Abstract
Staphylococcus aureus commonly infects medical implants or devices, with devastating consequences for the patient. The infection begins with bacterial attachment to the device, followed by bacterial multiplication over the surface of the device, generating an adherent sheet of bacteria known as a biofilm. Biofilms resist antimicrobial therapy and promote persistent infection, making management difficult to futile. Infections might be prevented by engineering the surface of the device to discourage bacterial attachment and multiplication; however, progress in this area has been limited. We have developed a novel nanoscale plasma coating technology to inhibit the formation of Staphylococcus aureus biofilms. We used monomeric trimethylsilane (TMS) and oxygen to coat the surfaces of silicone rubber, a material often used in the fabrication of implantable medical devices. By quantitative and qualitative analysis, the TMS/O2 coating significantly decreased the in vitro formation of S. aureus biofilms; it also significantly decreased in vivo biofilm formation in a mouse model of foreign-body infection. Further analysis demonstrated TMS/O2 coating significantly changed the protein adsorption, which could lead to reduced bacterial adhesion and biofilm formation. These results suggest that TMS/O2 coating can be used to effectively prevent medical implant-related infections.
INTRODUCTION
Staphylococcus aureus frequently causes skin, soft-tissue, respiratory, bone, joint, and endovascular infections (1). Although S. aureus employs multiple virulence mechanisms to promote these infections (1), the formation of a bacterial biofilm appears to be the crucial stage in staphylococcal infection of implanted medical devices, such as intravascular catheters, artificial heart valves, orthopedic devices, and prosthetic joints (2, 3). Staphylococcal biofilms consist of bacterial cells cemented to the medical device surface and to each other by an extracellular matrix composed of polysaccharides produced by the bacteria and by serum and tissue proteins produced by the patient (2, 3).
The biofilm protects the bacterial cells from antimicrobial therapy and host defenses (4). The resting metabolism of the biofilm bacteria resists the action of antimicrobial agents, while the extracellular matrix also serves as a diffusion barrier of antimicrobial agents into the encased bacterial cells (5). However, some studies disputed the relevance of extracellular matrix as a diffusion barrier to account for antibiotic resistance, because the difference in the diffusion coefficients of antibiotics between biofilms and microcolonies could not account for the increased resistance fully (6, 7). The mechanism of the antibiotic resistance is still not clearly defined.
The combination of the unique biofilm physiology and the enhanced virulence of pathogenic staphylococci make medical device-associated infections caused by S. aureus particularly difficult to treat. Management often requires the removal of the implant at great cost to the patient's health and wealth (2). It was estimated that in the United States the annual cost of medical device-associated infections was in excess of 3 billion dollars (2). For these reasons, we need new approaches to prevent device-related infections.
One way would be to apply antimicrobial agents to the surface of devices to impair bacterial survival and proliferation (8–10). For example, some investigators have coated biomaterial surfaces with the combination of the antiseptics chlorhexidine and silver sulfadiazine or the combination of the antibiotics minocycline and rifampin (11). Using a combination of multiple antimicrobial reagents is meant to prevent resistance for singular agents and has been shown to be highly effective in preventing resistance. Antimicrobial-impregnated catheters have demonstrated efficacy in preventing catheter-related infections (12). However, there still are risks that the extended and widespread use of bactericidal agents could lead to the emergence of bacterial resistance to the antibiotics (13). Loss of antimicrobial function also could occur by covering the surface of the biomaterial with a layer of organic macromolecules and dead microorganisms (14).
Another way would be to modify the biomaterial surface in a manner that does not alter the bulk properties of the material nor utilize licensed pharmaceutical agents but at the same time discourages bacterial surface adhesion and growth. Reports of success with this tactic include coating the material with peptide-functionalized poly(l-lysine)-grafted-poly(ethylene glycol) copolymers (15), grafting long-chain zwitterionic poly(sulfobetaine methacrylate) onto the surface (16), and manufacturing the biomaterial with a submicron-textured, antiadhesive surface (17). Because animal studies have not yet been reported for these approaches, the in vivo efficacy of such antibiofilm techniques remains unclear. One possible exception is the recent report by Tran et al. (18). These investigators coated hemodialysis catheters with an organoselenium compound through a wet coating process consisting of multiple steps and taking 3 days to complete; the organoselenium-coated catheters inhibited S. aureus biofilms both in vitro and in vivo.
In our previous study, we developed a novel low-temperature, nanoscale plasma-coating technology using TMS alone as a monomer to modify the surfaces of stainless steel and titanium to prevent Staphylococcus epidermidis biofilm formation (19). In the current study, we demonstrate that further modification of this coating technology with the addition of oxygen at an optimal ratio in the deposition process inhibits S. aureus biofilm formation on silicone rubber (also known as polydimethylsiloxane [PDMS]) both in vitro and in vivo.
MATERIALS AND METHODS
Silicone rubber.
Silicone rubber (polydimethylsiloxane [PDMS]) (catalog no. 1227609; Bentec Medical Inc., Wakefield, MA) coupons of 10 mm by 10 mm by 1 mm or 5 mm by 5 mm by 1 mm were used as substrates for plasma coating to study its antibiofilm properties. Silicone substrates were cleaned by soaking them in 100% alcohol (200 proof) for 1 h at room temperature and then blot-dried with Kimwipes paper.
Plasma coating on silicone substrate.
The plasma reactor used in this study has been described in our previous publication (19). Trimethylsilane (TMS) (SIT8570.0; >97.0% pure; Gelest Inc., Morrisville, PA) and its mixture with oxygen (O2) (OX UHP300; 99.994% pure; Airgas, Bowling Green, KY) were used for coating deposition. Plasma surface pretreatment using argon (Ar) (AR UPC300; 99.999% pure; Airgas, Bowling Green, KY) as a working gas was believed to provide a clean and reproducible starting condition for further plasma-coating deposition to form a well-controlled surface layer. Specifically, it was used to introduce reactive sites on the silicone substrate surface for covalent chemical bonding to the subsequent TMS/O2 plasma coating. The argon plasma pretreatment step was carried out at a flow rate of 1 standard cubic centimeter per minute (sccm), a working pressure of 50 mTorr, and a power of 20 W for 5 min. Following plasma pretreatment, the samples were coated at 1 sccm of TMS plus 4 sccm of O2, 30 W, and 50 mTorr for 10 min. Si wafers were included in each plasma coating batch so as to ascertain coating thickness.
