Abstract
A paucity of effective, currently available antibiotics and a lull in antibiotic development pose significant challenges for treatment of patients with multidrug-resistant (MDR) Acinetobacter baumannii infections. Thus, novel therapeutic strategies must be evaluated to meet the demands of treatment of these often life-threatening infections. Accordingly, we examined the antibiotic activity of gallium protoporphyrin IX (Ga-PPIX) against a collection of A. baumannii strains, including nonmilitary and military strains and strains representing different clonal lineages and isolates classified as susceptible or MDR. Susceptibility testing demonstrated that Ga-PPIX inhibits the growth of all tested strains when cultured in cation-adjusted Mueller-Hinton broth, with a MIC of 20 μg/ml. This concentration significantly reduced bacterial viability, while 40 μg/ml killed all cells of the A. baumannii ATCC 19606T and ACICU MDR isolate after 24-h incubation. Recovery of ATCC 19606T and ACICU strains from infected A549 human alveolar epithelial monolayers was also decreased when the medium was supplemented with Ga-PPIX, particularly at a 40-μg/ml concentration. Similarly, the coinjection of bacteria with Ga-PPIX increased the survival of Galleria mellonella larvae infected with ATCC 19606T or ACICU. Ga-PPIX was cytotoxic only when monolayers or larvae were exposed to concentrations 16-fold and 1,250-fold higher than those showing antibacterial activity, respectively. These results indicate that Ga-PPIX could be a viable therapeutic option for treatment of recalcitrant A. baumannii infections regardless of the resistance phenotype, clone lineage, time and site of isolation of strains causing these infections and their iron uptake phenotypes or the iron content of the media.
INTRODUCTION
Immediately following the advent of antibiotics as therapeutic agents, resistance to these drugs emerged among pathogenic bacteria. Drug-resistant bacterial strains are selected for immediately after initiation of antibiotic regimens, and as a consequence of continued selective pressure, very few treatment options now exist for infections caused by resistant strains of some pathogens (1, 2). The continuing battle with resistance has led to the initial emergence of pathogens displaying multidrug-resistant (MDR) phenotypes, which has since been followed by the emergence of extremely drug-resistant (XDR) or totally drug-resistant (TDR) strains, an outcome that has recreated the preantibiotic era (3, 4). This crisis has involved major Gram-positive and Gram-negative pathogens, including Enterococcus spp., Staphylococcus aureus, members of the family Enterobacteriaceae, Neisseria gonorrhoeae, Pseudomonas aeruginosa, and Acinetobacter spp. (4). The emergence of MDR strains of each of these microorganisms has contributed to increased morbidity and mortality among patients, leading to extended lengths of hospital stay and exorbitant health care financial burdens that often go unremitted. Compounding this continuum of resistance evolution and treatment failures is the stagnation in the development of new antimicrobial agents used to treat infectious diseases caused by multidrug-resistant organisms (MDROs) (3, 4). All of the aforementioned factors have resulted in a call to action from the Infectious Diseases Society of America (5) and highlight the critical need for alternative therapeutic options that could be used alone or in combination with standard antimicrobials for the treatment of infections caused by MDROs.
Iron acquisition has been considered an alternate target of antimicrobial agents, largely because of the critical role that iron plays in the physiology of bacteria, including pathogens that cause severe human infections (6, 7). Thus, siderophore-mediated iron acquisition processes have been targeted for the development of sideromycins, derivatives in which siderophores have been covalently linked to antibiotics, such as albomycin, salmycin, ferrymicins, the siderophore monosulfactam BAL30072, and a biscatecholate-monohydroxamate-carbacephalosporin conjugate (8–10). This “Trojan Horse” strategy has also included the use of gallium(III) as an anti-infective agent. Gallium binds to virtually all biological complexes that normally contain Fe(III), but it is not reduced to Ga(II) under physiological conditions and thus disrupts essential redox-driven biological processes (11). Accordingly, Ga has been used as simple inorganic and organic salts or complexed with organic compounds, including bacterial siderophores and porphyrins, such as protoporphyrin IX (12, 13). For example, gallium nitrate and gallium desferrioxamine showed antimicrobial activity against relevant human pathogens such as P. aeruginosa by killing free-living bacteria, blocking biofilm formation, and targeting iron metabolism (14, 15). Noniron metalloporphyrins have also been tested as antibacterials with Ga-protoporphyrin IX (Ga-PPIX) effecting the most potent action against Gram-positive and Gram-negative bacteria. The biological activity of this derivative (Fig. 1) seems to involve high-affinity uptake pathways, which depend on the expression of hemin/hemoglobin receptors, or low-affinity transport processes, whose mechanism of action are unknown (16). Furthermore, Ga-PPIX showed antimicrobial activity against N. gonorrhoeae when used topically in a murine vaginal infection model (17).
FIG 1.
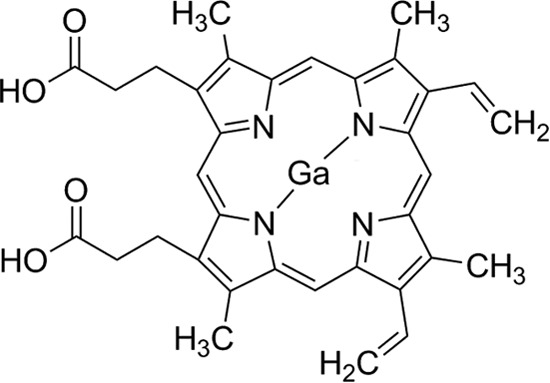
Molecular structure of gallium protoporphyrin IX (Ga-PPIX).
The Gram-negative obligate aerobe Acinetobacter baumannii is an opportunistic pathogen capable of causing a wide range of infections in humans, particularly in those with impaired immune systems (18, 19). The inherent and acquired resistance of clinical isolates of this pathogen complicates the severity of A. baumannii infections and their treatment because of the emergence of isolates that are resistant to all commercial antibiotics during the course of treatment (20, 21). This necessitates the investigation into novel chemotherapeutics needed for the treatment of infections, particularly those caused by MDR A. baumannii isolates obtained from patients with nosocomial infections and wounded patients. In spite of the increased occurrence of these infections, little is known about the pathobiology of this relevant pathogen. One of the best-understood interactions of this pathogen with the human host is its ability to acquire essential iron during the infection process. The results of genetic and functional analyses of the A. baumannii ATCC 19606 type strain showed that it acquires iron via the acinetobactin-mediated siderophore system, which proved to be critical for the infection of human alveolar epithelial cells, Galleria mellonella caterpillars, and mice (22). Furthermore, preliminary experimental data (23) and in silico genomic studies (24) indicate that A. baumannii expresses hemin acquisition systems that remain to be characterized experimentally. These observations suggest that Ga-containing derivatives could be effective microbiocides against A. baumannii MDR strains. Accordingly, gallium maltolate and gallium nitrate showed antibacterial activity in mice and G. mellonella larvae experimentally infected with different A. baumannii clinical strains, including MDR isolates, although the in vitro activity of these derivatives depended on the free-iron content of the media (25–27). Furthermore, the analysis of A. baumannii LAC-4, which is considered a hypervirulent clinical isolate, showed that it is resistant to gallium nitrate except when it is in the presence of hemin, which it can use as an alternative iron source. Interestingly, the hemin utilization phenotype of the LAC-4 strain is abrogated when Ga-PPIX is added to medium containing serum (28). In addition, the formation of Ga-siderophore complexes, such as those formed with staphyloferrin, could not result in effective antimicrobial activity (13). On the basis of these observations, we studied the effect of Ga-PPIX as an antimicrobial agent against a collection of A. baumannii clinical isolates that include strains obtained from military personnel with wound infections. Our results show that Ga-PPIX is an effective antimicrobial agent when tested under in vitro, ex vivo, and in vivo conditions independently of the antimicrobial resistance phenotype of the tested strains.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All strains used in this work, which are listed in Table S1 in the supplemental material, were stored at −80°C as Luria-Bertani (LB) glycerol stocks. All strains were routinely cultured in LB agar or broth at 37°C. For antimicrobial susceptibility determinations, strains were passaged on 5% sheep blood agar prior to growth in cation-adjusted Mueller-Hinton broth (CAMHB; Oxoid, Basingstoke, Hampshire, England) as previously described (29). Iron-rich and iron-chelated conditions were achieved by adding 100 μM FeCl3 and the synthetic iron chelator 2,2′-dipyridyl (DIP), respectively, to the culture media. The identities of the A. baumannii isolates listed in Table 1 were confirmed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) using a Bruker microflex analyzer. The MALDI Biotyper v3.1 software package (Bruker Daltonic, Billerica, MA) was used to characterize MS fingerprints comprised of the means of 240 mass spectra profiles for each isolate analyzed. Score values greater than 2.000 were considered acceptable for species-level identification. All A. baumannii strains provided by D. Zurawski (Walter Reed Army Institute of Research [WRAIR]) were described previously (30). A 10-mg/ml stock solution of Ga-PPIX (Frontier Scientific, Logan, UT), for use whenever Ga-PPIX was required, was prepared using cell culture-grade dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO) and then sterilized using 0.2-μm nylon membrane filters (Pall Corp., Port Washington, NY).
