Abstract
Dalbavancin is a novel lipoglycopeptide with activity against Staphylococcus aureus, including glycopeptide-resistant isolates. The in vivo investigation reported here tested the effects of this antibiotic against seven S. aureus isolates with higher MICs, including several vancomycin-intermediate strains. Results of 1-log kill and 2-log kill were achieved against seven and six of the isolates, respectively. The mean free-drug area under the concentration-time curve (fAUC)/MIC values for net stasis, 1-log kill, and 2-log kill were 27.1, 53.3, and 111.1, respectively.
TEXT
The increasing rates of resistance among hospital- and community-acquired bacterial pathogens such as Staphylococcus aureus, coagulase-negative staphylococci, and enterococci have prompted attempts to discover new antimicrobials with activities against multidrug-resistant Gram-positive pathogens (1–6). Dalbavancin is a new lipoglycopeptide antibiotic with activity against multidrug-resistant Gram-positive organisms (4, 7–9). In addition to enhanced antimicrobial potency, the compound possesses a unique pharmacokinetic (PK) profile that includes an extremely long elimination half-life of more than 1 week (10–12). Clinical development of the compound has thus far demonstrated success for the treatment of skin and soft tissue infections and catheter-related bloodstream infections (13–18). Once-weekly administration of the doses used in these trials has been shown to produce free-drug trough concentrations exceeding the MIC90s of Gram-positive pathogens from large surveillance databases (17, 19–23).
The current studies were designed to define the pharmacodynamic (PD) target for dalbavancin against S. aureus strains with dalbavancin MICs at or above the current FDA breakpoint (≥0.12 μg/ml), some of which were vancomycin-intermediate S. aureus (VISA) strains (24–29). The results from these studies provide a pharmacodynamic rationale in support of the current clinical dosing regimens. Furthermore, the data provide a starting point for the development of revised susceptibility breakpoints for this new compound.
Seven strains of Staphylococcus aureus (including four vancomycin-intermediate S. aureus [VISA] strains) were studied (Table 1). The dalbavancin and vancomycin MIC values were determined in triplicate using CLSI reference broth microdilution methods, in the presence of polysorbate 80 (30). The dalbavancin MIC range for the S. aureus isolates was 0.12 to 0.50 μg/ml. Animals were maintained in accordance with the criteria of the Association for Assessment and Accreditation of Laboratory Animal Care. All animal studies were approved by the Animal Research Committee of the William S. Middleton Memorial Veterans Hospital. The neutropenic murine thigh infection model was used for all studies. Mice were inoculated with 107 CFU/ml of each strain. Single-dose plasma pharmacokinetic studies were performed with thigh-infected mice given intraperitoneal doses (0.2 ml/dose) of dalbavancin (2.5, 10, 40, 80, or 160 mg/kg). Dalbavancin plasma concentrations were measured with a liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay (Fig. 1); the lower limit of quantification for the assay was 0.05 μg/ml. Sample analysis precision (coefficient of variation [CV]) ranged from 5% to 6.4%, and accuracy (bias) ranged from −3.5% to −10.0%. Peak levels were observed by 2 to 6 h. Dalbavancin exhibited relatively linear pharmacokinetics, based on the dose-area under the concentration-time curve (AUC) relationship. The half-life was long and varied from 4.1 to 9.31 h. A protein binding value of 98.4%, based on prior studies in this model (31), was used.
TABLE 1.
Study strains and dalbavancin in vitro susceptibility
| S. aureus isolate | MIC (mg/liter) |
|
|---|---|---|
| Dalbavancin | Vancomycin | |
| LSI653 | 0.25 | 2 |
| LSI1848 | 0.12 | 2 |
| LSI1854 | 0.5 | 2 |
| LSI1856 | 0.25 | 4 (VISA) |
| LSI1857 | 0.25 | 4 (VISA) |
| LSI1861 | 0.25 | 4 (VISA) |
| LSI1862 | 0.5 | 4 (VISA) |
FIG 1.
