Abstract
An Enterobacter cloacae isolate was recovered from a rectal swab from a patient hospitalized in France with previous travel to Switzerland. It was resistant to penicillins, narrow- and broad-spectrum cephalosporins, aztreonam, and carbapenems but remained susceptible to expanded-spectrum cephalosporins. Whereas PCR-based identification of the most common carbapenemase genes failed, the biochemical Carba NP test II identified an Ambler class A carbapenemase. Cloning experiments followed by sequencing identified a gene encoding a totally novel class A carbapenemase, FRI-1, sharing 51 to 55% amino acid sequence identity with the closest carbapenemase sequences. However, it shared conserved residues as a source of carbapenemase activity. Purified β-lactamase FRI-1 hydrolyzed penicillins, aztreonam, and carbapenems but spared expanded-spectrum cephalosporins. The 50% inhibitory concentrations (IC50s) of clavulanic acid and tazobactam were 10-fold higher than those found for Klebsiella pneumoniae carbapenemase (KPC), IMI, and SME, leading to lower sensitivity of FRI-1 activity to β-lactamase inhibitors. The blaFRI-1 gene was located on a ca. 110-kb untypeable, transferable, and non-self-conjugative plasmid. A putative LysR family regulator-encoding gene at the 5′ end of the β-lactamase gene was identified, leading to inducible expression of the blaFRI-1 gene.
INTRODUCTION
Carbapenem resistance in Enterobacteriaceae may be related to two mechanisms: (i) overexpression of a β-lactamases possessing no (or weak) activity against carbapenems (e.g., extended-spectrum beta-lactamase [ESBL] and cephalosporinases) combined with decreased outer membrane permeability and (ii) expression of enzymes able to hydrolyze carbapenems, namely, the carbapenemases (1). The most clinically relevant carbapenemases are classified into three groups according to protein sequence identity: (i) the Klebsiella pneumoniae carbapenemase (KPC)-type enzymes (Ambler class A), first described in the United States but now found worldwide (2, 3); (ii) the VIM, IMP, and NDM metallo-β-lactamases (Ambler class B) (1, 4); and (iii) the OXA-48-type enzymes (Ambler class D), widespread among Mediterranean countries and progressively disseminating to other geographical areas (5).
Ambler class A carbapenemases hydrolyze a large variety of β-lactams, including penicillins, cephalosporins, carbapenems, and aztreonam (6). Their hydrolytic activity is in vitro inhibited by clavulanic acid and tazobactam. Four main types of class A carbapenemases are known: NmcA/IMI, SME, KPC, and several variants of the GES type (GES-2, -4, -5, -6, and -11) (7).
The SME family includes three variants (SME-1 to -3). These enzymes have all been chromosome encoded in S. marcescens isolates (8–11). The chromosome-encoded NmcA and IMI enzymes have been detected in rare isolates of Enterobacter spp. (12, 13). The gene encoding the IMI-2 variant has been identified sporadically as plasmid located in environmental strains of Enterobacter spp. (14). The GES-type family includes 27 variants, only a few of which possess carbapenemase activity (7). All the GES variants possess the ability to hydrolyze broad-spectrum cephalosporins, but only some variants (mainly GES-2, GES-4, and GES-5 in Enterobacteriaceae) possess amino acid substitutions within their active sites (positions 104 and 170 according to the Ambler classification) that enlarge their spectra of activity against carbapenems (7, 15). Although still rare, GES enzymes have been identified worldwide. The most prevalent Ambler class A carbapenemase is KPC. This plasmid-encoded enzyme was first identified in 2001 in the United States (16). Since then, KPC-producing Enterobacteriaceae have spread worldwide, mostly due to the clonal dissemination of KPC-producing K. pneumoniae isolates of sequence type 258 (ST258) (2, 3). While this study was being completed, another class A carbapenemase, BKC-1, was identified from K. pneumoniae in Brazil (17).
The aim of this study was to characterize at the genetic and biochemical levels the molecular mechanisms of resistance to carbapenems from an Enterobacter cloacae isolate.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The E. cloacae isolate DUB was recovered from a urine sample from a patient, hospitalized in a suburb of Paris, with a previous history of travel (without hospitalization) in Switzerland. Identification of the clinical isolate was done by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (MALDI Biotyper CA system; Bruker Daltonics, Billerica, MA, USA). Escherichia coli TOP10 (Invitrogen, Saint-Aubin, France) was used for cloning experiments and azide-resistant E. coli J53 for conjugation assays. The kanamycin-resistant pBK-CMV (Invitrogen, Saint-Aubin, France) was used as the cloning vector. Bacterial cultures were grown in Trypticase soy (TS) broth at 37°C for 18 h unless otherwise indicated.
Susceptibility testing.
