Abstract
Cryptococcal antigen screening is recommended among people living with AIDS when entering HIV care with a CD4 count of <100 cells/μl, and preemptive fluconazole monotherapy treatment is recommended for those with subclinical cryptococcal antigenemia. Yet, knowledge is limited of current antimicrobial resistance in Africa. We examined antifungal drug susceptibility in 198 clinical isolates collected from Kampala, Uganda, between 2010 and 2014 using the CLSI broth microdilution assay. In comparison with two previous studies from 1998 to 1999 that reported an MIC50 of 4 μg/ml and an MIC90 of 8 μg/ml prior to widespread human fluconazole and agricultural azole fungicide usage, we report an upward shift in the fluconazole MIC50 to 8 μg/ml and an MIC90 value of 32 μg/ml, with 31% of isolates with a fluconazole MIC of ≥16 μg/ml. We observed an amphotericin B MIC50 of 0.5 μg/ml and an MIC90 of 1 μg/ml, of which 99.5% of isolates (197 of 198 isolates) were still susceptible. No correlation between MIC and clinical outcome was observed in the context of amphotericin B and fluconazole combination induction therapy. We also analyzed Cryptococcus susceptibility to sertraline, with an MIC50 of 4 μg/ml, suggesting that sertraline is a promising oral, low-cost, available, novel medication and a possible alternative to fluconazole. Although the CLSI broth microdilution assay is ideal to standardize results, limit human bias, and increase assay capacity, such assays are often inaccessible in low-income countries. Thus, we also developed and validated an assay that could easily be implemented in a resource-limited setting, with similar susceptibility results (P = 0.52).
INTRODUCTION
Cryptococcus neoformans is an opportunistic fungal pathogen, and it causes the disease cryptococcal meningitis. Cryptococcal meningitis is a common fungal infection of immunocompromised individuals, causing 15% to 20% of AIDS-related mortality, with a prevalence of cryptococcal antigenemia averaging 7% in persons with CD4 counts of <100 cells/μl (1, 2). A large cause of the high death rate in resource-limited settings is due to the lack of a correct initial diagnosis, inadequate treatment, and, possibly, emerging resistance to antifungal drugs (3).
The World Health Organization (WHO) guidelines recommend an optimal treatment for cryptococcal meningitis comprised of a 2-week course of amphotericin B in conjunction with flucytosine (5FC) followed by fluconazole as consolidation therapy (4, 5). Amphotericin B is a fungicidal drug which binds to ergosterol, forming pores in the membranes of yeasts (6). Flucytosine is a fungistatic drug, a base analog that intercalates into fungal RNA, preventing protein synthesis from occurring (7). Different mechanisms of action for these drugs make it difficult for the cells to develop resistance to both drugs during the course of treatment (8–10). However, flucytosine is prohibitively expensive (>$500/day) and not licensed in many countries (11). The WHO guidelines then recommend the use of fluconazole in place of flucytosine, as it is much more readily available (4). Fluconazole is also fungistatic and acts by binding to and inhibiting the 14-α demethylase, preventing a cell from producing ergosterol for the cell membrane, a mechanism of action which targets the same cell process as amphotericin B (7). In settings where amphotericin B is unavailable, fluconazole monotherapy is used for 10 weeks at a high dose (800 to 1,200 mg/day), with worse survival than amphotericin-based regimens (12, 13). The WHO also recommends fluconazole for preemptive treatment of asymptomatic cryptococcal antigen-positive persons with CD4 counts of <100 cells/μl who have early subclinical infection (2, 4). Fluconazole monotherapy relies on the assumption that the organism is not resistant to fluconazole, yet drug susceptibility testing is uncommon in sub-Saharan Africa. Additionally, low-dose preemptive therapy over multiple weeks could lead to the selection of resistant organisms, with limited alternative treatment options available (14). Recently, it has been shown that the antidepressant sertraline (Zoloft; Pfizer, New York) may exhibit antifungal properties against C. neoformans by inhibiting protein translation (15). On this basis, we examined the MIC values for sertraline in our clinical isolates to determine the current levels of sertraline susceptibility and to determine if sertraline could be a viable adjunctive preemptive treatment option.
The current guidelines for antifungal drug resistance were established based on research performed in Candida species and have since been adapted for use with Cryptococcus (16, 17). Previous researchers have shown that the resistance breakpoints established for Candida species may be valid for use in Cryptococcus species drug resistance assays (18–22). Breakpoints for Candida have recently been changed (17), and it is unclear whether these new breakpoints are appropriate for Cryptococcus. For the studies presented here, we used the older fluconazole breakpoints previously shown to be relevant to Cryptococcus: MICs of ≤8 μg/ml as susceptible, 16 to 32 μg/ml as dose-dependent susceptible, and ≥64 μg/ml as resistant. For amphotericin B, the MIC breakpoint for susceptibility is ≤1 μg/ml. There are currently no standard clinical breakpoints for susceptibility or resistance to sertraline.
Historical data on antifungal susceptibility of Ugandan clinical isolates were reported in 1998 and in 1999, with an MIC50 of 4 μg/ml for fluconazole and an amphotericin B MIC50 of 0.25 μg/ml, both of which are considered susceptible (18, 20). To determine current rates of C. neoformans antifungal resistance in Uganda, we analyzed patient isolates obtained prior to treatment from HIV-infected individuals presenting with cryptococcal meningitis. Additionally, the effectiveness of an assay which could easily be performed in a resource-limited setting was assessed. Finally, the drug resistance of the isolates was compared to their genotype as well as to clinical outcome data.
MATERIALS AND METHODS
Ethical statement and data.
Clinical isolates were collected from participants screened for the Cryptococcal Optimal Antiretroviral therapy Timing (COAT) trial and Adjunctive Sertraline for the Treatment of HIV-associated Cryptococcal Meningitis (ASTRO-CM) trial at Mulago hospital in Kampala, Uganda (ClinicalTrials.gov registration no. NCT01802385) (23). Each participant was HIV infected and was presenting with his or her first episode of cryptococcal meningitis. Antecedent fluconazole therapy was exceedingly rare. The clinical studies were approved by the institutional review boards of the University of Minnesota, Makerere University, and the Uganda National Council of Science and Technology. Written informed consent was obtained from all subjects or their proxy, and all data were deidentified.
