Abstract
There is growing interest in biomaterials that can cure bone infection and also regenerate bone. In this study, two groups of implants composed of 10% (wt/wt) teicoplanin (TEC)-loaded borate bioactive glass (designated TBG) or calcium sulfate (TCS) were created and evaluated for their ability to release TEC in vitro and to cure methicillin-resistant Staphylococcus aureus (MRSA)-induced osteomyelitis in a rabbit model. When immersed in phosphate-buffered saline (PBS), both groups of implants provided a sustained release of TEC at a therapeutic level for up to 3 to 4 weeks while they were gradually degraded and converted to hydroxyapatite. The TBG implants showed a longer duration of TEC release and better retention of strength as a function of immersion time in PBS. Infected rabbit tibiae were treated by debridement, followed by implantation of TBG or TCS pellets or intravenous injection with TEC, or were left untreated. Evaluation at 6 weeks postimplantation showed that the animals implanted with TBG or TCS pellets had significantly lower radiological and histological scores, lower rates of MRSA-positive cultures, and lower bacterial loads than those preoperatively and those of animals treated intravenously. The level of bone regeneration was also higher in the defects treated with the TBG pellets. The results showed that local TEC delivery was more effective than intravenous administration for the treatment of MRSA-induced osteomyelitis. Borate glass has the advantages of better mechanical strength, more desirable kinetics of release of TEC, and a higher osteogenic capacity and thus could be an effective alternative to calcium sulfate for local delivery of TEC.
INTRODUCTION
Bacterial infection resulting from orthopedic surgery is a serious complication. The pathogenic organisms responsible for such infections are often resistant to multiple drugs. Methicillin-resistant Staphylococcus aureus (MRSA) has become the most common osteomyelitis-inducing microorganism clinically (1, 2). Currently, the main treatment for chronic osteomyelitis includes surgical debridement and prolonged systemic antibiotic therapy over a course of several weeks. As an antibiotic, teicoplanin (TEC) has a long serum half-life and broad-spectrum antibacterial activity against most Gram-positive aerobic and anaerobic organisms such as MRSA and methicillin-resistant coagulase-negative S. aureus (3). Compared to vancomycin (a more widely used antibiotic), the use of TEC also results in less ototoxicity, nephrotoxicity, and gastrointestinal side effects. Because of its favorable pharmacokinetics, TEC is commonly used in Europe to treat osteomyelitis (4).
Even after thorough debridement, residual infection often remains in the bone. The surgical procedure also creates a bone defect that usually requires subsequent reconstruction (5). Furthermore, nidus formation considerably limits the efficacy of systemic antibiotic therapy because of the compromised vascular perfusion at the infected site. High concentrations of antibiotics in the blood resulting from long-term intravenous treatment also carry the risks of systemic toxicity and development of antibiotic-resistant bacterial strains. Consequently, an alternative to the use of systemic antibiotics is the use of osteoconductive biomaterials to locally deliver high doses of antibiotics (6).
Poly(methyl methacrylate) (PMMA) cement has been widely used as a carrier for antibiotics for the treatment of bacterial infection in orthopedic surgery. However, PMMA is not biodegradable, and it provides a surface upon which secondary bacterial infection can occur. Consequently, PMMA must be removed upon completion of antibiotic treatment. Also, the rate of antibiotic release from PMMA can be low. Because of these difficulties, considerable effort has been made to develop local antibiotic delivery vehicles as alternatives to PMMA cement. Biocompatible inorganic materials that stimulate bone formation, such as hydroxyapatite (HA), tricalcium phosphate (TCP), calcium sulfate (CaSO4), and silicate-based bioactive glass (e.g., 45S5 glass), have been widely studied (7). Antibiotic-loaded CaSO4 is commercially available for clinical use in the treatment of osteomyelitis (8–10). CaSO4 has shown predictable antibiotic release rates in vitro and a high release rate due to its high degradation rate, but its ability to stimulate bone regeneration is limited.
Bioactive glass-based materials are emerging as alternative antibiotic carriers for the treatment of bone infection (11). Bioactive glasses are unique in their ability to be converted to HA in vivo, in addition to their proven osteoconductivity and their ability to form a strong bond with bone and soft tissues (12, 13). Borate bioactive glasses have been attracting greater interest than the more widely known silicate bioactive glasses, such as 45S5 and S53P4, because of their ability to be converted more rapidly and completely to HA (14, 15). However, the potential of borate bioactive glass as an antibiotic carrier in the treatment of bone infection is currently unclear due to limited data. In particular, there are few data on the effectiveness of borate bioactive glass in treating bone infection compared to more conventional biomaterials that are used clinically.
The objective of the present study was to compare the capacity of borate bioactive glass implants to deliver TEC and to cure MRSA-induced osteomyelitis in a rabbit tibial model with that of CaSO4, a biomaterial that is used clinically. The conversion of the two carrier materials to HA and antibiotic release from the carriers were measured as a function of immersion time in phosphate-buffered saline (PBS). The capacity of the antibiotic-loaded implants to cure osteomyelitis in rabbit tibiae was evaluated at 6 weeks postimplantation by using radiography, histology, micro-computed tomography (micro-CT), and microbiological techniques.
MATERIALS AND METHODS
Preparation of TEC-loaded implants.
Borate bioactive glass particles (size, <50 μm) with the composition 6Na2O, 8K2O, 8MgO, 22CaO, 54B2O3, and 2P2O5 (mol%) were prepared as described previously (16). The CaSO4 used in this study was medical-grade calcium sulfate hemihydrate (OsteoSet resorbable minibead kit; Wright Medical Technology, Inc., Arlington, TN). TEC powder (Gruppo Lepetit SPA, Anagni, Italy) was mixed with each biomaterial and PBS (pH 7.2 to 7.4), with a final concentration of 10 wt%. The mixtures of TEC-loaded borate glass (designated TBG) and TEC-loaded CaSO4 (designated TCS) were each placed into polyethylene molds (4.7 mm diameter by 3.5 mm height) and allowed to harden overnight, without any externally applied pressure. The prepared pellets were sterilized by using gamma irradiation (25 Gy).
Evaluation of implant properties and TEC release profile in vitro.
The amount of TEC in each pellet was determined from the mass of the TBG and TCS implants. Four TBG or TCS implants were immersed in 10 ml of PBS (pH 7.4) in sterile polyethylene centrifuge tubes and kept in a water bath at 37°C. The experiments were performed in triplicate, and PBS was replaced every 48 h. While there is no standard protocol for measuring the release of antibiotics from a carrier material in vitro, the parameters of the system used in the present study are consistent with those described previously (17, 18). The amount of TEC released from the implants was determined by using high-performance liquid chromatography (HPLC) (detection limit of 0.2 μg/ml), as described previously (19). The experiments were performed under aseptic conditions until no measurable TEC could be detected in PBS. The amount of TEC released from each implant group at each time point was expressed as an average ± standard deviation (SD) and normalized to the total amount of TEC initially loaded into the implants.