Coating thickness assessment.
The thicknesses of plasma coatings on silicone rubber substrates were determined by measuring coatings deposited on Si wafers for each coating batch. The thicknesses of coatings on Si wafers were assessed using an AutoEL-II automatic ellipsometer (Rudolph Research Corporation, Flanders, NJ) as described previously (19).
Contact angle measurement.
The contact angle formed between a sessile drop and its support indicates the hydrophilic or hydrophobic characteristics of the surface. The water droplet size used on silicone surfaces in the contact measurements was 1 μl, and the measurements were performed and recorded using a computer-aided VCA-2500XE video contact angle system (AST Products Inc., Billerica, MA).
Surface chemistry analysis.
All of the plasma-coated and uncoated substrates were analyzed using X-ray photoelectron spectroscopy (XPS) at the Material Research Center, Missouri University of Science & Technology, Rolla, MO, as previously described (19). The takeoff angle of the X-ray source was fixed at 90° to the substrate surface, for an area of 200 μm by 200 μm to be analyzed at a depth of 1 to 10 nm from the top surface of the substrates.
Bacterial strains.
The two strains used in this study, RN6390 and NRS234, were the subjects of a previous report from us regarding the inhibition of biofilm formation (20). RN6390 is a laboratory strain originally derived from RN1 (21). It is known to be a relatively weak former of biofilm based on a previous report (22). In contrast, NRS234 is a wild-type, clinical isolate from a patient with native valve endocarditis; our prior investigations have found that it was capable of forming biofilm (20). Both RN6390 and NRS234 strains were obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus program (NARSA), which is supported by NIAID, NIH contract no. HHSN272200700055C.
Biofilm formation measurement.
Biofilm formation was measured, under static conditions, as previously reported (19, 20). We examined uncoated and coated 5-mm by 5-mm silicone coupons in triplicate. Those silicone coupons were sterilized by exposing them to UV lamps at a wavelength of 253.7 nm for 20 min on each side. The sterilized coupons then were coated by overnight incubation in a 20% (vol/vol) dilution of human plasma (Innovative Research, Novi, MI) in phosphate-buffered saline (PBS). Coupons coated with human plasma were incubated in 1 ml Todd-Hewitt broth containing 0.2% yeast extract (THY) medium, 0.5% glucose, and 1:200 diluted bacterial culture at 37°C overnight. The coupons were washed gently three times with PBS to remove nonadherent cells and air dried briefly. The coupons were put in 1.5-ml Eppendorf tubes with 1 ml PBS. The biofilms formed on the substrates were detached and disaggregated with ultrasonic bath treatment (23, 24). The number of bacterial cells in PBS was quantified using the spread plate technique. The percentage of biofilm formation on coated silicone surfaces was calculated against the mean of biofilms on uncoated controls. Experiments were repeated three times. Student's t tests were performed. A P value of <0.05 was considered statistically significant.
Biofilm response to vancomycin treatment was studied with a protocol established previously, with modifications (25). Biofilm was grown on uncoated coupons as described above for 8 h. The coupons were gently washed with PBS to remove nonadherent bacterial cells. Fresh medium with or without 100 μg/ml vancomycin was added to the wells containing coupons and cultured at 37°C overnight. The coupons then were washed and biofilm formation was analyzed as described above.
SEM.
Biofilms of S. aureus formed on two groups of silicone coupons (uncoated control and TMS/O2 coated) were visualized by scanning electron microscopy (SEM) as described previously (19, 20).
Protein adsorption on silicone coupons.
The protein adsorption on silicone coupons was measured by an enzyme-linked immunosorbent assay (ELISA) approach (26, 27). The protein binding of fibrinogen, fibronectin, and albumin to biomaterial surfaces was measured by adopting the protocol established previously, with modifications.
Silicone coupons (10 mm by 10 mm) with and without TMS/O2 plasma coating were coated by 20% (vol/vol) human plasma in PBS at 37°C overnight as a test group. Another group of coupons with and without TMS/O2 plasma coating was coated with 1% bovine serum albumin (BSA) as a blank for fibrinogen or fibronectin adsorption evaluation. A third group of coupons with and without TMS/O2 plasma coating coated with 5% low-fat milk was used as a blank for measurement of albumin adsorption. After washing in PBS 3 times, triplicate coupons of each condition from the test group were incubated in PBS containing 1% BSA and a 1:500 dilution of either goat anti-human fibrinogen (Nordic Immunology Laboratory, Tilburg, The Netherlands) or rabbit anti-human fibronectin (Sigma-Aldrich, St. Louis, MO) as the primary antibody. For the evaluation of albumin adsorption, coupons of the test group were incubated in 5% low-fat milk with rabbit anti-human albumin (Cell Signaling Technology, Danvers, MA) as the primary antibody. The blank group coupons also were incubated in triplicate with the respective antibody. All of the coupons, including the test group and the blank groups (1% BSA blank group and 5% low-fat milk blank group), were incubated at 37°C for 1 h and washed with PBS. Those coupons then were moved to a fresh 24-well plate, incubated in PBS-BSA or PBS-milk containing 1:250 dilutions of the respective horseradish peroxidase (HRP)-conjugated polyclonal IgG for 1 h, and washed with PBS. The coupons then were incubated with the chromogenic substrate 2, 2′-azinobis(3-ethylbenzothiazoline-6-sulfinic acid) (ABTS; Thermo Fisher Scientific Inc., Rockford, IL) for 10 min at room temperature, and the absorbance at a wavelength of 410 nm was measured using a SpectraMax spectrophotometer (Molecule Probes, Sunnyvale, CA). The final values of the optical density at 410 nm (OD410) of either fibrinogen or fibronectin adsorption were calibrated by subtracting the average OD410 of the respective 1% BSA blank group. The final OD410 values of albumin adsorption were calibrated by subtracting the average OD410 of the 5% low-fat milk blank group.