Strains and media.
A total of 198 clinical isolates were obtained from HIV-infected participants with cryptococcal meningitis prior to initiation of antifungal drug treatment. All isolates were stored as glycerol stocks and grown on yeast peptone dextrose medium prior to use (24). ATCC strains 22019 (Candida parapsilosis) and 6258 (Candida krusei), and C. neoformans strain H99, were used as control strains for all susceptibility assays.
Drug resistance assays.
The CLSI broth microdilution assay was performed according to CLSI guidelines using 2.5 × 103 CFU/ml (16). Fluconazole dilutions tested were from 512 μg/ml to 0.125 μg/ml as 2-fold serial dilutions. Amphotericin B dilutions tested were from 8 μg/ml to 0.125 μg/ml as 2-fold serial dilutions. Sertraline dilutions tested were 1, 2, 4, 6, 8, 12, 16, 32, and 64 μg/ml. As indicated by the CLSI guidelines, a final volume of 200 μl per well was used. Spectrophotometric analysis of well turbidity at 600 nm was used to determine the MIC for each strain. Plates were scanned in a Biotek Synergy H1 hybrid reader (Winooski, VT) prior to and after 72 h of incubation at 35°C. The amphotericin B MIC was defined as the drug concentration at which no growth was observed at 72 h (100% inhibition of growth). A 50% reduction in growth (turbidity) compared to the no-drug control was used to define the fluconazole MIC. The sertraline MIC was determined using both a 50% reduction in growth and 100% inhibition of growth.
The resource-limited assay (RLA) was similar to the CLSI broth microdilution assay, with the following modifications: (i) all dilutions/solutions were made using volumes measurable with a 1,000-μl pipette; (ii) the assay was performed in a 24-well plate with a final volume of 1 ml; and (iii) the cryptococcal concentration was 5 × 103 cells/ml. Well turbidity was analyzed visually, with fluconazole MIC defined as the first drug dilution with an optically clear well and a lack of RPMI medium color change.
A subset of strains was tested using fluconazole and amphotericin B Etest strips (bioMérieux, Inc., Durham, NC) following the manufacturer's protocols.
Multilocus sequence typing.
Eight gene loci were amplified, sequenced, and analyzed, as described previously (24). Briefly, genomic DNA extraction was performed, as described previously (25). Eight loci—International Society for Human and Animal Mycology (ISHAM) consensus loci (n = 7; CAP59, GPD1, IGS1, LAC1, PLB1, SOD1, and URA5) (26) and the optional TEF1 locus (27)— were amplified and sequenced. Locus alleles and subsequent sequence types (STs) were numbered based on the fungal multilocus sequence typing (MLST) database for Cryptococcus neoformans (http://mlst.mycologylab.org), and novel alleles/sequence types were deposited into the database.
Analysis.
In vitro growth comparisons were analyzed using Mann-Whitney rank sum tests performed with SigmaPlot software (Systat Software, Inc., San Jose, CA). Fungal clearance was assessed with the use of the early fungicidal activity (EFA) method, and measures between susceptibility groups were compared with generalized linear models. Proportions of participants who were cerebrospinal fluid (CSF) culture positive after cryptococcal meningitis treatment, who experienced a cryptococcus-related immune reconstitution inflammatory syndrome (IRIS) event, and who died within 10 weeks of diagnosis were compared with Fisher's exact tests.
RESULTS
Drug resistance determination using CLSI assay.
To determine the MICs of clinical isolates to antifungal drugs, as well as the best drugs to be used during treatment, the CLSI developed a standardized broth microdilution assay (16, 17, 21). The assay was initially developed for determining MIC values in Candida species, with susceptible and resistant breakpoints for C. neoformans based on Candida species data. Since the initial standardization, the assay has been adapted successfully for use in C. neoformans (18–20, 28). While this assay was developed for a wide range of antifungal drugs, we examined the MICs of fluconazole and amphotericin B, as they are the primary antifungal medications used in sub-Saharan Africa (11). Additionally, we examined the MIC values for sertraline, which recently had been shown to exhibit antifungal effects on C. neoformans (15). Cultures from 198 patients admitted to Mulago Hospital in Kampala, Uganda, between 2010 and 2014 were analyzed for their growth in the presence of fluconazole, amphotericin B, and sertraline (Table 1 and 2).
TABLE 1.
MICs to fluconazole and amphotericin B of Cryptococcus neoformans clinical isolates from Uganda
| Drug | No. (%) of isolatesa with MIC (μg/ml) of: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | |
| Fluconazole | 1 (1) | 2 (2) | 8 (5) | 20 (16) | 20 (25) | 39 (44) | 49 (69) | 37 (87) | 17 (97) | 5 (100) |
| Amphotericin B | 27 (14) | 35 (31) | 82 (73) | 53 (99) | 1 (100) | |||||
A total of 198 isolates were screened, and data are presented as the number (cumulative percentage) of isolates with growth inhibition at (or below, for cumulative percentage) the indicated drug concentration.
The MIC50 for fluconazole was 8 μg/ml, and the MIC90 was 32 μg/ml (Table 1). Based on susceptibility breakpoints used in other cryptococcal studies (18–20, 28), only 69% of the cultures would be classified as susceptible to fluconazole (ie, MIC, ≤8 μg/ml). Moreover, 28% of the cultures were classified as dose-dependent susceptible (ie, MIC, 16 to 32 μg/ml), and 3% of cultures exhibited an MIC of ≥64 μg/ml, categorized as fluconazole resistant (Table 1). Both Candida krusei and Candida parapsilosis control strains had fluconazole MICs consistent with their expected values (64 and 2 μg/ml, respectively), showing that the assay worked as expected. Additionally, assays performed with Etest strips confirmed the validity of the CLSI broth microdilution assay.
The MIC50 for amphotericin B was 0.5 μg/ml, and the MIC90 was 1.0 μg/ml (Table 1). For amphotericin B, both the MIC50 and the MIC90 were within the susceptible range (ie, MIC ≤1.0 μg/ml) (17–19, 22). Both the Candida krusei and Candida parapsilosis control strains had expected amphotericin MIC values (2 and 1 μg/ml, respectively).
