Abstract
The objective of this study was to investigate the risk of attenuated efficacy due to adaptive resistance for the siderophore-conjugated monocarbam SMC-3176 in Pseudomonas aeruginosa by using a pharmacokinetic/pharmacodynamic (PK/PD) approach. MICs were determined in cation-adjusted Mueller-Hinton broth (MHB) and in Chelex-treated, dialyzed MHB (CDMHB). Spontaneous resistance was assessed at 2× to 16× the MIC and the resulting mutants sequenced. Efficacy was evaluated in a neutropenic mouse thigh model at 3.13 to 400 mg/kg of body weight every 3 h for 24 h and analyzed for association with free time above the MIC (fT>MIC). To closer emulate the conditions of the in vivo model, we developed a novel assay testing activity mouse whole blood (WB). All mutations were found in genes related to iron uptake: piuA, piuC, pirR, fecI, and pvdS. Against four P. aeruginosa isolates, SMC-3176 displayed predictable efficacy corresponding to the fT>MIC using the MIC in CDMHB (R2 = 0.968 to 0.985), with stasis to 2-log kill achieved at 59.4 to 81.1%. Efficacy did not translate for P. aeruginosa isolate JJ 4-36, as the in vivo responses were inconsistent with fT>MIC exposures and implied a threshold concentration that was greater than the MIC. The results of the mouse WB assay indicated that efficacy was not predictable using the MIC for JJ 4-36 and four additional isolates, against which in vivo failures of another siderophore-conjugated β-lactam were previously reported. SMC-3176 carries a risk of attenuated efficacy in P. aeruginosa due to rapid adaptive resistance preventing entry via the siderophore-mediated iron uptake systems. Substantial in vivo testing is warranted for compounds using the siderophore approach to thoroughly screen for this in vitro-in vivo disconnect in P. aeruginosa.
INTRODUCTION
There has been a renewal of discovery and development efforts of siderophore-conjugated antibiotics intended for the treatment of Gram-negative infections, including those caused by the ever-challenging Pseudomonas aeruginosa (1–7). Coupling antibiotics to siderophores is an attractive strategy, as passage across the bacterium's poorly permeable outer membrane is facilitated via the iron uptake pathways (Trojan horse concept) (8, 9). However, a large number of siderophore systems are available for iron transport in P. aeruginosa, as well as complex regulatory mechanisms to govern their expression (10). Consequently, P. aeruginosa has the ability to adapt its methods for iron acquisition and potentially prevent conjugated agents from utilizing this mode of entry.
Such adaptive behavior was first reported with the siderophore-conjugated monobactam MB-1, for which in vivo failures (i.e., bacterial regrowth instead of reduction) were observed to be in discordance with the in vitro activity indicated by standard MIC testing (11). The attenuation in efficacy was determined not to be a result of insufficient drug exposure, as measured according to the pharmacokinetic/pharmacodynamic (PK/PD) index of β-lactams. Antimicrobial pharmacodynamics has long been reliably expressed as a function of the MIC, which is linked to the pharmacokinetics to constitute an exposure-response relationship (e.g., area under the concentration-time curve [AUC]-to-MIC ratio, peak concentration [Cmax]-to-MIC ratio, or the percentage of time above the MIC) (12); however, concern has now been raised over the predictability of the in vitro MIC for similar siderophore conjugates.
Our primary objective was to assess for differentiation in the risk of attenuated efficacy in P. aeruginosa for a new hydroxypyridinone siderophore-conjugated monocyclic β-lactam, SMC-3176 (a monocarbam) (Fig. 1). We sought to accomplish this by in-depth PK/PD profiling and characterization of resistance mechanisms using a collection of clinically relevant isolates. While a body of in vivo work across numerous P. aeruginosa isolates was performed for MB-1, PK/PD information at the isolate level was limited to single assessments, given the nature of the study design (11). We opted for a dose-ranging design to obtain estimates spanning the range of drug exposures and in vivo responses for each individual isolate. Correspondingly, alternative in vitro assays (as opposed to the standard broth microdilution MIC test in standard medium) were also explored in an attempt to bridge both sides of the possible in vitro-in vivo disconnect. These involved manipulating test conditions (e.g., using various medium types [broth, agar, whole blood, or supplementing with an iron chelator]) to more closely resemble in vivo iron-limited conditions, in the presence of which the siderophore uptake systems are activated.
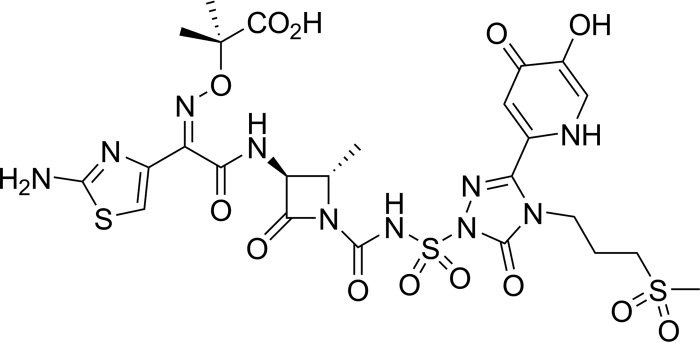
FIG 1.
Chemical structure of SMC-3176.
(This work was presented in part at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, 5 to 9 September 2014, Washington, DC, abstracts A-684 [13] and F-1566 [14]).
MATERIALS AND METHODS
Antimicrobial agents.
SMC-3176 and comparator siderophore-conjugated β-lactams (MB-1 and MC-8) were synthesized at AstraZeneca (see Methods S1 in the supplemental material). MC-8 is related to the siderophore-conjugated monocarbam MC-1 and was selected for testing because of its improved hydrolytic stability over MC-1 (3). For non-siderophore-conjugated reference compounds, analytical-grade versions of aztreonam, ceftazidime, meropenem, and colistin sulfate (as the representative non-β-lactam agent) were used. All compounds were formulated according to published literature or as supported by internal stability data for in vitro and/or in vivo use.
Bacterial isolates.
Clinical isolates of P. aeruginosa were obtained from the culture collections at AstraZeneca, Hartford Hospital (Hartford, CT), or the International Health Management Associates, Inc. (Schaumburg, IL) for use in all studies. All isolates were grown and tested in cation-adjusted Mueller-Hinton broth (MHB), unless otherwise indicated. Select P. aeruginosa isolates (n = 12) for key in vitro and in vivo experiments were profiled using the Illumina whole-genome sequencing (WGS) platform for β-lactamases, the ampC regulon, and siderophore-uptake machinery, as shown in Table 1 (see Methods S2 in the supplemental material).
TABLE 1.
Key P. aeruginosa isolates studied in resistance selection and animal efficacy experimentsa
| Isolate | Acquired β-lactamaseb | Amp regulonc | Iron relatedd |
|---|---|---|---|
| ARC545 (PAO1) | None | No predicted LOF alteration | No predicted LOF alteration |
| ARC3483 (PAO1 ΔampC ΔpoxB) | None | No predicted LOF alteration | No predicted LOF alteration |
| ARC3502 | VIM-1 | No predicted LOF alteration | No predicted LOF alteration |
| ARC3506 | VEB-1, OXA-10 | No predicted LOF alteration | No predicted LOF alteration |
| ARC3514 | KPC-2 | No predicted LOF alteration | No predicted LOF alteration |
| ARC4659 | None | No predicted LOF alteration | No predicted LOF alteration |
| AZ 8-18 | None | No predicted LOF alteration | No predicted LOF alteration |
| AZ 32-13 | None | No predicted LOF alteration | No predicted LOF alteration |
| JJ 4-36 | None | No predicted LOF alteration | No predicted LOF alteration |
| JJ 5-35 | None | No predicted LOF alteration | No predicted LOF alteration |
| JJ 8-16 | None | E163* in AmpD | No predicted LOF alteration |
| JJ 11-54 | None | No predicted LOF alteration | No predicted LOF alteration |
See Table S5 in the supplemental material for a detailed description of the genotypes. LOF, loss of function.
All isolates contained chromosomal ampC and poxB genes.
Amp regulon includes ampD, ampR, and dacB.