The compressive strength of the TBG and TCS implants was measured as a function of immersion time in PBS by using a mechanical testing machine (CMT6104; Sans Test Machine, Inc., China) at a crosshead speed of 0.5 mm min−1. The surface morphology and composition of the samples were studied as a function of immersion time by using a field emission scanning electron microscope (FE-SEM) (Quanta 200 FEG; FEI Co., The Netherlands) equipped with an energy-dispersive X-ray (EDS) spectrometer. X-ray diffraction (XRD) (model D, maximum excitation voltage of 2,550 V; Rigaku International Corp., USA) was performed by using Cu Kα radiation (λ = 0.15406 nm) at a scanning rate of 1.8° 2θ per min.
In vivo implantation.
All animal surgical procedures were performed according to the guidelines of the Animal Welfare Committee of the Shanghai Jiaotong University Affiliated Sixth People's Hospital. Fifty-five adult female pathogen-free New Zealand White rabbits (2.7 ± 0.2 kg) were used in this study. Osteomyelitis was induced in rabbit tibiae by using a method modified from the one described previously by Norden et al. (20). In these experiments, 0.1 ml of 5% sodium morrhuate (Eli Lilly, Indianapolis, IN) and 0.1 ml of a bacterial suspension (MRSA strain ATCC 43300; 1 × 108 CFU/ml) were injected into the medullary cavity of the right tibiae. Sodium morrhuate was used in the present study for inducing local ischemia and, therefore, increasing the rate of success of bone infection, in accordance with data from previous studies (21, 22). After 4 weeks, 51 rabbits were diagnosed with osteomyelitis according to a radiographic scoring system described previously by Norden et al. (20). Five rabbits were sacrificed for microbiological and histological examination to confirm chronic osteomyelitis, while the remaining animals were randomly divided into 4 groups for further treatment.
Forty-six rabbits were thoroughly debrided and implanted with TBG implants (n = 12) or TCS implants (n = 12) or treated intravenously with TEC (n = 12; 6 mg/kg of body weight administered every 12 h for 3 doses and then every 24 h for a period of up to 4 weeks). The remaining animals were left untreated and served as negative controls (n = 10). A cortical bone window (∼2.0 by 0.8 cm) was made in the anteromedial surface of the proximal tibia for thorough removal of all necrotic tissue according to the different severities of bone infection. Debridement was performed until punctate bleeding from healthy tissue was noted (referred to as the paprika sign) in order to ensure that all necrotic tissue was removed (23). The animals were monitored daily to observe their general condition, including activity, body temperature, weight, and wound appearance. At 6 weeks postimplantation, the rabbits were euthanized by injection with an overdose of pentobarbital sodium, and the tibiae were harvested.
Evaluation of TEC concentrations in rabbit blood.
Prior to the implantation and every week postimplantation, venous blood samples were drawn from the rabbits to determine the number of leukocytes and to assess liver and renal functions, as measured by the levels of glutamate-pyruvate transaminase, glutamic oxaloacetic transaminase, urea, and creatinine. The TEC concentration in the blood of the rabbits implanted with the TBG and TCS implants was determined by using HPLC 0, 1, 2, 4, 8, 12, 24, 48, and 72 h after implantation and every 2 days thereafter. Serum samples of the rabbits treated intravenously were also analyzed to determine the peak level of TEC (just after the administration of a dose) and the trough level (just before a dose).
Radiography and micro-CT analysis.
Radiographs of the rabbit tibiae, taken before and after debridement and at autopsy, were scored by an orthopedic surgeon in a blind manner according to the radiographic scoring system described previously by Norden et al. (20). Sequestral bone formation (SBF), periosteal new bone formation (PNBF), destruction of bone (DB), soft tissue calcification (STC), and soft tissue swelling (STW) were scored. Rabbits with scores of 3 or higher were diagnosed with osteomyelitis. Synchrotron X-ray micro-computed tomography (SR micro-CT) was used to construct three-dimensional (3D) images of the tibial specimens after the rabbits were sacrificed. Scanning was conducted at the Advanced Light Source (ALS-LBNL, Berkeley, CA) with 22-keV monochromatic X rays and a 4.4-μm voxel size (resolution). The data sets were reconstructed by using Octopus software, while 3D visualization of the images was performed by using Avizo software.
Histological evaluation.
The tibial samples were fixed in 10% (wt/wt) formaldehyde, decalcified in EDTA, dehydrated in a graded series of ethanol, and embedded in paraffin. Longitudinal sections with a thickness of 5 μm were stained with hematoxylin and eosin (H&E) for light microscopy. The stained sections were assessed by a pathologist in a blind manner according to the scoring system described previously by Smeltzer et al. (24). Intraosseous acute inflammation (IAI), intraosseous chronic inflammation (ICI), periosteal inflammation (PI), and bone necrosis (BN) were measured. Animals with at least six stained sections with scores of 4 or higher were diagnosed as having osteomyelitis. The amounts of new bone and residual implant material in the bone defects were measured by using a computerized image analysis system (QWin Plus; Leica, Wetzlar, Germany) and expressed as a percentage of each measurable area in the bone histological section.
Microbiological examination.
At debridement before implantation and at autopsy after 6 weeks, the tissue samples collected from the surgical site were inoculated onto blood agar (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom) and incubated at 37°C for 48 h. For recovery of slow-growing microorganisms, the specimens were also inoculated into brain heart infusion (BHI) broth (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom) at 37°C for 7 days. In addition, portions of the bone samples were weighed and homogenized in 10 ml PBS for 5 min at 10,000 rpm by using a tissue homogenizer. A series of 10-fold dilutions was prepared in saline, and 10 μl of each dilution was plated onto blood agar plates and incubated at 37°C for 48 h. Colony counts were performed to obtain the amount of CFU per gram of bone tissue. For statistical analysis, negative cultures were conservatively calculated as having 2 × 103 CFU/g (the detection limit value). All of the tests were carried out in triplicate under aseptic conditions. The identification of S. aureus was based on a coagulase tube test and the API Staph system (ATB 32 Staph; bioMérieux, Marcy l'Etoile, France). Resistance to methicillin was further confirmed by detection of the mecA gene using PCR as previously reported (25).
Statistical analysis.
The data were expressed as means ± SD and analyzed by using the Statistical Package for the Social Sciences version 11.0 (SigmaStat; SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was performed to assess significant differences among the radiographic and histological scores. A chi-square test was used to analyze the rates of MRSA infection in bone samples, and nonparametric tests for independent samples (Mann-Whitney tests) were performed for comparison of the CFU counts among groups. Differences were considered to be significant at a P value of <0.05.
RESULTS
Characteristics of implants in vitro.
The compressive strengths of the fabricated TBG and TCS implants were 23 ± 5 MPa and 19 ± 4 MPa, respectively (Table 1). After immersion in PBS for 24 h, the strength of each group of implants showed a significant decrease, to 15 ± 3 MPa and 9 ± 2 MPa for the TBG and TCS implants, respectively. Thereafter, the TBG implants showed a decrease in their average strength, but the decrease was not significant. The compressive strength of the TBG implants was 10.8 ± 0.4 MPa at an immersion time of 7 days. In comparison, the TCS implants continued to show a significant decrease in strength up to 5 days, and at 7 days, the average strength was 1.4 ± 0.7 MPa.