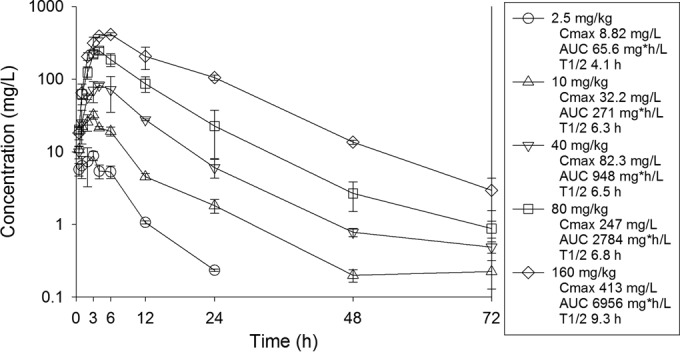
Plasma pharmacokinetics of dalbavancin in mice following intraperitoneal administration. Each symbol represents the mean and standard deviation from three mice. The drug concentration values presented represent total (protein-bound and unbound) drug. The AUC values represent 0 to infinity. Cmax, maximal drug concentration; T1/2, half-life.
The in vivo virulence of the S. aureus isolates was similar in the untreated control mice, based on the increase in thigh burden over the treatment period, i.e., 2.30 ± 0.14 log10 CFU/thigh. Two hours after infection, dalbavancin was administered via the intraperitoneal route, with one of seven 2-fold-escalating doses of dalbavancin (2.5, 5, 10, 20, 40, 80, and 160 mg/kg) being administered every 12 h for a 6-day treatment period. Untreated control groups were sampled at the start of therapy and at the end of the study. The thighs were removed from the animals and immediately processed for CFU determination. The results of these studies were analyzed by using a sigmoidal dose-effect model (32). The magnitude of the PK/PD index associated with each endpoint dose was calculated with the following equation: log10 D = log10 [E/(Emax − E)]/(N + log10 ED50), where E is the control growth for the static dose (D), E is the control growth − 1 log unit for D for 1-log kill, and E is the control growth − 2 log units for D for 2-log kill.
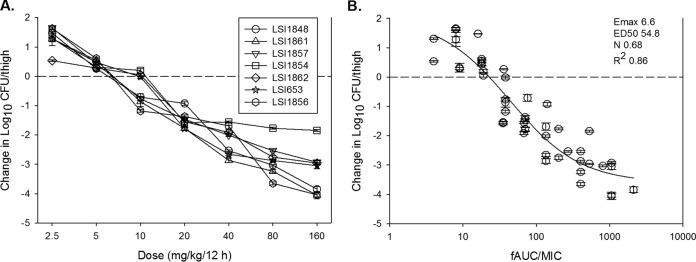
Results of 1-log kill and 2-log kill were achieved against seven and six of the isolates, respectively (Fig. 2A and Table 2). The dalbavancin in vivo exposure-response data were also considered relative to the PK/PD-linked driver AUC/MIC, using concentrations of free drug. Drug accumulation was calculated and included in AUC estimates. Using a sigmoidal Emax model, the data fit was strong for the seven-strain data set (R2 = 0.86), as shown in Fig. 2B. The numerical AUC/MIC values associated with each of the three treatment endpoints are also shown in Table 2. Net stasis was observed with a dalbavancin free-drug AUC (fAUC)/MIC value near 25. fAUC/MIC values near 50 and 100 were associated with 1-log and 2-log reductions, respectively, in organism burdens in the neutropenic mice.
FIG 2.
In vivo dose-dependent effects of dalbavancin against seven select S. aureus isolates in a neutropenic mouse thigh model. (A) Dalbavancin exposure expressed at dose level (mg/kg/12 h). (B) Exposure expressed as fAUC/MIC. Each symbol represents the mean and standard deviation from four thighs. Dalbavancin exposure is expressed as the 24-h fAUC/MIC. The burden of organisms was measured at the start and end of therapy. The horizontal dashed line at 0 represents the burden of organisms in the thighs of mice at the start of therapy. Data points below the line represent killing, and points above the line represent growth. R2 represents the coefficient of determination. The 50% effective dose (ED50) represents the AUC/MIC associated with 50% of the maximal effect (Emax), and N is the slope of the relationship or the Hill coefficient. The line drawn through the data points is the best-fit line based on the sigmoidal Emax formula.
