Abstract
Coccidioidomycosis, or valley fever, is a growing health concern endemic to the southwestern United States. Safer, more effective, and more easily administered drugs are needed especially for severe, chronic, or unresponsive infections. The novel fungal CYP51 inhibitor VT-1161 demonstrated in vitro antifungal activity, with MIC50 and MIC90 values of 1 and 2 μg/ml, respectively, against 52 Coccidioides clinical isolates. In the initial animal study, oral doses of 10 and 50 mg/kg VT-1161 significantly reduced fungal burdens and increased survival time in a lethal respiratory model in comparison with treatment with a placebo (P < 0.001). Oral doses of 25 and 50 mg/kg VT-1161 were similarly efficacious in the murine central nervous system (CNS) model compared to placebo treatment (P < 0.001). All comparisons with the positive-control drug, fluconazole at 50 mg/kg per day, demonstrated either statistical equivalence or superiority of VT-1161. VT-1161 treatment also prevented dissemination of infection from the original inoculation site to a greater extent than fluconazole. Many of these in vivo results can be explained by the long half-life of VT-1161 leading to sustained high plasma levels. Thus, the efficacy and pharmacokinetics of VT-1161 are attractive characteristics for long-term treatment of this serious fungal infection.
INTRODUCTION
Coccidioidomycosis, commonly known as valley fever, is caused by Coccidioides sp. fungi endemic to the southwestern United States (1). An estimated 150,000 people in the United States are infected annually with Coccidioides, with incidence and hospitalization rates increasing over the past 2 decades (2–4). While the majority of illnesses are self-resolving, chronic pulmonary and disseminated coccidioidomycosis, especially coccidioidal meningitis, may require long-term to lifetime therapy with antifungal drugs to control infection and prevent death (5–7).
Current antifungal treatments for patients with progressive or life-threatening coccidioidomycosis primarily include amphotericin B and azole antifungals. Amphotericin B is recommended for severe, life-threatening coccidioidomycosis, but toxicity and intravenous or intrathecal administration are significant drawbacks (7–9). Azole antifungals are usually orally administered and are the backbone of long-term treatment for coccidioidomycosis (7). Azole antifungals diminish ergosterol synthesis by inhibiting fungal sterol 14α-demethylase cytochrome P450 (CYP51), thereby compromising the integrity of the fungal cell membrane. All marketed azoles employ either an imidazole or triazole moiety that binds the active-site heme iron in not only fungal CYP51 but also mammalian cytochrome P450s (CYPs). Consequently, azole antifungals have a propensity to inhibit human CYPs, directly contributing to many of their toxicities and drug-drug interactions (10–12).
VT-1161 is a novel fungal CYP51 inhibitor that was rationally designed through the use of a tetrazole moiety for greater selectivity against binding mammalian CYP enzymes while retaining the same or greater potency for the fungal CYP51 target (13). The potency of VT-1161 against Candida albicans CYP51 in a cellular assay was ≤0.5 nM compared to in vitro 50% inhibitory concentration (IC50) values of ∼100 μM or greater against human CYP51 and key xenobiotic-metabolizing CYPs present in human liver microsomes (e.g., CYP2C9, 2C19, and 3A4) (14). VT-1161 has recently completed two trials for the treatment of superficial and mucosal mycoses (NCT01891305 and NCT01891331, respectively [www.clinicaltrials.gov]). The studies reported herein extend the spectrum of the therapeutic potential for VT-1161 by demonstrating in vitro antifungal activity against Coccidioides spp. and efficacy in treating both respiratory and central nervous system (CNS) infections in lethal murine models of this disease.
MATERIALS AND METHODS
Mice.
Eight-week-old, female Swiss-Webster mice were purchased from Harlan Laboratories (Indianapolis, IN) and housed according to National Institutes of Health (NIH) standards. All procedures were performed with approval of the Institutional Animal Care and Use Committee.
Fungal strains.
All growth and manipulation of Coccidioides were performed at biosafety level 3 (BSL3), and colonies were grown on glucose-yeast extract (GYE) agar, with modifications specified where relevant. Coccidioides posadasii strain Silveira arthroconidia for animal studies were grown to maturity and harvested at the University of Arizona as previously described (15). Clinical isolates for the fungal susceptibility testing were from the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio, TX, and were grown to maturity for harvest of arthroconidia. For DNA extraction to determine species of the clinical isolate collection, cultures were grown on 2× GYE agar with penicillin (100 IU/ml) and streptomycin (100 μg/ml) (Corning Cellgro penicillin-streptomycin solution; Mediatech/VWR International, Radnor, PA) at the Translational Genomics Institute, Pathogen Genomics Division, Flagstaff, AZ.
Species determination of clinical isolates.