Serial dilutions of the commercially available fibrinogen, fibronectin, or albumin protein (Sigma-Aldrich, St. Louis, MO) in PBS were incubated with uncoated coupons, and OD410 values were obtained to construct standard curves for each protein (28). The amounts of proteins adsorbed to the coupons were calculated based on the standard curves. The percentage of protein adsorption to coated samples was calculated against the mean of uncoated samples, which was set as 100%. Experiments were repeated three times. Student's t tests were performed. A P value of <0.05 was considered statistically significant.
Cytotoxicity test on silicone surfaces.
CCL-1 fibroblasts (ATCC, Manassas, VA) (10,000 cell/well) were cultured with silicone coupons (10 mm by 10 mm) in 24-well plates in 5% CO2 at 37°C, and the medium was changed every 2 days. At day 3, cell medium was aspirated and cells were incubated with 1 ml 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) solution (0.5 mg/ml; Roche, Indianapolis, IN) for 4 h at 37°C. The MTT solution was aspirated and the precipitate dissolved in dimethyl sulfoxide (DMSO)-ethanol (1:1) solution, and the OD540 was used as a measurement of cell viability. One hundred percent viability was set as the absorbance of the cells incubated with uncoated coupons. The assay was performed three times to obtain the means and standard errors of means of cell viability.
Biofilm infection mouse mode.
Our model of foreign-body infection is a modification of the model originally introduced by Christensen et al. (29). The biofilm infection mouse procedure was approved by the University of Missouri Institutional Biosafety Committee (IBC) and Animal Care and Use Committee (ACUC). We used 8-week-old BALB/cJ female mice (The Jackson Laboratory, Bar Harbor, ME) and housed the mice in the University of Missouri animal facility prior to performing the foreign-body infection study. After disinfecting the skin, we implanted 5-mm by 5-mm silicone coupons subcutaneously into the back and closed the incision with surgical sutures. One day after implantation, we injected 100 μl of a bacterial suspension (1.7 × 108 to 2.8 × 108 CFU) of S. aureus NRS234 subcutaneously into the area next to the implanted coupon. Three days later we harvested the implanted silicone coupons and rinsed the coupons with sterile PBS three times. Bacterial numbers were counted by the spread plate method after sonication (19).
In order to assess the soft-tissue damage caused by biofilm infection on implants, we infected mice with 1.2 × 109 to 2.0 × 109 CFU of S. aureus NRS234 and observed the mice for 2 weeks. The skin lesion size around the area of implant was measured daily. Once the mouse lost the implanted coupon due to skin necrosis, the mouse was excluded from further skin lesion measurement.
We analyzed bacterial count data by the Mann-Whitney rank test and skin lesion size by the student t test. The time of the mouse losing the implanted coupon due to skin necrosis was analyzed by the Kaplan-Meier method and log-rank test. The SigmaStat program (Systat Software, Inc., Chicago, IL) was used to perform the analysis.
RESULTS
Inhibition of biofilm formation by TMS/O2 coating.
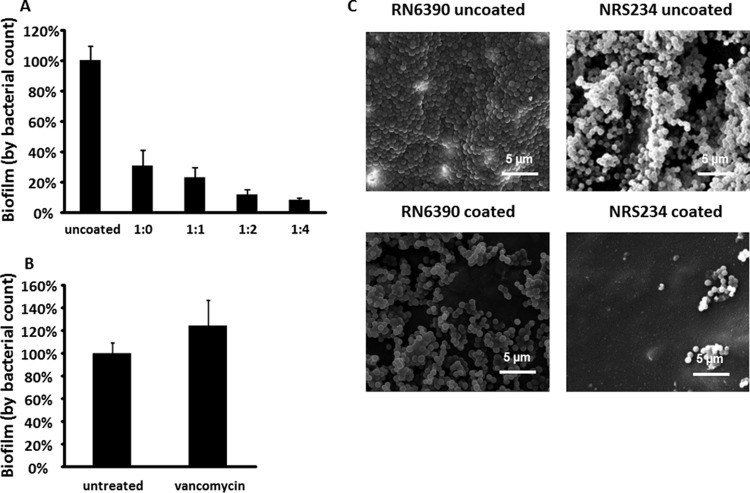
We measured bacterial density on PDMS coupons by counting the number of CFU released from the coupons by ultrasound disaggregation; with this method, we found that adding oxygen to the low-temperature plasma-coating process significantly decreased biofilm formation by S. aureus NRS234. NRS234 is a wild-type strain isolated from a patient with native valve endocarditis (20). The inhibition of biofilm formation increased with the increase of oxygen level with TMS. When expressed as a percentage of biofilm on the uncoated PDMS, coating PDMS with TMS alone significantly reduced biofilm formation (69.5% ± 10.1%; P < 0.001), while coating PDMS with TMS and oxygen at a 1:4 ratio (termed TMS/O2 1:4) yielded the most potent inhibition (92.7% ± 0.95%; P < 0.001) (Fig. 1A). Thus, we selected TMS/O2 1:4 for detailed analysis. As a comparison, we also examined how biofilm responded to antibiotic treatment, since antibiotics are used to treat biofilm-related infections in the clinic with unsatisfactory results. Treating biofilm on uncoated PDMS with 100 μg/ml vancomycin led to no reduction of biofilm (Fig. 1B), supporting previous observations of the high resistance of biofilm to antibiotics (30).
FIG 1.
TMS/O2 coating inhibits S. aureus biofilm formation on silicone surfaces. (A) Biofilm formation on silicone coupons (uncoated or coated with different ratios of TMS/O2) by NRS234. The mean of uncoated samples was set as 100%. Data were pooled from nine samples (three independent experiments with triplicates) and are presented as means ± standard errors of means. **, P < 0.01. (B) Biofilm response to vancomycin (100 μg/ml) treatment. (C) Scanned electron microscope studies of biofilm formation. One representative picture is presented from duplicate samples. Scale bar, 5 μm.
