Abstract
Currently, the World Health Organization recommends addition of a 0.25-mg base/kg single dose of primaquine (PQ) to artemisinin combination therapies (ACTs) for Plasmodium falciparum malaria as a gametocytocidal agent for reducing transmission. Here, we investigated the potential interactions of PQ with the long-lasting components of the ACT drugs for eliminating the asexual blood stages and gametocytes of in vitro-cultured P. falciparum strains. Using the SYBR green I assay for asexual parasites and a flow cytometry-based assay for gametocytes, we determined the interactions of PQ with the schizonticides chloroquine, mefloquine, piperaquine, lumefantrine, and naphthoquine. With the sums of fractional inhibitory concentrations and isobolograms, we were able to determine mostly synergistic interactions for the various PQ and schizonticide combinations on the blood stages of P. falciparum laboratory strains. The synergism in inhibiting asexual stages and gametocytes was highly evident with PQ-naphthoquine, whereas synergism was moderate for the PQ-piperaquine, PQ-chloroquine, and PQ-mefloquine combinations. We have detected potentially antagonistic interactions between PQ and lumefantrine under certain drug combination ratios, suggesting that precautions might be needed when PQ is added as the gametocytocide to the artemether-lumefantrine ACT (Coartem).
INTRODUCTION
Asexual multiplication of the malaria parasites in human blood is associated with the morbidity and mortality due to the disease. The gametocyte, the sexual stage of the parasites, is the obligatory link perpetuating the parasite's life cycle into the Anopheles vectors. While most antimalarial drugs target the asexual intraerythrocytic stages of the malaria parasites, it has been increasingly recognized that drugs with actions on the gametocyte stages are critical for severing this transmission link (1, 2). In particular, interruption of malaria transmission is a major challenge for malaria elimination (3). Among the currently used antimalarial drugs, primaquine (PQ) is the only one with gametocytocidal activity on late-stage gametocytes (4). This drug has been used since the 1950s primarily in combination with chloroquine (CQ) as a radical cure for preventing relapses due to Plasmodium vivax. Presently, it is used in combination with CQ or artemisinin combination therapies (ACTs) for radical cure of relapsing malaria parasites due to P. vivax and Plasmodium ovale. In 2012, the World Health Organization (WHO) recommended the addition of a single dose of 0.74 mg/kg PQ as a gametocytocidal agent to reduce P. falciparum transmission in low-transmission settings, particularly in areas under the threat of artemisinin resistance (5). Later in the same year, the WHO Malaria Advisory Committee modified their recommendation to a single 0.25-mg/kg PQ dose to alleviate concerns of serious toxicity in patients with glucose-6-phosphate dehydrogenase deficiency and in consideration of the benefits of disseminating PQ as a transmission blocking drug to a high proportion of patients within a population. In vivo studies conducted in Southeast Asia and Africa showed that PQ added to ACTs for treating P. falciparum malaria exhibited effective gametocyte clearance in patients (6–9). Even when added to non-artemisinin-based regimens, PQ (>0.4 mg/kg) was able to drastically reduce the proportions of people with detectable gametocytemia (10–14).
The ACT policies have been adopted in almost all regions of the world where malaria is endemic, with the exception of parts of South America where CQ is still efficacious (15). ACT contains a potent artemisinin component, which rapidly reduces the asexual stage population with its fast therapeutic response, plus a longer acting partner drug, which eliminates the remaining asexual stage parasites left in circulation (16). Additionally, some of these drugs also have activities against early-stage gametocytes, thus limiting transmission of the parasites into the mosquitoes (17–20). Some of the ACTs used in different countries include those recommended by the WHO such as artemether-lumefantrine, artesunate-amodiaquine, dihydroartemisinin-piperaquine, and artesunate-mefloquine, as well as artemisinin-naphthoquine. In theory, PQ added to ACTs would target gametocytes formed from asexual parasites that have not been cleared by ACTs and mature gametocytes already present upon ACT treatment, thereby reducing the density and duration of transmissible gametocytes and concomitantly the duration of infectiousness to mosquitoes. PQ is added on the first day of ACT treatment when persisting concentrations of the longer-acting partner drugs are high; thus, drug-drug interactions are expected. Therefore, this study aimed to determine the interactions between PQ and commonly used ACT partner drugs on both P. falciparum asexual blood stages and gametocytes using two in vitro assays.
MATERIALS AND METHODS
Materials and chemicals.
Routine media, solvents, and chemicals were purchased from Fisher Scientific (Newark, DE, USA) or Sigma-Aldrich (St. Louis, MO, USA). The antimalarial drugs PQ, CQ, and mefloquine (MQ) were purchased from Sigma-Aldrich. Piperaquine (PPQ) was from Chongqing Kangle Pharmaceutical Co. (Chongqing, China), while lumefantrine (LMF) and naphthoquine (NQN) were from Kunming Pharmaceutical Co. (Kunming, Yunnan, China). SYBR green I PCR master mix was purchased from Invitrogen (Eugene, OR, USA).
Parasite cultures.