Siderophore-related genes were piuA, piuC, pirA, pirR, fecI, pvdA, pvdS, and fpvA.
Susceptibility testing.
MICs were determined in MHB (Becton, Dickinson and Company, Franklin Lakes, NJ) by broth microdilution, according to CLSI guidelines (15, 16). Against a subset of 10 isolates available for in vivo testing, MICs were additionally determined by broth microdilution in medium treated with an iron chelator by using Chelex-treated, dialyzed MHB (CDMHB) (11). CDMHB was prepared, as previously described, on a large-batch scale (see Methods S3 in the supplemental material). For isolates evaluated in the animal efficacy studies, ≥10 MIC replicates in MHB and ≥5 MIC replicates in CDMHB were performed to obtain the modal MIC. Modal MICs were used for reporting and in PK/PD analyses of SMC-3176 efficacy.
Resistance assessment.
Spontaneous resistance studies for SMC-3176 were conducted against five P. aeruginosa isolates (PAO1, ARC3483, ARC3502, ARC3506, and ARC3514), which were chosen based on β-lactamase content. The MICs were first determined in MHB agar (Becton, Dickinson and Company, Franklin Lakes, NJ), as per CLSI guidelines (15, 16). Spontaneous resistance was then assessed by plating 1 × 109 CFU in triplicate on compound-containing MHB agar plates at concentrations of up to 16× the MIC. The plates inoculated with 100 μl of bacterial suspension were incubated at 37°C for 24 h, after which the colonies were counted. The resistance frequency was calculated as the ratio of average CFU in the presence of compound versus that in the absence of compound. Resistance of the isolated mutants was verified by MIC determinations in MHB after passaging twice on nonselective medium. WGS was performed using the Illumina MiSeq V2 instrument (Illumina, Inc., San Diego, CA) to determine the genetic basis of resistance (see Methods S2 in the supplemental material).
Isogenic mutants constructed in the background of PAO1 were also assessed by MIC determinations in MHB. The following deletion isolates were constructed by Microbiotix, Inc. (Worcester, MA) using a modified version of splicing by overlap extension (SOE) PCR: ΔpiuA, ΔpirA, ΔpiuA + ΔpirA, and ΔtonB1 as the comparator for disruption of multiple siderophore systems. An additional deletion isolate, ΔpiuC, was constructed at AstraZeneca (see Methods S4 in the supplemental material).
Animal infection model.
Female ICR mice weighing approximately 20 g (Charles River Laboratories, Inc., Wilmington, MA) or 25 g (Harlan Sprague Dawley, Inc., Indianapolis, IN) were prepared for the neutropenic mouse thigh infection model using P. aeruginosa isolates (17). The animals were maintained in accordance with the American Association for Accreditation of Laboratory Animal Care, and the studies were executed under protocols approved by the Institutional Animal Care and Use Committees at AstraZeneca and Hartford Hospital. The mice were rendered neutropenic by intraperitoneal injections of cyclophosphamide at 150 mg/kg of body weight on day −4 and 100 mg/kg on day −1 prior to inoculation. Thigh infection was produced by intramuscular injection of the inoculum, prepared from a fresh subculture, to target 1 × 106 CFU/thigh. Treatment was initiated at 2 h postinoculation, and all doses were administered subcutaneously.
In vivo efficacy and PK/PD.
The mice were prepared as described for the neutropenic thigh infection model for all pharmacokinetic and efficacy studies. The pharmacokinetics of SMC-3176 was evaluated following single doses of 6.25, 25, and 100 mg/kg. Blood samples were collected from 6 mice per time point for 8 time points per dose at 0.08, 0.25, 0.5, 1, 1.5, 2, 3, and 4 h postdose. Plasma samples were separated after centrifugation in the presence of EDTA and stored at −20°C until bioanalysis. The concentrations of SMC-3176 in mouse plasma were measured by liquid chromatography-tandem mass spectrometry (LC/MS/MS), for which the assay was linear over a range of 0.005 to 10 μg/ml (R2 = 0.995), and quality control accuracy was 100% ± 15%. In brief, plasma samples were extracted in acetonitrile containing 10% trichloroacetic acid and 250 ng/ml carbutamide as an internal standard, centrifuged, and loaded onto a Polaris C18 analytical column (20 by 2.1 mm, 3 μm pore size) (Agilent Technologies, Santa Clara, CA). The high-performance liquid chromatography (HPLC) system consisted of mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in acetonitrile), which was increased in a linear fashion from 5% to 95% over 2 min at a flow rate of 0.8 ml/min. The samples were analyzed using the Applied Biosystems API4000 triple quadrupole mass spectrometer (AB Sciex, Framingham, MA) tuned to the most intense mass transition using multiple-reaction monitoring mode. Pharmacokinetic parameters in the neutropenic thigh-infected mouse were determined from single-dose data for each dose level by compartmental analysis (Phoenix WinNonlin 6.3; Certara, St. Louis, MO), with model selection based on the visual inspection of fit, Akaike's information criterion, and correlation coefficient value (R2 ≥ 0.920). Plasma exposures following multiple-dose administration in the efficacy studies were simulated by mean parameters and verified to correspond between observed and simulated concentrations. To reflect free (i.e., unbound, microbiologically active) drug, simulated plasma concentration-time data were corrected for SMC-3176 protein binding in mouse plasma (55.6%).
SMC-3176 was examined for in vivo efficacy using the neutropenic mouse thigh infection model against five P. aeruginosa isolates (PAO1, ARC3502, ARC3506, ARC4659, and JJ 4-36) chosen with consideration as to β-lactamase content. Multiple doses ranging from 25 to 3,200 mg/kg/day were divided and administered every 3 h (q3h) for 3 mice per dose group. Growth control animals received vehicle solution in the same manner as SMC-3176-treated groups. Mice were euthanized by CO2 exposure, followed by cervical dislocation just prior to antibiotic initiation (pretreatment) and 24 h after treatment initiation (vehicle and treatment groups). For certain isolates, the vehicle control animals were sacrificed at an earlier time point prior to expiry due to the aggressively virulent features of the pseudomonal infection. Infected thigh muscles were aseptically removed, individually homogenized, and plated in serial dilutions on Trypticase soy agar for bacterial counts. Efficacy was assessed by the bacterial density in thigh tissue and was defined as the change in log10 CFU per thigh or per gram of thigh for antibiotic-treated groups versus the pretreatment group. The relationship between efficacy and the PK/PD index of interest for SMC-3176, the free time above the MIC (fT>MIC), was analyzed by the inhibitory sigmoid maximum effect (Emax) model (Phoenix WinNonlin 6.3; Certara, St. Louis, MO), and magnitudes of the exposure index associated with 80% of the maximal effect (EI80), net bacteriostasis, and 1- and 2-log reductions were determined. Thigh tissue samples from the vehicle control group and select treatment groups of SMC-3176 were additionally cultured on 8× the MIC MHB agar plates to assess for potentially resistant colonies.
CDMHB agarose growth assay.
In a follow-up to the SMC-3176 efficacy results, an in vitro CDMHB agarose growth assay was employed, as previously described (11). Bacterial isolates were first grown in Luria broth (LB) (Sigma-Aldrich, St. Louis, MO) to mid-log phase and then plated at 5 × 106 CFU on 100-mm diameter CDMHB agarose plates (poured using CDMHB and 1.5% UltraPure agarose [Life Technologies, Grand Island, NY]) containing SMC-3176 at 0 to 32 μg/ml in doubling dilutions for a target bacterial density of 9.1 × 104 CFU/cm2 per plate. CDMHB agarose plates inoculated with 100 μl of bacterial suspension were incubated at 37°C for 40 h and then inspected visually under UV light to determine the highest SMC-3176 concentration that supported bacterial growth (as opposed to the lowest concentration that inhibited growth). A minimum of two independent tests were performed for each isolate to assess the reproducibility of the assay.
Pyoverdine quantification.