Using scanning electron microscopy (SEM), we examined the microscopic biofilm structures of NRS234 and RN6390, which is a well-characterized laboratory strain (20), on both coated and uncoated PDMS coupons. On uncoated coupons, RN6390 formed tight multilayered structures with dense bacterial cells that covered the surface, while NRS234 formed a multilayered biofilm with numerous channels and spaces between cell clusters (Fig. 1C). The colonization of coated coupons by both strains also was greatly reduced, exhibiting only scattered cells or cell clusters in SEM images (Fig. 1C). The inhibition was more evident with strain NRS234, which only sparsely colonized TMS/O2-coated surfaces (Fig. 1C).
Surface characterization.
With ellipsometric measurements, we found the thickness of the resultant TMS/O2 plasma coatings deposited on Si wafers to be 50 to 60 nm, with a refractive index of about 1.58. Bare PDMS substrates without plasma coating exhibited a hydrophobic surface with contact angles over 105° (106° ± 5°). The PDMS substrate with TMS/O2 plasma coating greatly reduced surface hydrophobicity initially, generating a contact angle of 51° ± 3° measured at day 1 after plasma coating. Plasma reduction of surface hydrophobicity degraded over time; 1 week after plasma coating, the contact angle of coated PDMS changed to 99° ± 7°, nearly identical to that of uncoated PDMS.
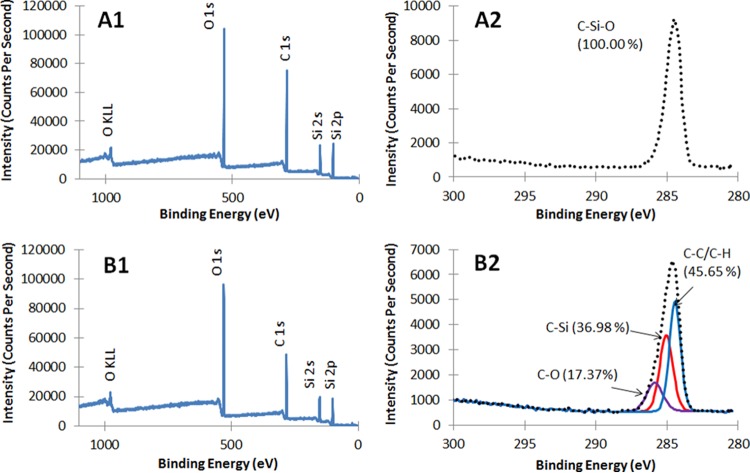
We used X-ray photon spectroscopy (XPS) to detect surface elemental concentrations for uncoated PDMS containing a repeating unit of –O-Si-(CH3)2 in the polymer chain and on the TMS/O2 plasma coatings (Fig. 2 and Table 1). The atomic composition of PDMS, as determined by XPS, was similar to theoretical values (carbon, 50%; oxygen, 25%; silicon, 25%) (note that XPS could not detect hydrogen incorporated within methyl groups of PDMS). Compared to the uncoated PDMS, the TMS/O2 plasma-coated surface showed a different composition, with both higher silicon and oxygen and lower carbon concentrations at the surface. Surfaces coated with TMS only demonstrated 17% O, 56% C, and 26% Si at the surface (19); in contrast, the TMS/O2 plasma-coated surface demonstrated a higher atomic oxygen concentration (about 32%) (Table 1), indicating oxygen incorporation into the coating during the TMS/O2 plasma deposition process. XPS further revealed distinct chemical states of carbon or functional groups on the plasma-coated surface through high-resolution deconvolution of the C1s peaks (Fig. 2A2 and B2 and Table 1). Three distinct subpeaks were fitted to the C1s peak associated with the plasma coating. C—C/C—H bonding was prevalent within the coating, along with C—Si and C—O bonding at higher binding energies (31). Other researchers also found C—C/C—H, C—O, and C—Si on the surfaces of TMS-based plasma coatings using XPS and spectral deconvolution of C1s (31–33). The presence of the oxides was due to the reactive nature of the TMS/O2 plasma glow discharge as well as subsequent surface oxidation by atmospheric oxygen (32, 34–37).
FIG 2.
XPS survey spectra of uncoated PDMS (A1) and TMS/O2 1:4 plasma-coated PDMS (B1) and high-resolution C1s spectra of uncoated (A2) and plasma-coated PDMS (B2).
TABLE 1.
Surface elemental compositiona
| Sample | Survey analysis |
High-resolution scan of C1s |
|||
|---|---|---|---|---|---|
| Element | Atomic % | Binding energy (eV) | Chemical state | %b | |
| PDMS (control) | C | 54.63 | 284.6 | C—Si—O | 100 |
| O | 26.55 | ||||
| Si | 18.82 | ||||
| PDMS (plasma coated) | C | 46.83 | 284.4 | C—C/C—H | 45.65 |
| O | 32.45 | 285.0 | C—Si | 36.98 | |
| Si | 20.72 | 285.9 | C—O | 17.37 | |
Surface elemental composition was determined by XPS surface scan and carbon functional groups from C1s peaks as determined from high-resolution scan for uncoated and TMS/O2 plasma-coated PDMS.
Proportion of chemical bonds in C1s.
Inhibition of biofilm-related infection by nanocoating.
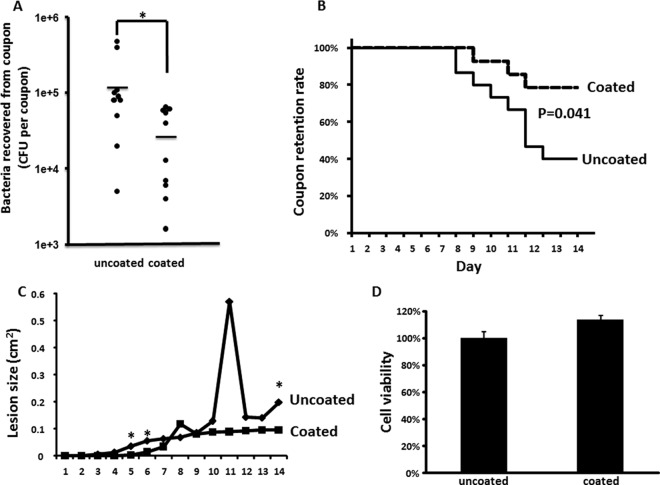
Because NRS234 is a clinic isolate and TMS/O2 1:4 inhibition of biofilm formation in vitro was more evident with NRS234, we used NRS234 to test the capacity of TMS/O2 coating to prevent biofilm formation in vivo using a mouse model of device-related infection. The model consisted of the subcutaneous insertion of coupons on day 0, challenging the coupons with an injection of 1.7 × 108 to 2.8 × 108 CFU bacteria on day 1, recovery of the coupons on day 4, and then counting the number of CFU in the biofilm. Compared to the bacterial density on uncoated coupons (1.4 × 105 ± 0.5 × 105 CFU), we observed a significant reduction in the number of CFU on the surfaces of TMS/O2-coated coupons (3.1 × 104 ± 0.9 × 104 CFU) (P = 0.014) (Fig. 3A). In order to further assess the soft-tissue damage of biofilm infection, mice were infected with higher doses of bacteria and monitored for longer times to observe the skin lesion around the implant area.
