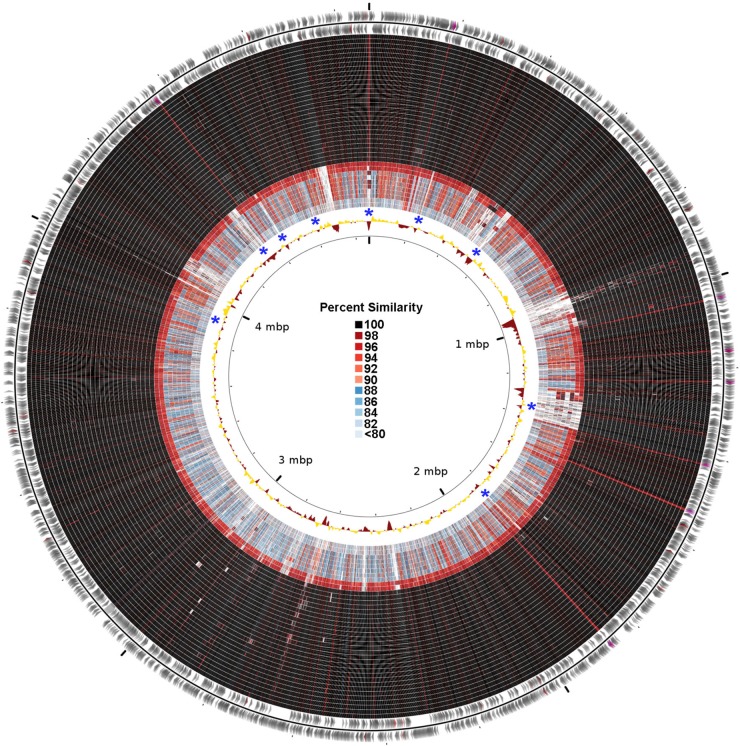
Gene content comparisons were performed between strains of the circulating lineage and other nonrelated E. cloacae isolates to identify regions of interest that possibly contribute to the success of this clone (Fig. 5). From this analysis, 4,545 out of 4,718 coding regions in index strain MNCRE09 (96.3%) were identified as core to all strains within the circulating lineage, including all plasmids and the chromosome. The core genome of all E. cloacae isolates examined, including non-CP isolates from this study and database reference genomes, contained 2,435 of 4,718 coding regions (51.6%). A total of 1,482 coding regions were identified and were present in 100% of the strains of the circulating lineage but were absent from >50% of remaining isolates. Of these, 384 coding regions were unique to the circulating lineage and absent from all other strains. Of the regions that were unique or nearly unique to the circulating lineage, many were associated with heavy-metal resistance, transport of large molecules, production of fimbriae, and biofilm formation (Table 3).
FIG 5.
Circular map visualizing chromosomal nucleotide similarities between strains of the circulating lineage and unrelated reference/KPC-negative E. cloacae genomes. The outer two rings represent the genome of strain NDA, with coding regions in forward and reverse orientations and tRNA genes colored purple. The next 28 rings depict nucleotide similarities between strain NDA and other sequenced E. cloacae isolates belonging to the circulating lineage (some strains were excluded to eliminate redundancy). Similarities were calculated using nucleotide BLAST in 1,000-bp increments across the chromosome. The remaining rings are unrelated E. cloacae reference/KPC-negative strains in the following order: MNCRE15, MNCRE55, ATCC 13047, MNCRE04, MNCRE16, MNCRE03, MNCRE20, MNCRE14, MNCRE46, and MNCRE47. The innermost ring depicts deviation from the average G+C content for the chromosome, with gold representing higher G+C content and maroon representing lower G+C content. Asterisks depict regions of interest, as defined in Table 3. The legend for BLAST similarity colors is in the center of the circle.
TABLE 3.