TABLE 2.
In vivo efficacy of dalbavancin against select S. aureus isolates, using fAUC/MIC as the predictive pharmacodynamic index
| Strain | Stasis |
1-log kill |
2-log kill |
|||
|---|---|---|---|---|---|---|
| 24-h dose (mg/kg) | 24-h fAUC/MIC | 24-h dose (mg/kg) | 24-h fAUC/MIC | 24-h dose (mg/kg) | 24-h fAUC/MIC | |
| LSI1848 | 15.17 | 56.49 | 31.45 | 112.81 | 62.75 | 214.21 |
| LSI1861 | 13.55 | 25.00 | 24.63 | 45.35 | 44.38 | 77.35 |
| LSI1857 | 14.34 | 26.59 | 26.72 | 48.74 | 60.05 | 102.72 |
| LSI1854 | 15.00 | 13.95 | 35.80 | 31.73 | ||
| LSI1862 | 12.64 | 11.60 | 32.92 | 29.39 | 85.46 | 76.66 |
| LSI653 | 14.93 | 27.77 | 27.20 | 49.52 | 54.09 | 93.07 |
| LSI1856 | 15.21 | 28.32 | 30.88 | 55.49 | 60.05 | 102.73 |
| Mean | 14.41 | 27.10 | 29.94 | 53.29 | 61.13 | 111.12 |
| Median | 14.93 | 26.59 | 30.88 | 48.74 | 60.05 | 97.90 |
| SDa | 0.98 | 14.62 | 3.93 | 27.93 | 13.62 | 51.81 |
SD, standard deviation.
These PK/PD targets are lower than those observed previously with wild-type S. aureus strains in the same model (31). This is partly due to lower pharmacokinetic values measured in the present study, perhaps due to differences in the drug assay method. Of note, the present kinetic study included a robust sampling scheme and a more sensitive and accurate drug assay method, compared to the prior animal model investigation; we used a specific LC-MS/MS assay, in contrast to the prior bioassay. The treatment studies were otherwise similar with respect to animal species, neutropenia, antibiotic (dalbavancin), drug preparation, route of administration, treatment duration, study endpoints, and data analysis.
The present studies were designed to discern the PK/PD impact of infection with less common S. aureus strains that had dalbavancin MICs at or above the current dalbavancin FDA breakpoint (≥0.12 μg/ml). Dalbavancin demonstrated potent in vivo activity against S. aureus strains with higher MICs, including those exhibiting a VISA phenotype. While it will be important to corroborate these preclinical findings with data from patients, consideration of the AUC/MIC targets from these studies in the context of human pharmacokinetics suggests a safe treatment margin against these higher-MIC isolates. If the steady-state kinetics of dalbavancin in patients are considered relative to the stasis, 1-log kill, and 2-log kill AUC/MIC targets in this study, then the MIC breakpoints would be revised to 4, 2, and 1 μg/ml, respectively.