FIG 3.
Biofilm formation on implanted coupons in BALB/cJ female mice. (A) Biofilm on implanted coupons. Data were pooled from 2 independent experiments (with an NRS234 inoculation dose of 2.8 × 108 and 1.7 × 108 CFU per mouse, respectively). A total of 10 mice are represented in each group. Means are represented by horizontal lines. *, P < 0.05. (B) Rate of implanted coupon retention of infected mice. Data were pooled from 3 independent experiments (with an NRS234 inoculation dose of 1.2 × 109, 1.2 × 109, and 2.0 × 109 CFU per mouse, respectively). A total of 15 mice were represented in the uncoated group, and 14 mice were in the coated group. (C) Skin lesion size of infected mice as described for panel B. (D) L-929 mouse fibroblast cell viability (as determined by mitochondrial reduction of MTT substrate) when cultured with TMS/O2 1:4 coupons normalized to the value of cells cultured with uncoated controls, which was defined as 100%. The data are presented as means ± standard errors of the means for a total of 9 samples (pooled from 3 independent experiments in triplicates).
Skin lesions started to appear in mice implanted with uncoated coupons at day 3 postinfection, while skin lesions started to appear in mice with coated coupons at day 5. At day 8, mice implanted with uncoated coupons started to lose the coupon due to skin necrosis, while mice with coated coupons started to lose coupon at day 9. At the end of the experiment, only 40% of mice with uncoated coupon still retained their implants while 78.6% of mice with coated coupons retained their implants (P = 0.041) (Fig. 3B), suggesting there was a significant difference in soft-tissue damage caused by infections on uncoated and coated coupons. A significant difference in skin lesion size was observed between uncoated and coated groups at days 5, 6, and 14 (Fig. 3C). Of note, once a mouse lost its implant, its skin lesion no longer would be included in skin lesion analysis, because it had lost the source of foreign-body biofilm infection. As a result, the uncoated group had lost the severely affected members much faster than the uncoated group after day 6, which could bias the test of skin lesion after day 6. However, a significant difference in skin lesion sizes still was observed at day 14 between the two groups. Overall, our in vivo data support the hypothesis that coating the implant with TMS/O2 1:4 will significantly reduce biofilm formation and cause less soft-tissue damage.
Cytotoxicity of TMS/O2 coating on mammalian cells.
The effect of the coated coupons on the viability of L-929 mouse fibroblast cells was tested using a protocol based on ISO 10993-5 and MTT assay. The TMS/O2 1:4 coating had no deleterious effect on cell viability, and it may slightly increase the cell viability (P = 0.034) (Fig. 3D).
Protein adsorption affected by TMS/O2 coating.
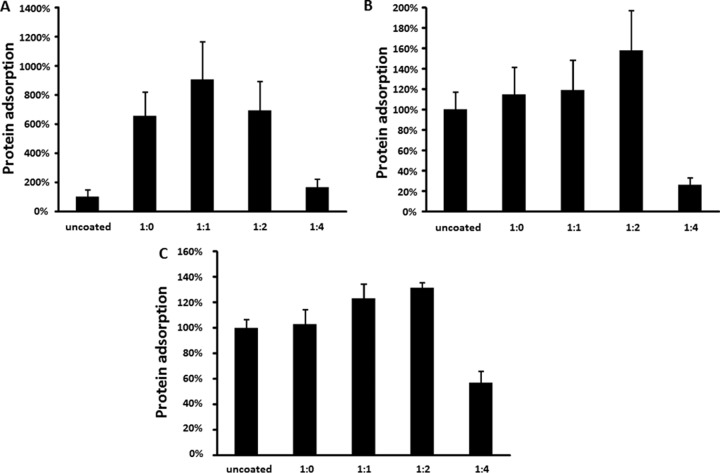
We used a modified ELISA technique to measure the binding of fibrinogen, fibronectin, and albumin to coated and uncoated PDMS surfaces. Although coated and uncoated surfaces exhibited similar hydrophobicity, we found that TMS/O2 plasma coating had a specific effect on the adhesion of human plasma proteins to PDMS. The albumin binding was increased on TMS-coated surfaces with different oxygen mixtures (Fig. 4A). However, only the TMS/O2 1:4 coating was able to reduce surface fibronectin by 73.9% ± 6.9% (P < 0.003) (Fig. 4B) and surface fibrinogen by 43.1% ± 9.3% (P < 0.002) (Fig. 4C).
FIG 4.
Plasma protein adsorption on silicone coupons. The mean of uncoated samples was set as 100%. Data were pooled from nine samples (three independent experiments with triplicates) and are presented as means ± standard errors of the means. *, P < 0.05; **, P < 0.01. (A) Albumin adsorption on uncoated and coated silicone coupons. (B) Fibronectin adsorption on uncoated and coated silicone coupons. (C) Fibrinogen adsorption on uncoated and coated silicone coupons.
DISCUSSION
In our previous studies, a trimethylsilane (TMS) plasma nanocoating was developed to coat surfaces of metallic biomaterials, such as stainless steel and titanium, for reduced bacterial adhesion and biofilm formation (19). The TMS plasma nanocoatings have the characteristics of chemical inertness and surface smoothness. TMS coating demonstrated significant inhibition of S. epidermidis biofilm on stainless steel and titanium surfaces (19).