TABLE 1.
Antibiotic susceptibility profile of nonmilitary A. baumannii strainsa
| Strain | Penicillins |
Cephalosporins |
Carbapenems |
Fluoroquinolones |
Aminoglycosides |
Tetracyclines |
Miscellaneous |
|||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP |
SAM |
TZP |
CFZ |
FEP |
FOX |
CAZ |
CRO |
IMP |
MEM |
CIP |
LVX |
AMK |
GEN |
TOB |
TGC |
DOX |
CST |
SXT |
Ga-PPIX |
|||||||||||||||||||||
| MICb,d | CIc | MICd | CI | MICd | CI | MICd | CI | MICe | CI | MICd | CI | MICe | CI | MICd | CI | MICf | CI | MICe | CI | MICe | CI | MICe | CI | MICf | CI | MICe | CI | MICe | CI | MICe | CI | MICh | CI | MICe | CI | MICe | CI | MICi | CI | |
| ATCC 19606T | ≥32 | R | ≤2 | S | ≤4 | S | ≥64 | R | 24 | *g | ≥64 | R | 12 | * | 16 | I | ≤1 | S | 1.5 | S | 0.8 | S | 0.5 | S | 16 | S | 12 | R | 3 | S | 2 | * | 27 | S | 0.13 | S | >640 | R | 15 | * |
| ATCC 17978 | ≥32 | R | ≤2 | S | ≤4 | S | ≥64 | R | 3 | S | ≥64 | R | 6 | S | 16 | I | 1 | S | 0.8 | S | 0.3 | S | 0.2 | S | ≤4 | S | 1.5 | S | 0.5 | S | 0.38 | * | 26 | S | 0.38 | S | >640 | R | 17 | * |
| LUH07672 | ≥32 | R | ≤2 | S | ≤4 | S | ≥64 | R | 32 | R | ≥64 | R | >256 | R | ≥64 | R | ≤1 | S | 1.5 | S | >32 | R | 24 | R | >32 | R | >256 | R | 64 | R | 6 | * | 12 | I | 0.13 | S | >640 | R | 18 | * |
| LUH08809 | ≥32 | R | 4 | S | ≥128 | R | ≥64 | R | 96 | R | ≥64 | R | 192 | R | ≥64 | R | ≤1 | S | 8 | I | >32 | R | >32 | R | >32 | R | >256 | R | >256 | R | 8 | * | 12 | I | 0.13 | S | >640 | R | 20 | * |
| LUH05875 | ≥32 | R | 4 | S | ≥128 | R | ≥64 | R | >256 | R | ≥64 | R | 64 | R | ≥64 | R | ≤1 | S | 8 | I | >32 | R | >32 | R | >32 | R | >256 | R | 96 | R | 12 | * | 14 | S | 0.13 | S | >640 | R | 19 | * |
| LUH13000 | ≥32 | R | 4 | S | ≥128 | R | ≥64 | R | 16 | I | ≥64 | R | ≥256 | R | ≥64 | R | 2 | S | 1 | S | >32 | R | >32 | R | >32 | R | >256 | R | 96 | R | 4 | * | 6 | R | 0.3 | S | >640 | R | 20 | * |
| RUH00134 | ≥32 | R | ≥32 | R | 8 | S | ≥64 | R | 12 | I | ≥64 | R | 6 | S | 16 | I | ≤1 | S | 0.8 | S | 0.4 | S | 0.3 | S | 8 | S | ≥256 | R | 1.5 | S | 3 | * | 6 | R | 0.3 | S | ≥32 | R | 20 | * |
| RUH00875 | ≥32 | R | ≥32 | R | 16 | S | ≥64 | R | 24 | I | ≥64 | R | 16 | I | 16 | I | ≤1 | S | 2 | S | 0.8 | S | 0.4 | S | 16 | S | >256 | R | >256 | R | 3 | * | 10 | I | 0.19 | S | >640 | R | 19 | * |
| AYE | ≥32 | R | 8 | S | >128 | R | ≥64 | R | >256 | R | ≥64 | R | >256 | R | ≥64 | R | ≤1 | S | 2 | S | >32 | R | >32 | R | >32 | R | >256 | R | 48 | R | 3 | * | 15 | S | 0.38 | S | >640 | R | 14 | * |
| SDF | ND | - | ND | - | ND | - | ND | - | 2 | S | ND | - | 4 | S | ND | - | ≤1 | S | 1 | S | 0.3 | S | 0.3 | S | <4 | S | 0.5 | S | 0.2 | S | 0.5 | * | 26 | S | 0.09 | S | 5 | S | 11 | * |
| A118 | 16 | R | ≤2 | S | 8 | S | ≥64 | R | 3 | S | 4 | S | 6 | S | 16 | I | ≤1 | S | 0.8 | S | 0.2 | S | 0.1 | S | ≤4 | S | 1.5 | S | 0.8 | S | 0.38 | * | 27 | S | 0.13 | S | 0.8 | S | 18 | * |
| ACICU | ≥32 | R | ≥32 | R | ≥128 | R | ≥64 | R | ≥256 | R | ≥64 | R | ≥256 | R | ≥64 | R | >8 | R | ≥32 | R | ≥32 | R | ≥32 | R | ≥32 | R | 16 | R | 16 | R | 4 | * | 15 | S | 0.13 | S | ≥32 | R | 18 | * |
Antibiotic abbreviations: AMK, amikacin; AMP, ampicillin; CAZ, ceftazidime; CFZ, cefazolin; CIP, ciprofloxacin; COX, ceftriaxone; CST, colistin; FEP, cefepime; FOX, cefoxitin; Ga-PPIX, gallium protoporphyrin IX; GEN, gentamicin; IPM, imipenem; LVX, levofloxacin; MEM, meropenem; SAM, ampicillin-sulbactam; SXT, trimethoprim-sulfamethoxazole; TGC, tigecycline; TOB, tobramycin; TZP, piperacillin-tazobactam.
The MIC values are shown in micrograms per milliliter.
The categorical interpretations (CIs) are as follows: R, resistant; S, susceptible; I, intermediate; ND, not determined. Asterisks indicate the absence of interpretive standards.
