Abstract
Coadministration of nevirapine-based antiretroviral therapy (ART) and artemether-lumefantrine is reported to result in variable changes in lumefantrine exposure. We conducted an intensive pharmacokinetic study with 11 HIV-infected adults who were receiving artemether-lumefantrine plus nevirapine-based ART, and we compared the results with those for 16 HIV-negative adult historical controls. Exposure to artemether and lumefantrine was significantly lower and dihydroartemisinin exposure was unchanged in subjects receiving nevirapine-based ART, compared with controls. Nevirapine exposure was unchanged before and after artemether-lumefantrine administration.
TEXT
Malaria and HIV affect millions of children and adults in sub-Saharan Africa, and drug-drug interactions between antiretroviral therapy (ART) and antimalarial agents are clinically important to characterize. Nevirapine-based ART represents 50% of first-line ART in regions where malaria coinfection occurs (1). Artemether-lumefantrine, an artemisinin derivative combined with a longer-acting partner drug, represents the most common antimalarial therapy (2, 3). Metabolism of these HIV and antimalarial agents occurs primarily via cytochrome P450 (CYP) enzymes. Artemether is metabolized to an active metabolite, dihydroartemisinin (DHA), predominately by CYP3A4/5 and to a lesser extent by CYP2B6, CYP2C9, and CYP2C19 (4). DHA undergoes glucuronidation by uridine diphosphoglucuronosyltransferases. Lumefantrine is metabolized by CYP3A4 into active desbutyl-lumefantrine (2, 4, 5). Nevirapine is metabolized by CYP3A4 and CYP2B6 while inducing CYP3A4 (6, 7). Studies report reduced artemisinin exposure in the presence of nevirapine-based ART, but the effects on lumefantrine concentrations are variable, with studies showing increased, decreased, or unchanged concentrations (8–14). Importantly, reduced antimalarial levels have been associated with therapeutic failure (12, 13, 15). The primary objective of this study was to evaluate the pharmacokinetics of artemether, DHA, and lumefantrine in HIV-infected Nigerian adults without clinical malaria who were receiving nevirapine-based ART.
(Data were presented at the 15th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy, Washington, DC, 19 May 2014.)
HIV-infected subjects (≥18 years of age) who had been receiving nevirapine-based ART for ≥4 weeks (nevirapine-based ART group) were eligible after informed consent was obtained. Exclusion criteria were current pregnancy; intolerance to study drugs; use of antimalarials or CYP substrates, inducers, or inhibitors within 4 weeks; and clinical symptoms of malaria. The historical control group included healthy adults (n = 16). The same laboratory analyzed all plasma drug concentrations (16). The University College Hospital Ethics Committee approved this study (protocol NHREC/05/01/2008a).
Participants received coformulated nevirapine-zidovudine-lamivudine (200/300/150 mg; Aurobindo Pharma, India) twice daily. On day 0, participants underwent venous sampling for nevirapine predose and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 h postdose. Following pharmacokinetic sampling, participants began to receive coformulated artemether-lumefantrine (80/480 mg, Coartem; Novartis Pharmaceuticals) twice daily for 3 days, along with nevirapine. Participants received a standard Nigerian meal 30 to 60 min postdose. The control group received artemether-lumefantrine alone, following the same schedule but with food provided immediately postdose (16). Blood samples for quantification of artemether, DHA, lumefantrine, and nevirapine were collected around the sixth (last) dose on day 3, predose and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 48, 72, and 96 h postdose. Pharmacokinetic sampling was identical for the control group, although samples were collected through 296 h. Plasma was stored at −80°C within 30 min after collection.
Artemether and DHA concentrations were analyzed using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method (17). The lower limit of quantification (LLOQ) for artemether and DHA was 2 ng/ml, and the calibration range was 2 to 200 ng/ml. The coefficients of variation (CVs) ranged from 2 to 12% for artemether and from 1.8 to 8.2% for DHA. The accuracy of quality controls (QCs), expressed as percent deviation from nominal values, ranged from −13% to 9.1% for artemether and from −13% to 5.6% for DHA at low (6 ng/ml), medium (80 ng/ml), and high (170 ng/ml) concentrations. Lumefantrine concentrations were determined using high-performance liquid chromatography (HPLC)-UV analysis, with an LLOQ of 50 ng/ml, a calibration range of 50 to 10,000 ng/ml, and a CV range of 1.1 to 6.7% (18). The accuracy of QCs for lumefantrine ranged from −1.1% to 9.3% at low (120 ng/ml), medium (900 ng/ml), and high (9,000 ng/ml) concentrations. Nevirapine concentrations were determined using HPLC-UV analysis, with an LLOQ of 200 ng/ml, a calibration range of 200 to 10,000 ng/ml, and a CV range of 5 to 13% (19).