Recently, sertraline has been reported to exhibit antifungal effects against Cryptococcus species (15). Because of this, we also examined the sertraline MIC in this clinical isolate set. MICs were examined using the 50% reduction in growth used for azoles and the 100% inhibition of growth used for amphotericin. The MIC50 for sertraline was 4 μg/ml, and the MIC90 was 8 μg/ml, using both of these methods (Table 2).
TABLE 2.
Growth inhibition in response to increasing concentrations of sertraline
| Reduction or inhibition (%)b | No. (%) of isolatesa with MIC (μg/ml) of: |
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 6 | 8 | 12 | 16 | |
| 50 | 19 (10) | 36 (28) | 96 (76) | 16 (84) | 30 (99) | 1 (100) | 0 (100) |
| 100 | 19 (10) | 35 (27) | 95 (75) | 16 (83) | 32 (99) | 1 (100) | 0 (100) |
A total of 198 isolates were screened, and data are presented as the number (cumulative percentage) of isolates with growth inhibition at (or below) the indicated drug concentration.
50% reduction in growth or 100% inhibition of growth.
Growth rate correlates with fluconazole resistance.
Because fluconazole, a fungistatic drug, acts on an enzyme important for cell membrane synthesis, we analyzed the growth rate of the isolates to determine whether growth correlated with drug resistance. Absolute growth rate was determined by growth in RPMI 1640 in the absence of drug. Growth was inversely associated with fluconazole concentration (Fig. 1A). Isolates resistant to fluconazole grew slower (i.e., to a lower optical density in 72 h) in the absence of any inhibitory compounds compared with susceptible clinical isolates (P = 0.004).
FIG 1.
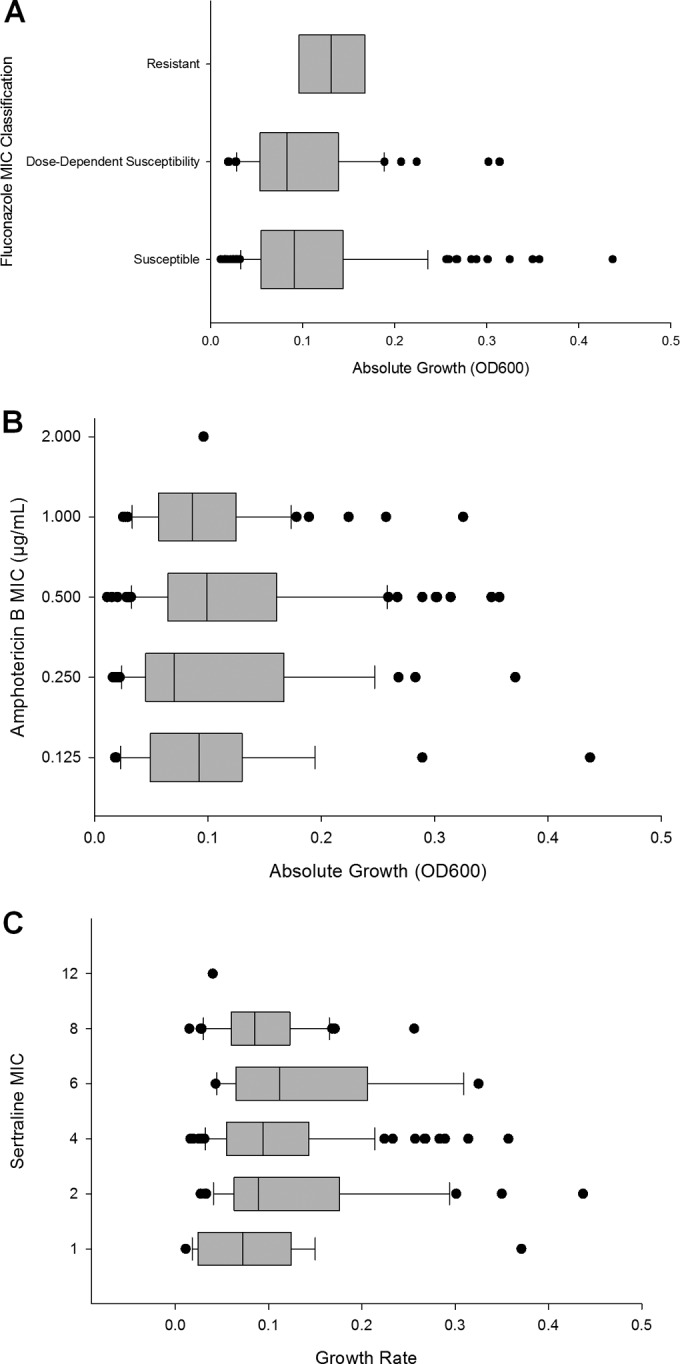
Effect of growth rate on fluconazole resistance. Relationship between growth rate and fluconazole (A), amphotericin B (B), or sertraline (C) resistance determined by MIC of drug. Growth of fluconazole-resistant isolates (MIC of ≥64 μg/ml) was lower than that of susceptible isolates (≤8 μg/ml) (P = 0.004). Growth did not correlate with increased resistance to amphotericin B or sertraline. Only 1 of the 103 isolates examined exhibited an MIC of ≥1 μg/ml to amphotericin B. OD600, optical density at 600 nm.
Conversely, there was no observable trend between growth and MIC for the fungicidal drug amphotericin B (Fig. 1B) or sertraline (Fig. 1C). It is important to note that only 1 of the 198 isolates tested was resistant to amphotericin B and that resistance breakpoints for sertraline are currently unknown.
RLA.
While the CLSI assay is widely used, a 96-well based assay is often not optimal in resource-limited environments due to the materials and equipment required to perform the assay, and visual determination of 50% reduction in growth can be subjective (21). Thus, we developed an assay—referred to as the RLA—utilizing 24-well plates, with all component volumes of ≥100 μl, room temperature incubation, and visual inspection of 100% growth inhibition instead of spectrophotometric analysis. This assay allows for analysis of multiple isolates at a time while keeping the required laboratory infrastructure and costs low. Presence of fungal growth upon drug exposure is based on color change of the RPMI medium and a lack of visible growth (Fig. 2A). The RLA was performed on a subset of 80 clinical isolates to determine fluconazole and amphotericin B resistance compared to the CLSI assay. With both fluconazole and amphotericin B, no significant differences between the CLSI assay and the RLA were detected (P = 0.52 for fluconazole, and P = 0.57 for amphotericin B) (Fig. 2B and C). We did note a tendency for some isolates to show increased fluconazole resistance. This could be due to differences in the assays. However, this could also be because some RLAs were performed at the time of primary culture, and previous studies have noted decreased resistance after laboratory passage (12).