MIC determined by Vitek-2 AST-GN73 card.
MIC determined by Etest.
MIC determined by BD Phoenix NMIC/ID-130 panel.
*, no interpretive standards available.
Susceptibility determined by Kirby-Bauer disk diffusion.
MIC determined by microdilution.
AST.
Strains were subjected to automated and manual antimicrobial susceptibility testing (AST) of standard antimicrobials and the experimental Ga-PPIX. MIC breakpoints and categorical interpretations for conventional antibiotics were mainly based on Clinical and Laboratory Standards Institute (CLSI) breakpoints (31). However, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints (http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_3.1.pdf) were used to interpret the MIC of gentamicin for A. baumannii 19606T. Currently, there are no breakpoints for tigecycline or Ga-PPIX.
Epsilometer (Etest)-based MIC tests (bioMérieux, Durham, NC) were performed and interpreted according to the manufacturer's instructions. Briefly, suspensions of the strains equivalent to a 0.5 McFarland standard were used to inoculate Mueller-Hinton agar plates (Remel, Lenexa, KS) prior to strip placement. The plates were incubated for 22 h in ambient air at 35°C prior to MIC interpretation. The MIC was interpreted by observing the concentration of the drug on the strip where the growth of the organism intersected the strip.
Isolate AST of Ga-PPIX and doxycycline was carried out by the Kirby-Bauer disk diffusion method according to standard procedures (32). Ga-PPIX stock solution was added to sterile 6-mm paper disks to deliver 50 μg or 100 μg of Ga-PPIX per disk and allowed to dry. Ga-PPIX paper disks and HardyDisk (Hardy Diagnostics, Franklin, OH) containing 30 μg doxycycline were applied to the surfaces of Mueller-Hinton agar plates inoculated with bacteria. Zones of inhibition (ZOI) were measured after incubation in ambient atmosphere at 37°C for 24 h. ZOI of doxycycline were classified as resistant, intermediate, or susceptible based on CLSI standards.
For automated AST determinations, the Vitek 2 XL (bioMérieux) microbial identification system and the BD Phoenix (BD Diagnostic Systems, Sparks, MD) automated microbiology system were used according to the manufacturer's instructions. The bioMérieux susceptibility card AST-GN73 and Phoenix NMIC/ID-130 panels were used for each platform, respectively. Interpretive criteria were based on CLSI breakpoints (31).
Broth microdilution MICs.
The MIC of Ga-PPIX was determined using the antimicrobial broth microdilution technique in the absence or presence of 10% heat-inactivated normal serum according to standard procedures (29). Briefly, broth microdilution plates were inoculated with 105 CFU/ml of each test organism in CAMHB and incubated for 24 h at 37°C in ambient air. Twofold dilutions of Ga-PPIX were performed; the concentrations ranged from 5 μg/ml to 166 μg/ml. A sample with no added antibiotic was included as a growth control on each plate. A more defined Ga-PPIX MIC was determined with 1-μg/ml resolution covering a range of 10 μg/ml to 20 μg/ml for selected strains. The wells on the plates were read for turbidity at an optical density at 595 nm (OD595) with a FilterMax F5 instrument using the multimode analysis software version 3.4.0.25 (Molecular Devices, Sunnyvale, CA). The MIC was determined as the microtiter plate's well with the lowest drug concentration at which there was no detectable growth. The MICs were determined at least three times in triplicate (n = 9) using fresh biological samples each time.
Ga-PPIX resistance frequency.
CAMH broth samples were inoculated with a 1:100 dilution of 24-h cultures of ATCC 19606T or ACICU strain and then incubated for 6 h at 37°C. Bacteria were plated onto CAMH agar plates containing 2× (40 μg/ml) and 4× (80 μg/ml) the MIC of Ga-PPIX. The population of bacteria treated with Ga-PPIX was quantified by plate count. Colonies were observed after 24 h and used to determine mutation frequency. The assay was performed twice in triplicate.
Time-kill assays.
Time-kill kinetics assays were performed to determine the rapidity of bactericidal activity of Ga-PPIX. The time-kill kinetic assays were performed basically as described previously (33). Briefly, after routine growth, 106 CFU/ml of A. baumannii ATCC 19606T or ACICU were inoculated into CAMHB. Cultures were then treated with 0, 10, 20, or 40 μg/ml Ga-PPIX, concentrations that corresponded to 0, 0.5, 1, or 2 times the MIC, respectively. The numbers of CFU were determined at 0, 2, 4, 6, and 24 h postinoculation by plate count. Assays were performed twice in triplicate (n = 6) using fresh biological samples each time.
A549 ex vivo assays.
To test the cytotoxicity of Ga-PPIX, an opaque white 96-well plate was seeded with 104 A549 human pulmonary adenocarcinoma cells and incubated for 24 h in Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Inc., Manassas, VA) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT), penicillin, and streptomycin at 37°C in a 5% CO2 atmosphere as previously described (22). Following incubation, the medium was exchanged for DMEM containing 10% heat-inactivated fetal bovine serum without antibiotics and supplemented with twofold dilutions ranging from 10 μg/ml to 640 μg/ml Ga-PPIX. The samples were incubated for 24 h, and cell viability was assayed using the CellTiter-Glo luminescent cell viability assay (Promega, Madison, WI). Luminescence was measured for 10 ms with a FilterMax F5 instrument. Experiments were performed twice in octuplet (n = 16). Groups were compared by analysis of variance (ANOVA) with Tukey's multiple-comparison test.
To test the antimicrobial effect of Ga-PPIX on the infection of submerged A549 cell monolayers, 24-well tissue culture plates were seeded with approximately 104 cells per well and then incubated for 16 h as described before (22). Bacteria were cultured for 24 h in CAMHB at 37°C with shaking at 200 rpm, collected by centrifugation at 21,000 × g for 10 min, washed, resuspended, and diluted in DMEM with 10% heat-inactivated fetal bovine serum without antibiotics or supplemented with 20 or 40 μg/ml Ga-PPIX. The A549 cell monolayers were infected with 104 CFU of the A. baumannii ATCC 19606T or ACICU strain. Inocula were estimated spectrophotometrically at OD600 and then confirmed by plate count. Infected-cell monolayers were incubated for 24 h at 37°C in 5% CO2. The tissue culture supernatants were collected, the A549 monolayers were lysed with sterile distilled H2O, and the lysates were added to the cognate tissue culture supernatants. Combined lysate samples were serially diluted and then plated on nutrient agar. After overnight incubation at 37°C, the numbers of CFU were counted, and the viable-cell plate count for each sample was calculated and recorded. Experiments were performed twice in octuplet (n = 16) using fresh biological samples each time and compared by ANOVA with Tukey's multiple-comparison test.
Galleria mellonella in vivo assays.
Ga-PPIX's toxicity and efficacy to treat experimental infections were tested using final instar larvae of the greater wax moth, G. mellonella (Grubco Inc., Fairfield, OH) as described before (22). Briefly, larvae weighing between 0.25 g and 0.35 g were randomly assigned to groups of 10 for each trial and then injected into the last left proleg using a syringe pump (New Era Pump Systems Inc., Farmingdale, NY) and 26.5-gauge needles. Larvae were inspected for survival every 24 h for 5 days. Dead larvae were removed at the time of inspection. If more than two larvae died in any control group, the results of the cognate trial were excluded from analysis, and the trial was repeated. Trials were performed in triplicate (n = 30).