Pharmacokinetic parameters were estimated using noncompartmental analysis via the linear up-log down trapezoidal rule in conjunction with first-order input, using WinNonlin (Pharsight Corp., Mountain View, CA). All data below the LLOQ, except at 0 h, were treated as missing data. Data from subjects with at least three samples in the elimination phase were used to calculate the half-life (t1/2). The DHA/artemether ratio of values of the area under the concentration-time curve from 0 to 6 h (AUC0–6) was calculated for each group, to explore potential CYP3A4 induction. Univariate analyses of demographic features were performed with Student's t test, the chi-square test, or Fisher's exact test, as appropriate.
The nevirapine-based ART group included 11 subjects, compared to 16 control subjects. There were more female subjects in the nevirapine-based ART group than in the control group (82% versus 25%; P < 0.01), although ages (median, 37 versus 33 years; P = 0.13) and weights (median, 66 versus 77 kg; P = 0.5) were similar for the groups. Subjects had been receiving nevirapine-based ART for a median of 3.5 years (range, 2 to 5.6 years), with a median CD4+ cell count of 388 cells/mm3 (range, 218 to 549 cells/mm3); five subjects (45.5%) received co-trimoxazole and one received dapsone prophylaxis.
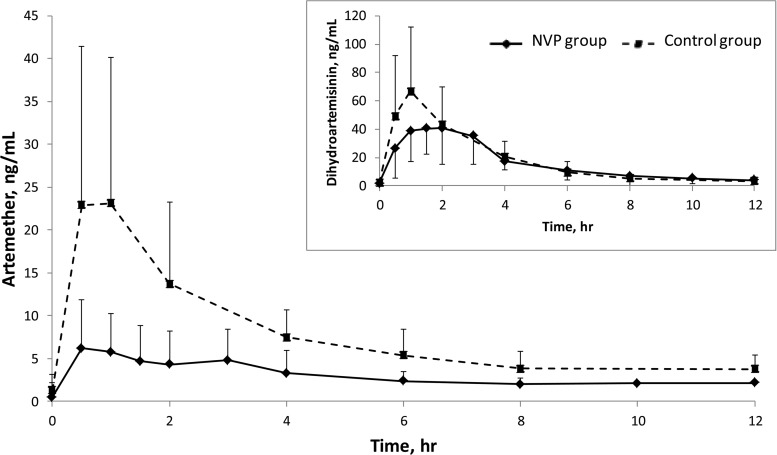
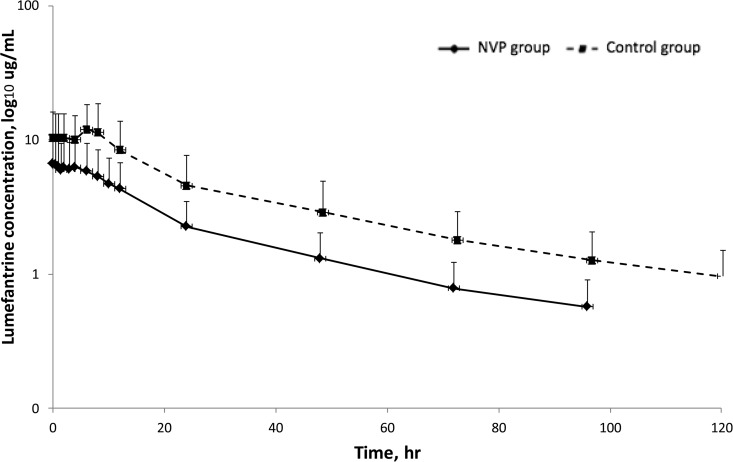
The artemether and DHA pharmacokinetics are described in Table 1 and Fig. 1. Artemether exposure (AUC0–6) was 68% lower in the nevirapine-based ART group than in the control group (19.9 versus 63.0 h · ng/ml; P < 0.01), while DHA parameters did not differ between the groups. The DHA/artemether AUC0–6 ratios were 2.5 in the control group and 7.2 in the nevirapine-based ART group, indicating that the proportion of DHA versus artemether was greater in the nevirapine-based ART group than in the control group. The pharmacokinetics of lumefantrine are described in Table 1 and Fig. 2. Lumefantrine exposure (AUC0–96) was 49% lower in the nevirapine-based ART group than in the control group (151 versus 295 h · μg/ml; P = 0.048). Extrapolation of lumefantrine concentrations from after the last dose to 120 h (day 7) suggests that 25% of participants who received nevirapine-based ART had day 7 concentrations below thresholds that correlate with the risk of recrudescent malaria (175 or 280 ng/ml) (20, 21). No differences in nevirapine pharmacokinetics with respect to treatment with artemether-lumefantrine were observed (AUC0–12 of 52 versus 54 h · μg/ml; P = 0.3). Other nevirapine pharmacokinetic parameters were not significantly different (geometric mean ratio [GMR] values of 0.98, 1.33, and 1.04 for the maximal concentration [Cmax], the time to Cmax [Tmax], and the concentration at 12 h [C12], respectively; data not shown).