Antimicrobial susceptibilities were determined by the disc diffusion technique on Mueller-Hinton agar (Bio-Rad, Marnes-La-Coquette, France) and interpreted according to the EUCAST breakpoints as updated in 2015 (http://www.eucast.org). MICs were determined using the Etest technique (bioMérieux, La Balme-Les-Grottes, France).
Detection of carbapenemase activity.
Carbapenemase activity was analyzed by using two techniques, namely, the biochemical Carba NP test (18) and UV spectrophotometry, as previously described (19). Discrimination between Ambler class A, B, and non-A non-B carbapenemases was assessed using the Carba NP test II results, as previously described (20).
Molecular detection of carbapenemase-encoding genes.
PCR screening for the most common class A carbapenemase genes (blaKPC, blaIMP, blaVIM, blaNDM, blaOXA-48, blaGES, blaSFC-1, and blaIMI/NMC-A) was done as previously described (21).
Plasmid extraction and conjugation assays.
Plasmid DNA of E. cloacae DUB was extracted and analyzed using the Kieser method, as described previously (22). Recombinant plasmid DNA was prepared using Qiagen maxi columns (Qiagen, Courtaboeuf, France). Transfer of the imipenem resistance marker into E. coli TOP10 was attempted by electroporation. Transformants were selected on ticarcillin (100 μg/ml)-containing TS agar plates (Oxoid, Dardilly, France). Transfer of the β-lactam resistance marker into azide-resistant E. coli J53 was also attempted by liquid mating-out assays at 37°C. Transformants were selected on azide (100 μg/ml)- and ticarcillin (100 μg/ml)-containing TS agar plates. Plasmid typing was performed on electrotransformant strains by using the PCR-based replicon-typing (PBRT) method, as described previously (23).
Cloning experiments, recombinant plasmid analysis, and DNA sequencing.
PCR amplification of the identified blaFRI-1 gene was performed by using the internal primers FRI-1A (5′-TGAACTCATTCGCCTCTCAG-3′) and FRI-1B (5′-CTGCTTCGTCATGTTTGTCG-3′). Whole-cell DNA of the E. cloacae isolate was extracted using a QIAamp DNA minikit (Qiagen, Courtaboeuf, France). Partially Sau3AI-restricted DNA was ligated into the BamHI-restricted pBK-CMV plasmid and introduced into E. coli TOP10 by electroporation. Recombinant plasmids were selected on ticarcillin (100 μg/ml)- and kanamycin (50 μg/ml)-containing Trypticase soy agar plates. The recombinant plasmid possessing the shortest insert, namely, pFRI, was retained for further analysis. Both strands of the cloned DNA inserts of recombinant plasmids were sequenced by using an Applied Biosystems sequencer (ABI 377). The nucleotide and deduced protein sequences were analyzed with software available on the Internet from the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/BLAST/).
Protein analysis.
β-Lactamase extracts from cultures of E. cloacae DUB and the E. coli TOP10 strain harboring the recombinant plasmid pFRI were subjected to analytical isoelectric focusing (IEF) analysis. Multiple nucleotide and protein sequence alignments were carried out online using the program ClustalW (http://www.ebi.ac.uk/Tools/clustalW2/index.html).
β-Lactamase purification.
Purification of the β-lactamase FRI-1 was carried out by ion-exchange chromatography. E. coli TOP10(pFRI) was grown overnight at 37°C in 2 liters of TS broth containing ticarcillin (100 μg/ml) and kanamycin (50 μg/ml). The bacterial suspension was resuspended and disrupted by sonication in 10 ml of 20 mM triethanolamine buffer (pH 7.2) (Sigma-Aldrich, Saint Quentin Fallavier, France) and cleared by ultracentrifugation. The protein extracts obtained were loaded onto a preequilibrated S-Sepharose column (Amersham Pharmacia Biotech) in the same buffer. The β-lactamase recovered in the flowthrough was subsequently dialyzed against triethanolamine buffer (pH 9.5), loaded onto a Q-Sepharose column preequilibrated with the same buffer, and eluted with a linear NaCl gradient (0 to 500 mM). The fractions containing the highest β-lactamase activity, as determined qualitatively using nitrocefin hydrolysis (Oxoid, Dardilly, France), were pooled and dialyzed overnight against 50 mM sodium phosphate buffer (pH 7). The protein content was measured by the Bio-Rad DC protein assay. The protein purification rate and the relative molecular mass of the FRI-1 β-lactamase were estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
Kinetic studies.