Ga-PPIX in vivo toxicity was tested by using mass-matched G. mellonella larvae (i.e., mean mass of each group was statistically indistinguishable by ANOVA from any other group). Larvae were injected with 5 μl of twofold dilutions ranging from 0.2 mM to 25 mM (15.7 mg/ml to 126 μg/ml) Ga-PPIX solubilized in 0.5 N NaOH and 1.5% DMSO. Control groups were either injected or not injected with carrier solution. Probit analysis was performed using R 3.0 (www.r-project.org). To test antimicrobial activity, larvae were injected with a 5-μl bolus of 105 CFU of A. baumannii ATCC 19606T or A. baumannii ACICU suspended in a phosphate-buffered saline (PBS) solution containing 2% DMSO (carrier) or carrier supplemented with either 20 μg/ml or 40 μg/ml Ga-PPIX. Uninjected larvae and larvae injected with the same volume of sterile carrier were used as controls. Kaplan-Meier survival analysis and odds ratios were determined using Prism 6.0 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Source and antibiotic susceptibility profiles of A. baumannii strains used in this study.
Ga-PPIX proved to be an effective antimicrobial when tested against Gram-negative bacteria (Yersinia enterocolitica), Gram-positive bacteria (Staphylococcus aureus), and acid-fast bacilli (Mycobacterium smegmatis) (16). In spite of initial promising results, the application of Ga-PPIX to other pathogens has not been systematically tested. Thus, we used an approach similar to that described in the aforementioned publication (16) to test the Ga-PPIX susceptibility of an A. baumannii strain collection that included 30 A. baumannii strains isolated from U.S. wounded military personnel (strains 3132 to 3160 and 3284 in Table S1 in the supplemental material) and 12 nonmilitary isolates representing type strains, different international clonal lineages, strains whose genomes were fully sequenced and annotated, and strains that were classified as antimicrobial susceptible or MDR (strains 406 to 3022 and 3161 in Table S1). We initially tested the antibiotic susceptibility of all non-WRAIR strains, the identities of which were confirmed using a combination of standard bacteriological methods and modern technology, such as MALDI-TOF MS. This analysis showed that this set of strains includes isolates, such as SDF, that are susceptible to all tested antibiotics, which included more than six different classes of antimicrobials; isolates that display resistance to some antibiotics, such as ATCC 19606T and ATCC 17978; MDR isolates, such as LUH07672, LUH08809, LUH05875, LUH13000, RUH00134, and RUH00875; and isolates that are resistant to practically all drugs, such as AYE and ACICU, with ACICU being the most resistant isolate used in this work (Table 1). The U.S. military strain collection of clinical isolates includes 30 strains, each of which are resistant to most of the 11 antibiotics tested (30).
Growth inhibitory effects of Ga-PPIX.
All 42 tested strains displayed apparent growth when cultured in CAMHB at 37°C for 18 h using 96-well microtiter plates, although there was some growth variability among the strains (see Table S2 in the supplemental material). The addition of Ga-PPIX produced a dose-dependent growth response with several strains growing to higher optical densities in the presence of 5 μg/ml to 20 μg/ml of this nonferric metalloporphyrin when assayed using an automated twofold microdilution method. However, bacterial growth was significantly reduced when the CAMHB contained more than 20 μg/ml Ga-PPIX, making 40 μg/ml the MIC for all 42 strains when tested using this automated approach, which resulted in inocula containing 106 bacteria per sample. A more detailed manual analysis of the nonmilitary isolates following the CLSI standards (using inocula of 105 bacteria and CAMHB) using a twofold Ga-PPIX serial dilution scheme resulted in a MIC value of 20 μg/ml for all of these isolates. Furthermore, a 1-μg/ml resolution scheme performed under the latter experimental conditions resulted in Ga-PPIX MIC values ranging between 11 μg/ml and 20 μg/ml (Table 1). The median and mean MIC of these nonmilitary isolates was 19 μg/ml and 17.4 μg/ml, respectively. The presence of 10% heat-inactivated normal human serum increased the MIC for the ATCC 19606T and ACICU strains to 62.5 μg/ml, a threefold increase compared with the values obtained in the absence of proteins, without causing the complete inactivation or sequestration of Ga-PPIX. The Ga-PPIX susceptibility of these isolates was also tested using disk diffusion assays. All strains tested produced detectable ZOI ranging between 13 mm and 23 mm in diameter when the seeded plates were exposed to sterile filter disks impregnated with 50 μg or 100 μg Ga-PPIX, respectively (Fig. 2), and there were no heteroresistant colonies noted in the ZOI, which is represented in the insets of Fig. 2A and B. This observation is in agreement with the failure to isolate Ga-PPIX-resistant colonies of ATCC 19606T and ACICU when a population of greater than 9.8 × 109 bacteria were challenged to 2× (40 μg/ml) or 4× (80 μg/ml) the MIC of Ga-PPIX (data not shown).
FIG 2.
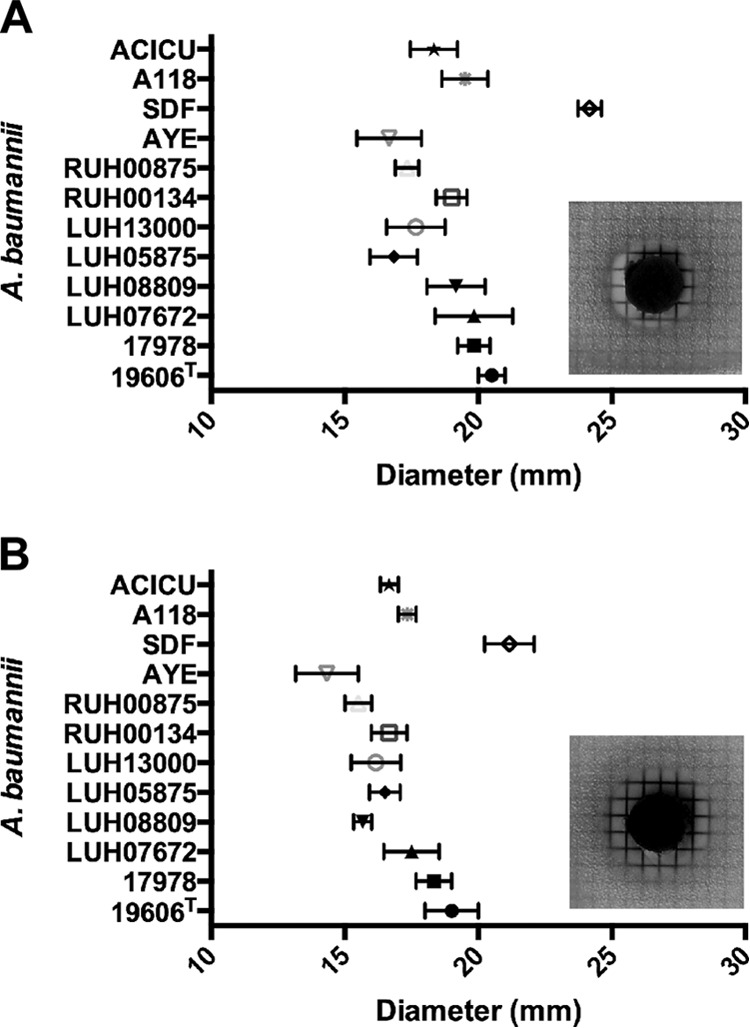
Ga-PPIX disk diffusion assays. A. baumannii bacteria were first seeded onto CAMH agar and then disks impregnated with 50 μg (A) or 100 μg (B) of Ga-PPIX were deposited on the surfaces of the plates. Growth inhibition halos were measured after incubation for 24 h at 37°C. Data are expressed as means ± standard errors of the means (SEM) (error bars) from experiments done twice in triplicate. The insets show the growth inhibition halos observed when the CAMH agar plates were seeded with A. baumannii ATCC 19606T and incubated for 24 h at 37°C.