Regions of interest present in the E. cloacae circulating lineage but absent from other isolates analyzed in this study
| Coordinatesa |
Description | |
|---|---|---|
| Start | Stop | |
| 1 | 7682 | Copper/heavy-metal resistance system |
| 234337 | 240546 | Beta-fimbria usher-chaperone system |
| 526119 | 531827 | Biofilm formation |
| 1287787 | 1311562 | Silver/copper/heavy-metal resistance |
| 1319321 | 1414914 | Integrative conjugative element |
| 1327053 | 1331503 | Mercury resistance |
| 1336017 | 1346424 | Multidrug transport/arsenic resistance |
| 1355500 | 1359153 | Mercury resistance |
| 1830841 | 1834731 | Fimbrial system |
| 3937401 | 3943271 | Heavy metal transport system |
| 4415512 | 4421439 | Iron-specific ABC transport system |
| 4434809 | 4440043 | Fimbrial usher-chaperone system |
| 4590130 | 4594396 | Beta-fimbria usher-chaperone system |
Coordinates correspond to those of strain NDA (NZ_JWRO01000001).
Plasmids conferring carbapenem resistance in E. cloacae.
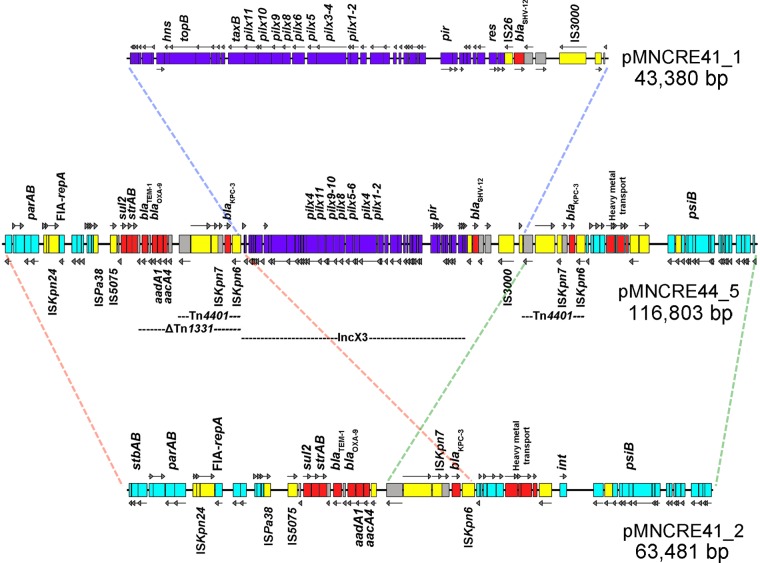
A PacBio instrument was used to complete the plasmid sequences of E. cloacae strain MNCRE41 belonging to the circulating lineage. The assembly yielded two circular plasmids, pMNCRE41_1 (43,380 bp) and pMNCRE41_2 (63,481 bp) (Fig. 6). This was further confirmed via S1 nuclease PFGE plasmid profiling (see Fig. S2 in the supplemental material), which yielded plasmids at their expected sizes using agarose gel electrophoresis. pMNCRE41_1 is an IncX3 plasmid harboring blaSHV-12 adjacent to IS26; pMNCRE41_2 is an IncFIA plasmid harboring blaTEM-1, blaOXA-9, and blaKPC-3 within a ΔTn1331 element which includes Tn4401 nested within. All strains in one of the sublineages also have acquired an additional IncX4 plasmid lacking any antibiotic resistance genes. Overall, the plasmids were highly conserved within the circulating lineage.
FIG 6.
Linear map of plasmids pMNCRE41_1 and pMNCRE41_2 from E. cloacae strain MNCRE41 and pMNCRE44_5 from E. coli ST131 strain MNCRE44. IncX3-associated genes are colored purple, and IncFIA-associated genes are colored light blue. Resistance-associated genes are colored red, and transposase-associated genes are colored yellow. Regions of similarity between plasmids are outlined by colored dashed lines.
The circulating lineage contributes to the dissemination of carbapenem resistance through plasmid transfer.