P. aeruginosa isolates evaluated in the animal efficacy model were also profiled for pyoverdine expression. Bacterial suspensions were prepared in LB by overnight incubation at 37°C and diluted in 25 ml of MHB or CDMHB to target absorbance at 600 nm (A600) of 0.1. P. aeruginosa cultures in MHB or CDMHB were then grown at 37°C with shaking for 3 h (over log-phase growth), during which 1-ml samples were collected for bacterial growth by A600 and for pyoverdine quantification. Following centrifugation of the samples, the pyoverdine content of the separated supernatant was determined by measuring absorbance at the characteristic color wavelength of pyoverdine, 405 nm (18). Pyoverdine concentrations were calculated based on a linear standard curve generated with pyoverdine purchased from Sigma-Aldrich (St. Louis, MO) and normalized to bacterial cell density, measured as milligrams per milliliter per A600.
Whole-blood MIC assay.
An alternative growth inhibition assay was developed using mouse whole blood (WB) to more closely emulate the animal efficacy model. Ninety-six-well plates containing 2 μl of 1 × 106 CFU/ml inoculum, 2 μl of serially diluted drug, and 26 μl of either freshly harvested, heparinized, and pooled mouse WB or LB medium (as control) per well were incubated at 37°C in 5% CO2 with shaking for 6 h. Next, 170 μl of distilled, deionized water was added to each well and incubated for another 5 min at room temperature to allow for red blood cell lysis. Lastly, 3 μl from each well was spotted onto LB agar in 86 by 128-mm microplate trays and incubated overnight at 37°C. The WB MIC was the lowest drug concentration at which no bacterial growth was observed following the final overnight incubation. A minimum of three independent tests were performed for each isolate to assess the reproducibility of the assay.
RESULTS
In vitro activity by MIC.
Against 100 contemporary, clinical isolates of P. aeruginosa, SMC-3176 displayed MIC50/90s of 0.12/1 mg/liter by standard broth microdilution in MHB. Conversely, the MIC50/90s for aztreonam, ceftazidime, and meropenem were 16/>64, 32/>64, and 8/>64 mg/liter, respectively. SMC-3176 was unaffected by a variety of β-lactamases in a characterized panel of 20 clinical P. aeruginosa isolates, as potent activity was maintained against isolates containing extended-spectrum β-lactamases, carbapenemases, and metallo-β-lactamases (Table 2).
TABLE 2.
In vitro activity of SMC-3176 against clinical isolates of P. aeruginosa (n = 20) containing various β-lactamases
| Isolate | Acquired β-lactamasea | MIC in MHB (mg/liter) |
|||
|---|---|---|---|---|---|
| SMC-3176 | Aztreonam | Ceftazidime | Meropenem | ||
| ATCC 27853 | None | 0.5 | 4 | 2 | 1 |
| ARC3502 | VIM-1 | 0.12 | 64 | >64 | >64 |
| ARC3504 | SHV-2 | 0.06 | 64 | 32 | 4 |
| ARC3505 | PER-1, OXA-10 | 0.5 | >64 | >64 | 4 |
| ARC3506 | VEB-1, OXA-10 | 0.12 | >64 | >64 | 8 |
| ARC3509 | None | 0.06 | >64 | >64 | 16 |
| ARC3511 | KPC-2 | 0.06 | >64 | 64 | 32 |
| ARC3514 | KPC-2 | 0.06 | >64 | >64 | >64 |
| ARC3606 | TEM-24 | 0.06 | 16 | 64 | 4 |
| ARC3607 | OXA-2 | 4 | 64 | >64 | 8 |
| ARC3611 | None | 0.5 | 32 | 32 | 4 |
| ARC3612 | None | 0.06 | 16 | 64 | 16 |
| ARC3727 | VIM-2, OXA-4 | 0.25 | 16 | 32 | >64 |
| ARC3729 | VEB-1, OXA-10 | 0.25 | >64 | >64 | 16 |
| ARC3756 | IMP-18, VIM-2, OXA-2 | 0.25 | 16 | >64 | >64 |
| ARC3916 | GES-1, OXA-2 | 0.12 | 16 | >64 | 16 |
| ARC3925 | VIM-2 | 0.12 | 32 | 32 | >64 |
| ARC4060 | PER-1, OXA-10 | 0.06 | >64 | >64 | 16 |
| ARC4063 | VEB-1 | 0.5 | >64 | >64 | 32 |
| ARC4068 | IMP-15, OXA-2 | 0.016 | 32 | >64 | >64 |
All isolates contained chromosomal ampC and poxB genes.
Against a subset of P. aeruginosa isolates fit for in vivo testing (n = 10), SMC-3176 MICs were within one dilution between determinations in standard MHB (0.12 to 0.5 mg/liter) and that in the iron-chelated medium, CDMHB (0.06 to 0.5 mg/liter), except for one isolate, ARC4659. A significant improvement in susceptibility was exhibited for SMC-3176 with this clinical isolate, from an elevated MIC of 8 mg/liter in MHB to a lower MIC of 0.5 mg/liter in CDMHB. Similar reductions in MIC were observed for the siderophore-conjugated comparators MC-8 and MB-1 (MIC in MHB, 8 to 16 mg/liter; MIC in CDMHB, 0.06 to 0.5 mg/liter). Accordingly, an in vivo efficacy experiment with ARC4659 was arranged to investigate whether efficacy could be achieved despite the nonsusceptible SMC-3176 MIC by standard MHB methods. Of note, we were unable to identify features in the siderophore-related genes of ARC4659 that could account for the large MIC difference across medium types (see Table S5 in the supplemental material).
Mechanisms of resistance.
The spontaneous resistance frequencies for SMC-3176 were 5.5 × 10−8, 9.4 × 10−8, 9.9 × 10−8, 5.1 × 10−7, and 2.0 × 10−7 at 4× the MIC in PAO1, ARC3483, ARC3502, ARC3506, and ARC3514, respectively. At 8× the MIC, the frequencies were 8.4 × 10−9, 7.9 × 10−9, 1.1 × 10−8, 1.5 × 10−7, and 3.0 × 10−8, respectively. The SMC-3176 MICs for stably resistant isolates were 16- to 128-fold higher than those of the parental isolates, as presented in Table 3. All SMC-3176 mutants displayed cross-resistance to siderophore-conjugated comparators, while the MICs were unchanged for siderophore-free reference β-lactams (Table 3).
TABLE 3.
Characterization and susceptibilities of mutants generated from in vitro spontaneous resistance studies for SMC-3176 in P. aeruginosa
| Parent/mutant (selection concn [×MIC]) | Mutationa | MIC in MHB (mg/liter) |
|||||
|---|---|---|---|---|---|---|---|
| SMC-3176 | MB-1 | MC-8 | Aztreonam | Ceftazidime | Meropenem | ||
| PAO1 | 0.25 | 0.5 | 0.5 | 4 | 2 | 1 | |
| A1-PAO1 (4) | Δ[PiuC(PA4515)…PA4521]b | 4 | 4 | 16 | 4 | 1 | 2 |
| A2-PAO1 (8) | pfecI (−44 T→C)c | 32 | 8 | 16 | 8 | 2 | 2 |
| ARC3483 | 0.25 | 0.5 | 0.5 | 2 | 1 | 0.5 | |
| B1-ARC3483 (2) | PiuC [Q177fs] | 8 | 16 | 16 | 2 | 0.5 | 0.5 |
| ARC3502 | 0.25 | 0.25 | 0.25 | 64 | >64 | >64 | |
| C1-ARC3502 (4) | PiuA [Q186fs] | 8 | 4 | 4 | 32 | >64 | >64 |
| C2-ARC3502 (4) | pfecI (−41 C→A)c | 4 | 4 | 8 | 32 | >64 | >64 |
| C3-ARC3502 (8) | PiuA [Q604fs] | 8 | 8 | 4 | >64 | >64 | >64 |
| ARC3506 | 0.25 | 1 | 0.25 | >64 | >64 | 4 | |
| D2-ARC3506 (8) | pfecI (−45 C→T)c | 32 | 32 | 32 | >64 | >64 | 16 |
| ARC3514 | 0.13 | 0.13 | 0.13 | >64 | >64 | >64 | |
| E1-ARC3514 (4) | PiuC [W175*] | 1 | 8 | 2 | >64 | >64 | >64 |
| E2-ARC3514 (8) | ppvdS (−88 T→A)c | 2 | >64 | 8 | >64 | >64 | >64 |
| E3-ARC3514 (16) | PirR [G132fs] | 1 | 2 | 1 | >64 | >64 | >64 |
Mutations noted by amino acid changes in coding regions or base pair changes in gene promoter. fs, frameshift; p, promoter.