P. falciparum laboratory strains 3D7, HB3, and Dd2 were obtained from MR4 (Manassas, VA, USA). A green fluorescent protein (GFP)-expressing transgenic line (3D7α-tubII/GFP) was constructed with GFP expression directed under the α-tubulin II promoter (17). This line has been used to establish a flow cytometry (FCM)-based drug assay for P. falciparum gametocytes. P. falciparum parasites were maintained in O+ human red blood cells (RBCs) using the method of Trager and Jensen with some modifications (21). O+ RBCs were purchased from Biological Specialty Co. (Colmar, PA, USA), and O+ human serum was from Interstate Blood Bank, Inc. (Memphis, TN, USA). Briefly, asexual stage parasites were grown in complete medium (CM) composed of RPMI 1640 (Gibco Life Technologies, Grand Island, NY, USA) with 25 mM NaHCO3, 25 mM HEPES (pH 7.4), 11 mM glucose, 0.367 mM hypoxanthine, and 5 μg/liter gentamicin as incomplete medium supplemented with 0.5% AlbuMAX (Gibco Life Technologies) for asexual stage parasites or 0.25% AlbuMAX plus 5% human serum for gametocyte induction and cultures. The parasites were routinely maintained in a humidified 5% CO2 incubator at 37°C. Asexual stage cultures were synchronized at the ring stage by 5% d-sorbitol treatment (22). Gametocyte induction was performed by using spent medium as described previously (17).
Asexual stage growth inhibition assay.
The 50% inhibitory concentrations (IC50s) were measured using a modified SYBR green I drug assay (23, 24) where synchronized ring stage parasites were exposed to serial dilutions of drugs at a final volume of 200 μl with 0.5% parasitemia and 1% hematocrit in 96-well flat-bottom plates. Wells without drugs were used as positive controls, while wells with only RBCs were used to subtract background. The plates were kept at 37°C in a humidified 5% CO2 incubator for 72 h. After 72 h, the plates were wrapped and stored at −20°C for at least 16 h. To measure growth inhibition, the plates were thawed at 37°C, and 100 μl of lysis buffer (20 mM Tris-HCl [pH 7.5], 5 mM EDTA, 0.08% Triton X-100, 0.008% saponin in phosphate-buffered saline [PBS], 0.2 μl SYBR green I) was added to each well and mixed thoroughly. The resulting mixture was incubated at 37°C for 1 h, and fluorescence intensity (FI) measurements were obtained using a FLUOstar Optima microplate fluorometer set to an excitation wavelength (λex) of 485 nm and emission wavelength (λem) of 520 nm. The background signal from RBCs was subtracted, and percent growth was calculated against the positive controls. Drug concentrations and percent growth were then imported into SigmaPlot version 12.0, where the curves were plotted in a log scale on the x axis, and the IC50s were determined from sigmoidal curve fits.
Gametocyte drug sensitivity assay.
The gametocyte viability of enriched-stage IV 3D7α-tubII/GFP gametocytes was quantified using FCM by measuring the FI of the GFP signal driven by the gametocyte-specific α-tubulin II promoter (17). Stage IV 3D7α-tubII/GFP gametocytes were purified on a Percoll gradient and mixed with erythrocytes in CM to 0.04% gametocytemia and 2% hematocrit. Equal volumes of these purified gametocytes were added to serial dilutions of 100 μl of drugs in 96-well plates. The plates were then kept in a humidified 5% CO2 incubator for 48 h. The gametocyte viability was measured with a Guava easyCyte 5HT (λex of 488 nm and λem of 525 nm/30 nm) flow cytometer wherein FI in the green channel was collected from at least 30,000 total events. Guava easyCyte 5HT FCS 2.0 files were imported into FlowJo version 10 wherein GFP+ cells were gated to obtain the mean FI (FImean). The FImean was normalized to the total number of events collected by [(FImean × number of gated GFP+ cells/total number of events collected]. The normalized FI was converted to percent viability against the negative controls. Drug concentrations and percent viability were then imported into SigmaPlot, where the curves were plotted in a log scale on the x axis, and the IC50s were determined from sigmoidal curve fits.
Drug combinations and isobolograms.
PQ and each schizonticide (X represents CQ, MQ, LMF, PPQ, and NQN) were combined in various fixed molar ratios. For asexual stage parasites, PQ and X were combined in fixed ratios of 8:0.15, 4:0.3, 2:0.6, and 1:1.25 (in micromolar concentrations) according to Bray et al. (25). Ratios of 1:3, 1:1, and 3:1 (in hundreds of micromolar concentrations) were used for gametocytes. Drug combination studies were performed with at least two biological replicates, each containing duplicate technical repeats. Asexual stage parasite cultures at 0.5% parasitemia and 2% hematocrit or gametocytes at 0.04% gametocytemia and 2% hematocrit were added to equal volumes of fixed ratios of the drug combinations, PQ-X, plated as 100 μl of 2-fold serial dilutions in a 96-well flat-bottom plate. The asexual stage parasites were treated the same way as in the SYBR green I drug assay and as the gametocytes for an FCM-based drug sensitivity assay. To ensure that the experimental variables are consistent, all five drug combinations (PQ-CQ, PQ-MQ, PQ-NQN, PQ-PPQ, and PQ-LMF) were prepared during each technical repeat. The apparent IC50s from percent growth and percent viability were calculated for both PQ and X in each combination as if each drug was added alone. Fractional inhibitory concentrations (FICs) for PQ and X were calculated from ratios of the apparent IC50 to the true IC50. FICPQ versus FICX values at the different molar ratios were plotted to generate the isobolograms. Sums of the FICs (SFIC = FICPQ + FICX) for each concentration ratio were used to determine the type of interaction for each PQ-X combination, where a sum of <1 or a concave curve was considered synergism, >1 or a convex curve was considered antagonism, and ∼1 or along the diagonal line was considered an additive interaction. All statistical analysis was done in SigmaPlot version 12.0.
RESULTS
Growth inhibition of antimalarial drugs on P. falciparum asexual stages.