TABLE 1.
Pharmacokinetic parameter estimates for artemether, dihydroartemisinin, and lumefantrine, with or without nevirapine-based antiretroviral therapy
| Pharmacokinetic parametera | Control group (n = 16) | Nevirapine-based ART group (n = 11) | Nevirapine-based ART control group | Pb |
|---|---|---|---|---|
| Artemether | ||||
| Cmax (GM [95% CI]) (ng/ml) | 19.1 (13.2–27.5) | 6.04 (4.07–8.97) | 0.32 | <0.01 |
| Tmax (median [IQR]) (h) | 1.0 (0.50–1.0) | 1.5 (0.50–3.0) | 1.5 | 0.12 |
| AUC0–6 (GM [95% CI]) (h · ng/ml) | 63.0 (47.4–83.6) (n = 13) | 19.9 (13.8–28.7) (n = 10) | 0.32 | <0.01 |
| AUC0–∞ (GM [95% CI]) (h · ng/ml) | 93.2 (66.3–131) (n = 12) | 30.8 (14.7–64.5) (n = 6) | 0.33 | <0.01 |
| t1/2 (median [IQR]) (h) | 3.9 (2.1–5.9) (n = 12) | 2.2 (1.2–4.5) (n = 6) | 0.56 | 0.16 |
| Dihydroartemesinin | ||||
| Cmax (GM [95% CI]) (ng/ml) | 61.4 (47.7–78.9) | 47.3 (35.5–63.1) | 0.77 | 0.37 |
| Tmax (median [IQR]) (h) | 1.0 (1.0–2.0) | 1.5 (1.0–3.0) | 1.5 | 0.54 |
| AUC0–6 (GM [95% CI]) (h · ng/ml) | 160 (129–198) | 143 (115–179) | 0.89 | 0.69 |
| AUC0–∞ (GM [95% CI]) (h · ng/ml) | 189 (151–237) | 193 (152–244) | 1.02 | 0.84 |
| t1/2 (median [IQR]) (h) | 1.9 (1.5–3.7) | 2.6 (2.0–3.8) | 1.37 | 0.37 |
| Lumefantrine | ||||
| Cmax (GM [95% CI]) (μg/ml) | 11.0 (8.0–15.0) | 5.81 (3.50–9.65) | 0.53 | 0.07 |
| Tmax (median [IQR]) (h) | 2.0 (2.0–6.0) | 2.0 (0.0–6.0) | 1.0 | |
| AUC0–96 (GM [95% CI]) (h · μg/ml) | 295 (208–419) | 151 (98–232) | 0.51 | 0.048 |
| AUC0–∞ (GM [95% CI]) (h · μg/ml)c | 426 (298–609) | 180 (117–278) | 0.42 | 0.02 |
| t1/2 (median [IQR]) (h) | 116 (80.4–153) | 39.2 (35.9–50.2) | 0.34 | <0.01 |
The maximal plasma concentration (Cmax) and the time to Cmax (Tmax) were estimated by visual inspection, whereas the area under the concentration-time curve (AUC) and the elimination half-life (t1/2) were determined by noncompartmental analysis. Unless noted otherwise, the n value used to calculate each pharmacokinetic measure was that for the full study group, as indicated in the column heading. GM, geometric mean; CI, confidence interval; IQR, interquartile range.