Indicates a 8,725-bp deletion (5056096..5064820 relative to PAO1) encompassing PiuC (PA4515) through PA4521 and the terminal 14 amino acids of AmpD [W174fs].
Point mutation in promoter at predicted Fur binding site.
Ten resistant mutants were profiled by WGS, and all contained changes in genes associated with iron uptake (Table 3). For five mutants, a variety of genetic changes were seen in the piuA and piuC genes, including insertions, deletions, and frameshifts all likely to result in a loss of function of the encoded proteins. PiuA is an outer membrane siderophore receptor with similarity to the Fiu catechol receptor, while PiuC is a predicted Fe(II)-dependent oxygenase with its gene located directly adjacent to PiuA (19, 20). The predicted effect of a piuA or piuC mutation is decreased expression of the PiuA receptor, which is one of two receptors that hydroxypyridinone-based siderophore conjugates are believed to exploit for entry, with the other being PirA (20, 21). A frameshift mutation was also found in the gene encoding PirR, which is a new resistance mechanism to be reported for a siderophore-conjugated β-lactam. PirR is the response regulator of a two-component regulatory system predicted to activate expression of the PirA receptor, and a mutation in PirR is expected to result in decreased expression of PirA (10).
Three isolates contained mutations in the predicted Fur-binding region of the fecI promoter (pfecI) at positions of −41, −44, and −45 nucleotides before the start of the gene. The fecI gene is an extracytoplasmic function (ECF) sigma-70 factor that acts as a transcriptional activator of the ferric citrate siderophore system (22). Mutations at this pfecI location have been reported for BAL30072-resistant isolates, and it was shown that by derepressing the fecIRA operon, a mutation in the Fur-binding promoter of fecI led to increased expression of the ferric citrate siderophore receptor FecA (20). Transcriptional analysis of our C2-ARC3502 pfecI mutant yielded the same result of elevated fecA expression relative to the parent (data not shown).
In addition to pfecI, another instance of a promoter mutation at a Fur repressor site was found upstream of pvdS, which is an ECF sigma-70 factor that controls the expression of synthesis genes for pyoverdine (23). The predicted effect of a ppvdS mutation is the derepression of pyoverdine synthesis, which would lead to enhanced expression of the cognate siderophore receptor for pyoverdine, FpvA. Both promoter mutations (pfecI and ppvdS) are expected to result in increased expression of siderophore receptors (FecA and FpvA), which would outcompete the availability of PiuA and PirA and thus decrease SMC-3176 entry (20).
Isogenic isolates with knockout mutations in implicated siderophore receptor genes were used to confirm that mutations from selection studies were indeed the cause of SMC-3176 resistance (Table 4). Single piuA and piuC deletions conferred large decreases in susceptibility for SMC-3176 and other siderophore-conjugated β-lactams. The pirA deletion conferred no susceptibility change on its own; however, in conjunction with the piuA deletion, it resulted in a loss of activity that was greater than that of ΔpiuA alone (11).
TABLE 4.
Susceptibility of isogenic P. aeruginosa isolates containing knockout mutations in genes related to siderophore receptors
| Mutation | MIC in MHB (mg/liter) |
|||||
|---|---|---|---|---|---|---|
| SMC-3176 | MB-1 | MC-8 | Aztreonam | Ceftazidime | Meropenem | |
| (PAO1, parent) | 0.25 | 0.5 | 0.5 | 4 | 2 | 1 |
| ΔpiuA | 4 | 16 | 8 | 16 | 4 | 2 |
| ΔpiuC | 16 | 32 | 8 | 16 | 4 | 1 |
| ΔpirA | 0.5 | 0.5 | 1 | 16 | 4 | 2 |
| ΔpiuA + ΔpirA | 32 | 64 | >64 | 8 | 4 | 2 |
| ΔtonB1a | >64 | >64 | >64 | 8 | 2 | 2 |
MIC determined in medium supplemented with 160 μM Fe(SO4).
In vivo efficacy and PK/PD.
A one-compartment model with first-order input and elimination was used to characterize the pharmacokinetics of SMC-3176 in the neutropenic thigh-infected mouse, with the following final parameters: volume of distribution (V), 899 ml/kg; clearance (CL), 2006 ml/h/kg; and elimination half-life (t1/2), 0.30 h. SMC-3176 displayed linear pharmacokinetics over the range of doses studied at 6.25 mg/kg (Cmax, 10.5 μg/ml; time to Cmax [Tmax], 0.13 h; AUC, 5.19 μg · h/ml), 25 mg/kg (Cmax, 29.3 μg/ml; Tmax, 0.10 h; AUC, 14.6 μg · h/ml), and 100 mg/kg (Cmax, 62.6 μg/ml; Tmax, 0.12 h; AUC, 39.8 μg · h/ml) (see Fig. S6 to S8 in the supplemental material, respectively). The mean ± standard deviation (SD) starting bacterial density in the model was 5.9 ± 0.3, 6.6 ± 0.2, 6.4 ± 0.3, 5.5 ± 0.4, and 5.8 ± 0.1 log10 CFU per thigh or gram of thigh for PAO1, ARC3502, ARC3506, ARC4659, and JJ 4-36, respectively. Bacterial growth was approximately 2 to 3 log, respectively, progressing to 7.6 ± 0.3, 8.5 ± 0.3, 8.1 ± 0.7, 8.2 ± 0.3, and 9.5 ± 0.2 log10 CFU per thigh or gram of thigh in vehicle controls by 24 h.
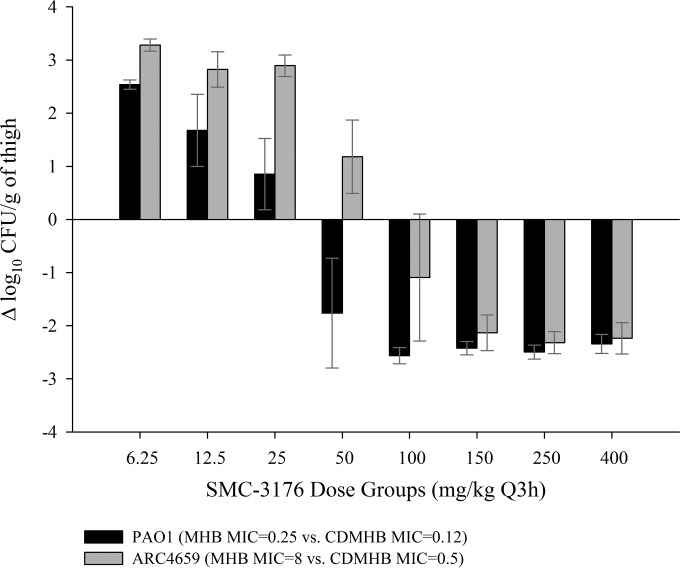
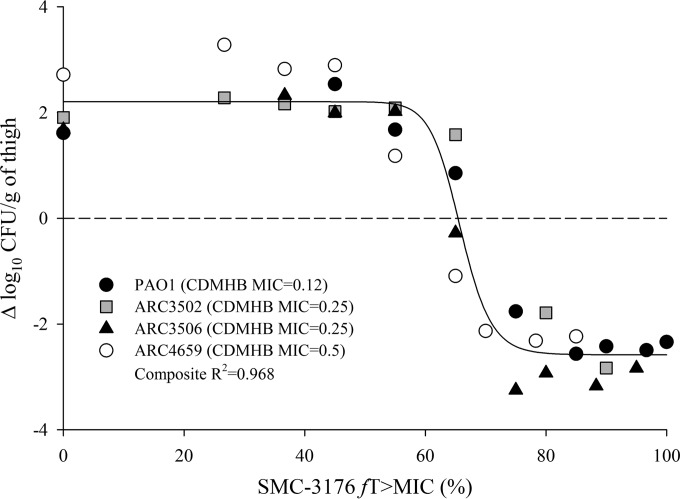
Efficacy was demonstrated in a dose-dependent fashion against PAO1 and clinical isolates ARC3502 and ARC3506, with a maximal reduction in bacterial load of approximately 3 log relative to that at the start of treatment. SMC-3176 was also efficacious against ARC4659 despite the elevated, nonsusceptible MIC in MHB of 8 mg/liter. Rather, efficacy was more appropriately described by the MIC in CDMHB of 0.5 mg/liter, as in vivo responses against ARC4659 paralleled those against PAO1, for which the MICs in MHB and CDMHB were 0.25 and 0.12 mg/liter, respectively (Fig. 2). Using the MIC in CDMHB, in vivo efficacy for SMC-3176 was predictable against the four isolates in association with the fT>MIC (R2 = 0.968 to 0.985) (Fig. 3). SMC-3176 exposures ranged from 59.4 to 81.1% fT>MIC for stasis to 2-log kill effects, consistent with those reported for historical β-lactams, such as aztreonam or cephalosporins (Table 5) (12, 24).