TABLE 1.
Compressive strengths of TBG and TCS pellets as a function of immersion time in PBS
| Pellet type | Mean compressive strength (MPa) ± SD at: |
|||||
|---|---|---|---|---|---|---|
| 0 h | 2 h | 24 h | 72 h | 120 h | 168 h | |
| TBG | 23 ± 5 | 22 ± 3 | 15 ± 3 | 11 ± 2 | 11 ± 1 | 10.8 ± 0.4 |
| TCS | 19 ± 4 | 17 ± 3 | 9 ± 2 | 5 ± 1 | 1.8 ± 0.4 | 1.4 ± 0.7 |
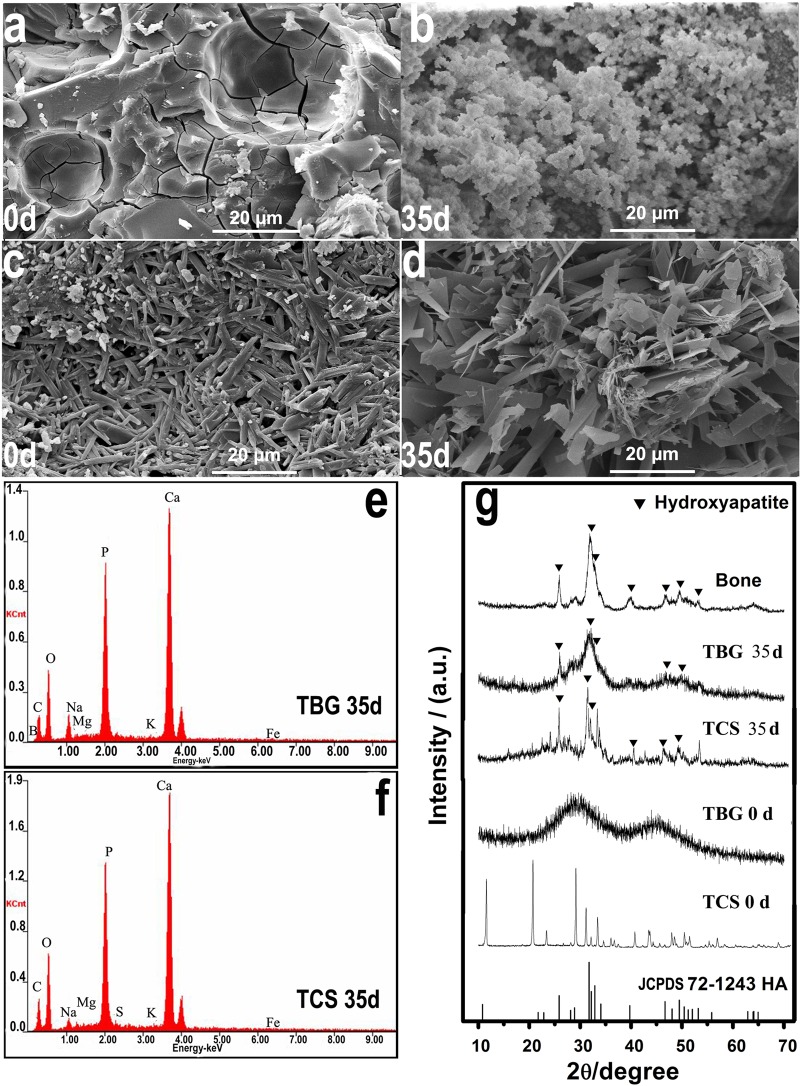
Scanning electron microscopy (SEM) images of the surface of the prepared TBG implants showed some pores embedded in the solid phase (Fig. 1a). The TCS implant was composed of fine rod-like particles typical of calcium sulfate dihydrate crystals (Fig. 1c). Immersion in PBS resulted in the formation of a fine particulate product on the surface of the TBG implants (Fig. 1b). In comparison, the surface of the TCS implants became more porous and was composed of coarser rod-like or plate-like particles (Fig. 1d).
FIG 1.
(a to d) SEM micrographs of the surfaces of TBG (a and b) and TCS (c and d) implants as fabricated (designated 0d [day 0]) (a and c) and after immersion in PBS for 35 days (35d) (b and d). (e and f) EDS spectra of the surface of the TBG implant (e) and the TCS implant (f) after immersion in PBS for 35 days. (g) XRD patterns of the TBG and TCS implants as fabricated (0 d) and after immersion in PBS for 35 days. For comparison, the XRD patterns of reference hydroxyapatite (HA) (JCPDS 72-1243) and dry rabbit bone are also shown. a.u., arbitrary units.
EDS analysis showed that after immersion in PBS for 35 days, the surfaces of both the TBG and TCS implants were composed essentially of a calcium phosphate material (Fig. 1e and f). The Ca/P atomic ratios of the product on the surfaces of the TBG and TCS implants were 1.62 and 1.74, respectively. For comparison, the Ca/P atomic ratio of stoichiometric HA is 1.67. XRD (Fig. 1g) of the prepared TBG implants showed a pattern typical of an amorphous glass, whereas the pattern of the prepared TCS implants corresponded to that of crystalline CaSO4·2H2O. After immersion in PBS for 35 days, the XRD pattern of the TBG implants showed peaks corresponding to those of a reference HA (JCPDS 72-1243) and to the main peaks in the pattern for dry rabbit bone. In comparison, the CaSO4·2H2O peaks found in the pattern for the prepared TCS implants disappeared after 35 days, and they were replaced by peaks that corresponded to those of HA.
Profile of release of TEC from implants in vitro.
Figure 2a shows the profile of release of TEC from the TBG and TCS implants as a function of immersion time in PBS. Both groups of implants showed a rapid release in the first 7 days followed by a more gradual release thereafter. At 7 days, the cumulative amounts of TEC released from the TBG and TCS implants were 73% and 79%, respectively, of the total amounts incorporated into the prepared implants (Fig. 2b). When the release effectively ceased, the cumulative amounts of TEC released from the TBG and TCS implants were 83% (at 37 days) and 87% (at 29 days), respectively. The amounts of TEC released from the TBG and TCS implants were above the MIC for MRSA (2 μg/ml) for immersion times of 31 days and 23 days, respectively. At these two immersion times, the TEC released from the TBG and TCS implants was just as effective against the standard MRSA strain as the TEC that was not incorporated into the implants, indicating that the incorporation of TEC into the implants did not affect its activity.
FIG 2.