The Mann-Whitney U test was used to evaluate differences in pharmacokinetic parameters between groups. A P value of <0.05 was considered significant.
The AUC0–∞ values include residual area from the previous dose, due to the long elimination half-life of lumefantrine.
FIG 1.
Artemether and dihydroartemesinin (inset) plasma concentration-time curves surrounding the last dose of artemether-lumefantrine in HIV-infected patients receiving nevirapine-based antiretroviral therapy, compared to healthy volunteers. Data are presented as geometric means. Error bars, standard deviations. NVP, nevirapine.
FIG 2.
Lumefantrine plasma concentration-time curve surrounding the last dose of artemether-lumefantrine in HIV-infected patients receiving nevirapine-based antiretroviral therapy, compared to healthy volunteers. Data are presented as geometric means. Error bars, standard deviations. NVP, nevirapine.
Our results describe lower artemether and lumefantrine exposure and no change in DHA exposure in Nigerian adults receiving nevirapine-based ART, compared to historical control subjects not receiving ART. The results are consistent with findings for artemether in HIV-infected Ugandan and South African adults, although different from findings for DHA, as decreases of 25 to 37% were reported (2, 15, 22). One explanation for the lack of change in DHA exposure may involve combined effects of reduced artemether bioavailability (as evidenced by the Cmax) and induction of CYP3A metabolism of artemether to DHA (as evidenced by the higher DHA/artemether ratio in the nevirapine-based ART group than in the control group, i.e., ratios of 7.2 and 2.5, respectively).
The reported effects of nevirapine-based ART on lumefantrine exposure are variable (Table 2) (8, 10, 11, 13, 14, 22). Our study reports ∼50% lower lumefantrine exposure, consistent with findings reported from a Ugandan nonlinear mixed-effects modeling study (8, 22). The overall lower lumefantrine exposure may be due in part to a decrease in oral bioavailability resulting from increased intestinal P-glycoprotein or CYP3A4 expression or to the 30- to 60-min delay in food intake in the nevirapine-based ART group (23). While reported studies were all conducted with adults, there were differences in study design (parallel groups, crossover, or historical controls), pharmacokinetic analysis (intensive sampling, population modeling, or day 7 sampling only), sample size, patient characteristics (malaria infection status, race, ethnicity, and gender), and food intake that may underlie these differences (8, 10, 11, 13, 14, 22, 24).
TABLE 2.
Summary of findings from studies evaluating changes in artemether-lumefantrine exposure in the setting of nevirapine-based ART
| Study location and reference(s) | Study design | Effectsa |
|||
|---|---|---|---|---|---|
| Artemether | DHA | Lumefantrine | Nevirapine | ||
| Nigeria (present study) | Two groups, rich artemether-lumefantrine PK sampling (0–96 h after last dose); non-HIV-infected, non-malaria-infected patients (n = 16) vs HIV-infected, non-malaria-infected patients on nevirapine-based ART for ≥2 yr | Decreased 67% (P < 0.01) | Unchanged | Decreased 49% (P = 0.048) | Unchanged |
| Uganda (8, 22)b | Crossover, rich artemether-lumefantrine PK sampling (0–120 h after last dose); HIV-infected, non-malaria-infected patients (n = 21) before ART and 4 wk after initiation of nevirapine-based ART | Decreased 70% (P < 0.01) | Decreased 37% (P < 0.01) | Decreased 21% (P = 0.4) | Decreased 46% (P < 0.01) |
| South Africa (10) | Parallel groups, rich artemether-lumefantrine PK sampling (0–504 h after last dose); HIV-infected, non-malaria-infected, ART-naive patients (n = 18) vs HIV-infected patients (n = 18) on nevirapine-based ART for ≥6 wk | Decreased 55% (P = 0.12) | Decreased 25% (P = 0.01) | Increased 56% (P < 0.01) | NA |
| Nigeria (11) | Parallel groups, single lumefantrine PK sampling on day 7 (120 h after last dose); malaria-infected, non-HIV-infected patients (n = 99) vs malaria/HIV-coinfected patients on NVP-based ART (n = 68) (duration of NVP treatment not reported) | NA | NA | Increased 29%c (P < 0.01) | NA |
| Tanzania (13, 14) | Parallel groups, lumefantrine PK sampling on day 7 (120 h after last dose); malaria-infected, non-HIV-infected patients (n = 60) vs malaria/HIV-coinfected patients on NVP-based ART for ≥8 wk (n = 121) | NA | NA | Increased 16%c,d (P = 0.06) | NA |
Results represent noncompartmental AUC comparisons after the last dose of artemether-lumefantrine unless otherwise noted. The reported changes in drug exposure were observed for patients receiving nevirapine-containing ART, compared to the control group. NVP, nevirapine; PK, pharmacokinetic; NA, not available.