FIG 2.
Efficacy defined by the change in bacterial burden for SMC-3176 against representative P. aeruginosa isolates PAO1 and ARC4659 in the neutropenic mouse thigh model. Each bar represents the mean ± SD from six data points.
FIG 3.
Composite relationship between fT>MIC and in vivo efficacy for SMC-3176 against four P. aeruginosa isolates in the neutropenic mouse thigh model. Each symbol represents mean value from six data points.
TABLE 5.
fT>MIC associated with various efficacy endpoints for SMC-3176 against P. aeruginosa in the neutropenic mouse thigh model
| Isolate | MIC (mg/liter) in: |
% fT>MIC (% CV) by CDMHB MIC ata: |
||||
|---|---|---|---|---|---|---|
| MHB | CDMHB | Stasis | 1-log kill | 2-log kill | EI80 | |
| PAO1 | 0.25 | 0.12 | 68.0 (1.1) | 71.3 (1.3) | 76.6 (2.0) | 76.5 (2.3) |
| ARC3502 | 0.12 | 0.25 | 72.9 (1.2) | 76.5 (1.1) | 81.1 (1.2) | 84.1 (2.3) |
| ARC3506 | 0.12 | 0.25 | 63.4 (1.2) | 66.9 (1.2) | 71.3 (1.9) | 74.3 (3.1) |
| ARC4659 | 8 | 0.5 | 59.4 (1.2) | 63.5 (1.5) | 71.4 (2.5) | 70.3 (3.3) |
| JJ 4-36 | 0.12 | 0.06 | Indeterminable | Indeterminable | Indeterminable | Indeterminable |
Indeterminable indicates that exposures for efficacy could not be appropriately determined against isolate JJ 4-36, due to the lack of a well-corresponding PK/PD relationship.
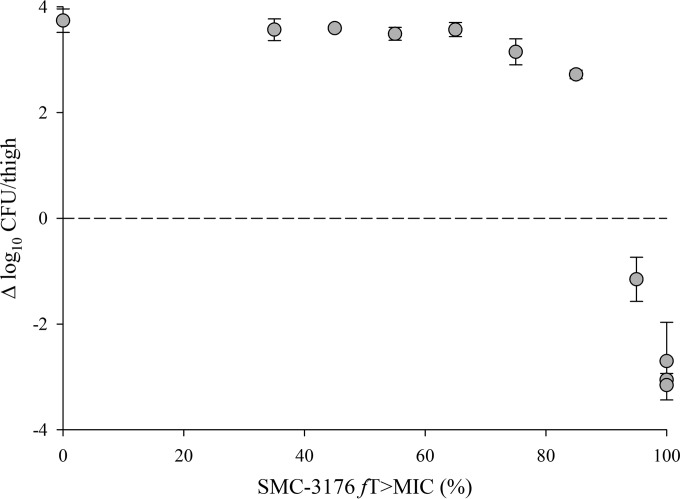
Against isolate JJ 4-36, however, in vivo responses for SMC-3176 were not predictable by MIC, be it in MHB (0.12 mg/liter) or CDMHB (0.06 mg/liter). While a maximal effect of 3-log kill was observed, it was achieved only at 100% fT>MIC, with consistent bacterial regrowth occurring over the 60 to 80% fT>MIC range (Fig. 4). This lack of predictable efficacy by the MIC was similarly reported for MB-1 against the same JJ 4-36 and four other P. aeruginosa isolates (AZ 32-13, JJ 5-35, JJ 8-16, and JJ 11-54) using a single humanized dosing regimen in a neutropenic mouse thigh model (11). With P. aeruginosa JJ 4-36, there appeared to be a threshold concentration greater than the MIC in MHB or CDMHB at which bacterial reduction was still achievable, if dosed high enough. From the multiple pharmacodynamic point estimates obtained, it was possible to back-extrapolate the in vivo threshold based on the PK/PD behavior of established β-lactams; this was estimated to be 0.5 mg/liter for SMC-3176. Likewise, when reexamined using our dose-ranging approach, in vivo responses for MB-1 failed to correlate by MIC (in MHB, 1 mg/liter; in CDMHB, 0.25 mg/liter) against JJ 4-36 and implied a higher threshold of 4 or 8 mg/liter (see Fig. S9 in the supplemental material).
FIG 4.
Lack of correlation between fT>MIC and in vivo response for SMC-3176 against P. aeruginosa JJ 4-36 (CDMHB MIC, 0.06 mg/liter) in the neutropenic mouse thigh model. Each symbol represents mean value ± SD from six data points.
Exploratory in vitro assays.
The CDMHB agarose growth assay was previously developed and reported to be highly correlated with in vivo outcomes for MB-1 (11). It should be emphasized that this assay is different and separate from the MIC determinations in CDMHB based on the experimental methods (e.g., agarose plates versus broth microtiter trays) and the final endpoint (i.e., the highest concentration at which bacterial growth was sustained versus the lowest concentration that inhibited growth). For SMC-3176, however, the assay was neither reproducible nor reliable for predicting the in vivo responses against the five P. aeruginosa isolates studied. The results for the maximum concentration with growth on CDMHB plates were varied upon repeat testing in PAO1 (0.12 and 1 mg/liter), ARC3502 (0.5 and 4 mg/liter), and ARC3506 (1 and 8 mg/liter). Values were more reproducible with ARC4659 (2 and 2 mg/liter) and JJ 4-36 (0.5, 0.5, and 1 mg/liter), and yet, there was no consistent trend associated with in vivo responses that held true across isolates. Although validated for MB-1 by the authors of that study (11) and by our own laboratory, the utility of the assay appeared to be compound specific and did not extend to SMC-3176.
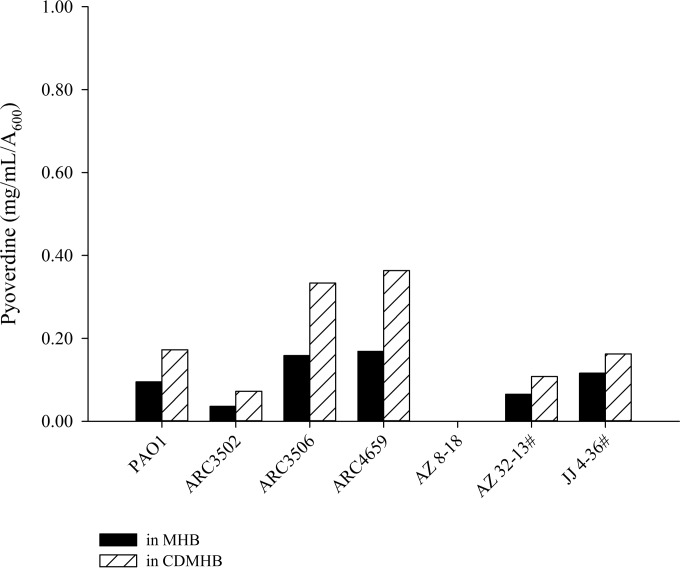
In the course of conducting the CDMHB growth assay, increased pyoverdine expression was detected, as evidenced by the fluorescent green haze surrounding colonies recovered on CDMHB agarose plates. Inability of the siderophore conjugate to outcompete pyoverdine, the major native siderophore of P. aeruginosa with high iron affinity, has been implicated as a possible cause of attenuated efficacy (11). Thus, an attempt was made to effectively quantify pyoverdine from isolates evaluated for efficacy in search of an in vivo correlation. When measured upon growth in liquid cultures, pyoverdine production was modestly higher in CDMHB than in MHB (Fig. 5). The amount of CDMHB-induced pyoverdine production, however, did not vary widely among isolates, despite contrasting outcomes in the animal efficacy model. Notably, P. aeruginosa JJ 4-36 (in CDMHB, 0.162 mg/ml/A600) did not produce more pyoverdine than the reference isogenic isolate, PAO1 (in CDMHB, 0.173 mg/ml/A600).