REFERENCES
- 1.Chambers HF. 2005. Community-associated MRSA: resistance and virulence converge. N Engl J Med 352:1485–1487. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- 2.Graber CJ, Wong MK, Carleton HA, Perdreau-Remington F, Haller BL, Chambers HF. 2007. Intermediate vancomycin susceptibility in a community-associated MRSA clone. Emerg Infect Dis 13:491–493. doi: 10.3201/eid1303.060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones RN, Sader HS, Flamm RK. 2013. Update of dalbavancin spectrum and potency in the USA: report from the SENTRY Antimicrobial Surveillance Program (2011). Diagn Microbiol Infect Dis 75:304–307. doi: 10.1016/j.diagmicrobio.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 4.Biedenbach DJ, Bell JM, Sader HS, Turnidge JD, Jones RN. 2009. Activities of dalbavancin against a worldwide collection of 81,673 Gram-positive bacterial isolates. Antimicrob Agents Chemother 53:1260–1263. doi: 10.1128/AAC.01453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moellering RC., Jr 2006. The growing menace of community-acquired methicillin-resistant Staphylococcus aureus. Ann Intern Med 144:368–370. doi: 10.7326/0003-4819-144-5-200603070-00014. [DOI] [PubMed] [Google Scholar]
- 6.Moellering RC., Jr 2008. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 46:1032–1037. doi: 10.1086/529445. [DOI] [PubMed] [Google Scholar]
- 7.McCurdy SP, Jones RN, Mendes RE, Puttagunta S, Dunne MW. 2015. In vitro activity of dalbavancin against drug-resistant Staphylococcus aureus from a global surveillance program. Antimicrob Agents Chemother 59:5007–5009. doi: 10.1128/AAC.00274-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malabarba A, Goldstein BP. 2005. Origin, structure, and activity in vitro and in vivo of dalbavancin. J Antimicrob Chemother 55(Suppl 2):ii15–ii20. [DOI] [PubMed] [Google Scholar]
- 9.Karlowsky JA, Adam HJ, Poutanen SM, Hoban DJ, Zhanel GG, Canadian Antimicrobial Resistance Alliance. 2011. In vitro activity of dalbavancin and telavancin against staphylococci and streptococci isolated from patients in Canadian hospitals: results of the CANWARD 2007–2009 study. Diagn Microbiol Infect Dis 69:342–347. doi: 10.1016/j.diagmicrobio.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Bradley JS, Puttagunta S, Rubino CM, Blumer JL, Dunne M, Sullivan JE. 2015. Pharmacokinetics, safety and tolerability of single dose dalbavancin in children 12–17 years of age. Pediatr Infect Dis J 34:748–752. doi: 10.1097/INF.0000000000000646. [DOI] [PubMed] [Google Scholar]
- 11.Dorr MB, Jabes D, Cavaleri M, Dowell J, Mosconi G, Malabarba A, White RJ, Henkel TJ. 2005. Human pharmacokinetics and rationale for once-weekly dosing of dalbavancin, a semi-synthetic glycopeptide. J Antimicrob Chemother 55(Suppl 2):ii25–ii30. [DOI] [PubMed] [Google Scholar]
- 12.Leighton A, Gottlieb AB, Dorr MB, Jabes D, Mosconi G, VanSaders C, Mroszczak EJ, Campbell KC, Kelly E. 2004. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob Agents Chemother 48:940–945. doi: 10.1128/AAC.48.3.940-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raad I, Darouiche R, Vazquez J, Lentnek A, Hachem R, Hanna H, Goldstein B, Henkel T, Seltzer E. 2005. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by Gram-positive pathogens. Clin Infect Dis 40:374–380. doi: 10.1086/427283. [DOI] [PubMed] [Google Scholar]
- 14.Seltzer E, Dorr MB, Goldstein BP, Perry M, Dowell JA, Henkel T. 2003. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin Infect Dis 37:1298–1303. doi: 10.1086/379015. [DOI] [PubMed] [Google Scholar]
- 15.Zervou FN, Zacharioudakis IM, Mylonakis E. 2014. Weekly dalbavancin was noninferior to daily vancomycin for acute bacterial skin infection in adults. Ann Intern Med 161:JC9. doi: 10.7326/0003-4819-161-8-201410210-02009. [DOI] [PubMed] [Google Scholar]