Cultures of the clinical isolates were incubated for 1 to 3 weeks at 28°C, and DNA was extracted from mycelia using the PowerSoil DNA isolation kit protocol (Mo Bio Laboratories, Inc., Carlsbad, CA) with a minor modification. Mycelia underwent an additional 1-min bead-beating step in Lysing Matrix D (MP Biomedicals, Santa Ana, CA) at 4,000 rpm in a BeadBug Microtube Homogenizer (Benchmark Scientific, Edison, NJ). The lysate was then transferred to the Mo Bio bead tubes, and the manufacturer's protocol was followed from that point to obtain DNA from each strain.
A CocciDif assay was performed on the isolated DNA as described previously (16). TaqMan minor groove binding (MGB) probes were purchased from Applied Biosystems (Waltham, MA). MGB probes incorporate a 5′ reporter dye and a 3′ nonfluorescent quencher. One probe hybridizes to a Coccidioides immitis-specific sequence (5′-6FAM-ATTGTCCAGTATGAGGAT-3′, where FAM is 6-carboxyfluorescein), and the other hybridizes to a C. posadasii-specific sequence (5′-VIC-ATTGTCCAGAATGAGGAT-3′). The reactions were performed in 10.0-μl reaction volumes containing 900 nM forward (5′-CGTGTGGCCTTGCAGTATAGC-3′) and reverse (5′-TTTACGCCGTAGCCTTTGATG-3′) primers, 125 nM each probe, 1× PerfeCTa quantitative PCR (qPCR) ToughMix, 6-carboxy-X-rhodamine (ROX), and 1.0 μl of template normalized to 0.05 ng/μl for initial amplification and to 5 ng/μl or undiluted for subsequent amplifications as needed. Thermal cycling was performed using an ABI 7900HT sequence detection system (Applied Biosystems, Waltham, MA) under the following conditions: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 58°C for 1 min.
In vitro susceptibility tests.
Antifungal susceptibility tests against Coccidioides spp. were performed using a broth macrodilution method according to the Clinical and Laboratory Standards Institute M38-A2 standard (17). Isolates were adjusted by a spectrophotometer (80 to 82% transmittance) to a starting inoculum of 1 × 104 to 5 × 104 arthroconidia/ml and then added to tubes containing serial 2-fold dilutions of VT-1161 (0.03 to 16 μg/ml) or fluconazole (0.125 to 64 μg/ml). The assay was conducted in 0.165 M morpholinepropanesulfonic acid (MOPS; pH 7.0), in RPMI 1640 medium with l-glutamine and without bicarbonate. The tubes were incubated at 35°C for 48 h, and the MIC was recorded as the lowest concentration that resulted in >80% inhibition of growth compared to growth of the drug-free control. Paecilomyces variotii (ATCC MYA-3630) served as the quality control organism, as recommended by M38-A2 (17). The MIC for the C. posadasii strain used in the murine models of coccidioidomycosis was determined independently using the broth macrodilution assay with incubation at 37°C for 48 h prior to animal infection studies.
Drug treatments.
VT-1161 was supplied by Viamet Pharmaceuticals, Inc. (Durham, NC). Fluconazole was obtained commercially and diluted in sterile water to desired concentrations. Cremophor EL ([CrEL] C5135; Sigma, St. Louis, MO), as a 20% solution in water, was used as an excipient for the suspension of VT-1161 and as placebo treatment. All treatments were administered by oral gavage.
Animal studies.
Mice and tissues were handled at an animal BSL3 (ABSL3) facility throughout the studies. Mice were infected with spores of C. posadasii suspended in sterile 0.9% saline. For the respiratory study, mice were infected intranasally with 500 spores in 30 μl of saline under ketamine-xylazine anesthesia as previously described (18). Mice were divided into groups of 16 and received VT-1161 at 10 mg/kg (VT10) or 50 mg/kg (VT50) once daily, fluconazole at 25 mg/kg twice daily (FLC), or CrEL once daily (placebo) beginning 120 h postinfection (p.i.) and continuing for 7 days. Eight mice per group were sacrificed 24 h after treatment was stopped (day 12 p.i.), and the other 8 mice were observed for 2 weeks before sacrifice (day 26 p.i.). Plasma from the VT-1161-treated mice was collected at sacrifice and frozen until analysis. Lung homogenates were 10-fold serially diluted (1:10 to 1:1,000) onto GYE plates for quantitative culture as previously described (19); additionally, for lungs with fewer than 10 ≤1-mm granulomas, the entire lung homogenate was plated to capture counts below 100 CFU (18). Spleens were cultured whole on GYE plates to detect disseminated disease. One mouse that was found to be uninfected at sacrifice as well as mice that died of gavage injury were excluded from statistical analysis of results.