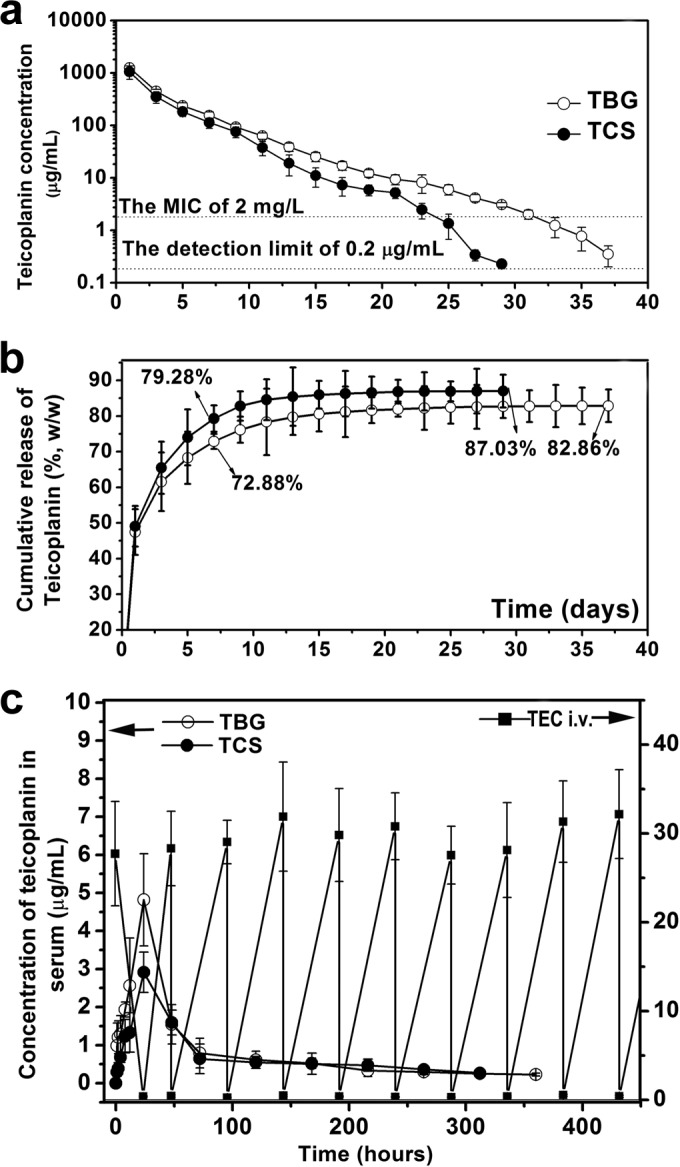
(a and b) Release of teicoplanin from TBG and TCS implants as a function of immersion time in PBS. (a) Amount released at different time points; (b) cumulative amount released (as a percentage of the amount incorporated into the fabricated implants). (c) Concentration of teicoplanin in serum of rabbits treated by implantation of TBG or TCS implants and by intravenous injection of teicoplanin (i.v.), as a function of time.
In vivo performance of TBG and TCS implants.
During the postimplantation period, 2 of the 12 rabbits in the untreated group died as a result of severe infection (determined at autopsy). No adverse effects were observed for the remaining rabbits.
TEC concentration in rabbit blood.
The TEC concentration in the serum of rabbits implanted with the TBG and TCS pellets remained at a low level during the postimplantation period (Fig. 2c). The concentrations reached peak values of 4.8 ± 1.7 μg/ml and 2.9 ± 0.5 μg/ml for the TBG and TCS groups, respectively, at 24 h postimplantation and decreased to a value below the detection limit (0.2 μg/ml) at 15 days for TBG group and at 13 days for the TCS group. For the rabbits treated intravenously, the average concentration of TEC in the blood varied from a high value of 29.8 ± 4.3 μg/ml to a low value of 0.4 ± 0.1 μg/ml. The assessment of liver and renal functions showed that there were no significant differences in the four treatment groups compared with the preoperative values (data not shown).
Radiographic analysis.
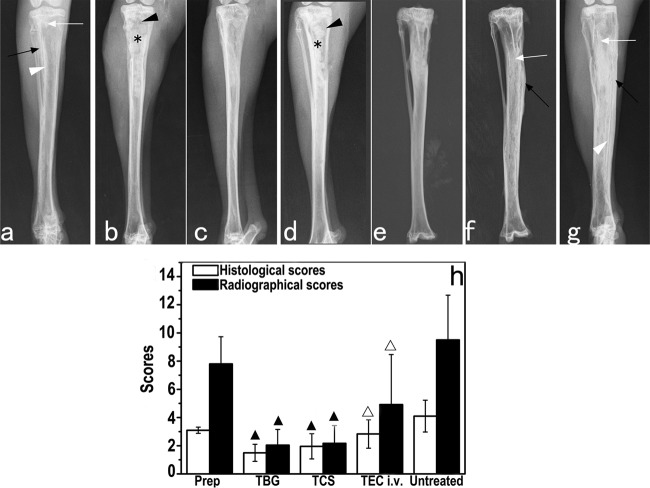
The animals selected for treatment were all diagnosed with chronic osteomyelitis according to the radiographic scoring system described previously by Norden et al. (20). Typical signs of bone infection, including SBF, PNBF, DB, STC, and STW, were observed in the images (Fig. 3a). At 6 weeks postsurgery, the radiographs showed that bone infections were mainly cured in rabbits implanted with the TBG (Fig. 3b and c) and TCS (Fig. 3d and e) pellets, with only 1 of 12 and 2 of 12 animals, respectively, having a score of 3. In comparison, a greater degree of infection remained in the rabbits treated intravenously (Fig. 3f) and in the untreated rabbits (Fig. 3g). Five of the 12 rabbits treated intravenously and all 10 of the untreated rabbits had scores of 3 or higher at autopsy. The radiographic scores for the TBG and TCS treatment groups decreased significantly compared to the preoperative values for these groups (P = 0.001 for the TBG group and P = 0.01 for the TCS group) (Fig. 3h). The radiographic scores for the TBG, TCS, and intravenous treatment groups were each significantly lower than that for the untreated group (P < 0.001 for the TBG group, P = 0.03 for the TCS group, and P = 0.01 for the intravenous group). The radiographic scores for the TBG and TCS groups were also significantly lower than that for the intravenous group (P < 0.001 for the TBG group and P = 0.03 for the TCS group). However, there was no significant difference between the radiographic scores for the TBG and TCS treatment groups (P = 0.17).
FIG 3.
(a to g) Representative radiographs of rabbit tibiae before and after treatment. (a) Experimental MRSA-induced tibia osteomyelitis shows bone destruction (white arrows), periosteal new bone formation (black arrows), and sequestral bone formation (white arrowheads). (b and d) Bone windows (black arrowheads) and implantation of TBG (b) or TCS (d) implants (stars) can be observed 1 day after debridement. (c and e to g) Healing of osteomyelitis was observed 6 weeks after implantation of TBG (c) or TCS (e) implants, but more progression was observed for the intravenous teicoplanin (f) and untreated (g) groups. (h) Radiographic and histological osteomyelitis scores for rabbit tibiae. ▲ indicates a significant difference compared with other groups, and △ indicates a significant difference compared with the untreated group (P < 0.05).