In order to determine the types of interactions between the two drugs in our drug combination assay, we determined the IC50s of each antimalarial drug (CQ, MQ, NQN, PPQ, LMF, and PQ) individually in the three laboratory strains, 3D7, HB3, and Dd2 (Table 1). The CQ IC50s in the CQ-sensitive (CQS) strains HB3 and 3D7 were 15.7 and 29.7 nM, respectively (analysis of variance [ANOVA], P > 0.05). The CQ IC50 in the CQ-resistant (CQR) strain Dd2 was 154.4 nM, ∼5- to 10-fold higher than those of the CQS strains (ANOVA, P < 0.05). All three strains displayed various degrees of sensitivity to the other tested antimalarials, MQ, NQN, PPQ, and LMF (1.1 to 102.0 nM). PQ is apparently a weak schizonticide with IC50s of micromolar concentrations for all three strains (Table 1). For each antimalarial drug with the exception of CQ, the IC50s were not significantly different between each strain (ANOVA, P > 0.05).
TABLE 1.
In vitro sensitivities of asexual stage parasites (strains HB3, 3D7, and Dd2) and 3D7α-tubII/GFP stage IV gametocyte to six antimalarials
| Drug | Mean (SEM) IC50 ofa: |
|||
|---|---|---|---|---|
| HB3 (nM) | 3D7 (nM) | Dd2 (nM) | 3D7α-tubII/GFP (μM) | |
| CQb | 15.7 (± 4.8) | 29.7 (± 6.0) | 154.4 (± 13.2) | 30.7 (± 4.0) |
| MQ | 12.9 (± 4.9) | 11.8 (± 1.9) | 22.1 (± 3.1) | 23.6 (± 3.5) |
| NQN | 13.6 (± 7.1) | 9.8 (± 4.8) | 19.8 (± 0.4) | 6.18 (± 1.1) |
| PPQ | 15.2 (± 3.5) | 18.6 (± 2.9) | 24.2 (± 1.4) | 271.8 (± 86.9) |
| LMF | 60.5 (± 18.6) | 29.5 (± 14.7) | 63.9 (± 0.79) | 559.0 (± 8.6) |
| PQ | 1,930.1 (± 440.0) | 1,016.5 (± 254.2) | 2,551.4 (± 948.0) | 18.9 (± 2.0) |
The IC50s of asexual stage parasites were measured using the SYBR green I assay, while the IC50s of gametocytes were determined using the flow cytometry-based method.
Significant differences in IC50s between CQS and CQR parasites (ANOVA, P < 0.05).
Drug-drug interactions in asexual stages.
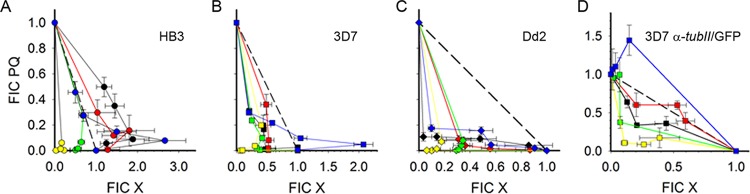
To assess the types of interactions between PQ and each schizonticide (CQ, MQ, NQN, PPQ, and LMF), PQ and each of the drugs were combined at molar ratios that encompass the in vitro IC50 of each antimalarial drug. With use of the SYBR green I assay, the apparent IC50 for each drug in the PQ-X combination along with the true IC50 was determined and used to calculate the FICs and plot the isobolograms (Fig. 1A to C). SFICs were calculated to determine whether PQ and X had synergistic, antagonistic, or additive interactions (Table 2). All PQ-X combinations were synergistic in the 3D7 strain (Fig. 1A) and the Dd2 strain (Fig. 1B) (average of all SFICs of <1). Of note, the effect of PQ-LMF in 3D7 shifted toward antagonism as [PQ] was decreased and [LMF] was increased (Fig. 1A). In the HB3 strain, PQ-CQ and PQ-MQ were antagonistic, whereas PQ-PPQ and PQ-NQN were synergistic (Fig. 1A). Similar to the effects in 3D7, PQ-LMF was additive in HB3 and had a trend toward antagonism at low [PQ] and high [LMF] (Fig. 1A).
FIG 1.
Isobolograms of in vitro sensitivities of asexual stages of three P. falciparum strains as well as the 3D7α-tubII/GFP stage IV gametocytes to the PQ-X drug combination at fixed drug concentration ratios. Fractional inhibitory concentrations (FICs) of PQ against various X (CQ,black; MQ, red; PPQ, green; NQN, yellow; LMF, blue) in asexual cultures of strains HB3 (A), 3D7 (B), and Dd2 (C) and gametocytes of 3D7α-tubII/GFP (D). Error bars indicate standard errors of the mean.
TABLE 2.
Summary of interactions between PQ and five schizonticides on the asexual stages of three P. falciparum strainsa
| Strain | Interaction with: |
||||
|---|---|---|---|---|---|
| CQ | MQ | PPQ | NQN | LMF | |
| 3D7 | Synergistic | Synergistic | Synergistic | Synergistic | Synergisticb |
| HB3 | Antagonistic | Antagonistic | Synergistic | Synergistic | Additiveb |
| Dd2 | Synergistic | Synergistic | Synergistic | Synergistic | Synergistic |
Interactions were based on sums of fractional inhibitory concentrations (SFICs).
Only at [PQ]high:[LMF]low, was the interaction shifting toward antagonism.
Effects of antimalarial drugs on P. falciparum gametocytes.
Using an FCM-based drug assay, we measured the IC50 of stage IV gametocytes in the 3D7α-tubII/GFP line to six antimalarial drugs (Table 1). The IC50s of the drugs on gametocytes ranged from low to high micromolar concentrations (2.4 to 813.2 μM). NQN was the most effective against late-stage gametocytes with the IC50 at 6.2 μM. PQ, MQ, and CQ had almost similar gametocytocidal activities at 18.9, 30.7, and 23.6 μM, respectively (ANOVA, P > 0.05). PPQ and LMF were significantly less effective with IC50s at 271.8 and 559.0 μM, respectively (ANOVA, P < 0.05).
