Abstract
Tuberculosis (TB) remains a major public health issue due to the increasing incidence of type 2 diabetes mellitus (T2DM), which exacerbates the clinical course of TB and increases the risk of poor long-term outcomes. The aim of this study was to characterize the pharmacokinetics of rifampin (RIF) and its relationship with biochemical and immunological parameters in patients with TB and T2DM. The biochemical and immunological parameters were assessed on the same day that the pharmacokinetic evaluation of RIF was performed. Factors related to the metabolic syndrome that is characteristic of T2DM patients were not detected in the TB-T2DM group (where predominant malnutrition was present) or in the TB group. Percentages of CD8+ T lymphocytes and NK cells were diminished in the TB and TB-T2DM patients, who had high tumor necrosis factor alpha (TNF-α) and low interleukin-17 (IL-17) levels compared to healthy volunteers. Delayed RIF absorption was observed in the TB and TB-T2DM patients; absorption was poor and slower in the latter group due to poor glycemic control. RIF clearance was also slower in the diabetic patients, thereby prolonging the mean residence time of RIF. There was a significant association between glycemic control, increased TNF-α serum concentrations, and RIF pharmacokinetics in the TB-T2DM patients. These altered metabolic and immune conditions may be factors to be considered in anti-TB therapy management when TB and T2DM are concurrently present.
INTRODUCTION
Tuberculosis (TB) is an infectious disease caused by the Mycobacterium tuberculosis complex (MTC) and remains a major cause of morbidity and mortality in developing countries. In 2013, an estimated 9 million people developed the disease, resulting in 1.5 million deaths (1). One-third of the population is infected with MTC, but only 5% to 10% develop active TB disease. The risk is increased by the presence of factors such as HIV, diabetes mellitus (DM), malnutrition, and other environmental factors (2). The increasing incidence of type 2 diabetes mellitus (T2DM) raises the risk of development of active TB in high-burden regions 2-fold to 8-fold over the baseline, especially in individuals with poor glycemic control (3–5). Moreover, an exacerbated TB clinical course and an increased risk of poor long-term outcomes have been reported for T2DM patients (6).
The cure rate reported after the directly observed treatment short course (DOTS) for TB was greater than 85%, with less than 5% of subjects relapsing (1). However, Jiménez-Corona et al. examined 1,262 TB patients from southern Mexico and found that the prevalence of T2DM was 29.63. T2DM was associated with more-severe clinical manifestations (adjusted odds ratio [OR] = 1.80), delayed sputum conversion (OR = 1.51), and a higher probability of treatment failure (OR = 2.93) (9). Differences in the pharmacokinetics (PK) of rifampin (RIF), the most important anti-TB concentration-dependent drug, may contribute to an increased risk of TB treatment failure for diabetic patients (10–14). Additionally, the study by Jiménez-Corona et al. showed that TB recurrence in diabetic individuals compared with nondiabetic individuals was caused by MTC of the same genotype in 81% of cases, indicating a predominance of relapse over exogenous reinfection (9). The molecular basis for this susceptibility to TB in diabetic patients is unclear. One possibility is a compromised immune response in diabetic patients that facilitates either primary progressive tuberculosis or the reactivation of latent tuberculosis infection. Chronic hyperglycemia is associated with dysfunctional immunity to MTC in T2DM patients (15) and hence is likely to reduce the efficacy of antimycobacterial treatment.
The immune response to MTC is complex and heterogeneous and has not been completely characterized. CD4+ T lymphocytes in combination with cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) are fundamental for control of the infection (7). However, active TB in patients with T2DM is characterized by an increased but delayed adaptive response involving CD4+ T cells and cytokines (8). Other types of cells, such as CD8+ T lymphocytes, monocytes, and natural killer (NK) cells, are also involved in anti-MTC immunity, but their role in T2DM susceptibility to TB infection is unclear (7, 8). Biochemical and immunological parameters reflect the pathophysiological status of TB patients with or without T2DM, and the pharmacokinetics (PK) of a drug may be modified according to a patient's condition. Therefore, the aim of this study was to characterize the PK of RIF with suitable time point sampling after a single standard dose. Furthermore, we evaluated biochemical and immunological parameters in TB patients with or without T2DM and examined their correlation with drug disposition.
MATERIALS AND METHODS
Ethics statement.
The study was conducted according to the principles expressed in the Declaration of Helsinki and was approved by the Research Ethics Committee of Health Services of San Luis Potosi, Mexico. All patients and volunteers provided written informed consent for the collection of samples and subsequent analysis.
Patients and healthy volunteers.
A longitudinal prospective study was performed in healthy volunteers, patients with a diagnosis of T2DM, TB patients, and patients with TB and T2DM. The ages of the participants ranged from 18 to 65 years. Patients were included consecutively and matched by gender. Patients were recruited from Infectology and Endocrinology Services in the Hospital Central Dr. Ignacio Morones Prieto in San Luis Potosi, Mexico. TB was diagnosed based on the clinical presentation and was confirmed by microscopic detection of acid-fast bacilli or microbiologic culture. T2DM diagnosis was based on standard guidelines: fasting glycemia glucose level, >126 mg/dl; hemoglobin A1c (HbA1c), >6.5% (16). Screenings for TB or T2DM were performed to sort each patient into the corresponding study group. For healthy volunteers, renal and hepatic function tests, urinalysis, and a chest X-ray were performed at the time of the study. Pregnant women, HIV-positive patients, and patients with inflammatory disorders, microvascular and macrovascular complications, abnormal liver, renal, or thyroid function, steroid therapy, and immunosuppressive medication were excluded. Blood counts and biochemical parameters, such as levels of glycemia, HbA1c, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides, were measured in the serum of patients and healthy volunteers after 10 to 12 h of fasting.