Kinetic measurements (kcat and Km) of purified β-lactamase FRI-1 were performed as described previously (24). The 50% inhibitory concentration (IC50) for FRI-1 was determined as the concentration of clavulanate or tazobactam that reduced the hydrolysis rate of 100 μM benzylpenicillin by 50% under conditions in which FRI-1 was preincubated with various concentrations of inhibitor for 3 min at 30°C before the substrate was added.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper have been submitted to the GenBank nucleotide database under accession no. KT192551.
RESULTS
Susceptibility testing, carbapenemase detection, and IEF analysis.
E. cloacae DUB was resistant to amino-, carboxy-, and ureidopenicillins; narrow-spectrum cephalosporins; aztreonam; and carbapenems (Table 1). It remained susceptible to broad-spectrum cephalosporins (cefotaxime, cefepime, and cefpirome), except for ceftazidime (MIC, 4 μg/ml). E. cloacae DUB was susceptible to non-β-lactam antibiotics, except for rifampin. Addition of tazobactam or clavulanic acid partially restored susceptibility to ceftazidime and carbapenems (Table 1). The positivity of the Carba NP test identified the expression of carbapenemase. PCR experiments carried out on purified DNA of whole-cell E. cloacae DUB with primers specific for the most common carbapenemase genes (blaKPC, blaIMP, blaVIM, blaNDM, and blaOXA-48) remained negative. The Carba NP test II was therefore performed to identify the type of carbapenemase. The inhibition of carbapenemase activity by tazobactam suggested that this carbapenemase belonged to the Ambler class A group. Accordingly, additional PCR experiments were performed using primers specific for rarer Ambler class A carbapenemase genes (blaGES, blaSFC-1, and blaIMI/NMC-A), but they also remained negative. IEF analysis revealed that strain DUB produced an acquired β-lactamase with a pI value of ca. 8.4.
TABLE 1.
MICs of β-lactams for E. cloacae DUB, E. coli TOP10 transformed with the natural blaFRI-1-bearing plasmid (pDUB), E. coli TOP10 harboring a recombinant plasmid (pFRI), and the E. coli TOP10 reference strain
| β-Lactam(s) | MIC (μg/ml) |
|||
|---|---|---|---|---|
| E. cloacae DUB | E. coli TOP10(pDUB)a | E. coli TOP10(pFRI)b | E. coli TOP10 | |
| Amoxicillin | >256 | 128 | >256 | 2 |
| Amoxicillin + CLAc | >256 | 64 | 96 | 2 |
| Ticarcillin | >256 | >256 | >256 | 2 |
| Ticarcillin + CLA | 256 | 96 | 96 | 2 |
| Piperacillin | 128 | 12 | 24 | 1 |
| Piperacillin + TZBd | 96 | 8 | 12 | 1 |
| Cefalotin | >256 | 256 | >256 | 4 |
| Cefoxitin | >256 | 2 | 2 | 2 |
| Ceftazidime | 4 | 2 | 2 | 0.12 |
| Ceftazidime + CLA | 0.78 | 0.5 | 0.75 | 0.12 |
| Ceftazidime + TZB | 4 | 1.5 | 1.5 | 0.12 |
| Cefotaxime | 1 | 0.38 | 0.5 | 0.06 |
| Cefotaxime + CLA | 0.5 | 0.12 | 0.19 | 0.06 |
| Cefotaxime + TZB | 1 | 0.09 | 0.5 | 0.06 |
| Cefepime | 0.5 | 0.19 | 0.19 | 0.02 |
| Cefepime + CLA | 0.06 | 0.06 | 0.06 | 0.02 |
| Cefepime + TZB | 0.25 | 0.06 | 0.06 | 0.02 |
| Cefpirome | 1.5 | 0.25 | 0.38 | 0.02 |
| Cefpirome + CLA | 1 | 0.06 | 0.09 | 0.02 |
| Cefpirome + TZB | 0.75 | 0.06 | 0.38 | 0.02 |
| Aztreonam | 256 | 256 | 256 | 0.09 |
| Aztreonam + CLA | 256 | 8 | 32 | 0.09 |
| Aztreonam + TZB | 256 | 64 | 256 | 0.09 |
| Imipenem | 8 | 0.75 | 4 | 0.06 |
| Imipenem + CLA | 4 | 0.38 | 2 | 0.06 |
| Imipenem + TZB | 4 | 0.5 | 1.5 | 0.06 |
| Meropenem | 3 | 0.12 | 0.38 | 0.02 |
| Meropenem + CLA | 2 | 0.03 | 0.12 | 0.02 |
| Meropenem +TZB | 4 | 0.09 | 0.25 | 0.02 |
| Ertapenem | 24 | 0.12 | 0.75 | 0.06 |
| Ertapenem + CLA | 8 | 0.03 | 0.12 | 0.06 |
| Ertapenem + TZB | 4 | 0.06 | 0.38 | 0.06 |
pDUB, natural plasmid carrying the blaFRI-1 gene.
pFRI, the blaFRI-1 gene cloned in the pBK-CMV plasmid.