Drug-drug interactions in late-stage gametocytes.
Using the FCM-based drug assay for gametocytes, we investigated the interactions of PQ with each schizonticide in 3D7α-tubII/GFP gametocytes using drug combinations at three molar ratios (26). FICs and SFICs were calculated and plotted in the isobolograms to assess the types of drug-drug interactions (Table 3; Fig. 1D). In the three molar ratios tested, synergism was readily apparent with PQ-NQN and PQ-CQ (Fig. 1D). PQ-MQ was additive, while PQ-PPQ was predominantly additive, but the interaction became synergistic when [PQ] was decreased and [PPQ] was increased (Fig. 1D). The only antagonistic effect on gametocytes was observed with the PQ-LMF combination (Fig. 1D).
TABLE 3.
Summary of interactions between PQ and five schizonticides on stage IV 3D7α-tubII/GFP gametocytesa
Interactions were based on sums of fractional inhibitory concentrations (SFICs).
Synergistic at [PQ]low-[X]high.
Antagonistic at [PQ]low-[X]high.
DISCUSSION
ACTs are the frontline treatment for falciparum malaria in most countries where malaria is endemic (15). The goal of the combination therapy is to circumvent the development and spread of resistance to both groups of drugs. The aim of adding the gametocytocidal agent PQ to an existing ACT is to limit the chances of transmission of resistant parasite strains (27). Here, we investigated the interactions between PQ and the longer-acting schizonticide in commonly used ACTs in both asexual parasites and late-stage gametocytes. Compared to the other antimalarials tested, PQ is clearly a weak blood stage schizonticide (17, 19, 25, 28, 29). However, besides its tissue schizonticide activity targeting relapsing malaria parasites, it has evident gametocytocidal activity and shortens gametocyte carriage times in vivo (6–8, 11, 12, 30). The mechanism of the antimalarial action of PQ is unknown, but it might be through perturbation of mitochondrial activity (31, 32). Further, the in vivo antimalarial activity of PQ might potentially be due to PQ metabolites (33).
The interactions between PQ and other antimalarials have been tested on a limited number of parasite strains, and all assays were performed on asexual stages. Ohrt et al. partnered PQ with azithromycin and showed a synergistic trend in strains W2 and C2B (34). Akoachere et al. tested PQ in combination with methylene blue and observed an antagonistic effect on the asexual stages of the CQR K1 strain (26). Bray et al. showed a synergistic effect when PQ was combined with CQ on CQR K1 and an additive effect on CQS D10 (26). More recently, Gorka et al. found a cytostatic additive effect of PQ with CQ on CQR Dd2 and an antagonistic effect on CQS HB3 (28). In this study, we systematically evaluated five combinations of PQ with the longer-acting schizonticides in ACTs on three parasite strains, 3D7, HB3 and Dd2, representing both CQS and CQR phenotypes. We focused on a range of concentrations of PQ and the schizonticides that encompassed the in vitro IC50s of the asexual parasites to the individual drugs and ratios where the PQ concentration is high when the X concentration is low. If we assume the dosing times of both PQ and X to be the same time on day 1 and consider the pharmacokinetics for these drugs, the maximum plasma drug concentration (Cmax) and the time it takes to reach that maximum concentration (tmax), our [PQ]low-[X]high combinations best fit the possible in vivo scenarios for CQ, MQ, and LMF, while our [PQ]high-[X]low concentrations are applicable to NQN and PPQ (see Table S1 in the supplemental material). Strong synergism was observed between PQ and all tested schizonticides in the CQR Dd2 strain. Furthermore, the synergisms observed were true of all the ratios of the schizonticide to PQ tested ([PQ]high-[X]low to [PQ]low-[X]high), which should correspond to PQ dosing at any time during schizonticide treatment (the interactions were not dependent on high or low plasma concentrations of one or the other drug). Of note, Dd2 was reported as hypersensitive to PQ when treated with very high bolus doses in a 50% lethal dose (LD50) assay format (28). Bolus drug exposures measure cytotoxic effects that are missed in growth inhibition assays due to the latter's use of lower drug concentrations that require longer periods of continuous exposure which can mask the cytotoxic effects of drugs. Coincidentally, our highest [PQ] in our PQ-X combinations on asexual stage parasites encompasses both PQ's IC50 and LD50 in Dd2, which might be responsible for our resulting synergistic interactions with all PQ-X combinations on Dd2 asexual stages.
Synergistic interactions were also apparent in the CQS 3D7 strain in all of the PQ-X ratios tested, except for the PQ-LMF combination which was synergistic at [PQ]high-[LMF]low but antagonistic at [PQ]low-[LMF]high. Intriguingly, such PQ-LMF interactions were also observed in the HB3 strain. This deviation from a completely synergistic curve for the PQ-LMF combination in HB3 and 3D7, or “anomalous” isoboles (showing a combination of two types of interactions), may be attributed to the chosen concentrations and paired molar ratios of the two compounds being combined. Ideally, the testing of drug-drug interactions in a combination assay requires titrating serial dilutions to find ratios of concentrations that will produce one specific effect (35, 36). This was not performed in our case because we were more interested in how a specific range of PQ-X combinations affects parasite growth. In this regard, the anomalous isoboles may be interpreted as the two limits of a drug dosing spectrum for 3D7 and HB3, where [PQ]high-[LMF]low produces synergism or additivity, whereas [PQ]low-[LMF]high is antagonistic. With a possible interference of PQ with LMF in clearing asexual parasitemia, the optimum PQ dosing for asexual parasitemia can be on the same day as the schizonticide dosage (PQ reaches Cmax in 2.8 h, while LMF takes 80 h to reach Cmax) or beyond 5 × LMF half-life (t1/2) (see Table S1 in the supplemental material). Similar to dosing for CQR Dd2, PQ may be dosed at the same time as the other schizonticides in CQS 3D7 since synergistic interactions were observed in all other PQ-X ratios.