FIG 2.
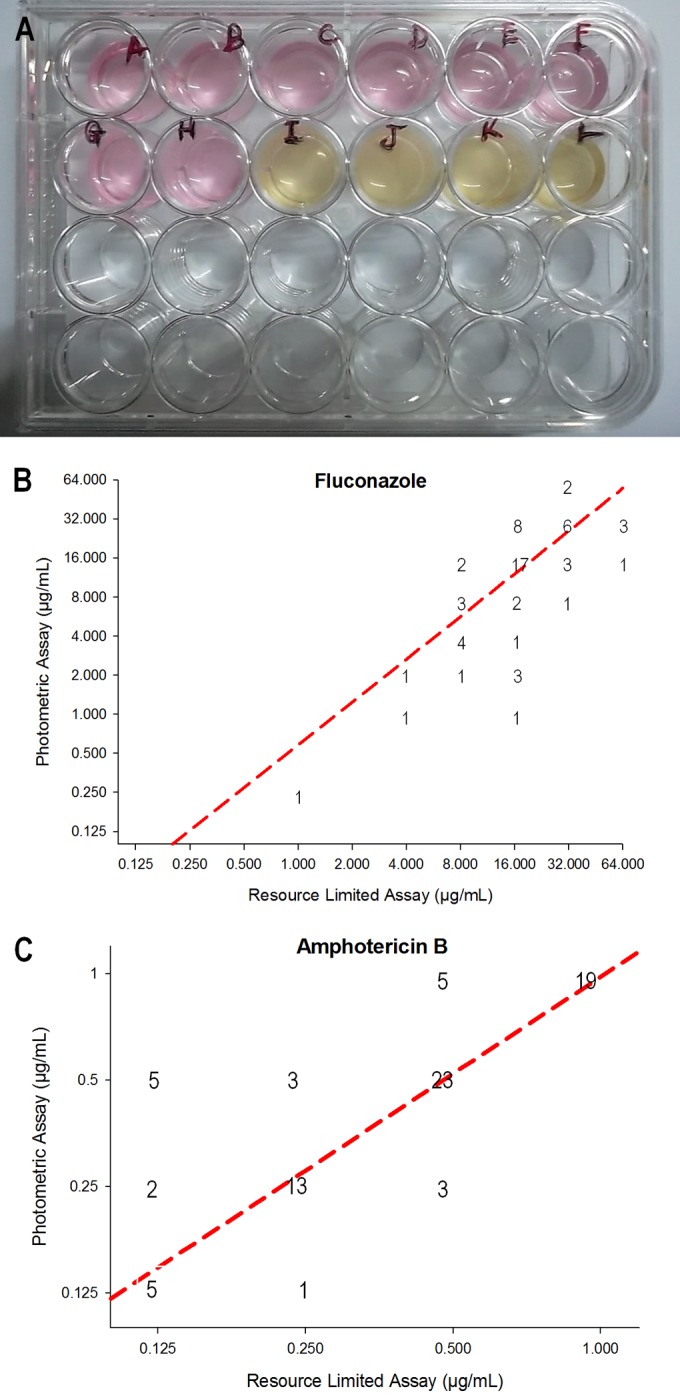
Resource-limited assay (RLA) validation. (A) Picture of RLA showing visual determination of fluconazole MIC. MIC determined by RLA compared to the CLSI method for fluconazole (B) and amphotericin B (C). Locations of numbers on the plot indicate the number of isolates which exhibited those MIC values. Dotted line indicates the line of best fit for the data set. There was no significant difference between the CLSI and RLA data (P = 0.521 for fluconazole, P = 0.571 for amphotericin B).
MLST genotype is not correlated with fluconazole resistance.
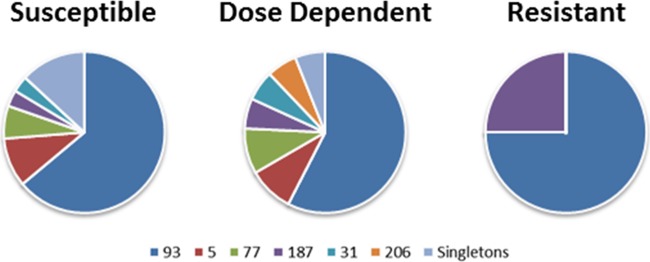
To determine the association between fluconazole resistance and genotype, we performed MLST on 98 of the isolates. Sixteen sequence types were observed in this population, but only 6 of the sequence types had multiple isolates (Table 3). As shown previously in Uganda (24), sequence type 93 predominated, with 61% of the isolates having this sequence type. Analysis of the relationship between sequence type and fluconazole resistance showed no significant correlation between genotype and fluconazole resistance (Table 3 and Fig. 3). Sequence type 206 had only dose-dependent susceptible strains, with no susceptible strains observed; however, with only 2 isolates, the significance of this trend could not be determined. The isolate with amphotericin B resistance belonged to sequence type 93, the most populated sequence type in our cohort.
TABLE 3.
Genotype and fluconazole resistance status for 98 clinical isolates
| Sequence typea | No. of susceptible isolates (MIC, ≤8 μg/ml) | No. of dose-dependent susceptible isolates (MIC, 16–32 μg/ml) | No. of resistant isolates (MIC, ≥64 μg/ml) | Total no. of isolates |
|---|---|---|---|---|
| 93 | 39 | 19 | 3 | 61 |
| 5 | 6 | 3 | 0 | 9 |
| 77 | 4 | 3 | 0 | 7 |
| 187 | 2 | 2 | 1 | 5 |
| 31 | 2 | 2 | 0 | 4 |
| 206 | 0 | 2 | 0 | 2 |
| 71 | 1 | 0 | 0 | 1 |
| 74 | 1 | 0 | 0 | 1 |
| 91 | 1 | 0 | 0 | 1 |
| 95 | 1 | 0 | 0 | 1 |
| 174 | 0 | 1 | 0 | 1 |
| 205 | 1 | 0 | 0 | 1 |
| 250 | 0 | 1 | 0 | 1 |
| 252 | 1 | 0 | 0 | 1 |
| 292 | 1 | 0 | 0 | 1 |
| 2000 | 1 | 0 | 0 | 1 |
| Total | 61 | 33 | 4 | 98 |
Sequence type designation is based on the mlst.mycologylab.org Cryptococcus neoformans database.