A temporal collection of isolates from a single patient with extensive health care exposure and multiple comorbidities provided an opportunity to examine plasmid transfer in vivo within the patient between isolates from the circulating E. cloacae lineage and cocolonizing Escherichia coli belonging to the globally emergent ST131 sequence type. Isolates were collected between 8 March 2012 and 1 May 2012 from a single patient and included two E. cloacae (n = 2) and four E. coli (n = 4) isolates from two body sites (Table 4). Both E. cloacae isolates possessed the blaKPC-3 carbapenemase gene. However, E. coli variants isolated from this patient included non-CP and CP strains. Sequencing was performed on all of the isolates, including PacBio sequencing on strains MNCRE41 (KPC-positive E. cloacae) and MNCRE44 (KPC-positive E. coli). Genomic analyses revealed that the E. cloacae isolates from the patient were clonal and belonged to the circulating lineage. All E. coli strains also were clonal and typed in silico as ST131 H30R, a fluoroquinolone-resistant globally circulating sublineage of ST131 (44). Upon initial submission, two of the E. coli strains were susceptible to carbapenems and the other two were carbapenem resistant. Due to an initial carbapenem-susceptible result in an otherwise highly drug-resistant profile, strain MNCRE44 was retested and subsequently found to be imipenem resistant (Table 4). All four strains were identical in their gene content with the exception of a single plasmid, pMNCRE44_5, 116,803 bp in size and present only in the three KPC-positive E. coli strains (Fig. 6).
TABLE 4.
Isolates temporally collected from a single patient
| Organism | Isolate | Date collected | Source | Imipenem MICa | blaKPC PCR |
|---|---|---|---|---|---|
| E. cloacae | MNCRE41 | March 10 | Sputum | 8, R | + |
| E. coli | MNCRE46 | April 11 | Urine | 2, I; 4, R (ms) | + |
| E. coli | MNCRE47 | April 11 | Sputum | ≤1, S | − |
| E. coli | MNCRE45 | April 11 | Urine | ≥16, R | + |
| E. coli | MNCRE44 | April 13 | Sputum | ≤1, S (≥16, Rb) | + |
| E. cloacae | MNCRE51 | May 1 | Sputum | 8, R | + |
MIC values (in micrograms per milliliter) were reported by the submitting clinical laboratories using Vitek 2 (bioMérieux) automated susceptibility analysis, with the exception of the entry labeled ms, which represents a MicroScan (Siemens Healthcare Diagnostics) automated susceptibility testing result. Letters after numbers indicate resistance status: R, resistant; I, intermediate; S, susceptible.
Due to the initial carbapenem-susceptible result in an otherwise highly drug-resistant MIC profile, isolate MNCRE44 was retested using broth microdilution to confirm a carbapenem-resistant phenotype.
Analysis of the two plasmids of E. cloacae and the single plasmid of E. coli from the same patient revealed that they were highly similar (Fig. 6). The single plasmid in E. coli was identified as a cointegrate formation of the two plasmids of E. cloacae, mediated by a duplication of Tn4401 containing blaKPC-3. The Illumina assemblies of MNCRE41 and MNCRE44 also confirmed this orientation; that is, in MNCRE44 the duplication of Tn4401 corresponded to an approximately 2-fold increase in Illumina sequencing coverage in this region, while the remainder of the plasmid was of similar fold coverage in the draft assembly. In contrast, in MNCRE41 the Illumina sequencing coverage of Tn4401 was equal to that of the remainder of the IncFIA plasmid on which it was contained. Furthermore, the IncX3 plasmid Illumina sequencing coverage was approximately twice that of the IncFIA plasmid coverage, supporting the possession of two plasmids of differing copy number by strain MNCRE41. S1 nuclease PFGE plasmid profiling confirmed this finding (see Fig. S2 in the supplemental material), along with PCR spanning the junctions of cointegrate formation (see Fig. S3), suggesting that a non-CP E. coli ST131 isolate (MNCRE44) acquired a cointegrate version of the plasmids of the E. cloacae circulating clone (Fig. 7).