Mice were infected intracerebrally according to a previously published model (20, 21) for the CNS studies. Briefly, anesthetized mice were inoculated through the calvarium with a target dose of 90 spores in 30 μl of saline. Drug treatment was initiated at 48 h p.i. In the fungal burden study (n = 11/group), mice were treated for 7 days with 25 mg/kg VT-1161 (VT25) once daily or with VT50, FLC, or placebo and sacrificed 48 h after treatment was discontinued. In the survival study (n = 13 mice/group), mice were treated with VT25, FLC, or placebo for 14 days and sacrificed at 28 days posttreatment. At sacrifice, the brains and spinal cords were cultured quantitatively. Lungs, liver, spleen, and terminal plasma were collected and processed as described above.
VT-1161 plasma level analysis.
VT-1161 was extracted from plasma samples with methyl tert-butyl ether. Samples were analyzed using high-performance liquid chromatography with tandem mass spectrometry using electrospray ionization. Quantification was achieved against an external calibration curve generated in the same matrix and using the signal response ratio between the sample and the internal standard.
Statistical analysis.
Statistical analysis was performed using Systat, version 8.0. Total lung fungal burdens and the number of CFU/gram of the brain and spinal cord were log transformed and analyzed by analysis of variance (ANOVA). Where a fungal burden was 0, it was changed to 1 before log transformation. Statistical analysis of survival was performed with a Kruskal-Wallis test. Differences were considered significant at a P value of 0.05.
RESULTS
VT-1161 in vitro antifungal activity against Coccidioides spp.
DNA was isolated from 52 clinical strains, and all but one of the isolates from patients from central California were identified as C. immitis while all the Texas and Arizona specimens as well as those from sundry other locations were determined to be C. posadasii by the CocciDif assay (16). VT-1161 was tested in macrodilution assays against 23 C. immitis and 29 C. posadasii isolates (Table 1). The MIC50, MIC90, and geometric mean (GM) MIC values for VT-1161 against all 52 isolates were 1, 2, and 1.45 μg/ml, respectively, with a range of 1 to 4 μg/ml. Consistent with approved azole antifungals (22), the MIC ranges, MIC50, MIC90, and GM MIC values for VT-1161 were essentially the same for the two sets of isolates, and these values were lower than those observed for fluconazole. (Table 1).
TABLE 1.
VT-1161 and fluconazole MICs (μg/ml) against Coccidioides spp. clinical isolates
| Parameter | Value for the indicated drug (μg/ml)a |
|||||
|---|---|---|---|---|---|---|
|
C. immitis (n = 23) |
C. posadasii (n = 29) |
Coccidioides spp. (n = 52) |
||||
| VT-1161 | Fluconazole | VT-1161 | Fluconazole | VT-1161 | Fluconazole | |
| MIC range | 1–4 | 4–16 | 1–4 | 4–16 | 1–4 | 4–16 |
| MIC50 | 2 | 8 | 1 | 8 | 1 | 8 |
| MIC90 | 2 | 16 | 2 | 16 | 2 | 16 |
| GMb MIC | 1.48 | 8.50 | 1.43 | 8.19 | 1.45 | 8.33 |
C. immitis strains were isolated from patients in central California, while C. posadasii strains were isolated from patients in Arizona (n = 10), California (n = 1), Texas (n = 14), and other miscellaneous locations (n = 4).
Geometric mean.
Prior to animal studies, VT-1161 was also tested against the C. posadasii Silveira strain used to infect the mice; the MIC was 2 μg/ml. The MIC of fluconazole against C. posadasii was 16 μg/ml.
Efficacy of VT-1161 in murine respiratory coccidioidomycosis.
Based on mouse pharmacokinetics (PK) (23) and in vitro antifungal potency of VT-1161, oral doses of 10 (VT10) and 50 (VT50) mg/kg once daily were chosen to test in the respiratory model of coccidioidomycosis, with FLC (25 mg/kg twice daily) as the positive comparator and CrEL (once daily) as the placebo control. All placebo-treated mice either died of progressive disease on day 12 or 13 (survival arm) or were sacrificed per schedule, though the scheduled mice were moribund as well. In contrast, all but one of the antifungal-treated mice appeared healthy and survived until the scheduled sacrifice on either day 12 or day 26; one mouse in the VT10 survival group died on day 15. Due to the early deaths of all placebo-treated mice, the placebo survival and fungal burden groups were combined for statistical analysis of fungal burden (Table 2). Both doses of VT-1161 and FLC were highly efficacious compared to placebo treatment in the reduction of lung fungal burden (P < 0.001, all comparisons). Though VT50 resulted in slightly lower mean fungal burdens than FLC or VT10 at 1 day posttreatment, these differences were not significant. However, VT50 continued to reduce fungal burden in the 2 weeks following treatment (from log 5.24 to log 3.99 CFU; P = 0.001) and significantly reduced lung fungal burdens compared to values for VT10 and FLC 14 days after therapy had stopped (P < 0.001, both comparisons). None of the treatments eradicated infection in the mice.