CLA, clavulanic acid at a fixed concentration of 4 μg/ml.
TZB, tazobactam at a fixed concentration of 4 μg/ml.
Cloning, conjugation, and transformation of the β-lactamase gene.
Shotgun cloning resulted in the selection of an E. coli TOP10(pFRI) recombinant strain that expressed a clavulanic acid-inhibited carbapenemase phenotype with resistance or reduced susceptibility to penicillins, ceftazidime, aztreonam, and carbapenems. The addition of clavulanic acid partially restored the activities of the β-lactams (Table 1). IEF analysis showed that E. coli TOP10(pFRI) produced a β-lactamase with a pI value of 8.4, identical to that identified in E. cloacae DUB (data not shown). Analysis of the plasmid DNA extract of the E. cloacae DUB isolate revealed a ca. 110-kb plasmid. Transfer of the imipenem resistance marker into E. coli TOP10 by electroporation was successful. In contrast, mating-out assays failed to give any transconjugant. PBRT analysis performed using a DNA extract of an E. coli TOP10 transformant (pDUB) (23) failed to identify a plasmid of any known incompatibility group.
Identification of β-lactamase FRI-1.
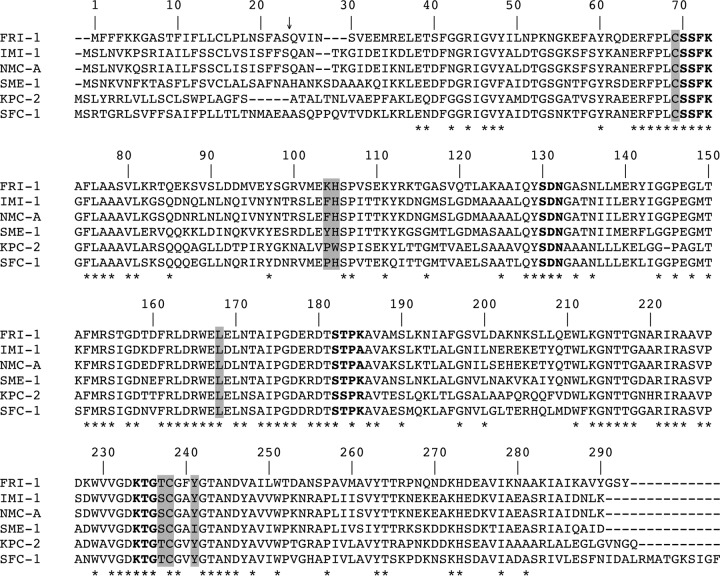
DNA sequence analysis of the 2,863-bp insert of pFRI revealed an open reading frame (ORF) of 885 bp encoding a 295-amino-acid preprotein, FRI-1 (French imipenemase), with a relative molecular mass of 32.5 kDa. The G+C content of this ORF was 39%. The signal peptide cleavage site was identified between the alanine and serine residues at positions 23 and 24 (AS-QV) of FRI-1 (Fig. 1). The β-lactamase FRI-1 contained four conserved motifs of class A serine β-lactamases, namely, 70SSFK73, 130SDN132, 166EXXXN170, and 234KTG236 (25) (Fig. 1). Interestingly, FRI-1 contains the amino acid residues that, associated, have been identified as a source of carbapenemase activity in class A β-lactamases; 69C, 105H, 167L, 237S, 238C, and 241Y (25–27). The β-lactamase FRI-1 shares 55%, 54%, 53%, 53%, and 51% amino acid identity with the class A carbapenemases NMC-A, IMI-1, SME-1, SFC-1, and KPC-2, respectively (Fig. 1). It shares 42% amino acid identity with the recently identified β-lactamase BKC-1 (data not shown). A dendrogram was generated from the amino acid sequence alignment of FRI-1 with main class A β-lactamases. It showed that FRI-1 is more closely related to the subgroup that includes SME-1, IMI-1, and NMC-A than to that of KPC-2, SFC-1, and BIC-1 (Fig. 2).
FIG 1.
Comparison of the amino acid sequence of FRI-1 with those of IMI-1, NMC-A, SME-1, KPC-2, and SFC-1. Dashes indicate the gaps that were inserted to optimize the alignment, and dashes indicate residues identical to those of BIC-1. The numbering is according to the method described by Ambler et al (33). The conserved domains of class A β-lactamases are in boldface. The residues shaded in gray are conserved among class A carbapenemases. The conserved residues are marked by asterisks. The arrow indicates the cleavage site for the leader peptide of FRI-1.
FIG 2.