Comparable results were obtained when the disk assays were conducted using LB agar plates that were supplemented with FeCl3 or DIP to generate iron-rich or iron-chelated conditions, respectively (data not shown). Furthermore, comparable Ga-PPIX susceptibility responses were detected with the ATCC 19606T strain and the isogenic derivatives ATCC 19606T t6 (strain 1653 in Table S1 in the supplemental material) and ATCC 19606T entA (strain 3069 in Table S1), which are affected in the uptake and biosynthesis of acinetobactin because of mutations in the genes coding for the BauA acinetobactin receptor protein and the EntA biosynthetic protein, respectively (34, 35). Additionally, the detailed analysis of A. baumannii AB5075 (strain 3156 in Table S1), a MDR isolate recently cultured from an infected wound that has been proposed as a model strain to study the pathobiology of this pathogen (30), showed a Ga-PPIX MIC of 20 μg/ml when cultured in CAMHB under standard laboratory conditions following CLSI guidelines (Fig. S1A and S1B in the supplemental material).
These experimental data collected using two different methods show that the susceptibility of different A. baumannii clinical strains to Ga-PPIX, with a standard MIC of 20 μg/ml, is independent of their time and site of isolation and their overall antimicrobial resistance phenotypes and clonal lineages. The data also indicate that the susceptibility to Ga-PPIX is independent of the free-iron content of the medium and the capacity of bacteria to produce or use acinetobactin, which seems to be the most common siderophore-mediated iron uptake system found in the genome of different A. baumannii clinical isolates (36).
Ga-PPIX time-kill kinetics.
The antibacterial activity of Ga-PPIX was tested by determining its time-kill kinetics using the ATCC 19606T and ACICU nonmilitary isolates, which are considered non-MDR and MDR strains, respectively, at a concentration of 0.5, 1, and 2 times the MIC as determined by the microdilution method. Both A. baumannii strains showed similar responses, with the addition of 10 μg/ml Ga-PPIX (0.5× MIC) causing only a small reduction in bacterial viability (Fig. 3). In contrast, the addition of 20 μg/ml (1× MIC) or 40 μg/ml (2× MIC) was effective in reducing the viable cells by >3 log units after 6 h of incubation. Furthermore, 1 × MIC did lead to a statistically significant reduction of 8 × 105- and 2.6 × 103-fold in viable cells after 24 h compared with the cognate untreated samples of ATCC 19606T and ACICU, respectively. Interestingly, 40 μg/ml of Ga-PPIX caused complete bacterial cell death for both the ATCC 19606T strain and ACICU MDR isolate. These results indicate that Ga-PPIX is both fast and effective in eliminating viable A. baumannii under in vitro conditions normally used to test the effectiveness of antimicrobial agents.
FIG 3.
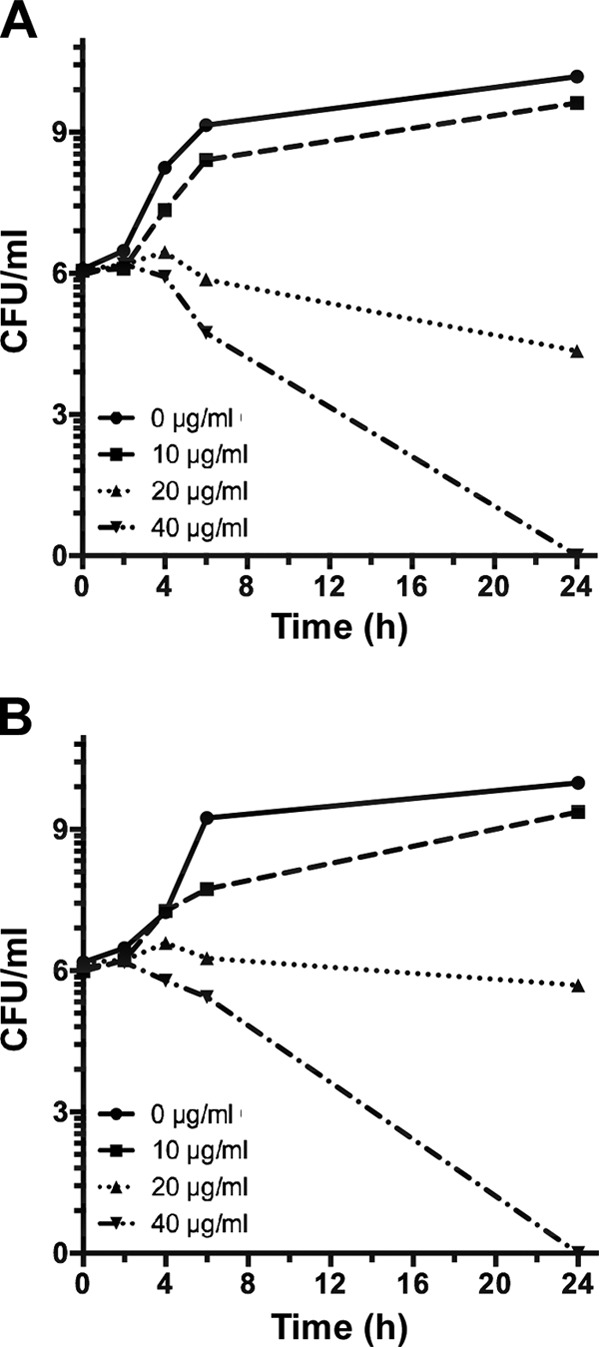
Ga-PPIX time-kill assays. Ga-PPIX time-kill kinetics for A. baumannii ATCC 19606T (A) and ACICU (B) was determined after 0, 2, 4, 6, and 24 h of incubation by CFU count after exposure to 0 μg/ml (0× MIC), 10 μg/ml (0.5× MIC), 20 μg/ml (1× MIC), or 40 μg/ml (2× MIC) Ga-PPIX.
Ga-PPIX toxicity to eukaryotic cells and G. mellonella larvae.
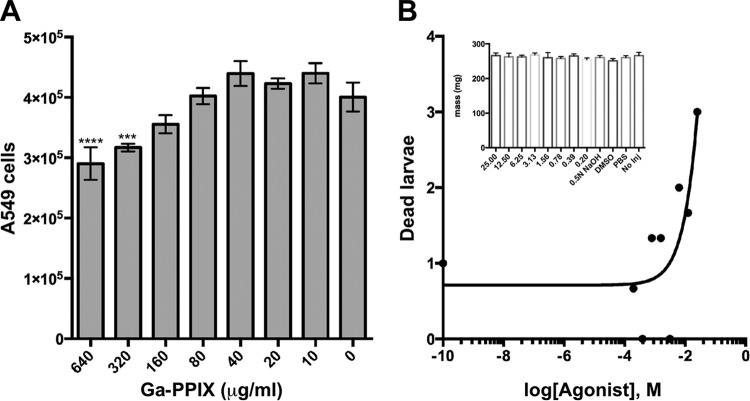
The toxicity of Ga-PPIX was tested using A549 alveolar epithelial tissue culture cells and G. mellonella caterpillars. Ga-PPIX exhibited no statistically significant toxicity for A549 cells at concentrations up to 160 μg/ml, 8 times the MIC, when tested using the CellTiter-Glo luminescent cell viability assay. This assay also showed that there was a statistically significant reduction in A549 viability when the culture medium contained 640 μg/ml (P ≤ 0.0001) or 320 μg/ml (P = 0.004) Ga-PPIX (Fig. 4A), concentrations that are far higher than the MIC values reported in the previous section. The ex vivo toxicity is corroborated by in vivo experiments using G. mellonella larvae (Fig. 4B) with comparable body mass (Fig. 4B, inset). After injection with Ga-PPIX over a 3-log-unit range (200 μM to 25 mM), the survival of larvae injected with 25 mM was significantly reduced than survival in the control group (P = 0.0363) (data not shown). The 50% lethal concentration (LC50) for Ga-PPIX in G. mellonella was extrapolated by probit analysis from the log dose-response curve to be 157 mg/ml (Fig. 4B). It was also observed that the injection of high concentrations of Ga-PPIX resulted in immediate coloration of the larvae, and subsequently excreted feces were noticeably pink (data not shown).