FIG 3.
Genotypic makeup of fluconazole MIC groupings based on sequence type. Proportion of each sequence type in the MIC grouping that was susceptible (n = 61) (A), dose-dependent susceptible (n = 33) (B), or resistant (n = 4) (C). Singletons were combined into one group for this analysis.
Clinical outcome following fluconazole and amphotericin B treatment.
The MIC values for a subset of isolates were also compared with clinical data collected from 72 participants in the COAT study and an additional 18 participants who died prior to enrollment. All participants were treated according to WHO guidelines with 0.7 to 1 mg/kg of body weight/day of amphotericin B for 2 weeks and 800 mg/day of fluconazole for 11 weeks. Clinical factors analyzed were EFA, presence of a positive CSF culture at 14 days, subsequent IRIS, and mortality at 10 weeks postdiagnosis. EFA is the rate of decrease of fungal burden in log10 CFU per milliliter of CSF over the course of treatment (5). Similarly, positive culture from CSF after 14 days of combination therapy indicates a lack of clearance during drug treatment (8, 29). IRIS is a paradoxical immune-mediated deterioration that occurs with recovery of the immune system upon initiating antiretroviral therapy (ART), and it is associated with poor antigen clearance or ongoing culture positivity (30–32).
Although our data set is likely too small to reveal statistically significant associations between clinical outcome and drug resistance, we observed a trend toward reduced culture positivity after treatment, less IRIS, and lower mortality in patients with fluconazole-resistant strains (Table 4). In contrast, patients with dose-dependent susceptible strains had a trend toward slightly increased culture positivity. These data indicate that the combination of amphotericin B and fluconazole was effective at reducing cryptococcal burden, clearance, and mortality, even in the context of fluconazole drug resistance, although analysis of a larger population is necessary due to low numbers of resistant strains.
TABLE 4.
Drug resistance does not impact clinical parameters of disease with combination therapy
| Parameter | CLSI susceptibility classification |
|||||
|---|---|---|---|---|---|---|
| Fluconazole |
Amphotericin B |
|||||
| Susceptible | Dose dependent | Resistant | Pa | Susceptible | Resistant | |
| CSF during follow-up (n = 73) | ||||||
| No. | 46 | 22 | 5 | 72 | 1 | |
| Mean (SD) EFA | −0.33 (0.35) | −0.26 (0.20) | −0.24 (0.15) | 0.59 | −0.31 (0.30) | |
| No. (%) culture positive after CM treatment | 26 (56.5) | 17 (77.3) | 1 (20.0) | 0.04 | 43 (59.7) | 1 |
| Initiated ART during follow-up (n = 62) | ||||||
| No. | 38 | 20 | 4 | 61 | 1 | |
| No. (%) of IRIS | 4 (10.5) | 3 (15.0) | 0 (0) | 0.81 | 7 (11.5) | 0 |
| Vital status at day 60 known (n = 90) | ||||||
| No. | 58 | 27 | 5 | 89 | 1 | |
| No. (%) of deaths | 29 (50.0) | 11 (40.7) | 1 (20.0) | 0.39 | 41 (46.1) | 0 |
P value for EFA is from the generalized linear model. P values for proportions are from Fisher's exact tests.
DISCUSSION
Previous studies in Uganda have reported MIC50 values for fluconazole of 4 μg/ml and MIC50 values for amphotericin B of 0.25 μg/ml (18–20). Our results indicate an increase in the MIC values for both fluconazole and amphotericin B in the decade since these previous studies from the same city were performed. Although the fluconazole MIC values have increased, with 31% of clinical isolates no longer susceptible to fluconazole, over 99% of isolates were still susceptible to amphotericin B. In addition, our results indicate that strains with higher growth rates exhibited greater susceptibility to fluconazole but not to amphotericin B or sertraline. Finally, while the standardized CLSI assay is ideal, it cannot be used in every setting due to equipment and financial restraints. Thus, we also developed and verified a similar assay, in which the MIC can be readily determined visually, that has been optimized for low-resource settings.
The CLSI M27-A3 protocol for broth microdilution antifungal susceptibility testing was developed using Candida species (21), so there are currently no standard breakpoints for antifungal testing for C. neoformans. However, previous studies have independently correlated a low MIC value (≤8 μg/ml) with successful treatment for cryptococcal meningitis (33, 34). Additionally, a recent study reported that fluconazole MICs determined by the CLSI method may be a potential predictor of therapeutic cure for patients with cryptococcal meningitis (28). For our analyses, we used these breakpoints previously shown to be correlated with treatment outcome data for cryptococcal meningitis. The most recent CLSI Candida albicans breakpoints for fluconazole have been lowered further (17), with susceptible strains designated by an MIC of ≤4 μg/ml. If these new breakpoints were to be used for C. neoformans, only 44% of our isolates would be considered susceptible to fluconazole. However, analysis of these new breakpoints compared to C. neoformans wild-type populations and clinical outcomes suggest that clinical susceptibility is most appropriately set at an MIC of ≤8 μg/ml and that clinical failure or recurrence is observed starting at an MIC of ≥16 μg/ml, based on epidemiological cutoff values and clinical breakpoints (22); 31% of our isolates showed MICs of ≥16 μg/ml. Thus, we observed an increase in fluconazole resistance in Uganda over the past decade that may impact the clinical utility of this drug.
Fluconazole monotherapy is already a suboptimal option for treatment of cryptococcal meningitis, with 10-week survival of only ∼40% (13, 35). Yet, fluconazole is the most common treatment given in Africa. With increasing resistance, outcomes may become even worse. However, the larger concern is for preemptive therapy of asymptomatic cryptococcal antigenemia, for which fluconazole is the recommended therapy of choice (2, 4, 36). Among persons with CD4 counts of <100 cells/μl, the prevalence of cryptococcal antigenemia averages 7% in low- and middle-income countries. If one-third of clinical isolates are not susceptible to fluconazole, fluconazole preemptive monotherapy may not be a viable option.