FIG 7.
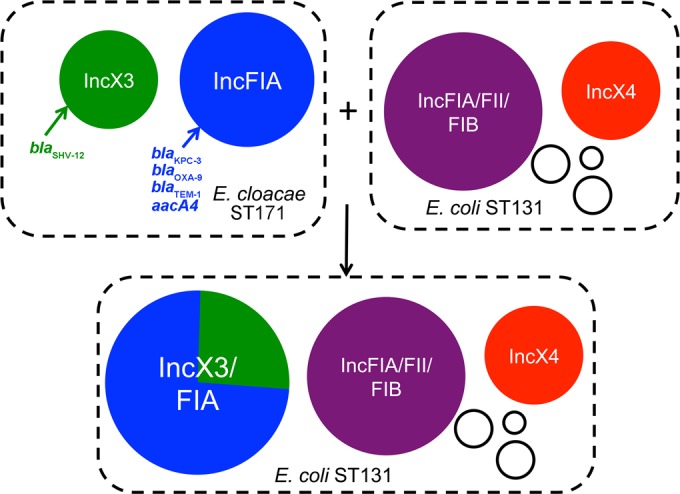
Diagram of proposed plasmid transfer between E. cloacae and E. coli within the same patient.
DISCUSSION
Other locations in the United States have reported K. pneumoniae as the dominant organism possessing blaKPC (14), but E. cloacae appears to be emerging in the upper midwestern United States as an additional prominent carrier of blaKPC (45). Here, we examined the genetic basis for carbapenem resistance in a subset of CP-E. cloacae strains circulating in Minnesota and eastern North Dakota health care facilities. The MDH-PHL performed PFGE on these isolates and identified numerous, closely related PFGE subtypes among the CP-E. cloacae strains examined. Whole-genome sequencing revealed that these strains belonged to a clonal lineage and possessed identical plasmids conferring a multidrug-resistant phenotype. PFGE subtyping correlated with whole-genome sequencing results. PFGE has been used to identify minor mutations in clonal outbreaks of E. cloacae, and the present study confirms the ability of PFGE to distinguish strains even within a circulating clade, albeit at lower resolution than whole-genome sequencing (46).
Based on more detailed phylogenetic analyses using whole-genome sequencing, this clone is circulating in eastern North Dakota and multiple regions of Minnesota, including the Minneapolis and St. Paul metropolitan area, although the full extent of its dissemination is not known. However, it is clear that multiple sublineages of the clone have emerged, and these sublineages are explained in geographical and temporal context yet suggest a mixing effect that could be explained by the movement of patients between health care facilities in the region. A previous study reported a CP-E. cloacae strain isolated from a health care system in Fargo, North Dakota (22). Our analysis suggests that these isolates share an ancestor with the circulating lineage. It is important to point out that the set of suspected CRE organisms analyzed in this study is limited because statewide surveillance was voluntary prior to June 2011; additionally, in June 2011 active surveillance was initiated in the Minneapolis and St. Paul metropolitan area. Therefore, we cannot say where the CP-E. cloacae clone originated because of sampling bias. Despite these limitations, the estimations obtained using synonymous SNP distribution are suggestive of recent emergence and spread of a drug-resistant clone with common ancestral origin. This clone has been in circulation since at least 2009 in Minnesota and continues to be isolated from multiple Minnesota and North Dakota health care facilities; its presence in other regions of the United States is unknown.
Some notable observations came from the analysis of the genomes of strains belonging to the circulating CP-E. cloacae lineage. Nonsynonymous changes have dominated in this clone as it has evolved over the period studied. These changes have occurred in proteins predicted to be involved in the regulation of antibiotic resistance and the physical transport of antibiotics and heavy metals. This is not surprising given that bacterial strains circulating in health care settings are under intense pressure to respond to a variety of chemicals and pharmaceutical agents in hospitals and other health care settings. When we analyzed gene content between this clone and other sequenced E. cloacae isolates, a number of novel systems were identified related to heavy-metal resistance, biofilm formation, iron transport, and fimbria formation. This again suggests adaptations to selective pressure in the hospital and host environments. It is possible that the acquisition of additional systems related to adhesion, biofilm formation, and iron acquisition would enhance this clone's fitness and pathogenic potential, although this remains to be determined.