Noncompartmental results are presented; however, nonlinear mixed-effects modeling of the same data found a statistically significant decrease in exposure and an increase in the clearance of lumefantrine.
Day 7 concentration.
Noncompartmental results are presented. Nonlinear mixed-effects modeling of sparse sampling from the same study estimated a 24.6% increase in lumefantrine AUC0–∞; no P value was reported (14).
A significant limitation of our study is the use of healthy historical controls as the comparator group. While the sampling protocols were nearly identical and assays were conducted in the same laboratory, differences in demographic features, ethnicity, and HIV status between subjects enrolled in the control and nevirapine-based ART groups are important (16, 18, 25). In addition, the shorter follow-up time for the nevirapine-based ART group versus the control group (96 h versus 296 h) may underestimate the t1/2 and AUC0–∞. Therefore, AUC0–96 values were used for the primary comparison between groups, to minimize the impact of the length of the follow-up period. Subjects self-administered antimalarial doses 2 through 5; therefore, although we provided instructions regarding the correct time to administer all doses, we did not observe the administration of all of the artemether-lumefantrine doses. However, administration of the final dose prior to pharmacokinetic sampling was observed by study staff members. Finally, we did not measure the metabolite desbutyl-lumefantrine. Some studies have shown that the metabolite has greater in vitro potency against Plasmodium falciparum and may have a role in antimalarial efficacy in humans, despite exposure levels that are substantially lower than those of lumefantrine itself (5, 22, 26). How coadministered nevirapine influences the balance between lumefantrine and desbutyl-lumefantrine concentrations and thus the overall antimalarial effects of the drug and the metabolite is not known.
In summary, these results support prior studies that demonstrated important drug-drug interactions between nevirapine-based ART and artemether-lumefantrine. Notably, published studies have been conducted exclusively in adults and are unable to assess developmental changes that may affect the metabolism of these drugs. Future studies should include HIV- and malaria-coinfected children, should consider quantification of metabolite levels, and should use population pharmacokinetic modeling to assess the effects of critical covariates on pharmacokinetic parameters.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health through the John E. Fogarty International Center (awards 1D43TW007995 and 1D43TW007991) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (award R01HD068174). Additional support was provided by the Centre for Population and Reproductive Health, College of Medicine, University of Ibadan, with funds from the Gates Institute, Johns Hopkins University School of Public Health.
We do not have any conflicts of interest related to this work to report.