For healthy volunteers and T2DM patients, blood samples were obtained randomly when all of the inclusion criteria were determined. For TB and T2DM-TB patients, samples were drawn on the first day of the DOTS scheme after the TB diagnosis was confirmed.
Isolation of peripheral blood mononuclear cells.
Blood samples were obtained by venipuncture and collected in tubes with EDTA as an anticoagulant. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Hypaque density gradient centrifugation (Sigma Chemicals, St. Louis, MO, USA). Cell viability was evaluated with trypan blue staining. Then, the cells were washed and counted in a Neubauer chamber and adjusted to 1 × 106 cells/ml–phosphate-buffered saline (PBS).
Flow cytometry.
PBMCs were immunostained with mouse anti-human CD4-fluorescein isothiocyanate (FITC), anti-human CD8-FITC, anti-human CD14-FITC, or anti-human CD56-FITC monoclonal antibodies (BD-Pharmingen, San Diego, CA, USA) for 20 min at 4°C. After incubation, the labeled PBMCs were washed and fixed with 1% paraformaldehyde. Cells were gated in a FACSCalibur flow cytometer using CellQuest software (Becton Dickinson, San Jose, CA, USA). The results were presented as the percentage of positive cells relative to the blood counts and were reported as the absolute number of cells per microliter.
Serum cytokine quantification.
Serum was collected and frozen (at below −20°C) prior to the cytokine assay. Levels of cytokines (IFN-γ, TNF-α, interleukin-17 [IL-17], and IL-10) were measured using a commercial enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA).
Pharmacokinetics assessment.
During the first day of DOTS, the TB and TB-T2DM patients received a standard dose of RIF, isoniazid, and pyrazinamide (Rifater; Sanofi-Aventis, Mexico, D.F.) under fasting conditions with the following weight criteria: three tablets for patients of <50 kg body weight and four tablets for patients of >50 kg. The formulation contained 150 mg of RIF, 75 mg of isoniazid, and 400 mg of pyrazinamide per tablet. A standard low-fat diet was provided 3, 6, and 12 h after medication intake; the patients were allowed to ingest water ad libitum (17, 18). After the drugs were administered, blood samples were collected 0.33, 0.66, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 h postdose. Plasma was immediately separated and frozen at −80°C prior to analysis.
Quantification of rifampin in plasma.
The plasma concentration of RIF was assessed using the high-performance liquid chromatography (HPLC) method that was previously described and validated (19). An HPLC Breeze system consisted of a 1525 multisolvent delivery system, a 717 Plus loop injector, a UV light/visible light 2487 detector, and software (Waters Corporation, Milford, MA, USA). RIF was extracted from human plasma by adding acetonitrile (Fermont). The method was previously validated in a range of 0.1 to 20 mg/liter with an accuracy of 99.7% using a USP reference standard for RIF. The inter- and intra-assay coefficients of variation (CV) were 1.41% and 8.71%, respectively. The limit of detection was 0.03 mg/liter, and the limit of quantification was 0.1 mg/liter (CV = 3.2%). Measurements below the assay range were assigned a value equal to the mean between the lower limit of quantification and the limit of detection.
Pharmacokinetic analysis.
Plasma concentration versus time for each volunteer was assessed using compartmental and noncompartmental approaches (WinNonlin version 6.3; Pharsight Corp., St. Louis, MO, USA). For the noncompartmental model, the maximum observed concentration (Cmax) and the time at which this occurred (Tmax) were determined directly from the pharmacokinetic profile. The area under the concentration-time curve from time 0 to the last observation point at 24 h (AUC0–24) was calculated with the linear trapezoidal rule. The point extrapolated to infinity (AUC0–∞) was based on the last time estimated as AUC0–∞ = AUC0–24 + C24/λz, where λz was the first-order rate constant associated with the terminal (log-linear) portion of the curve estimated by the linear regression of time versus log concentration. The mean residence time (MRT) was calculated as follows: MRT = AUMC0–∞/AUC0–∞, where area under the first moment curve (AUMC) was calculated with use of the trapezoidal rule.
One-compartment open models were performed with first-order absorption and elimination with a lag time (tlag) in the absorption phase. The parameters evaluated by the compartmental model were the volume of distribution (V), the absorption rate (Ka) and the absorption half-life (t1/2abs), the elimination rate (Ke), and the elimination half-life (t1/2e), the tlag as the time prior to the first measurable concentration, and the total body clearance (CL).
Statistical analysis.
Pharmacokinetic parameters and covariates were assessed using the Kolmogorov-Smirnov test to determine the normality of the data distribution. Differences in anthropometric, biochemical, immunological, and pharmacokinetic parameters between TB, TB-T2DM, and T2DM patients and healthy volunteers were assessed with a one-way analysis of variance (ANOVA) and Tukey's post hoc text or a Kruskal-Wallis test with Duncan's post hoc test for parametric or nonparametric data, respectively. Pearson correlations were performed between continuous variables and PK parameters according to the group analyzed. All statistical evaluations were performed with GraphPad Prism V.5. P values of less than 0.05 were considered statistically significant.
RESULTS
Patients and healthy volunteers.
The anthropometric and biochemical characteristics of the 24 TB patients, 24 TB-T2DM patients, 18 T2DM patients, and 24 healthy volunteers included in the current study are detailed in Table 1. The predominant pulmonary form was observed for 62.5% of the TB patients and 87% of the TB-T2DM patients. The diabetic TB patients were older than the nondiabetic TB patients (P < 0.001); the body mass index (BMI) data and the doses of RIF per kilogram of body weight were similar between these groups. Close to 70% of the diabetic TB patients were previously diagnosed with T2DM; the remainder of these patients were diagnosed as diabetic at the time of the study.
TABLE 1.