TABLE 2.
Lung fungal burdens at 1 and 14 days posttreatment with VT-1161, fluconazole, or placebo
| Treatment group (dose) | Log10 CFU of lung (mean ± SD)a |
|
|---|---|---|
| Day 1 | Day 14 | |
| VT-1161 (10 mg/kg) | 5.60 ± 0.63 | 5.58 ± 0.82 |
| VT-1161 (50 mg/kg) | 5.24 ± 0.29 | 3.99 ± 0.15 |
| Fluconazole (50 mg/kg) | 5.4 ± 0.21 | 5.32 ± 0.24 |
| Placebo | 7.57 ± 0.18b | 7.57 ± 0.18b |
Values are means ± standard deviations at the indicated days posttreatment.
Placebo groups from both days of evaluation were combined for purposes of statistical analysis.
All antifungal treatments prevented early dissemination, as determined by the absence of fungal growth in spleens of mice sacrificed 1 day posttreatment (data not shown); however, following withdrawal of treatment, dissemination occurred in 87% and 75% of VT10- and FLC-treated mice, respectively, but in only 13% (1/8) of VT50-treated mice. In contrast, dissemination was observed in all mice in the placebo group.
Efficacy of VT-1161 for murine CNS infection.
As a model for treating coccidioidal meningitis in the clinic, VT-1161 was tested in the murine model of CNS infection at doses of 25 (VT25) and 50 (VT50) mg/kg, with the same positive and negative controls as described above. All placebo-treated animals developed neurological signs and succumbed to disease on days 7 to 8 p.i., while drug-treated mice remained healthy through the treatment period and the following 2 days until sacrifice in the initial fungal burden study.
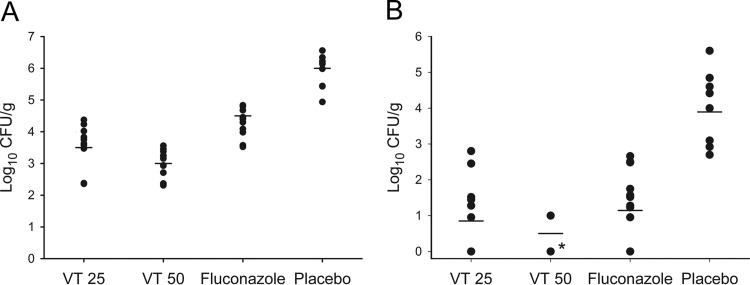
Figure 1 shows the number of CFU/gram of the brains and spinal cords. All antifungal treatments significantly lowered fungal burden in the brains and spinal cords compared to placebo treatment (P < 0.001, all comparisons). VT50 significantly lowered fungal burdens in both the brain and spinal cord compared to values for VT25 (P = 0.036 and P = 0.037, respectively) and FLC (P < 0.001, for both). No fungal growth was detected in the spinal cords from 10/11 VT50-treated mice. VT25 was superior to FLC in reducing the brain fungal burden (P = 0.007) but similar to FLC in the spinal cord measurements, with mean fungal burdens near 100 CFU/g for both groups.
FIG 1.
Fungal burdens in brain (A) and spinal cord (B) from mice with CNS coccidioidomycosis treated with VT-1161 or fluconazole for 7 days and sacrificed 2 days later. Horizontal bars represent mean values. All treatments significantly reduced brain and spinal cord CFU counts compared to treatment with placebo (P < 0.001). Activity of VT50 was superior to that of VT25 in both brain (P = 0.036) and spinal cord (P = 0.036) and also to that of fluconazole (P < 0.001, both comparisons). Activity of VT25 was superior to that of fluconazole in brain (P = 0.007) but not in spinal cord (P = 0.136). VT25, VT-1161 25 mg/kg; VT50, VT-1161 50 mg/kg (*, 10/11 mice had no fungal growth from spinal cord at time of sacrifice).
The lungs, liver, and spleen from all placebo-treated mice grew fungus, indicating extensive and early dissemination. In contrast, these organs were universally negative for fungal growth in the VT50-treated group. Among VT25- and FLC-treated animals, two mice had growth only from lungs, and two had growth from lungs and/or liver, respectively. Thus, whereas all antifungal treatments reduced dissemination outside the CNS, VT50 prevented it.