In addition to the opposing effects at some extreme molar ratios of the drug combinations tested, the drug-drug interactions might be modulated by molecular determinants of the drug sensitivities in the parasites. Although the molecular targets for many of the tested drugs are not known (37), the pfcrt K76T mutation is the main CQR determinant (38), while pfmdr1 mutations confer resistance to multiple drugs (39). Moreover, increased pfmdr1 copy numbers are associated with MQ resistance and that to a number of aminoalcohol drugs, including LMF (40–42). The three strains tested do possess quite different genotypes in pfcrt and pfmdr1. 3D7 and HB3 harbor wild-type pfcrt K76 and pfmdr1 N86, while 3D7 and Dd2 carry pfmdr1 N1042. In addition, Dd2 has four copies of pfmdr1 compared to one copy in 3D7 and HB3. These different genetic backgrounds may be partially responsible for the observed divergent PQ-X interactions. In clinical studies, artemether-LMF treatments have been found to select for wild-type pfcrt K76 (43, 44), pfmdr1 N86 (43, 45), and N1042 (45) alleles, as well as increased pfmdr1 copy numbers (40, 46). Our finding of the PQ-CQ synergistic interaction in the CQR Dd2 strain was different from the additive effect observed earlier in the same strain (28), but similar to another observation in the CQR K1 strain (25). As speculated earlier, the inconsistent results for PQ-CQ interactions in different parasite strains may be due to other unknown factors rather than to the pfcrt mutations (28).
Unlike some of the earlier gametocyte drug sensitivity assays where gametocyte stage-specific effects were not clearly distinguished (47–49), we determined PQ-X interactions using synchronized stage IV gametocytes (17). Late-stage gametocytes, with drastically increased resistance to most schizonticides as well as to artemisinin family drugs (17), are particularly targeted by PQ for transmission interruption. All schizonticides tested had IC50s in the lower micromolar range on stage IV gametocytes. Utilizing an intracellular ATP-dependent assay, Lelièvre et al. presented similar IC50s for mixed-stage IV to V gametocytes for some of these schizonticides (29). Consistent with an earlier finding (20), NQN appeared to be the most effective gametocytocidal agent for late-stage gametocytes. Collectively, most currently used schizonticides have weak activity on late-stage gametocytes (20, 50–52). In particular, PPQ and LMF, based on our in vitro assay results, are quite ineffective as gametocytocidal agents.
Similar to the drug combination design in the asexual stage parasites, we used concentration ratios that encompass the in vitro IC50 of each drug alone and the in vivo Cmax in humans for the gametocytes, with the notable exception of PPQ and LMF for the former. The very high in vitro IC50s of PPQ and LMF are drastically greater than their measured Cmax values in human plasma (see Table S1 in the supplemental material), and, hence, the in vitro concentrations were not considered physiologically relevant nor deemed necessary in these two drugs' fixed molar ratios. Among the various PQ-X combinations tested, PQ-NQN showed excellent synergism on 3D7α-tubII/GFP gametocytes. Considering that the same combination was quite effective in inhibiting growth of the three asexual stage strains tested, as well as the low NQN IC50s for each strain, our in vitro results prompt us to promulgate the overall effectivity of PQ-NQN in clearing the blood stages of the parasite. Although PPQ's high gametocytocidal IC50 was discouraging, PQ-PPQ showed good antimalarial activity on the gametocytes, recapitulating the synergism on the asexual stages of the three strains. In this scenario, PQ dosed with PPQ may alleviate each drug's weak gametocytocidal activity, and the two might become an overall effective combination treatment for the parasite blood stages but only if PPQ is dosed when the plasma PQ concentration is low, which can be achieved in vivo with a PQ dose on day 1 and subsequent PPQ dose at least 15 h after PQ (considering the PQ t1/2) (see Table S1 in the supplemental material). PQ-CQ and PQ-MQ were slightly less effective in comparison to PQ-PPQ. However, these two combinations appear to have almost the same synergistic/additive effects on the asexual and sexual stages, making them potential effective blood stage drug combinations in areas where these two drugs are still utilized in malaria treatment, with the curious exception of strain HB3. Intriguingly, PQ-LMF was antagonistic in the 3D7α-tubII/GFP gametocytes and only at [PQ]low-[LMF]high, while the opposite ratio spectrum of [PQ]high-[LMF]low was additive. Since these conditions are similar to those of the asexual stages, the same PQ and LMF dosing is probably also relevant to the gametocytes. Overall, PQ might still be effective in synergy with LMF for clearing surviving asexual stage parasites during ACT (e.g., Coartem). Furthermore, since the PQ-schizonticide interactions on gametocytes were based on the 3D7α-tubII/GFP line only, future studies are needed to compare the potential differences between strains.