FIG 5.
Pyoverdine production from P. aeruginosa isolates in MHB and CDMHB liquid cultures during log-phase growth at 3 h. #, isolates against which in vivo failures were previously reported for siderophore-conjugated monobactam MB-1 (11).
Having been unsuccessful in uncovering an informative in vitro tool for SMC-3176, the mouse WB MIC assay was retrospectively developed in a final attempt to identify isolates prone to adaptive resistance, like JJ 4-36. First, the results of the WB MIC assay were highly reproducible for SMC-3176; values were within 1 to 2 doubling dilutions upon repeat testing in all 10 isolates studied (Table 6). The MICs determined in LB under CO2 served as points of reference and were well matched to standard MICs in MHB. Second, the assay results were consistently in alignment with the in vivo responses for SMC-3176 across isolates. When interpreted based on the shift in MIC (and not the numerical value itself) from LB to mouse WB under CO2, significant increases of ≥16-fold were observed with JJ 4-36 and four other P. aeruginosa isolates against which in vivo failures were reported for MB-1. Minor to moderate shifts in MICs were seen in isolates with predictable in vivo efficacy, most notably including ARC4659, which demonstrated a decreasing trend in MIC, akin to the MIC reduction observed between the MHB and CDMHB media. This behavior of a ≥16-fold MIC increase in mouse WB was unique to siderophore-conjugated compounds, including MB-1, as the MICs were overall comparable to those generated by standard MHB methods for aztreonam and colistin sulfate (Table 7). The MICs for meropenem were not as comparable, but no significant shifts in the upward direction were observed with any isolate.
TABLE 6.
Correlation between results of the mouse whole-blood assay and in vivo response for SMC-3176 against P. aeruginosa
| Isolate | MIC (mg/liter) in: |
MIC shift in mouse WB assaya | In vivo response | |||
|---|---|---|---|---|---|---|
| MHB | CDMHB | LB CO2 | Mouse WB CO2 | |||
| PAO1 | 0.25 | 0.12 | 0.25, 0.25, 0.25, 0.25 | 0.25, 0.25, 0.5, 0.5 | 2-fold increase | Efficacy predicted by MIC |
| ARC3502 | 0.12 | 0.25 | 0.25, 0.25, 0.25, 0.5 | 1, 2, 2, 4 | 8-fold increase | Efficacy predicted by MIC |
| ARC3506 | 0.12 | 0.25 | 0.25, 0.25, 0.5 | 2, 2, 2 | 8-fold increase | Efficacy predicted by MIC |
| ARC4659 | 8 | 0.5 | 8, 8, 8, 16 | 2, 2, 2, 2 | 4-fold decrease | Efficacy predicted by MIC |
| AZ 8-18 | 0.5 | 0.25 | 0.12, 0.25, 0.25 | 0.5, 0.5, 0.5 | 2-fold increase | Not tested |
| AZ 32-13b | 0.5 | 0.5 | 0.5, 0.5, 0.5 | 8, 16, 16 | ≥16-fold increase | Not tested |
| JJ 4-36b | 0.12 | 0.06 | 0.06, 0.12, 0.12, 0.12 | 2, 2, 2, 4 | ≥16-fold increase | Efficacy not predicted by MIC |
| JJ 5-35b | 0.5 | 0.12 | 0.25, 0.25, 0.5 | 4, 8, 16 | ≥16-fold increase | Not tested |
| JJ 8-16b | 0.12 | 0.06 | 0.25, 0.25, 0.5, 0.5 | 4, 8, 8, 16 | ≥16-fold increase | Not tested |
| JJ 11-54b | 0.5 | 0.25 | 0.25, 0.5, 0.5, 0.5 | 4, 8, 8, 8 | ≥16-fold increase | Not tested |
Assay results interpreted by the fold increase between the modal or central MIC in LB CO2 and the modal or central MIC in mouse WB CO2 from three or more independent tests.
Isolates against which in vivo failures were previously reported for siderophore-conjugated monobactam MB-1 (11).
TABLE 7.
Activities of siderophore-free reference compounds against P. aeruginosa in the mouse whole-blood assay
| Isolate | MIC (mg/liter) in: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Aztreonam |
Meropenem |
Colistin sulfate |
|||||||
| MHB | LB CO2 | Mouse WB CO2a | MHB | LB CO2 | Mouse WB CO2a | MHB | LB CO2 | Mouse WB CO2a | |
| PAO1 | 4 | 4 | 2 | 0.25 | 0.12 | 0.03 | 0.5 | 1 | 2 |
| ARC3502 | 64 | 16 | 8 | >64 | 8 | 8 | 0.5 | 0.5 | 2 |
| ARC3506 | >64 | 256 | 128 | 8 | 4 | 4 | 0.5 | 0.5 | 2 |
| ARC4659 | 16 | 16 | 16 | 0.5 | 0.03 | 0.03 | 0.5 | 0.5 | 1 |
| AZ 8-18 | 8 | 16 | 16 | 8 | 4 | 4 | 0.5 | 1 | 1 |
| AZ 32-13 | 16 | 64 | 64 | 2 | 0.5 | 0.06 | 0.5 | 0.5 | 2 |
| JJ 4-36 | 32 | 64 | 32 | 32 | 8 | 4 | 1 | 2 | 2 |
| JJ 5-35 | 32 | 64 | 64 | 32 | 8 | 16 | 0.5 | 1 | 1 |
| JJ 8-16 | 16 | 64 | 64 | 2 | 0.5 | 0.12 | 0.5 | 1 | 2 |
| JJ 11-54 | >64 | 64 | 64 | 4 | 0.5 | 0.12 | 1 | 2 | 2 |
Assay results interpreted by the fold increase between the modal MIC in LB CO2 and the modal MIC in mouse WB CO2 from three independent tests.
DISCUSSION
In the face of the intrinsic impermeability and the many existing resistance mechanisms behind the multidrug-resistant nature of P. aeruginosa (from an array of β-lactamases to efflux pump systems and loss of outer membrane porin proteins) (25, 26), siderophore-antibiotic conjugates appear to be a promising endeavor during a dearth of truly novel agents. Screening cascades in early antibacterial discovery are typically launched with in vitro susceptibility testing of candidate compounds, and while standard MIC tests provided exquisitely potent values for SMC-3176, a report of a competitor siderophore conjugate with unanticipated loss of in vivo efficacy (11) served to caution investigators. Here, we determined the resistance mechanisms and PK/PD profile of the siderophore-conjugated monocarbam SMC-3176 in P. aeruginosa and further explored in vitro tools (broth microdilution MIC in MHB versus in CDMHB, CDMHB agarose growth assay, quantification of pyoverdine, and mouse WB MIC assay) to address the disconnect between in vitro measures of antibacterial activity and in vivo efficacy.