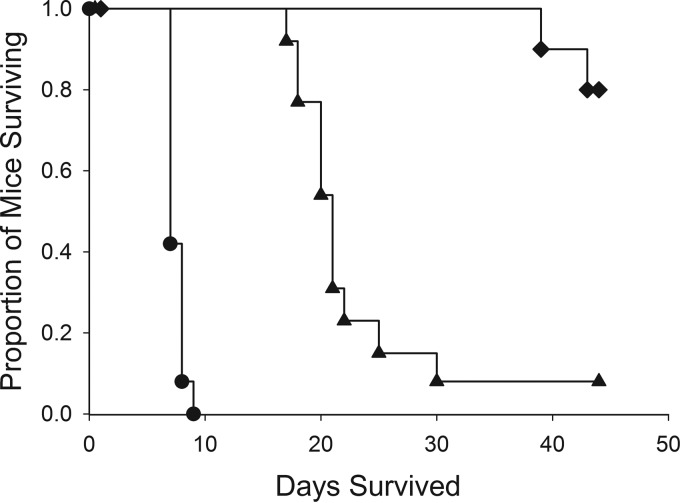
VT-1161 was subsequently tested at 25 mg/kg (VT25) once daily for 14 days and with the same control groups in a survival study of CNS coccidioidomycosis, with an extended observation period of 28 days posttreatment (Fig. 2). Similar to the results of the CNS fungal burden study, all placebo-treated mice succumbed to the infection at 7 to 9 days p.i. FLC-treated mice were clinically healthy throughout the treatment period and began to die with progressive neurological signs 2 days after the final dose, with only 1/13 surviving the duration of the study. All VT25-treated animals remained clinically healthy during the treatment period and the first 2 weeks posttreatment, with some neurological signs transiently appearing during the third week. Two of 13 mice succumbed to the infection, with clinical signs of weakness, weight loss, and dehydration, during the fourth week posttreatment, while the remaining 11 animals survived. Both drug treatments significantly enhanced survival compared to treatment with the placebo (P < 0.001), and results with VT25 were superior to those with FLC (P < 0.001).
FIG 2.
Survival of mice with CNS coccidioidomycosis. Starting 48 h after infection, mice were treated with VT-1161 and fluconazole for 14 days, after which they were observed for 28 days. Both treatments significantly lengthened survival compared to treatment with a placebo (P < 0.001), and VT-1161 prolonged survival significantly longer than fluconazole (P < 0.001; analysis by Kruskal-Wallis). Diamonds, VT-1161; triangles, fluconazole; circles, placebo.
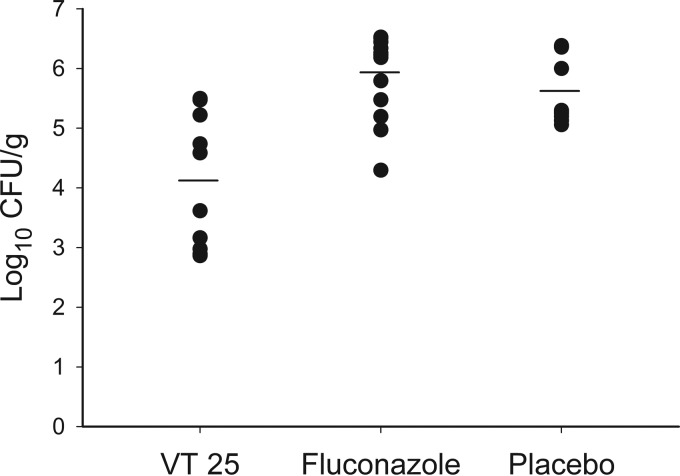
Brain fungal burden was also measured in the survival study, either at the time of sacrifice due to moribundity or at the end of the study (Fig. 3). Fungal burden was significantly reduced by VT25 treatment compared to treatment with the placebo and fluconazole (P < 0.001), while there was no difference between values for the last two groups (P = 0.197). VT-1161-treated animals had the lowest mean spinal cord fungal burden (16 CFU/g; range, 0 to 100 CFU/g), and 5/13 had no fungal growth. In contrast, colonies were recovered from the spinal cords of all mice in the placebo and FLC treatment groups. A total of 5/13 VT25-treated mice had fungal growth from at least one non-CNS organ (liver, lung, or spleen), while 13/13 and 12/13 mice in the FLC and placebo groups, respectively, had growth from at least one organ and usually from all three (data not shown).
FIG 3.
Brain fungal burdens of mice from the CNS coccidioidomycosis survival study. Mice were treated for 14 days, after which they were observed for 28 days. Fungal burden was quantitated when mice required sacrifice during the study or at the study termination. CFU counts for individual mice are shown in a scatterplot, with horizontal bars representing mean values. VT-1161 significantly reduced fungal burdens compared to placebo (P = 0.001) and fluconazole (P < 0.001) treatment. No statistically significant difference was observed between placebo and fluconazole results (P = 0.197). VT25, VT-1161 25 mg/kg per day. Fluconazole was given at 25 mg/kg twice daily.
VT-1161 plasma concentrations.