The drug combination in treatment regimens is not a novel concept and is an especially useful way to target one or more biochemical and/or molecular pathways of a disease. It has long been employed to reduce the likelihood of resistance in the treatment of tuberculosis, leprosy, and HIV infection and is strongly recommended by the WHO for treatment of malaria (53, 54). For malaria, the principle of combination therapy is the use of schizonticides with independent modes of action and different biochemical targets to circumvent and slow down selection for resistance (16, 53–56). Here, we tested how the 8-aminoquinoline PQ as a gametocytocidal drug interacts with the longer-acting ACT drugs on both asexual and sexual stages. For the latter, this is the first study of drug combinations in vitro on P. falciparum gametocytes. Whereas in most cases we identified synergism/additive effects between these drug combinations, there appeared to be some strain-specific differences that modulate the responses to the drug combinations, which warrants further studies on how P. falciparum strains from different geographical regions react to the drug combinations. Furthermore, the caveats due to the PQ versus the X dosing schedule and the pharmacokinetics/pharmacodynamics in vivo, which might not reflect the synergism we observed in vitro, must be addressed by further field studies (57). Taken as a whole, most of our PQ-X combinations, in both asexual and sexual stages, showed synergistic interactions in vitro, which supports the WHO recommendation of adding PQ as a gametocytocidal agent to ACTs, as well as the proposed PQ use in mass drug administration programs (2, 58, 59).
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (U19AI089672).
We thank Jun Miao for providing the parasite lines used in the gametocyte assays and The Huck Core Facility for fluorescence-activated cell sorting (FACS) assistance. We are also grateful to Sony Shrestha and Zenglei Wang for helpful insights on gametocyte induction and to Xiaolian Li for assisting in parasite culture preparations.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01948-15.
REFERENCES
- 1.Okell LC, Griffin JT, Kleinschmidt I, Hollingsworth TD, Churcher TS, White MJ, Bousema T, Drakeley CJ, Ghani AC. 2011. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS One 6:e20179. doi: 10.1371/journal.pone.0020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude RJ, Socheat D, Nguon C, Saroth P, Dara P, Li G, Song J, Yeung S, Dondorp AM, Day NP, White NJ, White LJ. 2012. Optimising strategies for Plasmodium falciparum malaria elimination in Cambodia: primaquine, mass drug administration and artemisinin resistance. PLoS One 7:e37166. doi: 10.1371/journal.pone.0037166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK, Greenwood B, Hall BF, Levine MM, Mendis K, Newman RD, Plowe CV, Rodriguez MH, Sinden R, Slutsker L, Tanner M. 2011. A research agenda to underpin malaria eradication. PLoS Med 8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White NJ. 2013. Primaquine to prevent transmission of falciparum malaria. Lancet Infect Dis 13:175–181. doi: 10.1016/S1473-3099(12)70198-6. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. 2012. The safety and effectiveness of single dose primaquine as a P. falciparum gametocytocide. http://www.who.int/malaria/mpac/sep2012/primaquine_single_dose_pf_erg_meeting_report_aug2012.pdf.
- 6.Shekalaghe S, Drakeley C, Gosling R, Ndaro A, van Meegeren M, Enevold A, Alifrangis M, Mosha F, Sauerwein R, Bousema T. 2007. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS One 2:e1023. doi: 10.1371/journal.pone.0001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, Sutherland C, Sauerwein R, Ghani AC, Drakeley C. 2010. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J 9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smithuis F, Kyaw MK, Phe O, Win T, Aung PP, Oo AP, Naing AL, Nyo MY, Myint NZ, Imwong M, Ashley E, Lee SJ, White NJ. 2010. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis 10:673–681. doi: 10.1016/S1473-3099(10)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eziefula AC, Bousema T, Yeung S, Kamya M, Owaraganise A, Gabagaya G, Bradley J, Grignard L, Lanke KH, Wanzira H, Mpimbaza A, Nsobya S, White NJ, Webb EL, Staedke SG, Drakeley C. 2014. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infect Dis 14:130–139. doi: 10.1016/S1473-3099(13)70268-8. [DOI] [PubMed] [Google Scholar]
- 10.Graves PM, Gelband H, Garner P. 2014. Primaquine or other 8-aminoquinoline for reducing P. falciparum transmission. Cochrane Database Syst Rev 6:CD008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamtekar KD, Gogtay NJ, Dalvi SS, Karnad DR, Chogle AR, Aigal U, Kshirsagar NA. 2004. A prospective study evaluating the efficacy of a single, 45-mg dose of primaquine, as a gametocytocidal agent, in patients with Plasmodium falciparum malaria in Mumbai, India. Ann Trop Med Parasitol 98:453–458. doi: 10.1179/000349804225003550. [DOI] [PubMed] [Google Scholar]
- 12.Lederman ER, Maguire JD, Sumawinata IW, Chand K, Elyazar I, Estiana L, Sismadi P, Bangs MJ, Baird JK. 2006. Combined chloroquine, sulfadoxine/pyrimethamine and primaquine against Plasmodium falciparum in Central Java, Indonesia. Malar J 5:108. doi: 10.1186/1475-2875-5-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rieckmann KH, McNamara JV, Frischer H, Stockert TA, Carson PE, Powell RD. 1968. Gametocytocidal and sporontocidal effects of primaquine and of sulfadiazine with pyrimethamine in a chloroquine-resistant strain of Plasmodium falciparum. Bull World Health Organ 38:625–632. [PMC free article] [PubMed] [Google Scholar]
- 14.Sutanto I, Suprijanto S, Kosasih A, Dahlan MS, Syafruddin D, Kusriastuti R, Hawley WA, Lobo NF, Ter Kuile FO. 2013. The effect of primaquine on gametocyte development and clearance in the treatment of uncomplicated falciparum malaria with dihydroartemisinin-piperaquine in South Sumatra, Western Indonesia: an open-label, randomized, controlled trial. Clin Infect Dis 56:685–693. doi: 10.1093/cid/cis959. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. 2014. World malaria report 2014. http://www.who.int/malaria/publications/world_malaria_report_2014/en/.