FIG 4.
Ga-PPIX toxicity toward A549 human cells and G. mellonella larvae. (A) Submerged A549 cell monolayers containing 4 × 105 cells were incubated with increasing concentrations of Ga-PPIX dissolved in DMSO and added to DMEM containing 10% heat-inactivated fetal bovine serum without antibiotics. Cell viability was determined after 24 h of incubation at 37°C in the presence of 5% CO2. Responses to the presence of Ga-PPIX were compared to those detected with cells cultured in the absence of this metalloporphyrin derivative. Values that are significantly different from the value for the control (0 μg/ml Ga-PPIX) are indicated by asterisks as follows: ***, P = 0.0004; ****, P < 0.0001. (B) Mass-matched G. mellonella larvae were injected with 5 μl of twofold dilutions ranging from 0.2 mM to 25 mM Ga-PPIX solubilized in 0.5 N NaOH and 1.5% DMSO. Larvae injected with 0.5 N NaOH, 1.5% DMSO, or PBS or not injected (No Inj) served as controls. The inset shows the mean masses of all animal groups with error bars showing standard errors.
These results demonstrate the ability of eukaryotic cells and organisms to tolerate Ga-PPIX at concentrations much higher than the MIC values determined using standard methods.
Ga-PPIX treatment of ex vivo and in vivo experimental infections.
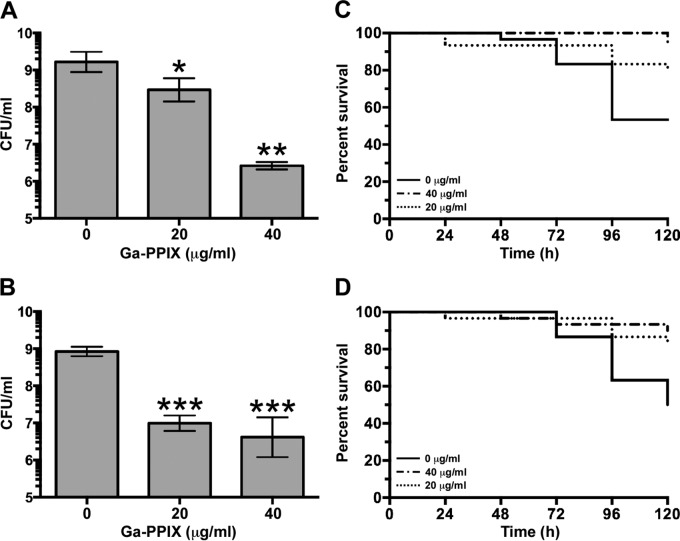
The antibacterial effectiveness of Ga-PPIX was determined by infecting A549 tissue cultures and G. mellonella larvae with the A. baumannii ATCC 19606T strain or the ACICU MDR strain in the presence or absence of this nonferric metalloporphyrin derivative. The presence of 20 μg/ml or 40 μg/ml of Ga-PPIX leads to a statistically significant reduction in CFU for ATCC 19606T (P = 0.037 or P = 0.014, respectively) and ACICU (P = 0.0005 or P = 0.0005, respectively) compared with the samples incubated in the absence of this nonferric metalloporphyrin derivative (Fig. 5A and B). Overall, the addition of 40 μg/ml of Ga-PPIX led to 701-fold and 145-fold reductions of ATCC 19606T and ACICU CFU recovered from the infected monolayers, respectively.
FIG 5.
Antibacterial activity of Ga-PPIX against experimental infections. Monolayers of A549 human alveolar epithelial cells (A and B) and G. mellonella larvae (C and D) were infected with A. baumannii ATCC 19606T strain (A and C) or ACICU strain (B and D) in the absence or the presence of either 20 μg/ml or 40 μg/ml of Ga-PPIX. Data shown in panels A and B represent the means (± SEM) of experiments performed twice in octuplet (n = 16) using fresh biological samples each time. Responses to the presence of Ga-PPIX were compared to those detected with bacteria cultured in the absence of this metalloporphyrin derivative. Values that are significantly different from the value for the control (0 μg/ml Ga-PPIX) are indicated by asterisks as follows: *, P = 0.037; **, P = 0.014; ***, P = 0.0005.
The G. mellonella in vivo infection model also showed the antibacterial activity of Ga-PPIX (Fig. 5C and D). Infection of larvae with an ATCC 19606T inoculum containing 20 μg/ml and 40 μg/ml Ga-PPIX significantly increased animal survival with P values of 0.03 and 0.0003, respectively, and log rank hazard ratios of 2.5 and 8.4, respectively, for each Ga-PPIX concentration tested compared with animals infected in the absence of this metalloporphyrin derivative (Fig. 5C). Similar results were obtained with the MDR ACICU strain (Fig. 5D), where injection with 20 μg/ml and 40 μg/ml of Ga-PPIX resulted in significant increases in survival with P = 0.008 and 0.001, respectively, and log rank hazard ratios of 3.4 and 5.0, respectively. Unfortunately, numerous attempts to test the antibacterial effect of Ga-PPIX by injecting it after infection failed to produce valid experimental data, since the death rates of caterpillars injected twice with 5 μl of sterile PBS, which were used as negative controls, were higher than those we consider acceptable—no more than two deaths per sample/per trial. This is one limitation of this otherwise convenient experimental virulence model we expect to address in the future by using vertebrate hosts where the volume of the inoculum is not as critical as is the case of G. mellonella.
Taken together, these observations indicate that Ga-PPIX has antibacterial activity when tested using experimental infection models previously used to show the role of iron acquisition functions in the virulence of A. baumannii (22).
DISCUSSION
Our previous work has shown that iron acquisition is a critical A. baumannii virulence trait when tested using ex vivo and in vivo experimental infection models (22). Furthermore, we observed that the presence of hemin promoted the growth of A. baumannii ATCC 19606T when cultured in the presence of the synthetic iron chelator 2,2′-dipyridyl. This response is independent of the expression of the acinetobactin-mediated system (23), which is the only complete siderophore-mediated system that allows this strain to prosper under iron-limiting conditions when tested using in vitro, ex vivo, and in vivo experimental conditions (22). All these observations strongly indicate that this strain expresses siderophore and hemin transport and utilization functions that could be targeted to treat A. baumannii infections, particularly those caused by MDR isolates. Our initial work (23) and unpublished preliminary observations indicate that the antibacterial activity of Ga nitrate is variable among all strains tested and depends on the free-iron content of the medium as has been reported before (26). These findings and the recent report that hypervirulent strains tolerate high concentrations of Ga nitrate (28) prompted us to study the antibacterial activity of Ga-PPIX against an A. baumannii strain collection that includes isolates obtained from different sources at different times, representing different clonal lineages and expressing different antibiotic resistance phenotypes. Our work demonstrated that all tested A. baumannii strains, including the A. baumannii AB5075 MDR military isolate recently proposed as a working model strain (30), displayed similar MIC values, which ranged between 11 and 20 μg/ml, when tested under standard laboratory conditions following CLSI guidelines. Furthermore, our data show that Ga-PPIX susceptibility is independent of the free-iron content of the media and the active expression of siderophore-mediated iron acquisition functions, while the presence of proteins in the in vitro, ex vivo, and in vivo susceptibility assays showed that they do not drastically affect the antibacterial activity of Ga-PPIX. Our data also showed that Ga-PPIX displays bactericidal activity relatively quickly, particularly when used at twice the MIC (40 μg/ml) under standard laboratory conditions, without causing any detectable cytotoxic effects on human cells and G. mellonella caterpillars. These findings are relevant since patients suffering from severe wound and soft tissue infections require immediate and effective antimicrobial chemotherapy. Equally concerning is the emergence of A. baumannii isolates with increased and/or altered virulence, such as the LAC-4 strain and isolates obtained from deadly cases of necrotizing fasciitis, respectively (37, 38). These infections require immediate and effective antimicrobial treatment not only because of the devastating nature of these infections but also because of the alarming MDR phenotype of the bacterial agents responsible for these diseases.