The path to resistance against SMC-3176 was multiple (via piuA, piuC, pirR, fecI, and pvdS genes), and all genes were linked to various components of the siderophore-mediated iron uptake systems in P. aeruginosa. It should be noted that no stably resistant colonies were recovered from any of the animal efficacy studies. This finding was indicated as being a distinct feature of the transient, adaptive resistance described for MB-1 by Tomaras and colleagues (11). Display of adaptive resistance (i.e., resistance induced in the presence of drug and reversed in the drug's absence) is not unfamiliar to P. aeruginosa and has been encountered with aminoglycosides and polymyxins (27–31). Although largely unnoticed due to the clinical practice of combining aminoglycosides with other antibiotics, adaptive resistance has been documented in time course experiments following multiple doses of drug in animal and human studies of P. aeruginosa infection (29, 30). In these observations, the susceptible MIC was reflected in the in vivo response that followed only the first-dose exposure to netilmicin in neutropenic thigh-infected mice and tobramycin in cystic fibrosis patients, and bacterial kill was considerably lessened or lacking with subsequent doses. Similar experiments may prove to be informative for siderophore conjugates; however, such studies were not feasible with SMC-3176 due to the substantial amount of compound required to enable multiple pharmacodynamic assessments at various time points following multiple-dose administration.
While a single pharmacodynamic point estimate (either regrowth or reduction) was only possible for MB-1 by the design of the study (11), the complete spectrum of in vivo response was profiled for SMC-3176, having tested a wide range of dose regimens against each isolate. This approach exposed that previously concluded treatment failures were not complete, clear-cut failures, but that efficacious bacterial kill could still be achieved in vivo if drug exposures surpassed a certain, but yet indeterminable, threshold. Despite our best efforts, it remains unclear as to why isolate JJ 4-36 behaved differently from the four other P. aeruginosa isolates tested (PAO1, ARC3502, ARC3506, and ARC4659), such that kill effects were observed only at SMC-3176 exposures exceeding the 60 to 80% fT>MIC mark. It would be inappropriate to imply that achieving 100% fT>MIC would evade the effects of adaptive resistance. That estimation is being driven by the denominator, i.e., the MIC value, which was incorrect and overpredicted the level of SMC-3176 activity against JJ 4-36. Moreover, the 100% fT>MIC descriptor does not capture the full extent of drug exposure; different dose regimens may provide the same 100% fT>MIC but vary significantly in other parameters, like Cmax and AUC. Such was the case with MB-1, for which four regimens that all provided 100% fT>MIC (using our dose-ranging approach) produced varied effects from 2-log growth to 2-log kill against JJ 4-36. The PK/PD index for SMC-3176 was reasonably assumed to be time driven (supported by in vitro time-kill results for SMC-3176 [data not shown]), as this has been well-established for various drug classes belonging to the β-lactam family (12, 24), and greater emphasis was placed on exploring the potential loss of in vivo efficacy.
The issue of adaptive resistance appeared to be isolate specific and was suspected to extend beyond JJ 4-36 to other clinical P. aeruginosa isolates. Tomaras and colleagues (11) observed in vivo regrowth against their MB-1 humanized dosing regimen in five out of nine isolates, suggesting a probability of encountering similarly adaptive isolates in the clinical setting. Further compounding this concern, unfavorable in vivo responses against JJ 4-36 occurred at standard inocula for both MB-1 and SMC-3176. Because the adaptive resistance phenomenon involves a phenotypic transformation and not the actual selection of resistant mutants, it can and did occur at the standard inoculum for SMC-3176, irrespective of the spontaneous mutation frequency. For aminoglycosides, the adaptive phenomenon along with concentration-dependent killing are fortuitously suited to the once-daily dosing regimen (32, 33), in which large dosages are separated by long intervals to take advantage of first-exposure effects while providing a drug-free period to allow reversal of the phenotype to the susceptible state (27–29). Whether a clinical dosing regimen can be engineered that would likewise circumvent adaptive resistance for siderophore-conjugated β-lactams remains to be determined.
Despite our findings that adaptive resistance can be overcome with increased drug exposure, it continues to be a question as to whether the in vivo threshold can be elucidated from a few isolates and effectively applied to the broader P. aeruginosa population. Several in vitro tests were designed in pursuit of a tool that would at best quantitatively predict the in vivo response for SMC-3176 across isolates, or at least qualitatively identify problematic adaptive isolates, like JJ 4-36. To start, broth microdilution MICs determined in iron-chelated MHB (i.e., in CDMHB) translated appropriately to in vivo efficacy against the four nonadaptive isolates tested versus values determined in unmodified MHB. Standard MIC methods have been rightly manipulated before for certain agents to more closely mimic the clinical situation as it relates to how the drug exerts its antibacterial activity, such as daptomycin requiring calcium supplementation to broth medium or fosfomycin requiring supplementation of glucose-6-phosphate to agar medium (16, 34, 35). However, simple MIC testing in CDMHB failed to convey the particular in vivo readout against JJ 4-36. Subsequently, we moved to adopt the CDMHB agarose growth assay that was conceived with MB-1, which disappointingly did not yield consistent or informative data for SMC-3176. Thereafter, an assay was conducted to quantify the endogenous siderophore, pyoverdine, in nonadaptive versus adaptive isolates, but this measurement also failed to provide insight. Hyperproduction of pyoverdine was visually detectable on CDMHB agarose plates but was not distinguishable among isolates; thus, by quantifying pyoverdine, we had aimed to identify meaningful differences with the increased expression by adaptively resistant isolates. In the end, we settled on the MIC assay in mouse WB (as opposed to another variation of broth medium), which was the only assay to correlate with the in vivo response for SMC-3176 across all examined isolates, including JJ 4-36. The utility of the assay was limited to isolate screening only and could not be leveraged for an estimation of SMC-3176's in vivo threshold concentration from the WB MIC value. As such, the question remains as to how an infected patient in the clinical setting may be treated with SMC-3176 (and possibly other siderophore conjugates) if there is no reliable in vitro susceptibility test to inform the likelihood of its treatment success.
Taken together, this work indicates that SMC-3176 is vulnerable to attenuated efficacy in P. aeruginosa due to rapid adaptive resistance against its siderophore-mediated mode of entry. While comprehensive PK/PD profiling led us to discover an in vivo threshold at which efficacy can be achieved, identifying such a threshold that can be applied across the pseudomonal population is not feasible with current in vitro tools, which includes the qualitative but not quantitative mouse WB assay. Combination therapy with another antipseudomonal agent that uses a non-siderophore-dependent route or with an agent that can potentiate the activity of the siderophore conjugate is a consideration (36, 37). Ultimately, extensive in vivo testing against numerous clinical isolates is warranted for siderophore-conjugated compounds to thoroughly screen for the potential disconnect between the in vitro MIC and in vivo response in P. aeruginosa.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by AstraZeneca Pharmaceuticals LP. All authors were financially compensated or employed by AstraZeneca Pharmaceuticals LP at the time of the study and may hold stock/stock options in the company.
Much appreciation is given to John Mueller, Boudewijn de Jonge, Joseph Newman, Thomas Durand-Réville, Sarah McLeod, and Maria Uria-Nickelsen for their valued scientific contributions and to Lena Grosser, Maryann San Martin, Jennifer Scafidi, Krista Farrington, Jianyan Wang, Wenzhan Yang, James Whiteaker, Catherine Gilbert, and Pamela Tessier for their dedicated technical assistance.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00831-15.