As previously noted, fluconazole reduces membrane synthesis; however, it is fungistatic. Because of this feature, we examined whether the growth rate of isolates, in the absence of any inhibitory compounds, correlated with the MIC of fluconazole. Isolates with a higher MIC to fluconazole grew to a lower optical density, suggesting that slower-growing isolates that require less membrane synthesis are more resistant to fluconazole. This same trend was not observed with amphotericin B or sertraline. Amphotericin B is a fungicidal drug, binding ergosterol and forming pores in cell membranes, destroying cells within 24 h (6). This provides little opportunity for cultures to adapt to the drug. Taken together, these results reiterate the importance of combination therapy of fungistatic drugs with fungicidal amphotericin B, as per the WHO recommended treatment (4, 8, 9).
Sertraline inhibits protein synthesis in C. neoformans (15), which is a vital process that, when inhibited, would limit cell adaptation. Based on tissue levels of sertraline and our MIC data, therapeutic levels of sertraline should be obtainable in humans (35). Combined with its current approval for use as an antidepressant in humans, these data make sertraline a promising new antifungal drug, and detailed clinical trials analyzing the efficacy of sertraline for the treatment of cryptococcal meningitis are warranted.
Relative to previous studies (18, 20), the MIC50 of amphotericin B has increased in our cohort; however, greater than 99% of the isolates were still susceptible to amphotericin B. All patients in our study received standard treatment, consisting of a 2-week course of amphotericin B and an 11-week course of fluconazole. Fluconazole resistance did not impact the rate of fungal clearance as measured by EFA, culture positivity at the end of amphotericin B treatment, mortality, or the development of IRIS. Previous studies have identified the importance of amphotericin B treatment in patient survival, and our results reiterate the effectiveness of amphotericin B as the drug of choice (4, 8, 9). However, a comparison of drug resistance prior to the widespread use of fluconazole and amphotericin B in Uganda (18, 20) to current resistance shows that levels of drug resistance are rising, indicating that the analysis of drug susceptibility testing prior to treatment should be prioritized in every setting, and especially if monotherapy is considered.
Our resource-limited assay results were not significantly different from the CLSI broth microdilution assay results for both antifungal drugs, indicating that the visual assay could be used successfully in place of the standard CLSI assay. This assay has several advantages over the CLSI assay. First, the assay is inspected visually, using a 100% reduction in growth and eliminating the difficulty in visually determining 50% reduction in growth (21), while still eliminating the need for a spectrophotometer. Second, the visual assay can be performed with a single P1000 pipette, as all the volumes that need to be measured are between 100 and 1,000 μl. Last, the RLA uses 24-well plates, which make it easier to view each well individually but which are large enough that the plates can be cleaned and sterilized for multiple uses. These features make this type of assay ideal for usage in settings with limited resources and when a spectrophotometer is not available.
While resistance to amphotericin B was rare in our study, resistance to fluconazole appeared to be emerging. Person-to-person transmission of cryptococcal meningitis is rare; thus, the treatment of an individual with antifungal drugs is likely to lead to the development of resistant isolates only within that individual—and antecedent fluconazole therapy was exceedingly rare in our cohort. However, it has also been shown in another environmentally acquired fungal pathogen, Aspergillus fumigatus, that resistance to fluconazole and voriconazole can develop in response to triazole fungicide exposure (37). Uganda has fertile soils and a subtropical climate, which make it ideal for agriculture. Agriculture is the most important aspect of the Ugandan economy, employing 80% of the work force (38). Coffee, tea, and sugar cane are major exports, while bananas, plantains, and potatoes constitute a large part of the diet of most Ugandans (38–40). All of these crops are susceptible to a wide variety of fungal diseases, which are most effectively prevented through the use of various fungicides. The use of fungicides not only prevents crop disease but also leads to an increased yield of cash crops (40–45). Due to the ubiquity of C. neoformans in the environment, the increasing use of triazole fungicides by farmers in Uganda could be a factor in the increased fluconazole resistance observed in our studies.
Interestingly, there was not a correlation between sequence type and MIC value. This is likely due in large part to the clonality observed in this region, with the majority of isolates having sequence type 93 (21). In each of the resistance groupings, the majority of isolates fell into this sequence type. The addition of more isolates with various genotypes could glean a trend between sequence type and fluconazole resistance, as there were low numbers of isolates in the dose-dependent and resistant categories compared to the sensitive category. Alternatively, finer whole-genome sequencing approaches may be necessary to identify genomic regions that correlate with drug resistance.
Our data showed that the fluconazole MIC values in Uganda are increasing compared to previously reported findings (18–20). Decreasing susceptibility to fluconazole monotherapy, used commonly in resource-limited settings, may lead to increasingly ineffective treatment of meningitis or asymptomatic cryptococcal antigenemia. Combination therapy with amphotericin B lessens the impact of fluconazole resistance, indicating that the recommended drug combination is effective at reducing the cryptococcal burden. The role of combination preemptive therapy is undefined, but the mortality rate with the WHO-recommended preemptive therapy regimen was 30% among asymptomatic persons in a recent trial (46). The contribution of resistance to failure and death is unclear. Due to the potential for the MIC values to increase further, rendering fluconazole monotherapy even less effective, fluconazole resistance screening should take place at sentinel surveillance sites in sub-Saharan Africa. In addition, novel oral drug treatments, such as adjunctive sertraline, need to be developed for use in rural areas where intravenous amphotericin B treatment may not be possible.
ACKNOWLEDGMENTS
We thank Henry Kajumbula for leadership of the Makerere University Microbiology Department. Additionally, we thank Benjamin Lueck and Joshua Kerkaert for their help with MIC assays.
This work was supported by NIH grants U01AI089244, R01NS086312, T32AI055433, R24TW008886, and R25TW009345 and by Grand Challenges Canada grant S40296.