Table 3 shows VT-1161 plasma levels measured from both the respiratory and CNS studies. Concentrations after 7 days of treatment were dose proportional within the standard deviation of the measurements. The concentrations determined at the end of each study were all ∼1 μg/ml or higher even at 4 weeks posttreatment, consistent with the long half-life of VT-1161 (23). The levels at the end of the respiratory study relative to the levels taken 1 day posttreatment can be used to compare the elimination rates of VT-1161 within this study. The levels in the 10-mg/kg dose group decreased approximately 10-fold during the 14 days, while the levels in the higher-dose group (50 mg/kg) decreased only 2-fold, indicating that the half-life of VT-1161 in this study was dose dependent.
TABLE 3.
VT-1161 plasma concentrations from murine coccidioidomycosis studies
| VT-1161 dose (mg/kg)a | Plasma concn (μg/ml)b |
|||
|---|---|---|---|---|
| Respiratory study |
CNS study |
|||
| Day 1 | Day 14 | Day 2 | Day 28 | |
| 10 | 11 ± 2 | 0.9 ± 0.5 | ||
| 25 | 26 ± 8 | 1.5 ± 0.5c | ||
| 50 | 46 ±14 | 23 ± 8 | 52 ± 24 | |
Dosing was once daily for 7 days except as otherwise noted.
Values are means ± standard deviations at the indicated days posttreatment.
Dosing was once daily for 14 days.
DISCUSSION
The present studies showed that the investigational agent VT-1161 possesses intrinsic in vitro antifungal activity against both species of Coccidioides and is highly efficacious compared to the vehicle control in both the reduction of fungal burden and prolongation of survival in lethal murine respiratory and CNS models of coccidioidomycosis. Additionally, VT-1161 was rationally designed to selectively inhibit fungal CYP51 versus human CYPs (13, 14), thus potentially reducing toxicity and drug-drug interactions. Azole antifungals, which target the same fungal CYP51 enzyme, are less selective and suffer from a range of toxicities directly related to the binding of human CYPs, including hepatotoxicity, drug-drug interactions due to induction or binding of liver microsome CYPs and other enzymes, limitations of metabolism, and some toxicities unique to the individual drugs (7, 12, 24–26). The VT-1161 preclinical and clinical safety profiles to date show few if any of these concerns and are consistent with a reduction in binding to mammalian CYPs (14). Given all of the above, VT-1161 represents a potential advancement in the treatment of coccidioidomycosis, especially for chronic disease requiring long-term dosing.
Efficacy of VT-1161 in these studies was dose dependent, with higher doses producing increased plasma levels correlating with greater reductions in fungal burden. Mean plasma levels of both 11 and 46 μg/ml, achieved with doses of 10 and 50 mg/kg, respectively, were sufficient to prevent mortality in the respiratory model. However, the lower dose can be viewed as suboptimal because the fungal burden and dissemination rates observed in mice after the extended observation period were significantly higher than levels with the higher dose. In addition, in the short-term fungal burden CNS study, mean plasma levels of 52 μg/ml reduced brain fungal burden to a greater extent than the mean 26 μg/ml level and resulted in minimal spread of infection to either spinal cords or distant organs compared to infection spread with the lower dose. Although an exhaustive search for potentially fungicidal doses was outside the scope of this work, the doses and plasma levels of VT-1161 explored herein did not eradicate coccidioidal infection. This apparent lack of eradication is consistent with VT-1161's mechanism of CYP51 inhibition, which has been shown to be fungistatic for this class of antifungal drugs (11, 12).
Fluconazole is the most common antifungal used to treat coccidioidomycosis and is the standard treatment for CNS disease (8). It is water soluble, not dependent on gastric acidity for absorption, and at least 70% excreted unchanged in the urine, and therapeutically relevant concentrations are found in cerebrospinal fluids and CNS tissues (11, 27). Consequently, this drug was selected as the comparator antifungal for these studies. At all doses, activity of VT-1161 was either equivalent or superior to that of fluconazole in both the respiratory and CNS studies. Increased efficacy of VT-1161 was primarily demonstrated when treatment was withdrawn and mice were observed for 2 or 4 weeks, presumably due to its long half-life. VT-1161 also significantly reduced brain fungal burdens compared to levels with fluconazole in the CNS study, which demonstrated an improvement in antifungal potency over fluconazole that may have important clinical implications for the treatment of coccidioidal meningitis.