The plasmids identified in this study belonged to the IncFIA and IncX3 incompatibility types. These plasmids, and their resistance genes, have been identified in other bacterial species (47, 48). The IncX3 plasmid has been identified in a nearly identical conformation in multiple strains of K. pneumoniae ST258 (49). The IncFIA plasmid has been found in multiple strains of K. pneumoniae associated with the NIH hospital CRE outbreak (3). Interestingly, a highly similar form of the cointegrate IncFIA/IncX3 plasmid described in this study (pMNCRE44_5) also was identified in a K. pneumoniae strain from the same NIH hospital outbreak, but it formed at different junctions within the two plasmids. This may highlight convergent plasmid evolution occurring in real time within health care settings, where these plasmids are actively recombining to form more stable cointegrate forms.
The paradigm that plasmid transfer is rare within the host or within hospital environments was challenged recently using single-molecule sequencing of plasmids in the NIH hospital outbreak (3). Here, we present additional evidence that plasmid transfer, and the dissemination of carbapenem resistance, can occur within the patient or within the health care environment. This specific example highlights a dangerous scenario in which a circulating, multidrug-resistant clone (E. cloacae) has transferred its plasmids to a globally emergent extraintestinal pathogen (E. coli ST131), rendering it nearly panresistant to medically relevant antibiotics. This example is alarming for multiple reasons. For one, the CP-E. cloacae clone likely is circulating among asymptomatic carriers in Minnesota and North Dakota and can serve as a reservoir for resistance genes. Second, E. coli ST131 also can exist within the fecal flora in asymptomatic hosts and appears to be a strong colonizer (50). If these strains coexist in the fecal flora, so does the opportunity for plasmid transfer and dissemination of antibiotic resistance. Disease may occur later due to either strain, or when the organism(s) is transferred to a host with risk factors for infection. The strains also may evolve and become more virulent and/or more extensively resistant to antimicrobial agents. Given the phylogeography observed in this study, it is likely that asymptomatic carriage of the CP-E. cloacae clone is an important factor in its dissemination. However, the extent to which this occurs compared to transmission between patients and health care workers is unknown.
This study confirms the unprecedented dissemination of a CP-E. cloacae clone that has emerged in the upper midwestern United States. This clone is under strong positive selection and is evolving to acquire changes in genes predicted to be involved in drug resistance, fitness, and virulence. This clone is contributing to the dissemination of carbapenem resistance in this region, although its current distribution in the broader community is unknown. This provides one example of how such extensively drug-resistant clones are emerging in response to selective pressures, and many of these types of clones currently may be circulating worldwide. Increased surveillance for such clones is an essential step toward understanding mechanisms of dissemination and informing appropriate infection prevention and control measures.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by University of Minnesota College of Veterinary Medicine funding. Computing resources were provided by the University of Minnesota Supercomputing Institute. MDH CRE surveillance was funded by CDC EIP Cooperative Agreement 1U50CK000204-01. This research was also supported in part by an appointment to the Emerging Infectious Diseases (EID) Fellowship Program administered by the Association of Public Health Laboratories (APHL) and funded by the Centers for Disease Control and Prevention (CDC).
We thank Cristian Flores Figueroa and Jeannette Munoz Aguayo for their assistance with the S1 PFGE.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01291-15.