ASTRO-CM and COAT team members are the following: Abdu Musubire, Henry W. Nabeta, Darlisha A. Williams, Bozena Morawski, Melissa A. Rolfes, Jane Francis Ndyetukira, Cynthia Ahimbisibwe, Florence Kugonza, Alisat Sadiq, Jonathan Dyal, Julie M. Neborak, Alexa M. King, Nathan Yueh, Sruti S. Velamakanni, Alice Namudde, Tadeo Kiiza Kandole, Julian Kaboggoza, Eva Laker, Tony Luggya, Liliane Tugume, Mahsa Abassi, Kate Birkenkamp, Elissa K. Butler, A. Wendy Fujita, Ryan Halupnick, Anna K. Strain, Priya Vedula, Radha Rajasingham, Andrew Kambugu, and Paul R. Bohjanen.
Contributor Information
Collaborators: Abdu Musubire, Henry W. Nabeta, Darlisha A. Williams, Bozena Morawski, Melissa A. Rolfes, Jane Francis Ndyetukira, Cynthia Ahimbisibwe, Florence Kugonza, Alisat Sadiq, Jonathan Dyal, Julie M. Neborak, Alexa M. King, Nathan Yueh, Sruti S. Velamakanni, Alice Namudde, Tadeo Kiiza Kandole, Julian Kaboggoza Eva Laker, Tony Luggya, Liliane Tugume, Mahsa Abassi, Kate Birkenkamp, Elissa K. Butler, A. Wendy Fujita, Ryan Halupnick, Anna K. Strain, Priya Vedula, Radha Rajasingham, Andrew Kambugu, and Paul R. Bohjanen
REFERENCES
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. 2009. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23:525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Rajasingham R, Meya DB, Boulware DR. 2012. Integrating cryptococcal antigen screening and preemptive treatment into routine HIV care. J Acquir Immune Defic Syndr 59:e85–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. 2011. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 5.Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, Phu NH, Nghia HD, Phong ND, Thai CQ. 2013. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med 368:1291–1302. doi: 10.1056/NEJMoa1110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray KC, Palacios DS, Dailey I, Endo MM, Uno BE, Wilcock BC, Burke MD. 2012. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A 109:2234–2239. doi: 10.1073/pnas.1117280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odds FC, Brown AJ, Gow NA. 2003. Antifungal agents: mechanisms of action. Trends Microbiol 11:272–279. doi: 10.1016/S0966-842X(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 8.Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker L-G, Harrison T. 2007. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 45:76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, White NJ, Harrison TS. 2004. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomized trial. Lancet 363:1764–1767. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 10.Vanden BH, Dromer F, Improvisi I, Lozano-Chiu M, Rex J, Sanglard D. 1998. Antifungal drug resistance in pathogenic fungi. Med Mycol 36:119–128. [PubMed] [Google Scholar]
- 11.Rajasingham R, Rolfes MA, Birkenkamp KE, Meya DB, Boulware DR. 2012. Cryptococcal meningitis treatment strategies in resource-limited settings: a cost-effectiveness analysis. PLoS Med 9:e1001316. doi: 10.1371/journal.pmed.1001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, Longley N, Muzoora C, Phulusa J, Taseera K, Kanyembe C, Wilson D, Hosseinipour MC, Brouwer AE, Limmathurotsakul D, White N, van der Horst C, Wood R, Meintjes G, Bradley J, Jaffar S, Harrison T. 2014. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 58:736–745. doi: 10.1093/cid/cit794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longley N, Muzoora C, Taseera K, Mwesigye J, Rwebembera J, Chakera A, Wall E, Andia I, Jaffar S, Harrison TS. 2008. Dose-response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis 47:1556–1561. doi: 10.1086/593194. [DOI] [PubMed] [Google Scholar]
- 14.Loyse A, Thangaraj H, Easterbrook P, Ford N, Roy M, Chiller T, Govender N, Harrison TS, Bicanic T. 2013. Cryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countries. Lancet 13:629–637. doi: 10.1016/S1473-3099(13)70078-1. [DOI] [PubMed] [Google Scholar]
- 15.Zhai B, Wu C, Wang L, Sachs MS, Lin X. 2012. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother 56:3758–3766. doi: 10.1128/AAC.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—3rd ed. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.CLSI. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts: fourth informational supplement, CLSI document M27-S4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.Jessup C, Pfaller M, Messer S, Zhang J, Tumberland M, Mbidde E, Ghannoum M. 1998. Fluconazole susceptibility testing of Cryptococcus neoformans: comparison of two broth microdilution methods and clinical correlates among isolates from Ugandan AIDS patients. J Clin Microbiol 36:2874–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller M, Messer S, Boyken L, Hollis R, Rice C, Tendolkar S, Diekema D. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn Microbiol Infect Dis 48:201–205. doi: 10.1016/j.diagmicrobio.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Pfaller M, Zhang J, Messer S, Brandt M, Hajjeh R, Jessup C, Tumberland M, Mbidde E, Ghannoum M. 1999. In vitro activities of voriconazole, fluconazole, and itraconazole against 566 clinical isolates of Cryptococcus neoformans from the United States and Africa. Antimicrob Agents Chemother 43:169–171. doi: 10.1093/oxfordjournals.jac.a020873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fothergill A. 2012. Antifungal susceptibility testing: Clinical Laboratory and Standards Institute (CLSI) methods, p 65–74. In Hall G. (ed), Interations of yeasts, moulds, and antifungal agents: how to detect resistance. Springer, New York, NY. doi: 10.1007/978-1-59745-135-5-2. [DOI] [Google Scholar]