Our recent observation that the Ga-PPIX-susceptible strain AYE produces the hydroxamate siderophore baumannoferrin (39), but not acinetobactin or any catechol-derived siderophore due to a natural entA natural mutation (35), shows that the antibacterial activity of this nonferric metalloporphyrin extends beyond those strains that produce and use acinetobactin to acquire iron. More recently, it was reported that the A. baumannii ATCC 17978 strain, which could rely on the production and use of acinetobactin and fimsbactins to grow under free-iron limiting conditions (40), is also susceptible to Ga-PPIX. These observations further demonstrate that the antibacterial activity of Ga-PPIX is independent of the type or number of high-affinity iron chelators produced by a particular isolate. Whether these conditions also apply to the LAC-4 hypervirulent strain, which proved to be susceptible to Ga-PPIX (28), is currently unknown, since the siderophore-mediated iron acquisition system or systems expressed by this isolate is/are unknown.
The fact that Ga-PPIX displays bactericidal activity even in a medium such as LB agar, which has enough free iron to inhibit the expression of the acinetobactin system in the ATCC 19606T strain (41), demonstrates the antibacterial effectiveness of this noniron metalloporphyrin derivative even under conditions that inhibit the expression of critical iron-regulated bacterial functions, including those required for iron acquisition via siderophore or hemin uptake processes. However, the mechanisms by which Ga-PPIX is transported into the bacterial cytoplasm and affects iron metabolism are not fully understood. Pioneering work done by Stoljiljkovic et al. showed that Gram-negative bacteria, such as Yersinia enterocolitica, are susceptible to Ga-PPIX when expressing hemin acquisition functions under low-iron conditions (16). Similarly, work done by Moriwaki et al. showed that the Staphylococcus aureus IsdH-NEAT3 hemin receptor interacts with Ga-PPIX in a fashion similar to that of the natural hemin ligand (42). However, these results contradict our observations showing that Ga-PPIX displays antibacterial activity against bacteria cultured in iron-rich media such as LB agar or broth, which should significantly inhibit the expression of hemin transport functions according to data collected with Y. enterocolitica and Escherichia coli (16). Taken together, these results argue in favor of the hypothesis that Ga-PPIX could reach the periplasmic and cytoplasmic spaces either by using unknown low-affinity or TonB-independent hemin receptors or passive diffusion through bacterial membranes (16). Those hypotheses are supported by our data showing that the A. baumannii strains AYE and ATCC 17978, which lack the eight-gene cluster coding for putative hemin oxygenase and hemin utilization proteins recently described by de Léséleuc et al. (28), and ATCC 19606T strain, which does not have an identifiable hemO ortholog and therefore lacks this cluster, were as susceptible as the ACICU, SDF, and AB0057 strains, all of which harbor the aforementioned eight-gene cluster. The Ga-PPIX susceptibility of AYE, ATCC 17978, and ATCC 19606T could be also explained by the presence of a 12-gene cluster (iron uptake gene cluster 2), which codes for predicted hemin uptake functions but lacks a canonical hemO ortholog, that is also present in the genomes of the ACICU, SDF, and AB0057 strains (24). Together, these observations indicate that the widespread susceptibility of A. baumannii to Ga-PPIX is mediated by more than one cellular process, a property that will most likely not favor the emergence of resistant derivatives at least in the short term as could be predicted from our work, which failed to detect a resistance phenotype using disk diffusion assays, broth MIC, or mutation rate studies.
In summary, our work shows that A. baumannii strains are susceptible to Ga-PPIX regardless of their clonal lineages, site and time of isolation, antimicrobial resistance phenotypes, expression of iron acquisition systems, iron content of the media, or presence of proteins in in vitro and ex vivo tissue culture susceptibility assays. All these properties make this nonferric metalloporphyrin derivative a potentially valuable and convenient antimicrobial agent that affects the viability of bacterial cells not only by interfering with critical physiological processes that depend on the redox properties of iron but also making bacterial cells more susceptible to oxidative stresses due to the presence of reactive oxygen species produced by bacteria growing under aerobic conditions as well as by the host in response to infection (16). However, the relatively low solubility of Ga-PPIX in aqueous solutions is one obstacle that must be overcome before this compound is introduced as a viable therapeutic option, either alone or in combination with current antibiotics, for the treatment of A. baumannii infections, particularly those caused by isolates that are highly resistant to current antimicrobial agents.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funds from Public Health AI070174 and Department of Defense W81XWH-12-2-0035 grants and Miami University research funds.
We are grateful to Daniel V. Zurawski from Walter Reed Army Institute of Research for providing the A. baumannii strains listed in Table S1 in the supplemental material.
The findings and opinions expressed herein belong to the authors and do not necessarily reflect the official views of the WRAIR, U.S. Army, or Department of Defense.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01472-15.