In this study, we focused on the development of new plasma-coating conditions targeting polymeric biomaterials, such as PDMS or silicone rubber of medical grade, which is widely used in the making of implantable catheters, by introducing oxygen into the TMS coating process at an optimal level. A variety of ratios of TMS to oxygen, e.g., 1:0, 1:1, 1:2, and 1:4, was studied using biofilm assay. It was found that the ratio of 1:4 for TMS to oxygen generated the most significant inhibitory effect on biofilm formation by S. aureus (Fig. 1A).
Analysis by biofilm formation and SEM demonstrated that TMS/O2 coating could significantly reduce S. aureus biofilm formation, in contrast to the ineffectiveness of vancomycin treatment. TMS/O2 1:4 also demonstrated in vivo efficacy at reducing biofilm formation in a mouse foreign-body biofilm infection model. For infections involving S. aureus biofilms in patients with surgical implants, conventional treatments of biofilm-related infections require removing the infected medical implants, followed by an extensive antibiotic regimen to clear the infection and then implanting new devices (2). The treatment regimens are highly risky and traumatic and incur high expense and high risks for selecting for antibiotic resistance pathogens. Thus, strategies of preventing S. aureus biofilm formation on medical devices are urgently needed to address the morbidity and mortality resulting from biofilm formation.
A key virulence mechanism for the pathogenesis of S. aureus infection is the display of bacterial surface proteins that specifically bind to human plasma and extracellular proteins, such as fibronectin and fibrinogen (38). Immediately upon insertion into the host, the surfaces of the implanted medical devices adsorb plasma and extracellular proteins; these adsorbed proteins are believed to mediate the targeted attachment of S. aureus to the implant surface (38).
We hypothesized that changes in surface chemistry due to the TMS/O2 plasma coating could cause changes in protein adsorption to the coated surface, which could in turn reduce bacterial adhesion to the surface, leading to the inhibition of biofilm formation and infection (19). The significantly increased albumin on TMS/O2-coated surfaces could lead to more inhibition of bacterial adhesion in all TMS-coated surfaces (39). It was found that modifying silicone surfaces with nanostructured carbon increased albumin adsorption and reduced S. aureus adhesion and proliferation (40). The effect of albumin in bacterial attachment to biomaterial surfaces is complicated. While there are many reports that demonstrated albumin inhibited bacterial adhesion (41–44), there also are reports that coating contact lenses with albumin could enhance bacterial attachment (45, 46). Thus, it remains a possibility that there are other factors contributing to the inhibition of biofilm formation other than albumin.
Interestingly, only TMS/O2 1:4-coated surfaces demonstrated a significant reduction of both fibrinogen and fibronectin adhesion, which could explain why the least biofilm was formed on TMS/O2 1:4 even though there was more albumin adsorption on other surfaces. Protein adsorptions have been reported to be more sensitive to the underlying surface chemistry than to surface hydrophobicity (47). In this study, the TMS/O2 plasma-coated surfaces exhibited fewer —CH3 groups than bare PDMS, and this change could contribute to the decreased binding of fibrinogen and fibronectin to the coated PDMS (48). Likewise, the increased C—O functional groups on the TMS/O2 1:4 plasma-coated PDMS also could lead to reduced fibrinogen adsorption (49). It also has been reported that albumin showed high binding affinity to hydrophobic surfaces (50). The substantially enhanced albumin adsorption on TMS/O2-coated surfaces could be attributed to the C—O functional group as well.
Just like the results from our in vitro studies, our findings from the mouse foreign-body infection study demonstrated that TMS/O2 1:4 coating significantly reduced staphylococcal biofilm in vivo. There also was reduced soft-tissue damage caused by the infection, as demonstrated by smaller skin lesion size and less skin necrosis, leading to the reduced loss of implant coupons in the group of mice implanted with coated coupons. This reduction could be due simply to a reduction in the initial bacterial attachment to the modified surface, but the reduction also could be due to interference with bacterial proliferation across the surface of the coupon, making the biofilm more susceptible to host defenses. For example, bacterial cells scattered over the surface could be more susceptible to host immunity than cells encased in the biofilm. It has been reported that bacteria adhering to polyethylene glycosylated surfaces exhibited a clear tendency to form clumps, which were similar to those of the bacteria on the TMS/O2 1:4-coated surfaces. This bacterial clumping suggests that in this setting, bacterium-to-bacterium interactions are stronger than bacterium-to-surface interactions, again possibly making the bacterial cells more susceptible to host defenses (15).
The biocompatibility of the coating also was assessed by examining how coating affected cell viability of L-929 mouse fibroblast cells based on ISO 10993-5 recommendations. The TMS/O2 coating demonstrated no deleterious effect on cell viability and even slightly increased cell viability, suggesting it is not cytotoxic to the host.
We expect the nanoscale plasma-coated surface developed in the current study to have stable and abrasion-resistant antibiofilm properties, because the TMS/O2 coating generates a covalent chemical bond to the biomaterial surface. In contrast to our method, other existing methods of coating biomaterials with antibiotics and other agents create surfaces that tend to degrade over time. Also unlike the wet chemistry processes of applying antibacterial agents to biomaterials (15, 16, 18), the nanoscale plasma coating technology is an environmentally friendly and cost-effective method for changing the biomaterial surface without affecting the properties of the bulk materials. While the TMS/O2 1:4 coating has yet to reduce the bacterial load by 3 log units (99.9% inhibition) as defined by biofilm minimum bactericidal concentrations, the in vivo efficacy demonstrated by our plasma-coating technology significantly boosts the potential for future clinical applications. In summary, our technology could offer a cost-effective and efficient way to coat implantable medical devices to prevent the development of S. aureus biofilms. The successful application of this technology could bring financial and medical benefits to the health care system.