As stated above, prolonged survival and suppressed fungal burdens in 14- and 28-day posttreatment assessments with VT-1161 treatment compared to fluconazole treatment are suspected to be primarily due to the ongoing high plasma levels measured for VT-1161 and the predicted elimination of fluconazole based on published data (26). With a half-life of ∼5 h in mice (28, 29), fluconazole concentrations likely fell below effective plasma levels shortly after dosing stopped, with fungal regrowth ensuing. In the respiratory study, plasma levels in the high-dose VT-1161 group remained above the MIC against the strain used to infect mice throughout the posttreatment period and likely led to the further 1- to 2-log reduction of lung fungal burden. In contrast, fungal burdens remained essentially unchanged in mice treated with fluconazole or the lower dose of VT-1161, the latter of which had plasma levels below the MIC at 14 days posttreatment. Similarly, mice given either antifungal were clinically indistinguishable during treatment in the CNS survival study, but after therapy was stopped, the fluconazole-treated mice began to die 2 days later while the majority of mice treated with VT-1161 survived the observation period and retained plasma concentrations near the MIC against the infecting strain 28 days after treatment ended.
These studies demonstrated that VT-1161 is at least as efficacious in treating coccidioidomycosis as fluconazole and possibly superior, providing prolonged suppression of fungal burdens, improved survival, and reduced dissemination of coccidioidal infection. VT-1161 has been evaluated in two phase 2a studies (NCT01891305 and NCT01891331 [www.clinicaltrials.gov]) and, including phase 1 studies, has been dosed in >150 individuals. So far, both the safety and PK data in humans have mirrored animal data. Relevant oral doses provide microgram/milliliter plasma levels that are sustained long after dosing with no serious drug-related adverse effects (T. Degenhardt and R. J. Schotzinger, unpublished data). These favorable safety and PK attributes are especially ideal for long-term therapy and, in conjunction with the data presented in this report, warrant consideration of future clinical studies of VT-1161 for coccidioidomycosis.
ACKNOWLEDGMENTS
Funding for the CNS model study was under an NIH NIAID R21 grant (NIH 1R21AI-101497-01). All other studies were supported by Viamet Pharmaceuticals, Inc.
All tissue drug level measurement was done at OpAns, LLC (Durham, NC).
REFERENCES
- 1.Brown J, Benedict K, Park BJ, Thompson GR III. 2013. Coccidioidomycosis: epidemiology. Clin Epidemiol 5:185–197. doi: 10.2147/CLEP.S34434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sondermeyer G, Lee L, Gilliss D, Tabnak F, Vugia D. 2013. Coccidioidomycosis-associated hospitalizations, California, USA, 2000–2011. Emerg Infect Dis 19:1590–1597. doi: 10.3201/eid1910.130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MMWR. 2013. Increase in reported coccidioidomycosis—United States, 1998–2011. MMWR Morb Mortal Wkly Rep 62:217–221. [PMC free article] [PubMed] [Google Scholar]
- 4.Hector RF, Rutherford GW, Tsang CA, Erhart LM, McCotter O, Anderson SM, Komatsu k, Tabnak F, Vugia DJ, Yang Y, Galgiani JN. 2011. The public health impact of coccidioidomycosis in Arizona and California. Int J Environ Res Public Health 8:1150–1173. doi: 10.3390/ijerph8041150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blair JE. 2009. Coccidioidal meningitis: update on epidemiology, clinical features, diagnosis, and management. Curr Infect Dis Rep 11:289–295. doi: 10.1007/s11908-009-0043-1. [DOI] [PubMed] [Google Scholar]
- 6.Huang JY, Bristow B, Shafir S, Sorvillo F. 2012. Coccidioidomycosis-associated deaths, United States, 1990–2008. Emerg Infect Dis 18:1723–1728. doi: 10.3201/eid1811.120752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen C, Barker BM, Hoover S, Nix DE, Ampel NM, Frelinger JA, Orbach MJ, Galgiani JN. 2013. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev 26:505–525. doi: 10.1128/CMR.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, Williams PL. 2005. Coccidioidomycosis. Clin Infect Dis 41:1217–1223. doi: 10.1086/496991. [DOI] [PubMed] [Google Scholar]
- 9.Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, Davies SF, Dismukes WE, Hage CA, Marr KA, Mody CH, Perfect JR, Stevens DA. 2011. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med 183:96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]
- 10.Fromtling RA. 1988. Overview of medically important antifungal azole derivatives. Clin Microbiol Rev 1:187–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maertens JA. 2004. History of the development of azole derivatives. Clin Microbiol Infect 10(Suppl 1):1–10. doi: 10.1111/j.1470-9465.2004.00841.x. [DOI] [PubMed] [Google Scholar]