Mathew Olatunde, N. K. Afolabi, and the entire laboratory and nursing staff of the Department of Clinical Pharmacology, University College Hospital (Ibadan, Nigeria), are appreciated for their dedication.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.World Health Organization. 2014. Meeting report of the joint WHO/UNAIDS annual consultation with pharmaceutical companies and stakeholders on forecasting global demand of antiretroviral drugs for 2013–2016: 25–26 November 2013, Geneva, Switzerland. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/111625/1/9789241506984_eng.pdf?ua=1. [Google Scholar]
- 2.German PI, Aweeka FT. 2008. Clinical pharmacology of artemisinin-based combination therapies. Clin Pharmacokinet 47:91–102. doi: 10.2165/00003088-200847020-00002. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2014. World malaria report 2014. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2014/en. [Google Scholar]
- 4.Coulibaly FH, Koffi G, Toure HA, Bouanga JC, Allangba O, Tolo A, Swandogo D, Sanogo I, Konate S, Prehu C, Sangare A, Galacteros F. 2000. Molecular genetics of glucose-6-phosphate dehydrogenase deficiency in a population of newborns from Ivory Coast. Clin Biochem 33:411–413. doi: 10.1016/S0009-9120(00)00078-3. [DOI] [PubMed] [Google Scholar]
- 5.Wong RP, Salman S, Ilett KF, Siba PM, Mueller I, Davis TM. 2011. Desbutyl-lumefantrine is a metabolite of lumefantrine with potent in vitro antimalarial activity that may influence artemether-lumefantrine treatment outcome. Antimicrob Agents Chemother 55:1194–1198. doi: 10.1128/AAC.01312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Moltke LL, Greenblatt DJ, Granda BW, Giancarlo GM, Duan SX, Daily JP, Harmatz JS, Shader RI. 2001. Inhibition of human cytochrome P450 isoforms by nonnucleoside reverse transcriptase inhibitors. J Clin Pharmacol 41:85–91. doi: 10.1177/00912700122009728. [DOI] [PubMed] [Google Scholar]
- 7.Erickson DA, Mather G, Trager WF, Levy RH, Keirns JJ. 1999. Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos 27:1488–1495. [PubMed] [Google Scholar]
- 8.Byakika-Kibwika P, Lamorde M, Mayito J, Nabukeera L, Namakula R, Mayanja-Kizza H, Katabira E, Ntale M, Pakker N, Ryan M, Hanpithakpong W, Tarning J, Lindegardh N, de Vries PJ, Khoo S, Back D, Merry C. 2012. Significant pharmacokinetic interactions between artemether/lumefantrine and efavirenz or nevirapine in HIV-infected Ugandan adults. J Antimicrob Chemother 67:2213–2221. doi: 10.1093/jac/dks207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehintola FA, Scarsi KK, Ma Q, Parikh S, Morse GD, Taiwo B, Akinola IT, Adewole IF, Lindegardh N, Phakderaj A, Ojengbede O, Murphy RL, Akinyinka OO, Aweeka FT. 2012. Nevirapine-based antiretroviral therapy impacts artesunate and dihydroartemisinin disposition in HIV-infected Nigerian adults. AIDS Res Treat 2012:703604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kredo T, Mauff K, Van der Walt JS, Wiesner L, Maartens G, Cohen K, Smith P, Barnes KI. 2011. Interaction between artemether-lumefantrine and nevirapine-based antiretroviral therapy in HIV-1-infected patients. Antimicrob Agents Chemother 55:5616–5623. doi: 10.1128/AAC.05265-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chijioke-Nwauche I, van Wyk A, Nwauche C, Beshir KB, Kaur H, Sutherland CJ. 2013. HIV-positive Nigerian adults harbor significantly higher serum lumefantrine levels than HIV-negative individuals seven days after treatment for Plasmodium falciparum infection. Antimicrob Agents Chemother 57:4146–4150. doi: 10.1128/AAC.02508-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh S, Mwebaza N, Kajubi R, Ssebuliba J, Kiconco S, Huang L, Gao Q, Kakuru A, Achan J, Aweeka F. 2014. Selection of antiretroviral treatment (ART) impacts antimalarial pharmacokinetics and treatment outcomes in HIV-malaria co-infected children in Uganda. Am J Trop Med Hyg 91(Suppl 1):272. [Google Scholar]