REFERENCES
- 1.Page MG, Dantier C, Desarbre E. 2010. In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant Gram-negative bacilli. Antimicrob Agents Chemother 54:2291–2302. doi: 10.1128/AAC.01525-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitt-Hoffman A, Roos B, Maares J, Sauer J, Spickermann J, Meyer I, Kaufhold A. 2011. Pharmacokinetics and safety of the novel sulfactam antibiotic BAL30072 after single ascending dose infusions in healthy volunteers, abstr A2-572 Abstr 51st Intersci Conf Antimicrob Agents Chemother, 17 to 20 September 2011, Chicago, IL. [Google Scholar]
- 3.Flanagan ME, Brickner SJ, Lall M, Casavant J, Deschenes L, Finegan SM, George DM, Granskog K, Hardink JR, Huband MD, Hoang T, Lamb L, Marra A, Mitton-Fry M, Mueller JP, Mullins LM, Noe MC, O'Donnell JP, Pattavina D, Penzien JB, Schuff BP, Sun J, Whipple DA, Young J, Gootz TD. 2011. Preparation, Gram-negative antibacterial activity, and hydrolytic stability of novel siderophore-conjugated monocarbam diols. ACS Med Chem Lett 2:385–390. doi: 10.1021/ml200012f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown MF, Mitton-Fry MJ, Arcari JT, Barham R, Casavant J, Gerstenberger BS, Han S, Hardink JR, Harris TM, Hoang T, Huband MD, Lall MS, Lemmon MM, Li C, Lin J, McCurdy SP, McElroy E, McPherson C, Marr ES, Mueller JP, Mullins L, Nikitenko AA, Noe MC, Penzien J, Plummer MS, Schuff BP, Shanmugasundaram V, Starr JT, Sun J, Tomaras A, Young JA, Zaniewski RP. 2013. Pyridone-conjugated monocarbam antibiotics with Gram-negative activity. J Med Chem 56:5541–5552. doi: 10.1021/jm400560z. [DOI] [PubMed] [Google Scholar]
- 5.Wencewicz TA, Miller MJ. 2013. Biscatecholate-monohydroxamate mixed ligand siderophore-carbacephalosporin conjugates are selective sideromycin antibiotics that target Acinetobacter baumannii. J Med Chem 56:4044–4052. doi: 10.1021/jm400265k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito A, Yoshizawa H, Nakamura R, Tsuji M, Yamano Y, Shimada J. 2014. S-649266, a novel siderophore cephalosporin: I. In vitro activity against Gram-negative bacteria including multidrug-resistant strains, abstr F-1562 Abstr 54th Intersci Conf Antimicrob Agents Chemother, 5 to 9 September 2014, Washington, DC. [Google Scholar]
- 7.Shimada J, Saisho Y, Katsube T, White S, Fukase H. 2014. S-649266, a novel cephalosporin for Gram-negative bacterial infection: pharmacokinetics (PK), safety and tolerability in healthy subjects, abstr F-1564 Abstr 54th Intersci Conf Antimicrob Agents Chemother, 5 to 9 September 2014, Washington, DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miethke M, Marahiel MA. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mislin GL, Schalk IJ. 2014. Siderophore-dependent iron uptake systems as gates for antibiotic Trojan horse strategies against Pseudomonas aeruginosa. Metallomics 6:408–420. doi: 10.1039/c3mt00359k. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis P, Matthijs S, Van Oeffelen L. 2009. Iron uptake regulation in Pseudomonas aeruginosa. Biometals 22:15–22. doi: 10.1007/s10534-008-9193-0. [DOI] [PubMed] [Google Scholar]
- 11.Tomaras AP, Crandon JL, McPherson CJ, Banevicius MA, Finegan SM, Irvine RL, Brown MF, O'Donnell JP, Nicolau DP. 2013. Adaptation-based resistance to siderophore-conjugated antibacterial agents by Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:4197–4207. doi: 10.1128/AAC.00629-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 13.Kim A, Crandon J, Gorseth E, Blinn C, Patey S, Kutschke A, Chen A, Rooney M, Benenato K, Ehmann D, Eakin A, Nicolau D. 2014. In vivo pharmacodynamics of a siderophore-conjugated monocarbam in Pseudomonas aeruginosa (PA): assessing the risk of attenuated efficacy, abstr A-684 54th Intersci Conf Antimicrob Agents Chemother, 5 to 9 September 2014, Washington, DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patey S, Blinn C, Kutschke A, McLaughlin R, Benenato K, Kim A. 2014. Susceptibility profiling of a siderophore-conjugated monocarbam in various iron-limited media against Pseudomonas aeruginosa, abstr F-1566 54th Intersci Conf Antimicrob Agents Chemother, 5 to 9 September 2014, Washington, DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob Agents Chemother 42:2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohnadel D, Haas D, Meyer JM. 1986. Mapping of mutations affecting pyoverdine production in Pseudomonas aeruginosa. FEMS Microbiol Lett 36:195–199. doi: 10.1111/j.1574-6968.1986.tb01695.x. [DOI] [Google Scholar]
- 19.Nikaido H, Rosenberg EY. 1990. Cir and Fiu proteins in the outer membrane of Escherichia coli catalyze transport of monomeric catechols: study with β-lactam antibiotics containing catechol and analogous groups. J Bacteriol 172:1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Delden C, Page MG, Köhler T. 2013. Involvement of Fe uptake systems and AmpC β-lactamase in susceptibility to the siderophore monosulfactam BAL30072 in Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:2095–2102. doi: 10.1128/AAC.02474-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McPherson CJ, Aschenbrenner LM, Lacey BM, Fahnoe KC, Lemmon MM, Finegan SM, Tadakamalla B, O'Donnell JP, Mueller JP, Tomaras AP. 2012. Clinically relevant Gram-negative resistance mechanisms have no effect on the efficacy of MC-1, a novel siderophore-conjugated monocarbam. Antimicrob Agents Chemother 56:6334–6342. doi: 10.1128/AAC.01345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Hove B, Staudenmaier H, Braun V. 1990. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J Bacteriol 172:6749–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochsner UA, Vasil AI, Vasil ML. 1995. Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophores and exotoxin A expression: purification and activity on iron-regulated promoters. J Bacteriol 177:7194–7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 17:479–501. doi: 10.1016/S0891-5520(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 25.Livermore DM. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis 34:634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 26.Bonomo RA, Szabo D. 2006. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin Infect Dis 43:S49–S56. doi: 10.1086/504477. [DOI] [PubMed] [Google Scholar]
- 27.Skiada A, Markogiannakis A, Plachouras D, Daikos GL. 2011. Adaptive resistance to cationic compounds in Pseudomonas aeruginosa. Int J Antimicrob Agents 37:187–193. doi: 10.1016/j.ijantimicag.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Daikos GL, Jackson GG, Lolans VT, Livermore DM. 1990. Adaptive resistance to aminoglycoside antibiotics from first-exposure down-regulation. J Infect Dis 162:414–420. doi: 10.1093/infdis/162.2.414. [DOI] [PubMed] [Google Scholar]
- 29.Daikos GL, Lolans VT, Jackson GG. 1991. First-exposure adaptive resistance to aminoglycoside antibiotics in vivo with meaning for optimal clinical use. Antimicrob Agents Chemother 35:117–123. doi: 10.1128/AAC.35.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barclay ML, Begg EJ, Chambers ST, Thornley PE, Pattemore PK, Grimwood K. 1996. Adaptive resistance to tobramycin in Pseudomonas aeruginosa lung infection in cystic fibrosis. J Antimicrob Chemother 37:1155–1164. doi: 10.1093/jac/37.6.1155. [DOI] [PubMed] [Google Scholar]
- 31.Fernández L, Gooderham WJ, Bains M, McPhee JB, Wiegand I, Hancock RE. 2010. Adaptive resistance to the “last hope” antibiotics polymyxin B and colistin in Pseudomonas aeruginosa is mediated by the novel two-component regulatory system ParR-ParS. Antimicrob Agents Chemother 54:3372–3382. doi: 10.1128/AAC.00242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore RD, Lietman PS, Smith CR. 1987. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 33.Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. 1995. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 39:650–655. doi: 10.1128/AAC.39.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barry AL, Fuchs PC, Brown SD. 2001. In vitro activities of daptomycin against 2,789 clinical isolates from 11 North American medical centers. Antimicrob Agents Chemother 45:1919–1922. doi: 10.1128/AAC.45.6.1919-1922.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barry AL, Fuchs PC. 1991. In vitro susceptibility testing procedures for fosfomycin tromethamine. Antimicrob Agents Chemother 35:1235–1238. doi: 10.1128/AAC.35.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofer B, Dantier C, Gebhardt K, Desarbre E, Schmitt-Hoffmann A, Page MG. 2013. Combined effects of the siderophore monosulfactam BAL30072 and carbapenems on multidrug-resistant Gram-negative bacilli. J Antimicrob Chemother 68:1120–1129. doi: 10.1093/jac/dks527. [DOI] [PubMed] [Google Scholar]
- 37.Tomaras AP, Crandon JL, McPherson CJ, Nicolau DP. 2015. Potentiation of antibacterial activity of the MB-1 siderophore-monobactam conjugate using an efflux pump inhibitor. Antimicrob Agents Chemother 59:2439–2442. doi: 10.1128/AAC.04172-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.