ACKNOWLEDGMENTS
The work was supported in part by NIH grant P01HL573461 and NIH grant R44HL097485. The Network on Antimicrobial Resistance in Staphylococcus aureus program (NARSA) is supported under NIAID, NIH contract no. HHSN272200700055C.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Darouiche RO. 2004. Treatment of infections associated with surgical implants. N Engl J Med 350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 3.Donlan RM. 2002. Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otto M. 2008. Staphylococcal biofilms. Curr Top Microbiol Immunol 322:207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies D. 2003. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 6.Nichols WW, Evans MJ, Slack MP, Walmsley HL. 1989. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol 135:1291–1303. [DOI] [PubMed] [Google Scholar]
- 7.Gordon CA, Hodges NA, Marriott C. 1988. Antibiotic interaction and diffusion through alginate and exopolysaccharide of cystic fibrosis-derived Pseudomonas aeruginosa. J Antimicrob Chemother 22:667–674. doi: 10.1093/jac/22.5.667. [DOI] [PubMed] [Google Scholar]
- 8.Pavithra D, Doble M. 2008. Biofilm formation, bacterial adhesion and host response on polymeric implants–issues and prevention. Biomed Mater 3:034003. doi: 10.1088/1748-6041/3/3/034003. [DOI] [PubMed] [Google Scholar]
- 9.Antoci V Jr, Adams CS, Parvizi J, Davidson HM, Composto RJ, Freeman TA, Wickstrom E, Ducheyne P, Jungkind D, Shapiro IM, Hickok NJ. 2008. The inhibition of Staphylococcus epidermidis biofilm formation by vancomycin-modified titanium alloy and implications for the treatment of periprosthetic infection. Biomaterials 29:4684–4690. doi: 10.1016/j.biomaterials.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hockenhull JC, Dwan KM, Smith GW, Gamble CL, Boland A, Walley TJ, Dickson RC. 2009. The clinical effectiveness of central venous catheters treated with anti-infective agents in preventing catheter-related bloodstream infections: a systematic review. Critical Care Med 37:702–712. doi: 10.1097/CCM.0b013e3181958915. [DOI] [PubMed] [Google Scholar]
- 11.Sampath LA, Tambe SM, Modak SM. 2001. In vitro and in vivo efficacy of catheters impregnated with antiseptics or antibiotics: evaluation of the risk of bacterial resistance to the antimicrobials in the catheters. Infect Control Hosp Epidemiol 22:640–646. doi: 10.1086/501836. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Dong Y, Qi XQ, Li YM, Huang CG, Hou LJ. 2013. Clinical review: efficacy of antimicrobial-impregnated catheters in external ventricular drainage–a systematic review and meta-analysis. Crit Care 17:234. doi: 10.1186/cc12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tambe SM, Sampath L, Modak SM. 2001. In vitro evaluation of the risk of developing bacterial resistance to antiseptics and antibiotics used in medical devices. J Antimicrob Chemother 47:589–598. doi: 10.1093/jac/47.5.589. [DOI] [PubMed] [Google Scholar]
- 14.Klibanov AM. 2007. Permanently microbicidal materials coatings. J Mater Chem 17:2479–2482. doi: 10.1039/b702079a. [DOI] [Google Scholar]
- 15.Harris LG, Tosatti S, Wieland M, Textor M, Richards RG. 2004. Staphylococcus aureus adhesion to titanium oxide surfaces coated with non-functionalized and peptide-functionalized poly(L-lysine)-grafted-poly(ethylene glycol) copolymers. Biomaterials 25:4135–4148. doi: 10.1016/j.biomaterials.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 16.Cheng G, Zhang Z, Chen S, Bryers JD, Jiang S. 2007. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 28:4192–4199. doi: 10.1016/j.biomaterials.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu LC, Siedlecki CA. 2012. Submicron-textured biomaterial surface reduces staphylococcal bacterial adhesion and biofilm formation. Acta Biomater 8:72–81. doi: 10.1016/j.actbio.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Tran PL, Lowry N, Campbell T, Reid TW, Webster DR, Tobin E, Aslani A, Mosley T, Dertien J, Colmer-Hamood JA, Hamood AN. 2012. An organoselenium compound inhibits Staphylococcus aureus biofilms on hemodialysis catheters in vivo. Antimicrob Agents Chemother 56:972–978. doi: 10.1128/AAC.05680-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y, Chen M, Jones JE, Ritts AC, Yu Q, Sun H. 2012. Inhibition of Staphylococcus epidermidis biofilm by trimethylsilane plasma coating. Antimicrob Agents Chemother 56:5923–5937. doi: 10.1128/AAC.01739-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Xu Y, Yestrepsky BD, Sorenson RJ, Chen M, Larsen SD, Sun H. 2012. Novel inhibitors of Staphylococcus aureus virulence gene expression and biofilm formation. PLoS One 7:e47255. doi: 10.1371/journal.pone.0047255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng HL, Novick RP, Kreiswirth B, Kornblum J, Schlievert P. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol 170:4365–4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beenken KE, Blevins JS, Smeltzer MS. 2003. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect Immun 71:4206–4211. doi: 10.1128/IAI.71.7.4206-4211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton M, Heersink J, Buckingham-Meyer K, Goeres D. 2004. The biofilm laboratory: step-by-step protocols for experimental design, analysis, and data interpretation. Cytergy, Bozeman, MT. [Google Scholar]
- 24.Kobayashi N, Bauer TW, Tuohy MJ, Fujishiro T, Procop GW. 2007. Brief ultrasonication improves detection of biofilm-formative bacteria around a metal implant. Clin Orthop Relat Res 457:210–213. [DOI] [PubMed] [Google Scholar]
- 25.Amorena B, Gracia E, Monzon M, Leiva J, Oteiza C, Perez M, Alabart JL, Hernandez-Yago J. 1999. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J Antimicrob Chemother 44:43–55. doi: 10.1093/jac/44.1.43. [DOI] [PubMed] [Google Scholar]