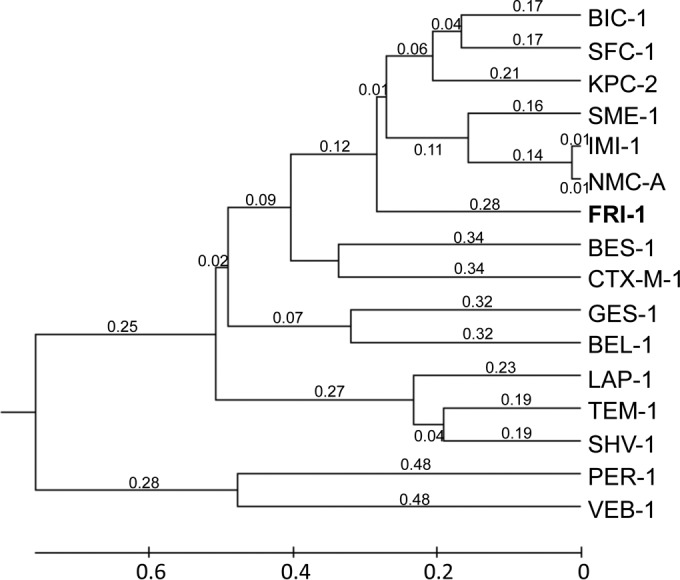
Dendrogram obtained for 16 representative class A β-lactamases by neighbor-joining analysis. The alignment used for the tree calculation was performed with the ClustalW program. Branch lengths are drawn to scale and are proportional to the number of amino acid changes. The distance along the vertical axis has no significance. The β-lactamases (GenBank accession numbers) are BIC-1 (GQ260093), SFC-1 (AY354402), KPC-2 (AY034847), SME-1 (Z28968), IMI-1 (U50278), NMC-A (Z21956), BES-1 (AF234999), CTX-M-1 (X92506), GES-1 (AF156486), BEL-1 (DQ089809), LAP-1 (EF026092), TEM-1 (AY458016), SHV-1 (AF148850), PER-1 (Z21957), and VEB-1 (AF010416).
Biochemical features of β-lactamase FRI-1.
The purification state of FRI-1 was estimated to be >95% by SDS-PAGE analysis (data not shown). Kinetic parameters of the purified β-lactamase FRI-1 showed that it possessed quite significant carbapenemase activity (Table 2). The highest kcat value for carbapenems was obtained with imipenem and was approximately 39- and 12-fold higher than those for meropenem and ertapenem, respectively (Table 2). The kcat/Km values obtained for FRI-1 were close to those obtained for NMC-A and IMI-1 (Table 2). Notably, the kcat value for aztreonam was very high (Table 2), which correlates with the high MIC values obtained with the E. coli TOP10 transformant and the E. coli TOP10(pFRI) recombinant strain (Table 1). Inhibition studies, as measured by IC50s, showed that the activity of FRI was weakly inhibited by clavulanic acid (IC50, 90 μM) and tazobactam (IC50, 15 μM). These values are in the same range as those found for SFC-1, 72.8 and 6.9 μM for clavulanic acid and tazobactam, respectively (28, 29). In addition, we observed that blaFRI-1 expression was inducible (∼8- to 10-fold) by imipenem (5 μg/ml) or by cefoxitin (50 μg/ml and 200 μg/ml).
TABLE 2.
Steady-state kinetic parameters of the β-lactamase FRI-1 and comparison of parameters obtained for the β-lactamases NMC-A (24), IMI-1 (13), SME-1 (11), KPC-2 (16), SFC-1 (28), and GES-4 (15)
| Parameter | β-Lactam | Valuea |
||||||
|---|---|---|---|---|---|---|---|---|
| FRI-1 | NMC-A | IMI-1 | SME-1 | KPC-2 | SFC-1 | GES-4 | ||
| kcat (s−1) | Benzylpenicillin | 1,060 | 260 | 36 | 19.3 | 63 | NA | 130 |
| Amoxicillin | >17,000 | 816 | 190 | 181 | NA | NA | 19 | |
| Ticarcillin | 120 | 81 | NA | NA | NA | NA | NA | |
| Piperacillin | >2,600 | NA | 6.1 | NA | NA | NA | NA | |
| Cefotaxime | >220 | 286 | 3.4 | <0.98 | 17 | 8.3 | 17 | |
| Cefepime | 28 | NA | NA | NA | 12 | NA | NA | |
| Ceftazidime | − | − | <0.01 | NA | 0.5 | 2.1 | 2.5 | |
| Aztreonam | >8,300 | 707 | 51 | 108 | 66 | 162 | NA | |
| Imipenem | 1,790 | 1,040 | 89 | 104 | 31 | 54 | 7.7 | |
| Ertapenem | 150 | NA | NA | NA | NA | NA | NA | |
| Meropenem | 46 | 12 | 10 | 8.9 | 3.6 | 6.5 | NA | |
| Km (μM) | Benzylpenicillin | 567 | 28 | 64 | 16.7 | 30 | NA | 160 |
| Amoxicillin | >5,000 | 90 | 780 | 488 | NA | NA | 62 | |
| Ticarcillin | 393 | 152 | NA | NA | NA | NA | NA | |
| Piperacillin | >3,000 | NA | 13 | NA | NA | NA | NA | |