REFERENCES
- 1.Mezzatesta ML, Gona F, Stefani S. 2012. Enterobacter cloacae complex: clinical impact and emerging antibiotic resistance. Future Microbiol 7:887–902. doi: 10.2217/fmb.12.61. [DOI] [PubMed] [Google Scholar]
- 2.Dalben M, Varkulja G, Basso M, Krebs VL, Gibelli MA, van der Heijden I, Rossi F, Duboc G, Levin AS, Costa SF. 2008. Investigation of an outbreak of Enterobacter cloacae in a neonatal unit and review of the literature. J Hosp Infect 70:7–14. doi: 10.1016/j.jhin.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Conlan S, Thomas PJ, Deming C, Park M, Lau AF, Dekker JP, Snitkin ES, Clark TA, Luong K, Song Y, Tsai YC, Boitano M, Dayal J, Brooks SY, Schmidt B, Young AC, Thomas JW, Bouffard GG, Blakesley RW, Mullikin JC, Korlach J, Henderson DK, Frank KM, Palmore TN, Segre JA. 2014. Single-molecule sequencing to track plasmid diversity of hospital-associated carbapenemase-producing Enterobacteriaceae. Sci Transl Med 6:254ra126. doi: 10.1126/scitranslmed.3009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo Y, Yang J, Ye L, Guo L, Zhao Q, Chen R, Chen Y, Han X, Zhao J, Tian S, Han L. 2014. Characterization of KPC-2-producing Escherichia coli, Citrobacter freundii, Enterobacter cloacae, Enterobacter aerogenes, and Klebsiella oxytoca isolates from a Chinese hospital. Microb Drug Resist 20:264–269. doi: 10.1089/mdr.2013.0150. [DOI] [PubMed] [Google Scholar]
- 5.Tijet N, Richardson D, MacMullin G, Patel SN, Melano RG. 2015. Characterization of multiple NDM-1-producing Enterobacteriaceae isolated from the same patient. Antimicrob Agents Chemother doi: 10.1128/AAC.04862-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang F, Jia XQ, Wang B, Li YH, Zhao QG. 2014. Control of an outbreak due to orthopedic infections caused by Enterobacteriaceae producing IMP-4 or IMP-8 carbapenemases. Pathol Biol 62:152–155. doi: 10.1016/j.patbio.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Novak A, Goic-Barisic I, Andrasevic AT, Butic I, Radic M, Jelic M, Rubic Z, Tonkic M. 2014. Monoclonal outbreak of VIM-1-carbapenemase-producing Enterobacter cloacae in intensive care unit, University Hospital Centre Split, Croatia. Microb Drug Resist 20:399–403. doi: 10.1089/mdr.2013.0203. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee P, Jaggi T, Haider M, Mishra B, Thakur A. 2014. Prevalence of carbapenemases and metallo-beta-lactamases in clinical isolates of Enterobacter cloacae. J Clin Diagn Res 8:DM01–DM02. doi: 10.7860/JCDR/2014/9485.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. [Google Scholar]
- 10.Tijet N, Sheth PM, Lastovetska O, Chung C, Patel SN, Melano RG. 2014. Molecular characterization of Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae in Ontario, Canada, 2008-2011. PLoS One 9:e116421. doi: 10.1371/journal.pone.0116421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain R, Walk ST, Aronoff DM, Young VB, Newton DW, Chenoweth CE, Washer LL. 2013. Emergence of carbapenemase-producing Klebsiella pneumoniae of sequence type 258 in Michigan, U S A. Infect Dis Rep 5:e5. doi: 10.4081/idr.2013.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Zulueta P, Silva-Sanchez J, Barrios H, Reyes-Mar J, Velez-Perez F, Arroyo-Escalante S, Ochoa-Carrera L, Delgado-Sapien G, Morales-Espinoza Mdel R, Tamayo-Legorreta E, Hernandez-Castro R, Garza-Ramos U. 2013. First outbreak of KPC-3-producing Klebsiella pneumoniae (ST258) clinical isolates in a Mexican medical center. Antimicrob Agents Chemother 57:4086–4088. doi: 10.1128/AAC.02530-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitchel B, Rasheed JK, Patel JB, Srinivasan A, Navon-Venezia S, Carmeli Y, Brolund A, Giske CG. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother 53:3365–3370. doi: 10.1128/AAC.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. 2013. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn C, Syed A, Hu F, O'Hara JA, Rivera JI, Doi Y. 2014. Microbiological features of KPC-producing Enterobacter isolates identified in a U.S. hospital system. Diagn Microbiol Infect Dis 80:154–158. doi: 10.1016/j.diagmicrobio.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koratzanis E, Souli M, Galani I, Chryssouli Z, Armaganidis A, Giamarellou H. 2011. Epidemiology and molecular characterisation of metallo-beta-lactamase-producing Enterobacteriaceae in a university hospital intensive care unit in Greece. Int J Antimicrob Agents 38:390–397. doi: 10.1016/j.ijantimicag.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Oteo J, Hernandez-Almaraz JL, Gil-Anton J, Vindel A, Fernandez S, Bautista V, Campos J. 2010. Outbreak of VIM-1-carbapenemase-producing Enterobacter cloacae in a pediatric intensive care unit. Pediatr Infect Dis J 29:1144–1146. doi: 10.1097/INF.0b013e3181efaa2d. [DOI] [PubMed] [Google Scholar]
- 18.Rozales FP, Ribeiro VB, Magagnin CM, Pagano M, Lutz L, Falci DR, Machado A, Barth AL, Zavascki AP. 2014. Emergence of NDM-1-producing Enterobacteriaceae in Porto Alegre, Brazil. Int J Infect Dis 25:79–81. doi: 10.1016/j.ijid.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Cremet L, Bourigault C, Lepelletier D, Guillouzouic A, Juvin ME, Reynaud A, Corvec S, Caroff N. 2012. Nosocomial outbreak of carbapenem-resistant Enterobacter cloacae highlighting the interspecies transferability of the blaOXA-48 gene in the gut flora. J Antimicrob Chemother 67:1041–1043. doi: 10.1093/jac/dkr547. [DOI] [PubMed] [Google Scholar]
- 20.Haraoui LP, Levesque S, Lefebvre B, Blanchette R, Tomkinson M, Mataseje L, Mulvey MR, Miller MA. 2013. Polyclonal outbreak of KPC-3-producing Enterobacter cloacae at a single hospital in Montreal, Quebec, Canada. J Clin Microbiol 51:2406–2408. doi: 10.1128/JCM.02480-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoesser N, Sheppard AE, Shakya M, Sthapit B, Thorson S, Giess A, Kelly D, Pollard AJ, Peto TE, Walker AS, Crook DW. 2015. Dynamics of MDR Enterobacter cloacae outbreaks in a neonatal unit in Nepal: insights using wider sampling frames and next-generation sequencing. J Antimicrob Chemother 70:1008–1015. doi: 10.1093/jac/dku521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiedrowski LM, Guerrero DM, Perez F, Viau RA, Rojas LJ, Mojica MF, Rudin SD, Hujer AM, Marshall SH, Bonomo RA. 2014. Carbapenem-resistant Enterobacter cloacae isolates producing KPC-3, North Dakota, U S A. Emerg Infect Dis 20:1583–1585. doi: 10.3201/eid2009.140344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute. 2011. Performance standards for antimicrobial susceptibility testing, 21st informational supplement Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 24.Ribot EM, Wierzba RK, Angulo FJ, Barrett TJ. 2002. Salmonella enterica serotype Typhimurium DT104 isolated from humans, United States, 1985, 1990, and 1995. Emerg Infect Dis 8:387–391. doi: 10.3201/eid0804.010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. 2011. Multiplex real-time PCR detection of Klebsiella pneumoniae carbapenemase (KPC) and New Delhi metallo-B-lactamase (NDM-1) genes. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/HAI/settings/lab/kpc-ndm1-lab-protocol.html. [Google Scholar]