- 16.Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. 2014. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med 370:2169–2179. doi: 10.1056/NEJMoa1310480. [DOI] [PubMed] [Google Scholar]
- 17.Buckwalter M, Dowell JA. 2005. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J Clin Pharmacol 45:1279–1287. doi: 10.1177/0091270005280378. [DOI] [PubMed] [Google Scholar]
- 18.Jauregui LE, Babazadeh S, Seltzer E, Goldberg L, Krievins D, Frederick M, Krause D, Satilovs I, Endzinas Z, Breaux J, O'Riordan W. 2005. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis 41:1407–1415. doi: 10.1086/497271. [DOI] [PubMed] [Google Scholar]
- 19.Salem AH, Zhanel GG, Ibrahim SA, Noreddin AM. 2014. Monte Carlo simulation analysis of ceftobiprole, dalbavancin, daptomycin, tigecycline, linezolid and vancomycin pharmacodynamics against intensive care unit-isolated methicillin-resistant Staphylococcus aureus. Clin Exp Pharmacol Physiol 41:437–443. doi: 10.1111/1440-1681.12195. [DOI] [PubMed] [Google Scholar]
- 20.Dowell JA, Goldstein BP, Buckwalter M, Stogniew M, Damle B. 2008. Pharmacokinetic-pharmacodynamic modeling of dalbavancin, a novel glycopeptide antibiotic. J Clin Pharmacol 48:1063–1068. doi: 10.1177/0091270008321273. [DOI] [PubMed] [Google Scholar]
- 21.Nicolau DP, Sun HK, Seltzer E, Buckwalter M, Dowell JA. 2007. Pharmacokinetics of dalbavancin in plasma and skin blister fluid. J Antimicrob Chemother 60:681–684. doi: 10.1093/jac/dkm263. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein BP, Jones RN, Fritsche TR, Biedenbach DJ. 2006. Microbiologic characterization of isolates from a dalbavancin clinical trial for catheter-related bloodstream infections. Diagn Microbiol Infect Dis 54:83–87. doi: 10.1016/j.diagmicrobio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Jones RN, Stilwell MG, Sader HS, Fritsche TR, Goldstein BP. 2006. Spectrum and potency of dalbavancin tested against 3322 Gram-positive cocci isolated in the United States Surveillance Program (2004). Diagn Microbiol Infect Dis 54:149–153. doi: 10.1016/j.diagmicrobio.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Mirza HC, Sancak B, Gur D. 2015. The prevalence of vancomycin-intermediate Staphylococcus aureus and heterogeneous VISA among methicillin-resistant strains isolated from pediatric population in a Turkish university hospital. Microb Drug Resist doi: 10.1089/mdr.2015.0048. [DOI] [PubMed] [Google Scholar]
- 25.Saito M, Katayama Y, Hishinuma T, Iwamoto A, Aiba Y, Kuwahara-Arai K, Cui L, Matsuo M, Aritaka N, Hiramatsu K. 2014. “Slow VISA,” a novel phenotype of vancomycin resistance, found in vitro in heterogeneous vancomycin-intermediate Staphylococcus aureus strain Mu3. Antimicrob Agents Chemother 58:5024–5035. doi: 10.1128/AAC.02470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinkraus G, White R, Friedrich L. 2007. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother 60:788–794. doi: 10.1093/jac/dkm258. [DOI] [PubMed] [Google Scholar]
- 27.Smith TL, Pearson ML, Wilcox KR, Cruz C, Lancaster MV, Robinson-Dunn B, Tenover FC, Zervos MJ, Band JD, White E, Jarvis WR. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N Engl J Med 340:493–501. [DOI] [PubMed] [Google Scholar]
- 28.Sieradzki K, Roberts RB, Haber SW, Tomasz A. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med 340:517–523. doi: 10.1056/NEJM199902183400704. [DOI] [PubMed] [Google Scholar]
- 29.John CC. 1999. Vancomycin resistance in Staphylococcus aureus. N Engl J Med 341:207–208. doi: 10.1056/NEJM199907153410314. [DOI] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility testing for bacteria that grow aerobically; approved standard—10th edition. CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 31.Andes D, Craig WA. 2007. In vivo pharmacodynamic activity of the glycopeptide dalbavancin. Antimicrob Agents Chemother 51:1633–1642. doi: 10.1128/AAC.01264-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]