Anthropometric data and biochemical measurements in patients and healthy volunteersa
| Parameter | Values for group |
P | |||
|---|---|---|---|---|---|
| Healthy volunteers | T2DM patients | TB patients | TB-T2DM patients | ||
| n (no. of females/no. of males) | 24 (15/9) | 18 (9/9) | 24 (11/13) | 24 (11/13) | >0.05 |
| Age (yrs) | 35.8 ± 6.6 | 45.4 ± 11.4 | 37.3 ± 14.8 | 52.2 ± 9.6 | <0.001d,g |
| BMI (kg/m2) | 23.7 ± 3.1 | 32.0 ± 7.5 | 21.6 ± 3.9 | 23.3 ± 3.8 | <0.0001b,e,f |
| Glucose (mg/dl) | 79.8 ± 8.3 | 178 ± 93 | 88.5 ± 10.8 | 188 ± 112 | <0.0001b,d,e,g |
| HbA1c (%) | 5.40 ± 0.3 | 8.27 ± 2.0 | 5.47 ± 0.7 | 9.02 ± 2.5 | <0.0001b,d,e,g |
| Total cholesterol (mg/dl) | 160 ± 28 | 188 ± 30 | 135 ± 26 | 157 ± 50 | 0.0001e,f |
| HDL cholesterol (mg/dl) | 60.0 ± 12.7 | 54.9 ± 17.8 | 41.1 ± 15.6 | 41.6 ± 15.0 | <0.0001b,c,e,f |
| LDL cholesterol (mg/dl) | 80.0 ± 22.8 | 96.2 ± 23.3 | 69.3 ± 21.2 | 85.6 ± 36.0 | 0.014e |
| Triglycerides (mg/dl) | 98 ± 58 | 182 ± 71 | 115 ± 50 | 148 ± 80 | <0.0001b,d,e |
Data are shown as median ± standard deviation. BMI, body mass index. Bold numbers are statistically significant (P < 0.05).
Data represent the differences in post hoc analysis for healthy volunteers versus T2DM patients.
Data represent the differences in post hoc analysis for healthy volunteers versus TB patients.
Data represent the differences in post hoc analysis for healthy volunteers versus TB-T2DM patients.
Data represent the differences in post hoc analysis for T2DM versus TB patients.
Data represent the differences in post hoc analysis for T2DM versus TB-T2DM patients.
Data represent the differences in post hoc analysis for TB versus TB-T2DM patients.
To confirm the diagnosis and management of T2DM in patients with or without TB, blood glucose and HbA1c levels were determined after 10 to 12 h of fasting (Table 1). The percentages of diabetic patients with and without TB with poor glycemic control (HbA1c > 8%) were 60% and 47%, respectively; no differences in the average glucose and HbA1c values were found between the TB-T2DM and T2DM groups. Most of the T2DM patients (90%) and T2DM-TB patients (63%) were under treatment at the time of the study: 50% were treated with oral antidiabetics (metformin/glyburide), and the remainder received insulin subcutaneously (s.c.).
Most of the T2DM patients had a metabolic disorder characterized by dyslipidemia, excess body weight, or obesity (P < 0.0001). In this study, the data corresponding to BMI, total cholesterol, LDL, and triglycerides were not altered in the patients who presented with both conditions, which was a significant difference from the group with T2DM alone (P < 0.0001). The HDL cholesterol levels were close to the lower limit in patients with TB and TB-T2DM and were significantly different from those seen with the healthy volunteers or the T2DM patients without evidence of infection (P < 0.001). Notably, none of the patients enrolled in the current study had severe liver damage, kidney failure, or other complications as a result of T2DM. Furthermore, none of these patients were on medication to control lipid levels or had received antihypertensive or immunosuppressive treatment.
Immune parameters.
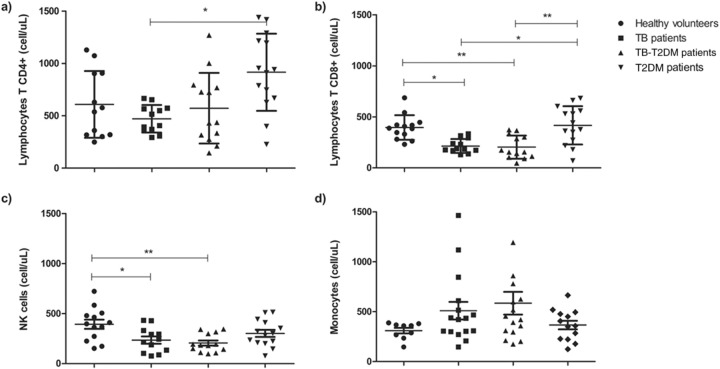
To characterize the immune system statuses of the T2DM patients with TB, T-cell (CD4+ and CD8+), NK cell (CD56+), and monocyte (CD14+) populations were measured with flow cytometry. As shown in Fig. 1a, the healthy controls, TB patients, and TB-T2DM patients had the same total CD4+ T-cell numbers. However, levels of CD8+ and CD56+ cells were diminished in the TB and TB-T2DM groups compared with the healthy controls (Fig. 1b and c). Increased numbers of both CD4+ and CD8+ T cells were found in the T2DM group compared with the TB and TB-T2DM groups. Conversely, no differences in the total levels of peripheral blood monocytes were found for any group (Fig. 1d).
FIG 1.
Flow cytometry subpopulations of CD4+ (a) and CD8+ (b) T lymphocytes, NK cells (c), and monocytes (d) in peripheral blood mononuclear cells (PBMC) from healthy volunteers and patients with T2DM without evidence of infection and patients with TB and TB-T2DM prior to the initiation of the DOTS scheme. The data represent means and standard deviations of the results, and the asterisks indicate significance as follows: *, P < 0.05; **, P < 0.001 (for comparisons between groups using ANOVA and post hoc tests).