| Cefotaxime | >5,000 | 956 | 190 | − | 100 | 89 | 700 | |
| Cefepime | 3,400 | NA | NA | NA | 540 | NA | NA | |
| Ceftazidime | − | − | 270 | − | 230 | 52 | 1,500 | |
| Aztreonam | >5,000 | 125 | 93 | 259 | 420 | 484 | − | |
| Imipenem | 1,614 | 92 | 170 | 202 | 90 | 82 | 4.7 | |
| Ertapenem | 98 | NA | NA | NA | NA | NA | NA | |
| Meropenem | 70 | 4.35 | 26 | 13.4 | 13 | 26 | NA | |
| kcat/Km (mM−1/s) | Benzylpenicillin | 1,870 | 9,300 | 560 | 1,160 | 2,100 | NA | 780 |
| Amoxicillin | 3,400 | 9,060 | 240 | 370 | NA | NA | 310 | |
| Ticarcillin | 305 | 530 | NA | NA | NA | NA | NA | |
| Piperacillin | 867 | NA | 470 | NA | NA | NA | NA | |
| Cefotaxime | 44 | 300 | 18 | − | 170 | 93 | 24 | |
| Cefepime | 8 | NA | NA | NA | 22 | NA | NA | |
| Ceftazidime | − | 52 | 0.02 | − | 2.1 | 40 | 1.7 | |
| Aztreonam | 1,660 | 5,600 | 550 | 420 | 160 | 3.5 | − | |
| Imipenem | 1,109 | 11,000 | 520 | 520 | 340 | 660 | 81 | |
| Ertapenem | 1,531 | NA | NA | NA | 280 | 250 | NA | |
| Meropenem | 657 | 2,700 | 380 | 660 | NA | NA | NA | |
−, no detectable hydrolysis; NA, no data available.
Genetic environment of the blaFRI-1 gene.
Part of the 5,750-bp insert of the recombinant plasmid pFRI was sequenced to identify the flanking sequences of the blaFRI-1 gene (Fig. 3). The blaFRI-1 gene was bracketed by two insertion sequences (IS). The closest IS shared 93% identity with ISRaq1 (GenBank accession no. AY528232), identified in Rahnella aquatilis. This ISRaq1-like IS was truncated by the insertion of another IS belonging to the IS66 family, sharing 87% identity with ISKpn24 (GenBank accession no. NC_014312). Upstream of blaFRI-1, a gene encoding FRI-R, a LysR transcriptional regulator sharing 63% amino acid identity with SmeR, the regulator of SME-1, was identified. The friR gene possessed a G+C content similar to that of the blaFRI-1 gene (39%).
FIG 3.
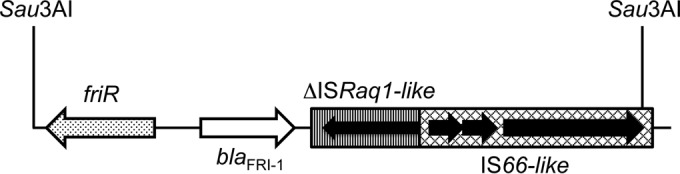
Schematic map of the structures surrounding blaFRI-1 identified in the E. cloacae DUB isolate. The genes and their corresponding transcriptional orientations are indicated by horizontal arrows. Sau3AI restriction sites that allowed cloning in the pBK-CMV plasmid (pFRI) are indicated.
DISCUSSION
Our study identified a novel plasmid-encoded class A carbapenemase from the urine of a French patient who had traveled in Switzerland. The β-lactamase FRI-1 shares the highest amino acid identity with the chromosome-encoded Ambler class A carbapenemases NMC-A from E. cloacae (12) and IMI-1 (13). Biochemical characterization of FRI-1 showed significant hydrolysis of carbapenemase, and its protein structure analysis identified conserved amino acid residues as a source of its carbapenemase activity. As observed for other class A β-lactamases, such as NMC-A (12), IMI-1 (13), and SFC-1 (30), FRI-1 confers a high level of resistance to aztreonam but does not confer significant resistance to broad-spectrum cephalosporins, such as ceftazidime, cefotaxime, and cefepime (Table 1). Therefore, its resistance profile differs from that of KPCs that hydrolyze all extended-spectrum β-lactams.