- 22.Espinel-Ingroff A, Aller A, Canton E, Castañón-Olivares L, Chowdhary A, Cordoba S, Cuenca-Estrella M, Fothergill A, Fuller J, Govender N. 2012. Cryptococcus neoformans-Cryptococcus gattii species complex: an international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole and voriconazole. Antimicrob Agents Chemother 56:5898–5906. doi: 10.1128/AAC.01115-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, Taseera K, Nabeta HW, Schutz C, Williams DA, Rajasingham R, Rhein J, Thienemann F, Lo MW, Nielsen K, Bergemann TL, Kambugu A, Manabe YC, Janoff EN, Bohjanen PR, Meintjes G, COAT Trial Team . 2014. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 370:2487–2498. doi: 10.1056/NEJMoa1312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiesner DL, Moskalenko O, Corcoran JM, McDonald T, Rolfes MA, Meya DB, Kajumbula H, Kambugu A, Bohjanen PR, Knight JF. 2012. Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. mBio 3:e00196-12. doi: 10.1128/mBio.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu D, Coloe S, Baird R, Pedersen J. 2000. Rapid mini-preparation of fungal DNA for PCR. J Clin Microbiol 38:471–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer W, Aanensen DM, Boekhout T, Cogliati M, Diaz MR, Esposto MC, Fisher M, Gilgado F, Hagen F, Kaocharoen S. 2009. Consensus multilocus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med Mycol 47:561–570. doi: 10.1080/13693780902953886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Litvintseva AP, Thakur R, Vilgalys R, Mitchell TG. 2006. Multilocus sequence typing reveals three genetic subpopulations of Cryptococcus neoformans var. grubii (serotype A), including a unique population in Botswana. Genetics 172:2223–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee C-H, Chang T-Y, Liu J-W, Chen F-J, Chien C-C, Tang Y-F, Lu C-H. 2012. Correlation of anti-fungal susceptibility with clinical outcomes in patients with cryptococcal meningitis. BMC Infect Dis 12:361. doi: 10.1186/1471-2334-12-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bicanic T, Meintjes G, Rebe K, Williams A, Loyse A, Wood R, Hayes M, Jaffar S, Harrison T. 2009. Immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis: a prospective study. J Acquir Immune Defic Syndr 51:130–134. doi: 10.1097/QAI.0b013e3181a56f2e. [DOI] [PubMed] [Google Scholar]
- 30.Wiesner DL, Boulware DR. 2011. Cryptococcus-related immune reconstitution inflammatory syndrome (IRIS): pathogenesis and its clinical implications. Curr Fungal Infect Rep 5:252–261. doi: 10.1007/s12281-011-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulware DR, Meya DB, Bergemann TL, Wiesner DL, Rhein J, Musubire A, Lee SJ, Kambugu A, Janoff EN, Bohjanen PR. 2010. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med 7:e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang CC, Dorasamy AA, Gosnell BI, Elliott JH, Spelman T, Omarjee S, Naranbhai V, Coovadia Y, Ndung'u T, Moosa MY, Lewin SR, French MA. 2013. Clinical and mycological predictors of cryptococcosis-associated immune reconstitution inflammatory syndrome. AIDS 27:2089–2099. doi: 10.1097/QAD.0b013e3283614a8d. [DOI] [PubMed] [Google Scholar]
- 33.Aller A, Martin-Mazuelos E, Lozano F, Gomez-Mateos J, Steele-Moore L, Holloway W, Gutierrez M, Recio F, Espinel-Ingroff A. 2000. Correlation of fluconazole MICs with clinical outcome in cryptococcal infection. Antimicrob Agents Chemother 44:1544–1548. doi: 10.1128/AAC.44.6.1544-1548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witt MD, Lewis RJ, Larsen RA, Milefchik EN, Leal MAE, Haubrich RH, Richie JA, Edwards JE, Ghannoum MA. 1996. Identification of patients with acute AIDS-associated cryptococcal meningitis who can be effectively treated with fluconazole: the role of antifungal susceptibility testing. Clin Infect Dis 22:322–328. doi: 10.1093/clinids/22.2.322. [DOI] [PubMed] [Google Scholar]
- 35.Gaskell KM, Rothe C, Gnanadurai R, Goodson P, Jassi C, Heyderman RS, Allain TJ, Harrison TS, Lalloo DG, Sloan DJ, Feasey NA. 2014. A prospective study of mortality from cryptococcal meningitis following treatment induction with 1,200 mg oral fluconazole in Blantyre, Malawi. PLoS One 9:e110285. doi: 10.1371/journal.pone.0110285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, Kambugu A, Kamya MR, Bohjanen PR, Boulware DR. 2010. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count ≤100 cells/μl who start HIV therapy in resource-limited settings. Clin Infect Dis 51:448–455. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet 9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 38.World Factbook. 2008. Uganda. Central Intelligence Agency, Washington, DC. [Google Scholar]
- 39.Tushemereirwe W, Kashaija I, Tinzaara W, Nankinga C, New S. 2003. Banana production manual: a guide to successful banana production in Uganda. Makerere University Printery, Kampala, Uganda. [Google Scholar]
- 40.Namanda S, Olanya O, Adipala E, Hakiza J, El-Bedewy R, Baghsari A, Ewell P. 2004. Fungicide application and host-resistance for potato late blight management: benefits assessment from on-farm studies in SW Uganda. Crop Protection 23:1075–1083. doi: 10.1016/j.cropro.2004.03.011. [DOI] [Google Scholar]
- 41.Bailey R, McFarlane S, Singh V, Kumar V. The incidence and effects of ratoon stunting disease of sugarcane in Southern and Central Africa, p 338–346. In Singh V, Kumar V (ed), Proceedings of the XXIII ISSCT Congress, New Delhi, India. [Google Scholar]
- 42.Gianessi L, Williams A. 2011. Fungicide spraying is critical for increasing potato production in Africa. CropLife Foundation, Washington, DC. [Google Scholar]
- 43.Mukiibi JK. 2001. Agriculture in Uganda. Fountain Publishers, Wageningen, Netherlands. [Google Scholar]
- 44.Mulumba J, Nankya R, Adokorach J, Kiwuka C, Fadda C, De Santis P, Jarvis D. 2012. A risk-minimizing argument for traditional crop varietal diversity use to reduce pest and disease damage in agricultural ecosystems of Uganda. Agric Ecosyst Environ 157:70–86. doi: 10.1016/j.agee.2012.02.012. [DOI] [Google Scholar]
- 45.Rutherford MA, Phiri N. 2006. Pests and diseases of coffee in Eastern Africa: a technical and advisory manual. CAB International, Wallingford, Oxon, United Kingdom. [Google Scholar]
- 46.Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, Chijoka C, Masasi A, Kimaro G, Ngowi B, Kahwa A, Mwaba P, Harrison TS, Egwaga S, Jaffar S, REMSTART trial team. 2015. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet 385:2173–2182. doi: 10.1016/S0140-6736(15)60164-7. [DOI] [PubMed] [Google Scholar]