- 12.Nivoix Y, Leveque D, Herbrecht R, Koffel JC, Beretz L, Ubeaud-Sequier G. 2008. The enzymatic basis of drug-drug interactions with systemic triazole antifungals. Clin Pharmacokinet 47:779–792. doi: 10.2165/0003088-200847120-00003. [DOI] [PubMed] [Google Scholar]
- 13.Hoekstra WJ, Garvey EP, Moore WR, Rafferty SW, Yates CM, Schotzinger RJ. 2014. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorg Med Chem Lett 24:3455–3458. doi: 10.1016/j.bmcl.2014.05.068. [DOI] [PubMed] [Google Scholar]
- 14.Warrilow AG, Hull CM, Parker JE, Garvey EP, Hoekstra WJ, Moore WR, Schotzinger RJ, Kelly DE, Kelly SL. 2014. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother 58:7121–7127. doi: 10.1128/AAC.03707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun SH, Cole GT, Drutz DJ, Harrison JL. 1986. Electron-microscopic observations of the Coccidioides immitis parasitic cycle in vivo. J Med Vet Mycol 24:183–192. doi: 10.1080/02681218680000281. [DOI] [PubMed] [Google Scholar]
- 16.Sheff KW, York ER, Driebe EM, Barker BM, Rounsley SD, Waddell VG, Beckstrom-Sternberg SM, Beckstrom-Sternberg JS, Keim PS, Engelthaler DM. 2010. Development of a rapid, cost-effective TaqMan real-time PCR assay for identification and differentiation of Coccidioides immitis and Coccidioides posadasii. Med Mycol 48:466–469. doi: 10.3109/13693780903218990. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.Shubitz LF, Trinh HT, Perrill RH, Thompson CM, Hanan NJ, Galgiani JN, Nix DE. 2014. Modeling nikkomycin Z dosing and pharmacology in murine pulmonary coccidioidomycosis preparatory to phase 2 clinical trials. J Infect Dis 209:1949–1954. doi: 10.1093/infdis/jiu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shubitz LF, Yu J, Hung C, Kirkland TN, Peng T, Perrill R, Simons J, Xue J, Herr RA, Cole GT, Galgiani JN. 2006. Improved protection of mice against lethal respiratory infection with Coccidioides posadasii using two recombinant antigens expressed as a single protein. Vaccine 24:5904–5911. doi: 10.1016/j.vaccine.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Hector RF, Zimmer BL, Pappagianis D. 1990. Evaluation of nikkomycins X and Z in murine models of coccidioidomycosis, histoplasmosis, and blastomycosis. Antimicrob Agents Chemother 34:587–593. doi: 10.1128/AAC.34.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pappagianis D, Zimmer BL, Theodoropoulos G, Plempel M, Hector RF. 1990. Therapeutic effect of the triazole Bay R 3783 in mouse models of coccidioidomycosis, blastomycosis, and histoplasmosis. Antimicrob Agents Chemother 34:1132–1138. doi: 10.1128/AAC.34.6.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramani R, Chaturvedi V. 2007. Antifungal susceptibility profiles of Coccidioides immitis and Coccidioides posadasii from endemic and non-endemic areas. Mycopathologia 163:315–319. doi: 10.1007/s11046-007-9018-7. [DOI] [PubMed] [Google Scholar]
- 23.Garvey EP, Hoekstra WJ, Schotzinger RJ, Sobel JD, Lilly EA, Fidel PL Jr. 2015. Efficacy of the clinical agent VT-1161 against fluconazole-sensitive and -resistant Candida albicans in a murine model of vaginal candidiasis. Antimicrob Agents Chemother 59:5567–5573. doi: 10.1128/AAC.00185-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bates DW, Yu DT. 2003. Clinical impact of drug-drug interactions with systemic azole antifungals. Drugs Today (Barc) 39:801–813. doi: 10.1358/dot.2003.39.10.799473. [DOI] [PubMed] [Google Scholar]
- 25.Wang JL, Chang CH, Young-Xu Y, Chan KA. 2010. Systematic review and meta-analysis of the tolerability and hepatotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob Agents Chemother 54:2409–2419. doi: 10.1128/AAC.01657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki Y, Tokimatsu I, Sato Y, Kawasaki K, Sato Y, Goto T, Hashinaga K, Itoh H, Hiramatsu K, Kadota J. 2013. Association of sustained high plasma trough concentration of voriconazole with the incidence of hepatotoxicity. Clin Chim Acta 424:119–122. doi: 10.1016/j.cca.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Charlier C, Hart E, Lefort A, Ribaud P, Dromer F, Denning DW, Lortholary O. 2006. Fluconazole for the management of invasive candidiasis: where do we stand after 15 years? J Antimicrob Chemother 57:384–410. doi: 10.1093/jac/dki473. [DOI] [PubMed] [Google Scholar]
- 28.Hughes CE, Beggs WH. 1987. Action of fluconazole (UK-49,858) in relation to other systemic antifungal azoles. J Antimicrob Chemother 19:171–174. doi: 10.1093/jac/19.2.171. [DOI] [PubMed] [Google Scholar]
- 29.Humphrey MJ, Jevons S, Tarbit MH. 1985. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob Agents Chemother 28:648–653. doi: 10.1128/AAC.28.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]