- 16.White N. 1999. Antimalarial drug resistance and combination chemotherapy. Philos Trans R Soc Lond B Biol Sci 354:739–749. doi: 10.1098/rstb.1999.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Liu M, Liang X, Siriwat S, Li X, Chen X, Parker DM, Miao J, Cui L. 2014. A flow cytometry-based quantitative drug sensitivity assay for all Plasmodium falciparum gametocyte stages. PLoS One 9:e93825. doi: 10.1371/journal.pone.0093825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, Richman A, Sim BK, Lee MC, Hoffman SL, Fidock DA. 2011. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci U S A 108:E1214-E1223. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duffy S, Avery VM. 2013. Identification of inhibitors of Plasmodium falciparum gametocyte development. Malar J 12:408. doi: 10.1186/1475-2875-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miguel-Blanco C, Lelievre J, Delves MJ, Bardera AI, Presa JL, Lopez-Barragan MJ, Ruecker A, Marques S, Sinden RE, Herreros E. 2015. Imaging-based high-throughput screening assay to identify new molecules with transmission-blocking potential against Plasmodium falciparum female gamete formation. Antimicrob Agents Chemother 59:3298–3305. doi: 10.1128/AAC.04684-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trager W, Jensen JB. 2005. Human malaria parasites in continuous culture. 1976. J Parasitol 91:484–486. doi: 10.1645/0022-3395(2005)091[0484:HMPICC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Lambros C, Vanderberg JP. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65:418–420. doi: 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 23.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother 48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett TN, Paguio M, Gligorijevic B, Seudieu C, Kosar AD, Davidson E, Roepe PD. 2004. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob Agents Chemother 48:1807–1810. doi: 10.1128/AAC.48.5.1807-1810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray PG, Deed S, Fox E, Kalkanidis M, Mungthin M, Deady LW, Tilley L. 2005. Primaquine synergises the activity of chloroquine against chloroquine-resistant P. falciparum. Biochem Pharmacol 70:1158–1166. doi: 10.1016/j.bcp.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Akoachere M, Buchholz K, Fischer E, Burhenne J, Haefeli WE, Schirmer RH, Becker K. 2005. In vitro assessment of methylene blue on chloroquine-sensitive and -resistant Plasmodium falciparum strains reveals synergistic action with artemisinins. Antimicrob Agents Chemother 49:4592–4597. doi: 10.1128/AAC.49.11.4592-4597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White NJ, Qiao LG, Qi G, Luzzatto L. 2012. Rationale for recommending a lower dose of primaquine as a Plasmodium falciparum gametocytocide in populations where G6PD deficiency is common. Malar J 11:418. doi: 10.1186/1475-2875-11-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorka AP, Jacobs LM, Roepe PD. 2013. Cytostatic versus cytocidal profiling of quinoline drug combinations via modified fixed-ratio isobologram analysis. Malar J 12:332. doi: 10.1186/1475-2875-12-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lelièvre J, Almela MJ, Lozano S, Miguel C, Franco V, Leroy D, Herreros E. 2012. Activity of clinically relevant antimalarial drugs on Plasmodium falciparum mature gametocytes in an ATP bioluminescence “transmission blocking” assay. PLoS One 7:e35019. doi: 10.1371/journal.pone.0035019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pukrittayakamee S, Chotivanich K, Chantra A, Clemens R, Looareesuwan S, White NJ. 2004. Activities of artesunate and primaquine against asexual- and sexual-stage parasites in falciparum malaria. Antimicrob Agents Chemother 48:1329–1334. doi: 10.1128/AAC.48.4.1329-1334.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aikawa M, Beaudoin RL. 1969. Morphological effects of 8-aminoquinolines on the exoerythrocytic stages of Plasmodium fallax. Mil Med 134:986–999. [PubMed] [Google Scholar]
- 32.Lanners HN. 1991. Effect of the 8-aminoquinoline primaquine on culture-derived gametocytes of the malaria parasite Plasmodium falciparum. Parasitol Res 77:478–481. doi: 10.1007/BF00928413. [DOI] [PubMed] [Google Scholar]
- 33.Vale N, Moreira R, Gomes P. 2009. Primaquine revisited six decades after its discovery. Eur J Med Chem 44:937–953. doi: 10.1016/j.ejmech.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Ohrt C, Willingmyre GD, Lee P, Knirsch C, Milhous W. 2002. Assessment of azithromycin in combination with other antimalarial drugs against Plasmodium falciparum in vitro. Antimicrob Agents Chemother 46:2518–2524. doi: 10.1128/AAC.46.8.2518-2524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tallarida RJ. 2011. Quantitative methods for assessing drug synergism. Genes Cancer 2:1003–1008. doi: 10.1177/1947601912440575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berenbaum MC. 1978. A method for testing for synergy with any number of agents. J Infect Dis 137:122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- 37.Petersen I, Eastman R, Lanzer M. 2011. Drug-resistant malaria: molecular mechanisms and implications for public health. FEBS Lett 585:1551–1562. doi: 10.1016/j.febslet.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 38.Bray PG, Martin RE, Tilley L, Ward SA, Kirk K, Fidock DA. 2005. Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance. Mol Microbiol 56:323–333. doi: 10.1111/j.1365-2958.2005.04556.x. [DOI] [PubMed] [Google Scholar]