- 26.Johnson JR, Stell AL. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis 181:261–272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- 27.McArthur AG, Waglechner N, Nizam F, Yan A, Azad MA, Baylay AJ, Bhullar K, Canova MJ, De Pascale G, Ejim L, Kalan L, King AM, Koteva K, Morar M, Mulvey MR, O'Brien JS, Pawlowski AC, Piddock LJ, Spanogiannopoulos P, Sutherland AD, Tang I, Taylor PL, Thaker M, Wang W, Yan M, Yu T, Wright GD. 2013. The comprehensive antibiotic resistance database. Antimicrob Agents Chemother 57:3348–3357. doi: 10.1128/AAC.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, Moller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Ponten T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361. doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marttinen P, Baldwin A, Hanage WP, Dowson C, Mahenthiralingam E, Corander J. 2008. Bayesian modeling of recombination events in bacterial populations. BMC Bioinformatics 9:421. doi: 10.1186/1471-2105-9-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cingolani P, Platts A, Wang le L, Coon LM, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drummond AJ, Ho SY, Phillips MJ, Rambaut A. 2006. Relaxed phylogenetics and dating with confidence. PLoS Biol 4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 38.Yang Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 39.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant JR, Arantes AS, Stothard P. 2012. Comparing thousands of circular genomes using the CGView comparison tool. BMC Genomics 13:202. doi: 10.1186/1471-2164-13-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal Biochem 226:235–240. doi: 10.1006/abio.1995.1220. [DOI] [PubMed] [Google Scholar]
- 42.Johnson TJ, Hargreaves M, Shaw K, Snippes P, Lynfield R, Aziz M, Price LB. 2015. Complete genome sequence of a carbapenem-resistant extraintestinal pathogenic Escherichia coli strain belonging to the sequence type 131 H30R subclade. Genome Announc 3:e00272-15. doi: 10.1128/genomeA.00272-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heled J, Drummond AJ. 2008. Bayesian inference of population size history from multiple loci. BMC Evol Biol 8:289. doi: 10.1186/1471-2148-8-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price LB, Johnson JR, Aziz M, Clabots C, Johnston B, Tchesnokova V, Nordstrom L, Billig M, Chattopadhyay S, Stegger M, Andersen PS, Pearson T, Riddell K, Rogers P, Scholes D, Kahl B, Keim P, Sokurenko EV. 2013. The epidemic of extended-spectrum-beta-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. mBio 4:e00377-13. doi: 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw KM, Harper JE, Vagnone PS, Lynfield R. 2014. Establishing surveillance for carbapenem-resistant Enterobacteriaceae in Minnesota, 2012. Infect Control Hosp Epidemiol 35:451–453. doi: 10.1086/675615. [DOI] [PubMed] [Google Scholar]
- 46.Stumpf AN, Roggenkamp A, Hoffmann H. 2005. Specificity of enterobacterial repetitive intergenic consensus and repetitive extragenic palindromic polymerase chain reaction for the detection of clonality within the Enterobacter cloacae complex. Diagn Microbiol Infect Dis 53:9–16. doi: 10.1016/j.diagmicrobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Johnson TJ, Bielak EM, Fortini D, Hansen LH, Hasman H, Debroy C, Nolan LK, Carattoli A. 2012. Expansion of the IncX plasmid family for improved identification and typing of novel plasmids in drug-resistant Enterobacteriaceae. Plasmid 68:43–50. doi: 10.1016/j.plasmid.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Chen L, Chavda KD, Melano RG, Hong T, Rojtman AD, Jacobs MR, Bonomo RA, Kreiswirth BN. 2014. Molecular survey of the dissemination of two blaKPC-harboring IncFIA plasmids in New Jersey and New York hospitals. Antimicrob Agents Chemother 58:2289–2294. doi: 10.1128/AAC.02749-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Fernandez A, Villa L, Carta C, Venditti C, Giordano A, Venditti M, Mancini C, Carattoli A. 2012. Klebsiella pneumoniae ST258 producing KPC-3 identified in Italy carries novel plasmids and OmpK36/OmpK35 porin variants. Antimicrob Agents Chemother 56:2143–2145. doi: 10.1128/AAC.05308-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicolas-Chanoine MH, Bertrand X, Madec JY. 2014. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev 27:543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.