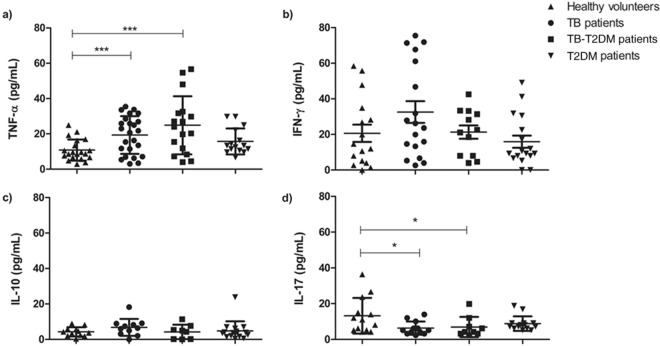
IFN-γ is an important cytokine for the TB immune protective response (20). Multifunctional T cells, which are producers of different cytokines, have also been reported to be associated with protective immunity to MTC (21). Therefore, TNF-α, IL-10, IL-17, and IFN-γ levels were determined in the sera of the healthy controls and of the TB, T2DM, and TB-T2DM patients. High levels of TNF-α (Fig. 2a) and low levels of IL-17 (Fig. 2d) were observed in the TB and TB-T2DM patients compared with the healthy controls. In contrast, the IFN-γ and IL-10 concentrations were comparable between the groups studied (Fig. 2b and c).
FIG 2.
Serum TNF-α (a), IFN-γ (b), IL-10 (c), and IL-17 (d) cytokine levels determined using ELISA in healthy volunteers, patients with T2DM without evidence of infection or previous exposure to TB, and TB-T2DM patients at the baseline of the DOTS scheme. The data represent means and standard deviations of the results, and the asterisks indicate significance as follows: *, P < 0.05; ***, P < 0.0001 (for comparisons between groups by ANOVA and post hoc tests).
Pharmacokinetics of rifampin.
The RIF average plasma concentrations versus time for each of the study groups are shown in Fig. 3. A wide variability in the plasma levels of the anti-TB drug was observed for up to 24 h after the single dose was detected. The same presentation of the drug was used to eliminate differences attributable to changes in the fraction absorbed due to the effects of excipients and formulation quality (19, 22).
FIG 3.
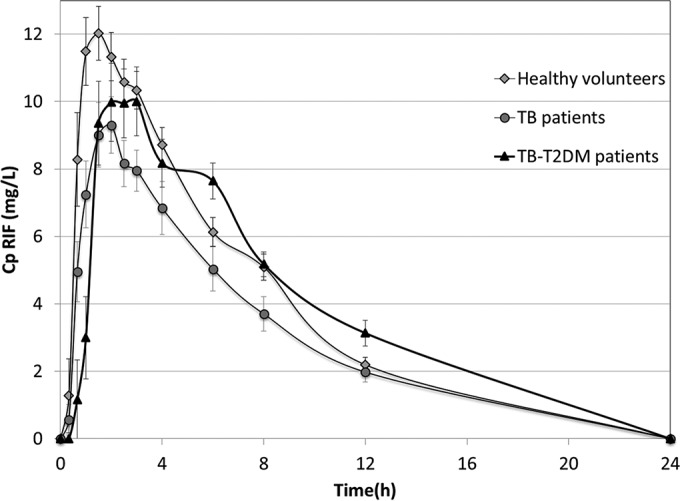
Average pharmacokinetic profiles (± standard error) of RIF in healthy volunteers and in TB and TB-T2DM patients obtained after administration of a single oral dose of a fixed-dose combination formulation quantified by HPLC.
Table 2 shows the pharmacokinetic parameters obtained from the noncompartmental and compartmental analyses and the results of the statistical analysis. The one-compartment open model with first-order elimination and a tlag in the absorption phase represented the evolution of RIF plasma concentrations.
TABLE 2.
Pharmacokinetic parameters obtained for RIF in healthy volunteers and in patients with TB and TB-T2DM determined from noncompartmental pharmacokinetics and the one-compartment open model after oral administration of a single dose of RIFa
| Parameter | Values for group |
P | ||
|---|---|---|---|---|
| Healthy volunteers | TB patients | TB-T2DM patients | ||
| Dose (mg/kg of body wt) | 9.41 ± 1.5 | 10.18 ± 1.7 | 9.72 ± 1.9 | 0.23 |
| Noncompartmental pharmacokinetics | ||||
| Cmax (mg/liter) | 13.07 ± 4.5 | 11.41 ± 3.8 | 12.10 ± 5.1 | 0.44 |
| Tmax (h) | 1.84 ± 0.9 | 2.32 ± 1.4 | 2.98 ± 1.9 | 0.03d |
| AUC0–24 (mg · h · liter−1) | 85.29 ± 27.1 | 82.60 ± 35.5 | 97.52 ± 36.7 | 0.17 |
| AUC0–∞ (mg · h · liter−1) | 87.53 ± 27.2 | 87.07 ± 39.9 | 107.73 ± 56.3 | 0.18 |
| MRT (h) | 6.34 ± 1.5 | 7.67 ± 2.7 | 9.07 ± 5.0 | 0.02c |
| One-compartment open model | ||||
| Ka (h−1) | 3.4 (1.3–8.5) | 1.79 (0.8–2.8) | 1.80 (0.4–3.4) | 0.01b,c |
| t1/2abs (h) | 0.20 (0.1–0.5) | 0.39 (0.2–0.9) | 0.39 (0.2–1.8) | 0.01b,c |
| tlag (h) | 0.34 ± 0.2 | 0.51 ± 0.4 | 0.65 ± 0.4 | 0.04c |
| V (liters) | 36.40 (29.6–45.8) | 45.19 (31.3–56.8) | 35.40 (28.3–44.4) | 0.13 |
| V/TBW (liters/kg) | 0.651 ± 0.23 | 0.840 ± 0.31 | 0.632 ± 0.21 | 0.01b,d |
| Ke (h−1) | 0.181 ± 0.06 | 0.208 ± 0.10 | 0.193 ± 0.08 | 0.58 |
| t1/2 (h) | 4.84 ± 1.2 | 4.31 ± 1.7 | 3.97 ± 1.5 | 0.48 |
| CL (liters/h) | 7.73 ± 3.2 | 8.62 ± 3.9 | 6.04 ± 1.8 | 0.04d |
Bold numbers are statistically significant (P < 0.05).