Histological evaluation.
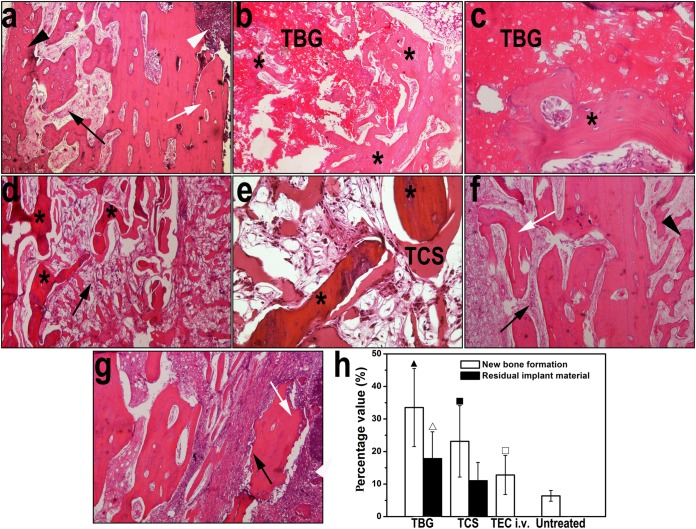
H&E-stained sections of rabbit tibiae before and after treatment showed differences in the capacities of the different treatments to cure bone infection. Experimental osteomyelitis in the tibiae, as determined by IAI, ICI, PI, and BN, occurred 4 weeks after it was induced by MRSA (Fig. 4a). Infection was mostly cured in rabbits implanted with the TBG and TCS pellets, with only 2 of the 12 rabbits in each treatment group having scores of 4 or higher according to the scoring system described previously by Smeltzer et al. (24). For the defects treated with the TBG implants, new bone formed around and within the implants as they were converted to HA, and there were no histological signs of infection (Fig. 4b and c). In comparison, only a small amount of the TCS implants remained in the defect sites, and there was new bone formation around the remaining material (Fig. 4d and e). The stained sections also showed large amounts of neutrophils and mononuclear cells and significant fibrosis, indicating an inflammatory response to the degradation product of the TCS implants. The stained sections of the tibiae for the intravenous treatment group (Fig. 4f) and the untreated group (Fig. 4g) showed various signs of infection. Six of the 12 rabbits in the intravenous treatment group and all 10 rabbits in the untreated group had scores of 4 or higher.
FIG 4.
Representative images of H&E-stained sections of rabbit tibiae before and after treatment. (a) Typical signs of bone infection were observed after induction of osteomyelitis, including the destruction of bone (white arrow), fibrosis (black arrow), periosteal new bone formation (black arrowhead), and intramedullary abscess (white arrowhead) (magnification, ×40). (b and c) Tibiae treated with the TBG implants show newly formed bone (stars) located intimately around and within the degraded TBG implant, with no signs of chronic inflammation (magnifications, ×40 and ×200 [c]). (d and e) Tibiae treated with the TCS implants show degraded CaSO4 surrounded by a moderate amount of new bone (stars), accompanied by obvious chronic fibrosis with proliferated foamy histiocytes and multinucleated granulocytes (black arrow) (magnifications, ×40 [d] and ×200 [e]). (f and g) Moderate to severe inflammation was observed in rabbits treated with teicoplanin intravenously (magnification, ×40) (f) and in the untreated group (magnification, ×40) (g), such as destruction of bone (white arrows), fibrosis (black arrows), and periosteal new bone formation (black arrowhead). (h) Quantitation of new bone and residual amounts of the implant from H&E-stained sections. ▲ indicates a significant difference compared with other groups, △ indicates a significant difference compared with the TCS group, ■ indicates a significant difference compared with the intravenous treatment and untreated groups, and □ indicates a significant difference compared with the untreated group (P < 0.05).
There was no significant difference between the histological scores for the TBG and TCS groups (P = 0.9) (Fig. 3h). However, the histological scores for the TBG and TCS treatment groups were significantly lower than the preoperative scores (P < 0.001) and those for the intravenous treatment group (P = 0.01 for TBG and P = 0.02 for TCS) and the untreated group (P < 0.001). The histological score for the intravenous treatment group was lower than that for the untreated group.
The percentages of newly formed bone in rabbit tibiae treated with the TBG and TCS implants were significantly higher than those in the intravenous treatment and untreated groups (P < 0.05) (Fig. 4h). The value for the TBG treatment group was also significantly higher than that for the TCS treatment group (P = 0.005). Intravenous treatment of infection also resulted in a significantly larger amount of new bone than that for the untreated group (P = 0.046). In addition, the amount of residual implant material in the tibiae treated with the TCS implants was lower than that for the TBG group (P = 0.01) (Fig. 4h).
Micro-CT evaluation.
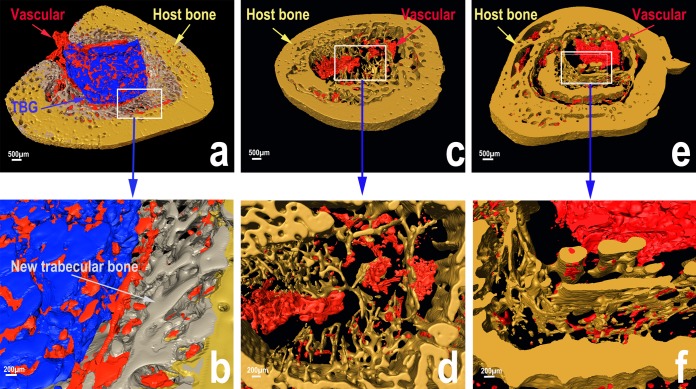
Reconstructed 3D micro-CT images showed considerable new bone regeneration in rabbit tibial defects implanted with the TBG pellets (Fig. 5a and b). A large amount of new trabecular bone was formed around the TBG implants, and it provided good integration between the implant and host bone. The TBG implant and new trabecular bone were infiltrated throughout with a large amount of vascular tissue. The defects implanted with the TCS pellets showed a smaller amount of new trabecular bone but a larger amount of cortical bone (Fig. 5c and d). Vascular tissue was found mainly in the center of the defect only, and the presence of a large amount of cavities indicated that the TCS implants had mostly degraded away. For the rabbits treated intravenously, a large cavity remained in the center of the tibiae due to debridement (Fig. 5e and f).
FIG 5.
Three-dimensional reconstructed SR micro-CT images of tibial defects showing a larger amount of vascular tissues infiltrating the TBG implants and new trabecular bone that formed in the region between the TBG implants and the host cortical bone (a and b) and a smaller amount of new bone in rabbits implanted with the TCS implants (c and d) or treated intravenously with teicoplanin (e and f) (blue areas, TBG implant; gray areas, new trabecular bone; yellow areas, host bone; red areas, vascular tissue).
Microbiological evaluation.