Analysis of the immediate upstream genetic environment of the blaFRI-1 gene identified a LysR-type transcriptional regulator, FriR, as previously observed for other class A carbapenemases, such as NMC-A, IMI-1, and SME (13, 31, 32). Notably, the G+C content of the blaFRI-1 gene and its regulator friR (39%) differed from that of E. cloacae genes (ca. 55%), suggesting the acquisition of the friR-blaFRI-1 locus through a horizontal gene transfer process. These results further emphasize that acquisition of carbapenemase genes by a group of Enterobacteriaceae results from acquisition “in block” of the β-lactamase gene and its regulator from a nonenterobacterial species acting as the reservoir. The presence of IS upstream and downstream of the DNA fragment friR-blaFRI-1 suggested that those elements might have been involved in the mobilization process. The plasmid location of the blaFRI-1 gene identified in an enterobacterial species adds to the list of carbapenemase genes able to disseminate worldwide.
Finally, this work highlights the need to use, not only molecular-based techniques, but also biochemical methods for the screening of carbapenemase-producing strains due to the growing diversity of carbapenemases.
ACKNOWLEDGMENTS
This work was financed by INSERM U914, Paris, France, and the University of Fribourg, Fribourg, Switzerland.
REFERENCES
- 1.Nordmann P, Dortet L, Poirel L. 2012. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med 18:263–272. doi: 10.1016/j.molmed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Cuzon G, Naas T, Truong H, Villegas MV, Wisell KT, Carmeli Y, Gales AC, Venezia SN, Quinn JP, Nordmann P. 2010. Worldwide diversity of Klebsiella pneumoniae that produce β-lactamase blaKPC-2 gene. Emerg Infect Dis 16:1349–1356. doi: 10.3201/eid1609.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dortet L, Poirel L, Nordmann P. 2014. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed Res Int 2014:249856. doi: 10.1155/2014/249856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67:1597–1606. doi: 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 6.Bush K. 2013. The ABCD's of β-lactamase nomenclature. J Infect Chemother 19:549–559. doi: 10.1007/s10156-013-0640-7. [DOI] [PubMed] [Google Scholar]
- 7.Kotsakis SD, Miriagou V, Tzelepi E, Tzouvelekis LS. 2010. Comparative biochemical and computational study of the role of naturally occurring mutations at Ambler positions 104 and 170 in GES β-lactamases. Antimicrob Agents Chemother 54:4864–4871. doi: 10.1128/AAC.00771-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naas T, Vandel L, Sougakoff W, Livermore DM, Nordmann P. 1994. Cloning and sequence analysis of the gene for a carbapenem-hydrolyzing class A β-lactamase, SME-1, from Serratia marcescens S6. Antimicrob Agents Chemother 38:1262–1270. doi: 10.1128/AAC.38.6.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poirel L, Wenger A, Bille J, Bernabeu S, Naas T, Nordmann P. 2007. SME-2-producing Serratia marcescens isolate from Switzerland. Antimicrob Agents Chemother 51:2282–2283. doi: 10.1128/AAC.00309-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Queenan AM, Shang W, Schreckenberger P, Lolans K, Bush K, Quinn J. 2006. SME-3, a novel member of the Serratia marcescens SME family of carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother 50:3485–3487. doi: 10.1128/AAC.00363-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Queenan AM, Torres-Viera C, Gold HS, Carmeli Y, Eliopoulos GM, Moellering RC Jr, Quinn JP, Hindler J, Medeiros AA, Bush K. 2000. SME-type carbapenem-hydrolyzing class A β-lactamases from geographically diverse Serratia marcescens strains. Antimicrob Agents Chemother 44:3035–3039. doi: 10.1128/AAC.44.11.3035-3039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordmann P, Mariotte S, Naas T, Labia R, Nicolas MH. 1993. Biochemical properties of a carbapenem-hydrolyzing β-lactamase from Enterobacter cloacae and cloning of the gene into Escherichia coli. Antimicrob Agents Chemother 37:939–946. doi: 10.1128/AAC.37.5.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasmussen BA, Bush K, Keeney D, Yang Y, Hare R, O'Gara C, Medeiros AA. 1996. Characterization of IMI-1 β-lactamase, a class A carbapenem-hydrolyzing enzyme from Enterobacter cloacae. Antimicrob Agents Chemother 40:2080–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aubron C, Poirel L, Ash RJ, Nordmann P. 2005. Carbapenemase-producing Enterobacteriaceae, U.S. rivers. Emerg Infect Dis 11:260–264. doi: 10.3201/eid1102.030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wachino J, Doi Y, Yamane K, Shibata N, Yagi T, Kubota T, Arakawa Y. 2004. Molecular characterization of a cephamycin-hydrolyzing and inhibitor-resistant class A β-lactamase, GES-4, possessing a single G170S substitution in the omega-loop. Antimicrob Agents Chemother 48:2905–2910. doi: 10.1128/AAC.48.8.2905-2910.