Data represent the differences in post hoc analysis for healthy volunteers versus TB patients.
Data represent the differences in post hoc analysis for healthy volunteers versus TB-T2DM patients.
Data represent the differences in post hoc analysis for TB-T2DM versus TB patients.
According to the value of Cmax, 65% of the subjects reached the reference value of RIF (8 to 24 mg/liter); these patients were equally distributed between the TB and TB-T2DM patients. Considerable variability was observed in the RIF plasma concentrations. The time to reach Cmax (Tmax) was 2 h for the TB patients and increased to 3 h in the TB-T2DM patients (P = 0.03). Likewise, the tlag obtained with the one-compartment open model was greater in the TB-T2DM patients (P = 0.04), and the absorption rate (Ka) was decreased in the diabetic and nondiabetic TB patients compared to the healthy volunteers (P = 0.01).
A wide interindividual variability in the areas under the time-concentration curves (AUC0–24 and AUC0–∞) was found between groups. Thus, it was not possible to detect significant differences in these parameters according to the disease status. The MRT expresses the permanency of RIF in the organism based on the processes of absorption (including tlag) and elimination; this value was higher for the TB-T2DM patients (P = 0.02) than for the other groups in this study.
The average volumes of distribution (V) were similar between groups; however, levels were significantly increased in the TB-T2DM patients (P = 0.01) when V was normalized to total body weight (V/TBW). In contrast, there was no difference in the rates of elimination (calculated as Ke and t1/2e) between patients. The mean value of clearance (CL), reported as a rate of 6 liters/h for the TB-T2DM patients, was significantly lower for the nondiabetic TB patients (P = 0.04).
Correlation between RIF's PK and biochemical or immunological parameters.
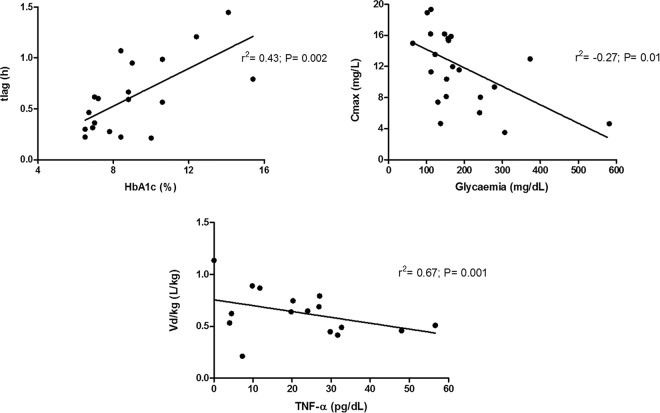
To assess clinically relevant characteristics that might be related to RIF's disposition, each anthropometric, biochemical, and immunological parameter was correlated with the evaluated pharmacokinetic parameters. As expected, the AUC, V, and CL of RIF were associated with the total body weight (P < 0.05) in healthy volunteers; this finding was not observed in the TB and TB-T2DM patients. In the TB-T2DM patients, clear trends were found for glycemic control (fast glycemia and HbA1c) with tlag and Cmax. Additionally, TNF-α levels were related to the V/TBW of RIF to a significant extent (P < 0.05) (Fig. 4).
FIG 4.
Correlation between pharmacokinetic parameters obtained after RIF oral administration with glycemic control and proinflammatory cytokine production in TB-T2DM patients. Plots show the relationships between the lag times of RIF absorption and the glycemic control represented as Hba1c (%) (top left panel), maximal concentrations of RIF and fast glycemia (top right panel), and the volume of distribution of RIF normalized by total body weight and the serum concentration of TNF-α (bottom panel). A Pearson correlation analysis was performed for each pair; the tendency line, correlation coefficient (r), and significance (P) are shown for each plot. A P value of <0.05 was considered significant.
DISCUSSION
The National Center for Epidemiological Surveillance and Control of Diseases (CENAVECE) in Mexico reported that TB was associated with T2DM in a greater proportion (35%) of patients over 50 years of age. The association of TB with HIV/AIDS was less prevalent (10% in the age range of 20 to 40 years) and reached 25% in patients with moderate to severe malnutrition. This finding was corroborated by the results obtained in the present study. The BMI, which is typically used to assess excess body weight or obesity (23), was higher in the group with T2DM. This was not observed in patients with TB-T2DM (P < 0.05). A loss of 20% of total body weight is a regular characteristic of TB patients, and this is reflected in a third of the patients, who had BMIs of <18.49 kg/m2. However, this state of malnutrition occurred in only 10% of the TB-T2DM patients.
Adverse effects of hyperglycemic conditions such as T2DM induce abnormal functions in cells involved in the innate and adaptive immune response, which are potentially relevant to host defense against TB (24). The CD4+ T cells involved in the protective immune response to MTC are key factors for the resolution of the infection, and low levels and abnormal functions of CD4+ T cells may result in clinically evident disease. In contrast, CD8+ T cells are generally thought to contribute to optimal immunity and protection, and a recent study demonstrated that these cells could be a better biomarker of the protective response to TB and the response to therapy than CD4+ T cells (25, 26). The relevance of innate cells such as monocytes/macrophages and NK cells has been well described (8). However, no studies on T-cell subpopulations, monocytes, and NK cell status have been performed in the context of RIF pharmacokinetics in TB-T2DM patients to date. Impaired host defenses in TB-T2DM patients might occur as a consequence of persistent hyperglycemia (27). Here, differences in the numbers of CD8+ and CD56+ cells in both TB and TB-T2DM patients compared with the control group were found, although the differences relative to the time evolution of T2DM in TB patients were not significant (data not shown). No significant differences in total cell numbers between TB and TB-T2DM patients were observed. The latter results are in agreement with a recent study by Kumar et al. (28), who compared T-cell frequencies in pulmonary TB patients with or without DM from a cohort in Chennai, India, and found no differences between the 2 groups in the absolute numbers of CD4+ and CD8+ T cells. However, the frequencies of subpopulations of immune cells, such as Th1, Th17, and regulatory T cells, were different between the diabetic and nondiabetic TB groups (28). Therefore, it would be of great interest to evaluate the behavior of these cell populations throughout the course of treatment because the success or failure of the treatment outcome may be related to the restoration of immunity.