At debridement, all 12 rabbits in the TBG treatment group, 11 of 12 rabbits each in the TCS and intravenous treatment groups, and 9 of 10 rabbits in the untreated group were positive for MRSA-induced bone infection. There were no significant differences among the groups before treatment (χ2 = 1.66; P = 0.89). Six weeks after treatment, MRSA was recovered from 1 bone specimen in the TBG group, 2 specimens in the TCS group, 6 specimens in the intravenous group, and all 10 specimens in the untreated group (including the two dead animals in the follow-up period). Differences among the groups were significant (χ2 = 23.9; P < 0.001). The rates of positive specimen cultures for the TBG group (8.3%) and the TCS group (16.7%) were significantly lower than those preoperatively (χ2 = 16.8 [P < 0.001] and χ2 = 10.7 [P = 0.001], respectively), but there was no significant difference between the two groups (P = 0.1).
Further quantitative data for bacterial culture (Fig. 6) showed significantly fewer viable bacteria in the TBG treatment group (median of 2.0 × 103 CFU/g bone) and in the TCS treatment group (median of 2.0 × 103 CFU/g bone) than those preoperatively (P = 0.001 and P < 0.001, respectively) and than those in the intravenous treatment (P = 0.004 and P = 0.003, respectively) and untreated (P < 0.001) groups. There was no significant difference between the TBG and TCS groups (P = 0.86). Significantly low scores were also observed for the intravenous treatment group compared to the preoperative scores (P = 0.045) and those for the untreated group (P < 0.001). The bacterial strains cultured from the bone samples with positive results were identical to those used for inducing osteomyelitis in rabbit tibiae.
FIG 6.
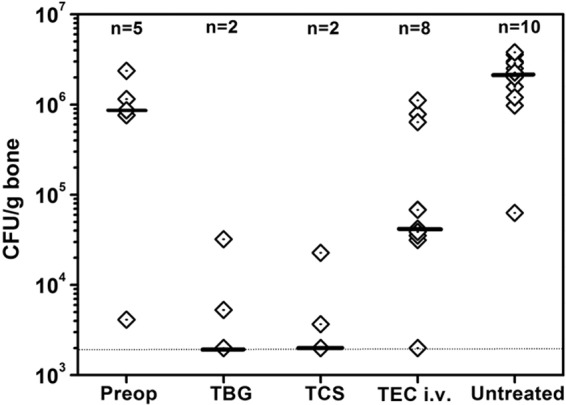
Quantitative microbiological data plotted as the number of CFU per gram of bone sample. The individual data (empty rhombi), the medians (black bars), the approximate detection limit (2.0 × 103 CFU/g) (dotted line), and the number of rabbits with detectable bacterial load are shown. The TBG and TCS groups had a significantly lower bacterial load at autopsy than that preoperatively (P = 0.001 and P < 0.001, respectively) and than those of the intravenous teicoplanin (P = 0.004 and P = 0.003, respectively) and untreated (P < 0.001) groups. Additionally, the intravenous teicoplanin group showed a significant lower CFU count than that preoperatively (P = 0.045) and than that of the untreated group (P < 0.001).
DISCUSSION
There is growing interest in new or improved treatments for osteomyelitis that can cure infection and also regenerate new bone after the infection has been cured. The use of osteoconductive biomaterials to locally deliver high doses of antibiotics is an alternative to the use of systemic antibiotics. In the present study, the capacity of an emerging biomaterial, borate bioactive glass, to deliver TEC and to cure MRSA-induced osteomyelitis in a rabbit tibial model was evaluated and compared with that of CaSO4, a biomaterial that is used clinically. The results showed that the borate bioactive glass implants (TBG) had an ability to release TEC in a sustained manner in vitro and to cure osteomyelitis at 6 weeks postimplantation which was comparable to that of CaSO4 implants (TCS). Furthermore, the TBG implants retained a higher fraction of their strength as a function of immersion time in PBS, and they showed a better capacity to stimulate new bone formation in the tibial defects. Based on these properties, the TBG implants could be considered an alternative to CaSO4 for the delivery of TEC in the treatment of osteomyelitis.
The cumulative amount of TEC released from TBG and TCS implants into PBS was 83 to 87% of the total amount incorporated into the prepared implants, and the release occurred over periods of 37 days and 29 days for the TBG and TCS implants, respectively. The mean effective concentration of TEC released from the implants over the MIC for MRSA was 76 to 81 μg/ml over periods of 31 days and 23 days for the TBG and TCS implants, respectively. While this sustained release of TEC at a therapeutically relevant level is important for curing osteomyelitis, it could also have other beneficial effects, such as inhibiting the development of antibiotic resistance (which normally results from slow antibiotic release at suboptimal concentrations) and preventing skeletal cell toxicity (26).
The sustained release of TEC from the TBG implants occurred over a longer period than those for the TCS implants (as described above) and antibiotic release from silicate bioactive glasses, which lasted from a few days to several weeks, depending on different protocols (5–7). The longer release period observed for the TBG implants could be attributed to the favorable ability of the borate glass to degrade and be converted to HA when immersed in an aqueous phosphate solution. The initial burst of release of TEC could be attributed to the rapid dissolution of TEC from the surface region of the TBG implants. Concurrently, the Ca2+ ions released from the borate glass can react with the (PO4)3− ions from the solution to form an HA layer on the surface of the TBG implant, which thickens with time (14, 27) and presumably contributes to a reduction in the rate of TEC release.
The formation of an HA product was also found on the surface of the TCS implants that were immersed in PBS. Presumably, the dissolution of the CaSO4 resulted in an ionic concentration above the solubility limit for HA, which resulted in the precipitation of HA. As the dissolution of CaSO4 is known to be rapid (28), presumably most of TEC was released in a short period. The more dynamic environment in vivo could facilitate the exchange of ions between the implants and the body fluids (29), thus further accelerating the degradation of CaSO4 and the release of TEC.
The TBG implants retained a larger fraction of their fabricated strength during the conversion process than did the TCS implants. Presumably, the amount of HA product formed in the conversion of the TCS implants was smaller, or the HA had a more porous microstructure, resulting in a more rapid degradation of strength. As fabricated, the TBG implants had a compressive strength of 23 ± 5 MPa, and at an immersion time of 7 days in PBS, the strength decreased to 10.8 ± 0.4 MPa, a value equal to the highest strength reported for human trabecular bone (2 to 12 MPa) (30). This indicates that the TBG implants could provide adequate mechanical strength for repairing bone defects even when some load-bearing ability is required (31).