- 26.Chen M, Zamora PO, Pena L, Som P, Osaki S. 2003. NH3/O2 mixed gas plasmas alter the interaction of blood components with stainless steel. J Biomed Mater Res A 67:994–1000. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Zamora PO, Som P, Pena LA, Osaki S. 2003. Cell attachment and biocompatibility of polytetrafluoroethylene (PTFE) treated with glow-discharge plasma of mixed ammonia and oxygen. J Biomater Sci Polym Ed 14:917–935. doi: 10.1163/156856203322381410. [DOI] [PubMed] [Google Scholar]
- 28.Whittle JD, Bullett NA, Short RD, Douglas CWI, Hollander AP, Davies J. 2002. Adsorption of vitronectin, collagen and immunoglobulin-G to plasma polymer surfaces by enzyme linked immunosorbent assay (ELISA). J Mater Chem 12:2726–2732. doi: 10.1039/b201471h. [DOI] [Google Scholar]
- 29.Christensen GD, Simpson WA, Bisno AL, Beachey EH. 1983. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun 40:407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakimura T, Kajiyama S, Adachi S, Chiba K, Yonekura A, Tomita M, Koseki H, Miyamoto T, Tsurumoto T, Osaki M. 2015. Biofilm-forming Staphylococcus epidermidis expressing vancomycin resistance early after adhesion to a metal surface. Biomed Res Int 2015:943056. doi: 10.1155/2015/943056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin YS, Liao YH, Hu CH. 2009. Effects of N2 addition on enhanced scratch resistance of flexible polycarbonate substrates by low temperature plasma-polymerized organo-silicon oxynitride. J Non-Crystalline Solids 355:182–192. doi: 10.1016/j.jnoncrysol.2008.10.015. [DOI] [Google Scholar]
- 32.Sabata A, van Ooij WJ, Yasuda HK. 1993. Plasma-polymerized films of trimethylsilane deposited on cold-rolled steel substrates. Part 1. Characterization by XPS, AES and TOF-SIMS. Surf Interface Anal 20:845–859. [Google Scholar]
- 33.Lin Y-S, Chen C-L. 2006. Wear resistance of low-temperature plasma-polymerized organosilica deposited on poly(ethylene terephthalate): the effect of O2 addition. Plasma Proc Polym 3:650–660. doi: 10.1002/ppap.200600034. [DOI] [Google Scholar]
- 34.Chen M, Osaki S, Zamora PO, Potekhin M. 2003. Effect of nitrogen and oxygen incorporated into TMSAA plasma coating on surface-bound heparin activity. J Appl Polym Sci 89:1875–1883. doi: 10.1002/app.12490. [DOI] [Google Scholar]
- 35.El-Agez TM, Wieliczka DM, Moffitt CE, Taya SA. 2011. Spectroscopic ellipsometry time study of low-temperature plasma-polymerized plain trimethylsilane thin films deposited on silicon. Phys Scripta 84:045302. doi: 10.1088/0031-8949/84/04/045302. [DOI] [Google Scholar]
- 36.Hayakawa T, Yoshinari M, Nemoto K. 2004. Characterization and protein-adsorption behavior of deposited organic thin film onto titanium by plasma polymerization with hexamethyldisiloxane. Biomaterials 25:119–127. doi: 10.1016/S0142-9612(03)00484-8. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda HK. 1985. Plasma polymerization. Academic Press, Orlando, FL. [Google Scholar]
- 38.Gotz F. 2002. Staphylococcus and biofilms. Mol Microbiol 43:1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 39.Katsikogianni M, Missirlis YF. 2004. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur Cells Mater 8:37–57. [DOI] [PubMed] [Google Scholar]
- 40.Nune C, Xu W, Misra RD. 2012. The impact of grafted modification of silicone surfaces with quantum-sized materials on protein adsorption and bacterial adhesion. J Biomed Mater Res Part A 100:3197–3204. doi: 10.1002/jbm.a.34260. [DOI] [PubMed] [Google Scholar]
- 41.Carballo J, Ferreiros CM, Criado MT. 1991. Influence of blood proteins in the in vitro adhesion of Staphylococcus epidermidis to teflon, polycarbonate, polyethylene and bovine pericardium. Rev Esp Fisiol 47:201–208. [PubMed] [Google Scholar]
- 42.Espersen F, Wilkinson BJ, Gahrn-Hansen B, Thamdrup Rosdahl V, Clemmensen I. 1990. Attachment of staphylococci to silicone catheters in vitro. APMIS 98:471–478. doi: 10.1111/j.1699-0463.1990.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 43.Zdanowski Z, Ribbe E, Schalen C. 1993. Influence of some plasma proteins on in vitro bacterial adherence to PTFE and Dacron vascular prostheses. APMIS 101:926–932. doi: 10.1111/j.1699-0463.1993.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 44.Kinnari TJ, Peltonen LI, Kuusela P, Kivilahti J, Kononen M, Jero J. 2005. Bacterial adherence to titanium surface coated with human serum albumin. Otol Neurotol 26:380–384. doi: 10.1097/01.mao.0000169767.85549.87. [DOI] [PubMed] [Google Scholar]
- 45.Taylor RL, Willcox MD, Williams TJ, Verran J. 1998. Modulation of bacterial adhesion to hydrogel contact lenses by albumin. Optom Vis Sci 75:23–29. doi: 10.1097/00006324-199801000-00021. [DOI] [PubMed] [Google Scholar]
- 46.Subbaraman LN, Borazjani R, Zhu H, Zhao Z, Jones L, Willcox MD. 2011. Influence of protein deposition on bacterial adhesion to contact lenses. Optom Vis Sci 88:959–966. doi: 10.1097/OPX.0b013e31821ffccb. [DOI] [PubMed] [Google Scholar]
- 47.Keselowsky BG, Collard DM, Garcia AJ. 2003. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res Part A 66:247–259. [DOI] [PubMed] [Google Scholar]
- 48.Allen LT, Tosetto M, Miller IS, O'Connor DP, Penney SC, Lynch I, Keenan AK, Pennington SR, Dawson KA, Gallagher WM. 2006. Surface-induced changes in protein adsorption and implications for cellular phenotypic responses to surface interaction. Biomaterials 27:3096–3108. doi: 10.1016/j.biomaterials.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Wang J, Li X, Tu Q, Sun H, Huang N. 2012. Interaction of platelets, fibrinogen and endothelial cells with plasma deposited PEO-like films. Appl Surf Sci 258:3378–3385. doi: 10.1016/j.apsusc.2011.11.013. [DOI] [Google Scholar]
- 50.Roach P, Farrar D, Perry CC. 2005. Interpretation of protein adsorption: surface-induced conformational changes. J Am Chem Soc 127:8168–8173. doi: 10.1021/ja042898o. [DOI] [PubMed] [Google Scholar]