Previous studies involving patients with TB and T2DM resulted in reports of significantly higher levels of IFN-γ production in response to purified protein derivative (PPD) in diabetic than in nondiabetic TB patients (15). Stalenhoef et al. (29) detected lower IFN-γ levels in PBMCs stimulated with MTC from TB patients with or without T2DM. These findings and the current results may appear to be contradictory because T2DM patients are more susceptible to development of active TB; however, these patients also show alterations in the downstream signal transduction of key Th1 and innate immune response cytokines, possibly due to an increase in the levels of advanced glycation end products that can bind and modify protein functions as described by Restrepo et al. (15, 30). The current work utilized fresh serum, which could explain why differences were not found in the levels of IFN-γ observed in TB patients with or without T2DM.
The IL-10 and TNF-α concentrations were in agreement with a previous report (28). Several studies have reported that IL-17 is involved in immune protection against MTC, primarily due to the function of this cytokine in attracting and activating neutrophils (31); Kumar et al. reported increased levels of this cytokine in TB-T2DM patients compared with the TB group, which was in contrast with our results (28). Other characteristics of the study population, such as the genetic background, may have contributed to these discrepancies.
No differences between TB and TB-T2DM patients were found in either the total numbers of cells or serum cytokine concentrations. Data from animal models indicate that the adverse impact of chronic hyperglycemia is seen in the initiation of the adaptive immune response rather than in its magnitude. However, variability between culture conditions or the populations studied has contributed to limiting knowledge regarding the biological basis of the association between TB and T2DM.
Previous studies in TB-T2DM patients resulted in reports of the noncompartmental pharmacokinetics of RIF (10, 11). Due to the sampling of suitable time points performed in the current study, a one-compartment open model with first-order elimination allowed us to characterize the absorption and elimination phases. The pharmacokinetic profiles showed a better fit by adding the tlag parameter. Although the Cmax average was >8 mg/liter (which was regarded as the lower limit of the plasma concentration expected at Tmax) (13, 32, 33), levels below this value were found in both groups of patients. This is relevant because there is evidence that RIF's effect is a function of the concentration found in the blood (13, 14). Additionally, this concentration is expected to decrease after at least 1 week of treatment, when the activity of liver enzymes and transporters is changed by RIF induction (34). We found a tlag of 30 min in TB patients; this is the time it took for the drug to appear in the blood. Conversely, the tlag increased to 40 min on average in patients with TB-T2DM (P = 0.04). This process is regulated by the duodenal efflux pump P-glycoprotein (Pgp). Thus, saturation of the system is required to facilitate RIF's entry into the bloodstream (22). The increase in tlag observed in patients with TB-T2DM could have a significant impact on the magnitude of the bioavailability of this antibiotic in these patients (33). This was reflected in the current study because poor glycemic control was associated with both the increase in tlag and the decrease in Cmax. Additionally, the rate of absorption (Ka) was decreased significantly in patients with TB-T2DM due to the increased tlag, and therefore the mean absorption time (t1/2abs) of RIF increased to up to 2 h in this group of patients.
RIF shows wide pharmacokinetic variability in the absorption process in patients with TB. Population studies identified a high interindividual variability of 66% to 93% that was attributed to the absorption process and specifically to the value of Ka (19, 35). However, this significant variation in TB patients can be explained by a possible reduction in the intestinal area based on the extent of malnutrition in these patients (36). For TB-T2DM patients, another important aspect to consider is the lower intestinal motility that has been previously reported in patients with diabetes; this effect reduces gastric emptying, changes the pH level, and therefore delays the absorption of some drugs (37). Furthermore, the distribution process was shown to be substantially diminished in patients with TB, which was determined by comparing the V/TBW ratios in patients with both pathologies.
Several studies in patients with T2DM reported a correlation between BMI and levels of inflammatory proteins and fibrinogen, angiotensinogen, and α1-glycoprotein. Here, the V/TBW ratio diminished as the proinflammatory cytokine TNF-α level increased in the TB-T2DM patients. One of the systemic effects of this cytokine is the induction of cachexia (38). It is possible that this situation could lead to a limitation in the distribution of RIF to other tissues in patients with T2DM. Additionally, this effect is facilitated by a decrease in blood volume because these patients have little water reabsorption in the kidney (39).
For the TB-T2DM patients, higher HbA1c correlated with increased tlag, and fasting glycemia was associated with a negative trend for Cmax. Drug binding to albumin and α1-glycoprotein may be reduced in the presence of poor glycemic control, possibly due to the glycosylation of plasma and/or displacement of the drug from the large amount of free fatty acids due to the lack of metabolic control proteins (37, 40). Otherwise, T2DM may damage the hepatic metabolism of certain drugs, although the mechanisms involved are unknown. Regardless, nearly complete drug removal was observed at 24 h postdose because the plasma concentrations were undetectable at the time of sampling.