The TEC dose used in the present study was comparable to the doses used in previous studies of experimental rabbit models of MRSA infection (32, 33). The TBG and TCS implants were found to be more effective in curing infection at 6 weeks postimplantation than intravenous injection of TEC. Furthermore, the rabbits treated with the TBG or TCS implants did not show any local or systemic adverse effects. This indicates that an effective bactericidal concentration of TEC was released from TBG and TCS implants into the infected site. The high local concentration of teicoplanin should eradicate the bacteria directly, in addition to providing an antimicrobial effect. The rabbit immune defense system could also contribute to the eradication of the residual of bacterial burden. The serum concentration of TEC in rabbits implanted with the TBG and TCS pellets was found to be maintained at a low level and lasted for no more than 2 weeks, which was far lower than that for the animals treated intravenously. In systemic antibiotic therapy, only a small fraction of the antibiotic dose will ultimately reach the infected site. The use of high doses may be required to reach poorly vascularized areas of bone infection, which can increase the risk of systemic toxicity (34). Although no adverse effects were observed for rabbits treated intravenously in the present study, the high cost and poor patient outcomes continue to limit the clinical use of systemic antibiotic treatment.
The creation of a bone defect is inevitable after thorough debridement, which would require subsequent reconstruction (5, 23). Although CaSO4 is the most commonly used antibiotic delivery material in experimental and clinical studies, it commonly shows variable osteogenic capacities and rapid antibiotic release in a more or less controlled manner (5). CaSO4 is known to be osteoconductive but not osteoinductive, and it supports the direct apposition of bone on its surface by mature osteoblasts (28). Borate bioactive glass has been shown to have a better capacity to stimulate bone regeneration than silicate bioactive glasses and calcium phosphate bioceramics (11–16). In vivo, bioactive glasses are degraded and converted to a carbonate-substituted HA that can remodel into bone. Calcium and other ions released during bioactive glass conversion activate osteogenic gene expression and stimulate osteogenesis (13). In comparison, synthetic HA with a nearly stoichiometric composition resorbs slowly or undergoes little remodeling into a bone-like material after in vivo implantation (35). The SR micro-CT images in the present study also showed greater infiltration of vascular tissue into the TBG implant and a larger amount of new trabecular bone surrounding the TBG implant. This indicates that borate glass might have an ability to simulate angiogenesis, a process that is required for the transport of oxygen and nutrients, and, thus, the growth and maintenance of new bone (36, 37).
In comparison, rapid degradation of CaSO4 can lead to the release of a high local concentration of calcium ions, which, in turn, can lead to changes in the local pH, the activation of a mild inflammatory response, and the inhibition of new bone formation (38–40). In the present study, it was observed that large amounts of inflammatory cells and fibrosis surrounded the newly formed bone and residual CaSO4 material in the defects implanted with the TCS pellets, which presumably contributed to inhibiting bone regeneration.
While the present study showed promising results for the use of TBG implants in curing osteomyelitis and regenerating bone, further studies are needed to evaluate the potential of the TBG implants for clinical applications. The local concentration of TEC in the bone in vivo should be studied because the in vitro medium (PBS) used to measure TEC release in the present study has little relation to the in vivo situation (bone defect). The number of pellets used to study TEC release in vitro was also different from the number implanted into infected bone in vivo. Furthermore, although the local delivery of TEC by the TBG and TCS implants showed positive results, the treatment did not cure bone infection in all of the animals. It is worth investigating whether the local delivery of TEC using TBG or TCS implants in conjunction with systemic antibiotic therapy could provide a more effective cure for MRSA-induced osteomyelitis.
Conclusions.
Implants composed of borate bioactive glass (TBG) or calcium sulfate (TCS) loaded with 10 wt% TEC were prepared and evaluated for their ability to cure osteomyelitis in a rabbit tibial model. When immersed in PBS, both groups of implants provided sustained TEC release for up to 3 to 4 weeks, and they were degraded and converted to hydroxyapatite within 5 weeks. The TBG implants showed a slower decrease in strength as a function of immersion time in PBS than did the TCS implants. When used to treat MRSA-induced osteomyelitis in a rabbit tibial model, the TBG and TCS implants showed a significantly better capacity to cure infection than intravenous treatment with TEC at 6 weeks postimplantation. While there was no significant difference in the capacities of the two implant groups to cure infection, the TBG implants showed a better capacity to regenerate bone in tibial defects. Together, the results indicate that these borate bioactive glass implants could be considered an alternative to calcium sulfate for the treatment of osteomyelitis.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant no. 81572105, 51072133, and 51372170) and the Science and Technology Commission of Shanghai Municipality (grant no. 12JC1408500).
REFERENCES
- 1.Lew DP, Waldvogel FA. 2004. Osteomyelitis. Lancet 364:369–379. doi: 10.1016/S0140-6736(04)16727-5. [DOI] [PubMed] [Google Scholar]
- 2.Sakoulas G, Moellering RC Jr. 2008. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis 46:S360–S367. doi: 10.1086/533592. [DOI] [PubMed] [Google Scholar]
- 3.Brogden RN, Peters DH. 1994. Teicoplanin. A reappraisal of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 47:823–854. [DOI] [PubMed] [Google Scholar]
- 4.Svetitsky S, Leibovici L, Paul M. 2009. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother 53:4069–4079. doi: 10.1128/AAC.00341-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaren AC. 2004. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin Orthop Relat Res 427:101–106. doi: 10.1097/01.blo.0000143554.56897.26. [DOI] [PubMed] [Google Scholar]
- 6.Hanssen AD. 2005. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop Relat Res 437:91–96. [DOI] [PubMed] [Google Scholar]
- 7.Paul W, Sharma CP. 2003. Ceramic drug delivery: a perspective. J Biomater Appl 17:253–264. doi: 10.1177/0885328203017004001. [DOI] [PubMed] [Google Scholar]
- 8.Chang W, Colangeli M, Colangeli S, Di Bella C, Gozzi E, Donati D. 2007. Adult osteomyelitis: debridement versus debridement plus Osteoset T pellets. Acta Orthop Belg 73:238–243. [PubMed] [Google Scholar]