- 13.Maganda BA, Minzi OM, Kamuhabwa AA, Ngasala B, Sasi PG. 2014. Outcome of artemether-lumefantrine treatment for uncomplicated malaria in HIV-infected adult patients on anti-retroviral therapy. Malar J 13:205. doi: 10.1186/1475-2875-13-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maganda BA, Ngaimisi E, Kamuhabwa AA, Aklillu E, Minzi OM. 2015. The influence of nevirapine and efavirenz-based anti-retroviral therapy on the pharmacokinetics of lumefantrine and anti-malarial dose recommendation in HIV-malaria co-treatment. Malar J 14:179. doi: 10.1186/s12936-015-0695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achan J, Kakuru A, Ikilezi G, Ruel T, Clark TD, Nsanzabana C, Charlebois E, Aweeka F, Dorsey G, Rosenthal PJ, Havlir D, Kamya MR. 2012. Antiretroviral agents and prevention of malaria in HIV-infected Ugandan children. N Engl J Med 367:2110–2118. doi: 10.1056/NEJMoa1200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang L, Parikh S, Rosenthal PJ, Lizak P, Marzan F, Dorsey G, Havlir D, Aweeka FT. 2012. Concomitant efavirenz reduces pharmacokinetic exposure to the antimalarial drug artemether-lumefantrine in healthy volunteers. J Acquir Immune Defic Syndr 61:310–316. doi: 10.1097/QAI.0b013e31826ebb5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang L, Jayewardene AL, Li X, Marzan F, Lizak PS, Aweeka FT. 2009. Development and validation of a high-performance liquid chromatography/tandem mass spectrometry method for the determination of artemether and its active metabolite dihydroartemisinin in human plasma. J Pharm Biomed Anal 50:959–965. doi: 10.1016/j.jpba.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, Lizak PS, Jayewardene AL, Marzan F, Lee MNT, Aweeka FT. 2010. A modified method for determination of lumefantrine in human plasma by HPLC-UV and combination of protein precipitation and solid-phase extraction: application to a pharmacokinetic study. Anal Chem Insights 5:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rezk NL, Tidwell RR, Kashuba AD. 2003. Simultaneous determination of six HIV nucleoside analogue reverse transcriptase inhibitors and nevirapine by liquid chromatography with ultraviolet absorbance detection. J Chromatogr B Anal Technol Biomed Life Sci 791:137–147. doi: 10.1016/S1570-0232(03)00224-1. [DOI] [PubMed] [Google Scholar]
- 20.Ezzet F, van Vugt M, Nosten F, Looareesuwan S, White NJ. 2000. Pharmacokinetics and pharmacodynamics of lumefantrine (benflumetol) in acute falciparum malaria. Antimicrob Agents Chemother 44:697–704. doi: 10.1128/AAC.44.3.697-704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price RN, Uhlemann AC, van Vugt M, Brockman A, Hutagalung R, Nair S, Nash D, Singhasivanon P, Anderson TJ, Krishna S, White NJ, Nosten F. 2006. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis 42:1570–1577. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoglund RM, Byakika-Kibwika P, Lamorde M, Merry C, Ashton M, Hanpithakpong W, Day NP, White NJ, Abelo A, Tarning J. 2015. Artemether-lumefantrine co-administration with antiretrovirals: population pharmacokinetics and dosing implications. Br J Clin Pharmacol 79:636–649. doi: 10.1111/bcp.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashley EA, Stepniewska K, Lindegardh N, Annerberg A, Kham A, Brockman A, Singhasivanon P, White NJ, Nosten F. 2007. How much fat is necessary to optimize lumefantrine oral bioavailability? Trop Med Int Health 12:195–200. doi: 10.1111/j.1365-3156.2006.01784.x. [DOI] [PubMed] [Google Scholar]
- 24.Maganda BA, Minzi OM, Ngaimisi E, Kamuhabwa AA, Aklillu E. 2015. CYP2B6*6 genotype and high efavirenz plasma concentration but not nevirapine are associated with low lumefantrine plasma exposure and poor treatment response in HIV-malaria-coinfected patients. Pharmacogenomics J doi: 10.1038/tpj.2015.37. [DOI] [PubMed] [Google Scholar]
- 25.Huang L, Olson A, Gingrich D, Aweeka FT. 2013. Determination of artemether and dihydroartemisinin in human plasma with a new hydrogen peroxide stabilization method. Bioanalysis 5:1501–1506. doi: 10.4155/bio.13.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salman S, Page-Sharp M, Griffin S, Kose K, Siba PM, Ilett KF, Mueller I, Davis TM. 2011. Population pharmacokinetics of artemether, lumefantrine, and their respective metabolites in Papua New Guinean children with uncomplicated malaria. Antimicrob Agents Chemother 55:5306–5313. doi: 10.1128/AAC.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]