REFERENCES
- 1.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. 2010. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 2.Hawser SP, Bouchillon SK, Hoban DJ, Badal RE, Hsueh PR, Paterson DL. 2009. Emergence of high levels of extended-spectrum-beta-lactamase-producing gram-negative bacilli in the Asia-Pacific region: data from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program, 2007. Antimicrob Agents Chemother 53:3280–3284. doi: 10.1128/AAC.00426-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 4.Rossolini GM, Arena F, Pecile P, Pollini S. 2014. Update on the antibiotic resistance crisis. Curr Opin Pharmacol 18:56–60. doi: 10.1016/j.coph.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J Jr, Infectious Diseases Society of America. 2008. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis 46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- 6.Bullen JJ, Rogers HJ, Spalding PB, Ward CG. 2005. Iron and infection: the heart of the matter. FEMS Immunol Med Microbiol 43:325–330. doi: 10.1016/j.femsim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Crosa JH, Mey AR, Payne SM. 2004. Iron transport in bacteria. ASM Press, Washington, DC. [Google Scholar]
- 8.Braun V, Pramanik A, Gwinner T, Koberle M, Bohn E. 2009. Sideromycins: tools and antibiotics. Biometals 22:3–13. doi: 10.1007/s10534-008-9199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Carvalho CC, Fernandes P. 2014. Siderophores as “Trojan Horses”: tackling multidrug resistance? Front Microbiol 5:290. doi: 10.3389/fmicb.2014.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mollmann U, Heinisch L, Bauernfeind A, Kohler T, Ankel-Fuchs D. 2009. Siderophores as drug delivery agents: application of the “Trojan Horse” strategy. Biometals 22:615–624. doi: 10.1007/s10534-009-9219-2. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein LR. 1998. Mechanisms of therapeutic activity for gallium. Pharmacol Rev 50:665–682. [PubMed] [Google Scholar]
- 12.Bonchi C, Imperi F, Minandri F, Visca P, Frangipani E. 2014. Repurposing of gallium-based drugs for antibacterial therapy. Biofactors 40:303–312. doi: 10.1002/biof.1159. [DOI] [PubMed] [Google Scholar]
- 13.Kelson AB, Carnevali M, Truong-Le V. 2013. Gallium-based anti-infectives: targeting microbial iron-uptake mechanisms. Curr Opin Pharmacol 13:707–716. doi: 10.1016/j.coph.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Banin E, Lozinski A, Brady KM, Berenshtein E, Butterfield PW, Moshe M, Chevion M, Greenberg EP, Banin E. 2008. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc Natl Acad Sci U S A 105:16761–16766. doi: 10.1073/pnas.0808608105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK. 2007. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J Clin Invest 117:877–888. doi: 10.1172/JCI30783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stojiljkovic I, Kumar V, Srinivasan N. 1999. Non-iron metalloporphyrins: potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol Microbiol 31:429–442. doi: 10.1046/j.1365-2958.1999.01175.x. [DOI] [PubMed] [Google Scholar]
- 17.Bozja J, Yi K, Shafer WM, Stojiljkovic I. 2004. Porphyrin-based compounds exert antibacterial action against the sexually transmitted pathogens Neisseria gonorrhoeae and Haemophilus ducreyi. Int J Antimicrob Agents 24:578–584. doi: 10.1016/j.ijantimicag.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 19.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doi Y, Husain S, Potoski BA, McCurry KR, Paterson DL. 2009. Extensively drug-resistant Acinetobacter baumannii. Emerg Infect Dis 15:980-982. doi: 10.3201/eid1506.081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishbain J, Peleg AY. 2010. Treatment of Acinetobacter infections. Clin Infect Dis 51:79–84. doi: 10.1086/653120. [DOI] [PubMed] [Google Scholar]
- 22.Gaddy JA, Arivett BA, McConnell MJ, Lopez-Rojas R, Pachon J, Actis LA. 2012. Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect Immun 80:1015–1024. doi: 10.1128/IAI.06279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimbler DL, Penwell WF, Gaddy JA, Menke SM, Tomaras AP, Connerly PL, Actis LA. 2009. Iron acquisition functions expressed by the human pathogen Acinetobacter baumannii. Biometals 22:23–32. doi: 10.1007/s10534-008-9202-3. [DOI] [PubMed] [Google Scholar]
- 24.Antunes LC, Imperi F, Towner KJ, Visca P. 2011. Genome-assisted identification of putative iron-utilization genes in Acinetobacter baumannii and their distribution among a genotypically diverse collection of clinical isolates. Res Microbiol 162:279–284. doi: 10.1016/j.resmic.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Antunes LC, Imperi F, Minandri F, Visca P. 2012. In vitro and in vivo antimicrobial activities of gallium nitrate against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 56:5961–5970. doi: 10.1128/AAC.01519-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Léséleuc L, Harris G, KuoLee R, Chen W. 2012. In vitro and in vivo biological activities of iron chelators and gallium nitrate against Acinetobacter baumannii. Antimicrob Agents Chemother 56:5397–5400. doi: 10.1128/AAC.00778-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLeon K, Balldin F, Watters C, Hamood A, Griswold J, Sreedharan S, Rumbaugh KP. 2009. Gallium maltolate treatment eradicates Pseudomonas aeruginosa infection in thermally injured mice. Antimicrob Agents Chemother 53:1331–1337. doi: 10.1128/AAC.01330-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Léséleuc L, Harris G, KuoLee R, Xu HH, Chen W. 2014. Serum resistance, gallium nitrate tolerance and extrapulmonary dissemination are linked to heme consumption in a bacteremic strain of Acinetobacter baumannii. Int J Med Microbiol 304:360–369. doi: 10.1016/j.ijmm.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically - 7th ed. Approved standard. CLSI document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 30.Jacobs AC, Thompson MG, Black CC, Kessler JL, Clark LP, McQueary CN, Gancz HY, Corey BW, Moon JK, Si Y, Owen MT, Hallock JD, Kwak YI, Summers A, Li CZ, Rasko DA, Penwell WF, Honnold CL, Wise MC, Waterman PE, Lesho EP, Stewart RL, Actis LA, Palys TJ, Craft DW, Zurawski DV. 2014. AB5075, a highly virulent isolate of Acinetobacter baumannii, as a model strain for the evaluation of pathogenesis and antimicrobial treatments. mBio 5:e01076–14. doi: 10.1128/mBio.01076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; twenty-third informational supplement M100-23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 32.Bauer AW. 1966. Current status of antibiotic susceptibility testing with single high potency discs. Am J Med Technol 32:97–102. [PubMed] [Google Scholar]
- 33.Petersen PJ, Jones CH, Bradford PA. 2007. In vitro antibacterial activities of tigecycline and comparative agents by time-kill kinetic studies in fresh Mueller-Hinton broth. Diagn Microbiol Infect Dis 59:347–349. doi: 10.1016/j.diagmicrobio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Dorsey CW, Tomaras AP, Connerly PL, Tolmasky ME, Crosa JH, Actis LA. 2004. The siderophore-mediated iron acquisition systems of Acinetobacter baumannii ATCC 19606 and Vibrio anguillarum 775 are structurally and functionally related. Microbiology 150:3657–3667. doi: 10.1099/mic.0.27371-0. [DOI] [PubMed] [Google Scholar]
- 35.Penwell WF, Arivett BA, Actis LA. 2012. The Acinetobacter baumannii entA gene located outside the acinetobactin cluster is critical for siderophore production, iron acquisition and virulence. PLoS One 7:e36493. doi: 10.1371/journal.pone.0036493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahl JW, Gillece JD, Schupp JM, Waddell VG, Driebe EM, Engelthaler DM, Keim P. 2013. Evolution of a pathogen: a comparative genomics analysis identifies a genetic pathway to pathogenesis in Acinetobacter. PLoS One 8:e54287. doi: 10.1371/journal.pone.0054287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charnot-Katsikas A, Dorafshar AH, Aycock JK, David MZ, Weber SG, Frank KM. 2009. Two cases of necrotizing fasciitis due to Acinetobacter baumannii. J Clin Microbiol 47:258–263. doi: 10.1128/JCM.01250-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris G, Kuo Lee R, Lam CK, Kanzaki G, Patel GB, Xu HH, Chen W. 2013. A mouse model of Acinetobacter baumannii-associated pneumonia using a clinically isolated hypervirulent strain. Antimicrob Agents Chemother 57:3601–3613. doi: 10.1128/AAC.00944-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penwell WF, DeGrace N, Tentarelli S, Gauthier L, Gilbert CM, Arivett BA, Miller AA, Durand-Reville TF, Joubran C, Actis LA. 2015. Discovery and characterization of new hydroxamate siderophores, baumannoferrin A and B, produced by Acinetobacter baumannii. Chembiochem 16:1896–1904. doi: 10.1002/cbic.201500147. [DOI] [PubMed] [Google Scholar]
- 40.Proschak A, Lubuta P, Grun P, Lohr F, Wilharm G, De Berardinis V, Bode HB. 2013. Structure and biosynthesis of fimsbactins A-F, siderophores from Acinetobacter baumannii and Acinetobacter baylyi. Chembiochem 14:633–638. doi: 10.1002/cbic.201200764. [DOI] [PubMed] [Google Scholar]
- 41.Nwugo CC, Gaddy JA, Zimbler DL, Actis LA. 2011. Deciphering the iron response in Acinetobacter baumannii: a proteomics approach. J Proteomics 74:44–58. doi: 10.1016/j.jprot.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moriwaki Y, Caaveiro JM, Tanaka Y, Tsutsumi H, Hamachi I, Tsumoto K. 2011. Molecular basis of recognition of antibacterial porphyrins by heme-transporter IsdH-NEAT3 of Staphylococcus aureus. Biochemistry 50:7311–7320. doi: 10.1021/bi200493h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.