- 39.Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906–909. doi: 10.1038/35002615. [DOI] [PubMed] [Google Scholar]
- 40.Sidhu AB, Uhlemann AC, Valderramos SG, Valderramos JC, Krishna S, Fidock DA. 2006. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J Infect Dis 194:528–535. doi: 10.1086/507115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alker AP, Lim P, Sem R, Shah NK, Yi P, Bouth DM, Tsuyuoka R, Maguire JD, Fandeur T, Ariey F, Wongsrichanalai C, Meshnick SR. 2007. Pfmdr1 and in vivo resistance to artesunate-mefloquine in falciparum malaria on the Cambodian-Thai border. Am J Trop Med Hyg 76:641–647. [PubMed] [Google Scholar]
- 42.Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, Phaipun L, Patel R, Laing K, Looareesuwan S, White NJ, Nosten F, Krishna S. 2004. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sisowath C, Petersen I, Veiga MI, Martensson A, Premji Z, Bjorkman A, Fidock DA, Gil JP. 2009. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J Infect Dis 199:750–757. doi: 10.1086/596738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valderramos SG, Valderramos JC, Musset L, Purcell LA, Mercereau-Puijalon O, Legrand E, Fidock DA. 2010. Identification of a mutant PfCRT-mediated chloroquine tolerance phenotype in Plasmodium falciparum. PLoS Pathog 6:e1000887. doi: 10.1371/journal.ppat.1000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Humphreys GS, Merinopoulos I, Ahmed J, Whitty CJ, Mutabingwa TK, Sutherland CJ, Hallett RL. 2007. Amodiaquine and artemether-lumefantrine select distinct alleles of the Plasmodium falciparum mdr1 gene in Tanzanian children treated for uncomplicated malaria. Antimicrob Agents Chemother 51:991–997. doi: 10.1128/AAC.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price RN, Uhlemann AC, van Vugt M, Brockman A, Hutagalung R, Nair S, Nash D, Singhasivanon P, Anderson TJ, Krishna S, White NJ, Nosten F. 2006. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis 42:1570–1577. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka TQ, Dehdashti SJ, Nguyen DT, McKew JC, Zheng W, Williamson KC. 2013. A quantitative high throughput assay for identifying gametocytocidal compounds. Mol Biochem Parasitol 188:20–25. doi: 10.1016/j.molbiopara.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka TQ, Williamson KC. 2011. A malaria gametocytocidal assay using oxidoreduction indicator, alamarBlue. Mol Biochem Parasitol 177:160–163. doi: 10.1016/j.molbiopara.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D'Alessandro S, Silvestrini F, Dechering K, Corbett Y, Parapini S, Timmerman M, Galastri L, Basilico N, Sauerwein R, Alano P, Taramelli D. 2013. A Plasmodium falciparum screening assay for anti-gametocyte drugs based on parasite lactate dehydrogenase detection. J Antimicrob Chemother 68:2048–2058. doi: 10.1093/jac/dkt165. [DOI] [PubMed] [Google Scholar]
- 50.Delves MJ, Ruecker A, Straschil U, Lelievre J, Marques S, Lopez-Barragan MJ, Herreros E, Sinden RE. 2013. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob Agents Chemother 57:3268–3274. doi: 10.1128/AAC.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruecker A, Mathias DK, Straschil U, Churcher TS, Dinglasan RR, Leroy D, Sinden RE, Delves MJ. 2014. A male and female gametocyte functional viability assay to identify biologically relevant malaria transmission-blocking drugs. Antimicrob Agents Chemother 58:7292–7302. doi: 10.1128/AAC.03666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanders NG, Sullivan DJ, Mlambo G, Dimopoulos G, Tripathi AK. 2014. Gametocytocidal screen identifies novel chemical classes with Plasmodium falciparum transmission blocking activity. PLoS One 9:e105817. doi: 10.1371/journal.pone.0105817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brunner R, Aissaoui H, Boss C, Bozdech Z, Brun R, Corminboeuf O, Delahaye S, Fischli C, Heidmann B, Kaiser M, Kamber J, Meyer S, Papastogiannidis P, Siegrist R, Voss T, Welford R, Wittlin S, Binkert C. 2012. Identification of a new chemical class of antimalarials. J Infect Dis 206:735–743. doi: 10.1093/infdis/jis418. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization. 2001. The use of antimalarial drugs: report of a WHO informal consultation. Roll Back Malaria, World Health Organization, Geneva, Switzerland. [Google Scholar]
- 55.White NJ, Olliaro PL. 1996. Strategies for the prevention of antimalarial drug resistance: rationale for combination chemotherapy for malaria. Parasitol Today 12:399–401. doi: 10.1016/0169-4758(96)10055-7. [DOI] [PubMed] [Google Scholar]
- 56.White NJ. 1999. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia 41:301–308. [PubMed] [Google Scholar]
- 57.Pukrittayakamee S, Tarning J, Jittamala P, Charunwatthana P, Lawpoolsri S, Lee SJ, Hanpithakpong W, Hanboonkunupakarn B, Day NP, Ashley EA, White NJ. 2014. Pharmacokinetic interactions between primaquine and chloroquine. Antimicrob Agents Chemother 58:3354–3359. doi: 10.1128/AAC.02794-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newby G, Koita K, Chen I, Poirot E, Wegbreit J, Gosling R, Hwang J, Ippolito M. 2014. Background paper: review of mass drug administration and primaquine use. UCSF Global Health Sciences, University of California, San Francisco, San Francisco, CA. [Google Scholar]
- 59.Lubell Y, White L, Varadan S, Drake T, Yeung S, Cheah PY, Maude RJ, Dondorp A, Day NP, White NJ, Parker M. 2014. Ethics, economics, and the use of primaquine to reduce falciparum malaria transmission in asymptomatic populations. PLoS Med 11:e1001704. doi: 10.1371/journal.pmed.1001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.