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S, Bush K, Tenover FC. 2001. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 45:1151–1161. doi: 10.1128/AAC.45.4.1151-1161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicoletti AG, Marcondes MF, Martins WM, Almeida LG, Nicolas MF, Vasconcelos AT, Oliveira V, Gales AC. 2015. Characterization of BKC-1 class A carbapenemase from Klebsiella pneumoniae clinical isolates in Brazil. Antimicrob Agents Chemother 59:5159–5164. doi: 10.1128/AAC.00158-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dortet L, Brechard L, Poirel L, Nordmann P. 2014. Impact of the isolation medium for detection of carbapenemase-producing Enterobacteriaceae using an updated version of the Carba NP test. J Med Microbiol 63:772–776. doi: 10.1099/jmm.0.071340-0. [DOI] [PubMed] [Google Scholar]
- 19.Bernabeu S, Poirel L, Nordmann P. 2012. Spectrophotometry-based detection of carbapenemase producers among Enterobacteriaceae. Diagn Microbiol Infect Dis 74:88–90. doi: 10.1016/j.diagmicrobio.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Dortet L, Poirel L, Nordmann P. 2012. Rapid identification of carbapenemase types in Enterobacteriaceae and Pseudomonas spp. by using a biochemical test. Antimicrob Agents Chemother 56:6437–6440. doi: 10.1128/AAC.01395-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dortet L, Brechard L, Cuzon G, Poirel L, Nordmann P. 2014. Strategy for rapid detection of carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 58:2441–2445. doi: 10.1128/AAC.01239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kieser T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19–36. doi: 10.1016/0147-619X(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 23.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Mariotte-Boyer S, Nicolas-Chanoine MH, Labia R. 1996. A kinetic study of NMC-A β-lactamase, an Ambler class A carbapenemase also hydrolyzing cephamycins. FEMS Microbiol Lett 143:29–33. [DOI] [PubMed] [Google Scholar]
- 25.Majiduddin FK, Palzkill T. 2005. Amino acid residues that contribute to substrate specificity of class A β-lactamase SME-1. Antimicrob Agents Chemother 49:3421–3427. doi: 10.1128/AAC.49.8.3421-3427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majiduddin FK, Palzkill T. 2003. Amino acid sequence requirements at residues 69 and 238 for the SME-1 β-lactamase to confer resistance to β-lactam antibiotics. Antimicrob Agents Chemother 47:1062–1067. doi: 10.1128/AAC.47.3.1062-1067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papp-Wallace KM, Taracila M, Hornick JM, Hujer AM, Hujer KM, Distler AM, Endimiani A, Bonomo RA. 2010. Substrate selectivity and a novel role in inhibitor discrimination by residue 237 in the KPC-2 β-lactamase. Antimicrob Agents Chemother 54:2867–2877. doi: 10.1128/AAC.00197-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca F, Sarmento AC, Henriques I, Samyn B, van Beeumen J, Domingues P, Domingues MR, Saavedra MJ, Correia A. 2007. Biochemical characterization of SFC-1, a class A carbapenem-hydrolyzing β-lactamase. Antimicrob Agents Chemother 51:4512–4514. doi: 10.1128/AAC.00491-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naas T, Dortet L, Iorga BI. Structural and functional aspects of class A carbapenemases. Curr Drug Targets, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henriques I, Moura A, Alves A, Saavedra MJ, Correia A. 2004. Molecular characterization of a carbapenem-hydrolyzing class A β-lactamase, SFC-1, from Serratia fonticola UTAD54. Antimicrob Agents Chemother 48:2321–2324. doi: 10.1128/AAC.48.6.2321-2324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naas T, Livermore DM, Nordmann P. 1995. Characterization of an LysR family protein, SmeR from Serratia marcescens S6, its effect on expression of the carbapenem-hydrolyzing β-lactamase SME-1, and comparison of this regulator with other β-lactamase regulators. Antimicrob Agents Chemother 39:629–637. doi: 10.1128/AAC.39.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naas T, Nordmann P. 1994. Analysis of a carbapenem-hydrolyzing class A β-lactamase from Enterobacter cloacae and of its LysR-type regulatory protein. Proc Natl Acad Sci USA 91:7693–7697. doi: 10.1073/pnas.91.16.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambler RP, Coulson AF, Frère JM, Ghuysen JM, Joris B, Forsman M, Levesque RC, Tiraby G, Waley SG. 1991. A standard numbering scheme for the class A beta-lactamases. Biochem J 276:269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]