- 9.Nelson CL, McLaren SG, Skinner RA, Smeltzer MS, Thomas JR, Olsen KM. 2002. The treatment of experimental osteomyelitis by surgical debridement and the implantation of calcium sulfate tobramycin pellets. J Orthop Res 20:643–647. doi: 10.1016/S0736-0266(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 10.McKee MD, Wild LM, Schemitsch EH, Waddell JP. 2002. The use of an antibiotic-impregnated, osteoconductive, bioabsorbable bone substitute in the treatment of infected long bone defects: early results of a prospective trial. J Orthop Trauma 16:622–627. doi: 10.1097/00005131-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Rahaman MN, Bal BS, Huang W. 2014. Review: emerging developments in the use of bioactive glasses for treating infected prosthetic joints. Mater Sci Eng C Mater Biol Appl 41:224–231. doi: 10.1016/j.msec.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 12.Hench LL, Wilson J. 1984. Surface active biomaterials. Science 226:630–636. doi: 10.1126/science.6093253. [DOI] [PubMed] [Google Scholar]
- 13.Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, Bonewald LF, Tomsia AP. 2011. Bioactive glass in tissue engineering. Acta Biomater 7:2355–2373. doi: 10.1016/j.actbio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang W, Rahaman MN, Day DE, Li Y. 2006. Mechanisms for converting bioactive silicate, borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solution. Phys Chem Glasses B 17:583–596. [DOI] [PubMed] [Google Scholar]
- 15.Fu Q, Rahaman MN, Fu H, Liu X. 2010. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. J Biomed Mater Res A 95:164–171. doi: 10.1002/jbm.a.32824. [DOI] [PubMed] [Google Scholar]
- 16.Jia WT, Zhang X, Luo SH, Liu X, Huang WH, Rahaman MN, Day DE, Zhang CQ, Xie ZP, Wang JQ. 2010. Novel borate glass/chitosan composite as a delivery vehicle for teicoplanin in the treatment of chronic osteomyelitis. Acta Biomater 6:812–819. doi: 10.1016/j.actbio.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Rauschmann MA, Wichelhaus TA, Stirnal V, Dingeldein E, Zichner L, Schnettler R, Alt V. 2005. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials 26:2677–2684. doi: 10.1016/j.biomaterials.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 18.Webb ND, McCanless JD, Courtney HS, Bumgardner JD, Haggard WO. 2008. Daptomycin eluted from calcium sulfate appears effective against Staphylococcus. Clin Orthop Relat Res 466:1383–1387. doi: 10.1007/s11999-008-0245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCann SJ, White LO, Keevil B. 2002. Assay of teicoplanin in serum: comparison of high-performance liquid chromatography and fluorescence polarization immunoassay. J Antimicrob Chemother 50:107–110. doi: 10.1093/jac/dkf067. [DOI] [PubMed] [Google Scholar]
- 20.Norden CW, Myerowitz RL, Keleti E. 1980. Experimental osteomyelitis due to Staphylococcus aureus or Pseudomonas aeruginosa: a radiographic-pathological correlative analysis. Br J Exp Pathol 61:451–460. [PMC free article] [PubMed] [Google Scholar]
- 21.Yin LY, Calhoun JH, Thomas JK, Shapiro S, Schmitt-Hoffmann A. 2008. Efficacies of ceftobiprole medocaril and comparators in a rabbit model of osteomyelitis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 52:1618–1622. doi: 10.1128/AAC.00638-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faber C, Stallmann HP, Lyaruu DM, Joosten U, von Eiff C, van Nieuw Amerongen A, Wuisman PI. 2005. Comparable efficacies of the antimicrobial peptide human lactoferrin 1-11 and gentamicin in a chronic methicillin-resistant Staphylococcus aureus osteomyelitis model. Antimicrob Agents Chemother 49:2438–2444. doi: 10.1128/AAC.49.6.2438-2444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazzarini L, Mader JT, Calhoun JH. 2004. Osteomyelitis in long bones. J Bone Joint Surg Am 86:2305–2318. [DOI] [PubMed] [Google Scholar]
- 24.Smeltzer MS, Thomas JR, Hickmon SG, Skinner RA, Nelson CL, Griffith D, Parr TR Jr, Evans RP. 1997. Characterization of a rabbit model of staphylococcal osteomyelitis. J Orthop Res 15:414–421. doi: 10.1002/jor.1100150314. [DOI] [PubMed] [Google Scholar]
- 25.Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, Watanabe S. 1991. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol 29:2240–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antoci V Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. 2007. Antibiotics for local delivery systems cause skeletal cell toxicity in vitro. Clin Orthop Relat Res 462:200–206. doi: 10.1097/BLO.0b013e31811ff866. [DOI] [PubMed] [Google Scholar]
- 27.Yao A, Wang D, Huang W, Fu Q, Rahaman MN, Day DE. 2007. In vitro bioactive characteristics of borate-based glasses with controllable degradation behavior. J Am Ceram Soc 90:303–306. doi: 10.1111/j.1551-2916.2006.01358.x. [DOI] [Google Scholar]
- 28.Hing KA, Wilson LF, Buckland T. 2007. Comparative performance of three ceramic bone graft substitutes. Spine J 7:475–490. doi: 10.1016/j.spinee.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson M, Wang JS, Wielanek L, Tanner KE, Lidgren L. 2004. Biodegradation and biocompatability of a calcium sulphate-hydroxyapatite bone substitute. J Bone Joint Surg Br 86:120–125. [PubMed] [Google Scholar]
- 30.Rho JY, Hobatho MC, Ashman RB. 1995. Relations of density and CT numbers to mechanical properties for human cortical and cancellous bone. Med Eng Phys 17:347–355. doi: 10.1016/1350-4533(95)97314-F. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein SA. 1987. The mechanical properties of trabecular bone: dependence on anatomic location and function. J Biomech 20:1055–1061. doi: 10.1016/0021-9290(87)90023-6. [DOI] [PubMed] [Google Scholar]
- 32.Cepeda JA, Whitehouse T, Cooper B, Hails J, Jones K, Kwaku F, Taylor L, Hayman S, Shaw S, Kibbler C, Shulman R, Singer M, Wilson AP. 2004. Linezolid versus teicoplanin in the treatment of Gram-positive infections in the critically ill: a randomized, double-blind, multicentre study. J Antimicrob Chemother 53:345–355. doi: 10.1093/jac/dkh048. [DOI] [PubMed] [Google Scholar]
- 33.Yenice I, Calis S, Kas H, Ozalp M, Ekizoglu M, Hincal A. 2002. Biodegradable implantable teicoplanin beads for the treatment of bone infections. Int J Pharm 242:271–275. doi: 10.1016/S0378-5173(02)00186-2. [DOI] [PubMed] [Google Scholar]
- 34.Mader JT, Landon GC, Calhoun J. 1993. Antimicrobial treatment of osteomyelitis. Clin Orthop Relat Res 295:87–95. [PubMed] [Google Scholar]
- 35.Klein C, Patka P, den Hollander W. 1989. Macroporous calcium phosphate bioceramics in dog femora: a histological study of interface and biodegradation. Biomaterials 10:59–62. doi: 10.1016/0142-9612(89)90011-2. [DOI] [PubMed] [Google Scholar]
- 36.Santos MI, Reis RL. 2010. Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci 10:12–27. doi: 10.1002/mabi.200900107. [DOI] [PubMed] [Google Scholar]
- 37.Kanczler JM, Oreffo RO. 2008. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater 15:100–114. [DOI] [PubMed] [Google Scholar]
- 38.Borrelli J Jr, Prickett WD, Ricci WM. 2003. Treatment of nonunions and osseous defects with bone graft and calcium sulfate. Clin Orthop 411:245–254. doi: 10.1097/01.blo.0000069893.31220.6f. [DOI] [PubMed] [Google Scholar]
- 39.Robinson D, Alk D, Sandbank J, Farber R, Halperin N. 1999. Inflammatory reactions associated with a calcium sulfate bone substitute. Ann Transplant 4:91–97. [PubMed] [Google Scholar]
- 40.Glazer PA, Spencer UM, Alkalay RN, Schwardt J. 2001. In vivo evaluation of calcium sulfate as a bone graft substitute for lumbar spinal fusion. Spine J 1:395–401. doi: 10.1016/S1529-9430(01)00108-5. [DOI] [PubMed] [Google Scholar]