Chigutsa et al. (13) recently demonstrated that an AUC of >35.4 mg · h/liter significantly improved the sterilizing activity of RIF. This value was not achieved by two T2DM-TB patients at the beginning of the DOTS scheme; both patients had poor glycemic control (HbA1c > 8%), and, as expected, the Cmax was below the threshold (8.2 mg/liter) proposed by the same study. Although the mean AUC values did not significantly differ between groups, the threshold value and the Cmax should be considered individually after at least 1 week of treatment, as mentioned above. The results reported by Chigutsa et al. suggested that the sterilizing effect could be increased by dosing with RIF and could be applied to design shorter treatments. Previous results obtained with diabetic patients (10, 11) and the results of the current study demonstrate the necessity of improving the glycemic control of TB-T2DM patients at the initiation of the DOTS scheme to achieve the desired threshold values and maintain them throughout the course of treatment.
The half-life of elimination (t1/2) is usually reported to be approximately 2 h after 2 weeks of treatment (10). Differences in the t1/2 and other RIF pharmacokinetic parameters in Mexican patients were observed but should be interpreted with caution because the sampling schedule was performed on the first day of the DOTS scheme. This is in contrast to previously published studies, where limited sampling strategies were applied during different TB treatment phases.
Diabetes mellitus may impair the hepatic metabolism of some drugs. Although the mechanisms underlying this effect are uncertain, they may reflect liver changes depending on the degree of glycemic control (41). In the current work, RIF's CL was shown to be lower for TB-T2DM patients than for TB patients. However, the effect of T2DM on hepatic metabolism most likely does not have major clinical significance in the presence of reasonable glycemic control (37).
In conclusion, in the current study, we characterized metabolic, immune, and RIF pharmacokinetic parameters in TB-T2DM patients. The differences in these parameters in the absence of one or both diseases were also established. Data related to metabolic syndrome in T2DM patients were not observed in the TB-T2DM patients. Levels of CD8+ T lymphocytes and NK cells were diminished in both the TB and TB-T2DM patients, who exhibited high concentrations of TNF-α but low levels of IL-17. Poor glycemic control without dyslipidemia accompanied by malnutrition was detected in most of the TB-T2DM patients; this was clearly related to the poor and slow absorption of RIF in terms of the lower Cmax and larger tlag, respectively. RIF clearance was also slower in the diabetic patients, thereby prolonging the MRT of the drug. These metabolic and immunologic alterations affecting RIF's pharmacokinetics should be taken into consideration when optimizing anti-TB therapy for patients with T2DM.
ACKNOWLEDGMENTS
We acknowledge the support given by the Technological Research Council of Science and Technology (CONACYT) through the subsidy FMSLP-2008-CO2-107406. S. E. Medellín-Garibay received a scholarship (309952) from CONACYT for the development of the current study.
We declare that we have no conflict of interest.
REFERENCES
- 1.World Health Organization (WHO). 2014. Global tuberculosis report, p 27. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Murray M, Oxlade O, Lin HH. 2011. Modeling social, environmental, and biological determinants of tuberculosis. Int J Tuberc Lung Dis 15(Suppl 2):S64–S70. [DOI] [PubMed] [Google Scholar]
- 3.Jeon CY, Murray MB. 2008. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duangrithi D, Thanachartwet V, Desakorn V, Jitruckthai P, Phojanamongkolkij K, Rienthong S, Chuchottaworn C, Pitisuttithum P. 2013. Impact of diabetes mellitus on clinical parameters and treatment outcomes of newly diagnosed pulmonary tuberculosis patients in Thailand. Int J Clin Pract 67:1199–1209. doi: 10.1111/ijcp.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapur A, Harries AD. 2013. The double burden of diabetes and tuberculosis—public health implications. Diabetes Res Clin Pract 101:10–19. doi: 10.1016/j.diabres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lonnroth K, Ottmani SE, Goonesekera SD, Murray MB. 2011. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. 2013. The immune response in tuberculosis. Annu Rev Immunol 31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 8.Martinez N, Kornfeld H. 2014. Diabetes and immunity to tuberculosis. Eur J Immunol 44:617–626. doi: 10.1002/eji.201344301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiménez-Corona ME, Cruz-Hervert LP, García-García L, Ferreyra-Reyes L, Delgado-Sánchez G, Bobadilla-Del-Valle M, Canizales-Quintero S, Ferreira-Guerrero E, Báez-Saldaña R, Téllez-Vázquez N, Montero-Campos R, Mongua-Rodriguez N, Martínez-Gamboa RA, Sifuentes-Osornio J, Ponce-de-León A. 2013. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax 68:214–220. doi: 10.1136/thoraxjnl-2012-201756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruslami R, Nijland HM, Adhiarta IG, Kariadi SH, Alisjahbana B, Aarnoutse RE, van Crevel R. 2010. Pharmacokinetics of antituberculosis drugs in pulmonary tuberculosis patients with type 2 diabetes. Antimicrob Agents Chemother 54:1068–1074. doi: 10.1128/AAC.00447-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nijland HM, Ruslami R, Stalenhoef JE, Nelwan EJ, Alisjahbana B, Nelwan RH, van der Ven AJ, Danusantoso H, Aarnoutse RE, van Crevel R. 2006. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis 43:848–854. doi: 10.1086/507543. [DOI] [PubMed] [Google Scholar]
- 12.Heysell SK, Moore JL, Staley D, Dodge D, Houpt ER. 2013. Early therapeutic drug monitoring for isoniazid and rifampin among diabetics with newly diagnosed tuberculosis in Virginia, USA. Tuberc Res Treat 2013:129723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chigutsa E, Pasipanodya JG, Visser ME, van Helden PD, Smith PJ, Sirgel FA, Gumbo T, McIlleron H. 2015. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother 59:38–45. doi: 10.1128/AAC.03931-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis 208:1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Restrepo BI, Fisher-Hoch SP, Pino PA, Salinas A, Rahbar MH, Mora F, Cortes-Penfield N, McCormick JB. 2008. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis 47:634–641. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association. 2013. Diagnosis and classification of diabetes mellitus. Diabetes Care 36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall RG, Leff RD, Gumbo T. 2009. Treatment of active pulmonary tuberculosis in adults: current standards and recent advances. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 29:1468–1481. doi: 10.1592/phco.29.12.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Ingen J, Aarnoutse RE, Donald PR, Diacon AH, Dawson R, Plemper van Balen G, Gillespie SH, Boeree MJ. 2011. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis 52:e194–e199. doi: 10.1093/cid/cir184. [DOI] [PubMed] [Google Scholar]
- 19.Milán-Segovia RC, Domínguez-Ramírez AM, Jung-Cook H, Magaña-Aquino M, Romero-Méndez MC, Medellín-Garibay SE, Vigna-Pérez M, Romano-Moreno S. 2010. Relative bioavailability of rifampicin in a three-drug fixed-dose combination formulation. Int J Tuberc Lung Dis 14:1454–1460. [PubMed] [Google Scholar]
- 20.Hang NT, Matsushita I, Shimbo T, Hong LT, Tam DB, Lien LT, Thuong PH, Cuong VC, Hijikata M, Kobayashi N, Sakurada S, Higuchi K, Harada N, Endo H, Keicho N. 21 June 2014, posting date Association between tuberculosis recurrence and interferon-gamma response during treatment. J Infect doi: 10.1016/j.jinf.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, Sanduzzi A, Franken WP, Ottenhoff TH, Dieli F. 2010. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol 40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal S, Panchagnula R. 2005. Implication of biopharmaceutics and pharmacokinetics of rifampicin in variable bioavailability from solid oral dosage forms. Biopharm Drug Dispos 26:321–334. doi: 10.1002/bdd.464. [DOI] [PubMed] [Google Scholar]
- 23.Flegal KM, Carroll MD, Kit BK, Ogden CL. 2012. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 24.Daoud AK, Tayyar MA, Fouda IM, Harfeil NA. 2009. Effects of diabetes mellitus vs. in vitro hyperglycemia on select immune cell functions. J Immunotoxicol 6:36–41. doi: 10.1080/15476910802604564. [DOI] [PubMed] [Google Scholar]
- 25.Prezzemolo T, Guggino G, La Manna MP, Di Liberto D, Dieli F, Caccamo N. 2014. Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis. Front Immunol 5:180. doi: 10.3389/fimmu.2014.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyendak MR, Park B, Null MD, Baseke J, Swarbrick G, Mayanja-Kizza H, Nsereko M, Johnson DF, Gitta P, Okwera A, Goldberg S, Bozeman L, Johnson JL, Boom WH, Lewinsohn DA, Lewinsohn DM; Tuberculosis Research Unit and the Tuberculosis Trials Consortium. 2013. Mycobacterium tuberculosis specific CD8(+) T cells rapidly decline with antituberculosis treatment. PLoS One 8:e81564. doi: 10.1371/journal.pone.0081564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. 2007. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol 37:518–524. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Fay MP, Nutman TB, Babu S. 2013. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Ann Am Thorac Soc 10:441–449. doi: 10.1513/AnnalsATS.201305-112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stalenhoef JE, Alisjahbana B, Nelwan EJ, van der Ven-Jongekrijg J, Ottenhoff TH, van der Meer JW, Nelwan RH, Netea MG, van Crevel R. 2008. The role of interferon-gamma in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis 27:97–103. doi: 10.1007/s10096-007-0395-0. [DOI] [PubMed] [Google Scholar]
- 30.Restrepo BI, Schlesinger LS. 2013. Host-pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis 93(Suppl):S10–S14. doi: 10.1016/S1472-9792(13)70004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ottenhoff TH. 2012. New pathways of protective and pathological host defense to mycobacteria. Trends Microbiol 20:419–428. doi: 10.1016/j.tim.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Peloquin CA. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62:2169–2183. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 33.McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. 2006. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob Agents Chemother 50:1170–1177. doi: 10.1128/AAC.50.4.1170-1177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. 2003. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet 42:819–850. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 35.Milán Segovia RC, Domínguez Ramírez AM, Jung Cook H, Magaña Aquino M, Vigna Pérez M, Brundage RC, Romano Moreno S. 2013. Population pharmacokinetics of rifampicin in Mexican patients with tuberculosis. J Clin Pharm Ther 38:56–61. doi: 10.1111/jcpt.12016. [DOI] [PubMed] [Google Scholar]
- 36.Pinheiro VG, Ramos LM, Monteiro HS, Barroso EC, Bushen OY, Facanha MC, Peloquin CA, Guerrant RL, Lima AA. 2006. Intestinal permeability and malabsorption of rifampin and isoniazid in active pulmonary tuberculosis. Braz J Infect Dis 10:374–379. doi: 10.1590/S1413-86702006000600003. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert RE, Cooper ME, Krum H. 1998. Drug administration in patients with diabetes mellitus. Safety considerations. Drug Safety 18:441–455. doi: 10.2165/00002018-199818060-00005. [DOI] [PubMed] [Google Scholar]
- 38.Dorhoi A, Kaufmann SH. 2014. Tumor necrosis factor alpha in mycobacterial infection. Semin Immunol 26:203–209. doi: 10.1016/j.smim.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Wada J, Makino H. 2013. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci 124:139–152. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 40.Gwilt PR, Nahhas RR, Tracewell WG. 1991. The effects of diabetes mellitus on pharmacokinetics and pharmacodynamics in humans. Clin Pharmacokinet 20:477–490. doi: 10.2165/00003088-199120060-00004. [DOI] [PubMed] [Google Scholar]
- 41.Salmela PI, Sotaniemi EA, Pelkonen RO. 1980. The evaluation of the drug-metabolizing capacity in patients with diabetes mellitus. Diabetes 29:788–794. [DOI] [PubMed] [Google Scholar]