Abstract
Plasmodium vivax resistance to chloroquine (CQ) is currently reported in almost all countries where P. vivax is endemic. In Vietnam, despite a first report on P. vivax resistance to chloroquine published in the early 2000s, P. vivax was still considered sensitive to CQ. Between May 2009 and December 2011, a 2-year cohort study was conducted in central Vietnam to assess the recommended radical cure regimen based on a 10-day course of primaquine (0.5 mg/kg/day) together with 3 days of CQ (25 mg/kg). Here we report the results of the first 28-day follow-up estimating the cumulative risk of P. vivax recurrences together with the corresponding CQ blood concentrations, among other endpoints. Out of 260 recruited P. vivax patients, 240 completed treatment and were followed up to day 28 according to the WHO guidelines. Eight patients (3.45%) had a recurrent P. vivax infection, at day 14 (n = 2), day 21 (n = 1), and day 28 (n = 5). Chloroquine blood concentrations, available for 3/8 recurrent infections (days 14, 21, and 28), were above the MIC (>100 ng/ml whole blood) in all of these cases. Fever and parasitemia (both sexual and asexual stages) were cleared by day 3. Anemia was common at day 0 (35.8%), especially in children under 10 years (50%), and hemoglobin (Hb) recovery at day 28 was substantial among anemic patients (median change from day 0 to 28, +1.7 g/dl; interquartile range [IQR], +0.7 to +3.2). This report, based on CQ blood levels measured at the time of recurrences, confirms for the first time P. vivax CQ resistance in central Vietnam and calls for further studies using standardized protocols for accurately monitoring the extent and evolution of P. vivax resistance to chloroquine in Vietnam. These results, together with the mounting evidence of artemisinin resistance in central Vietnam, further highlight the increasing threat of antimalarial drug resistance to malaria elimination in Vietnam.
INTRODUCTION
Plasmodium vivax is the most widely distributed malaria parasite species; an estimated 2.85 billion people were at risk of infection in 2009, the vast majority (2.59 billion [91.0%]) living in central and southeast Asia (1). Moreover, since malaria elimination has been on the global health agenda (2), the public health importance of vivax malaria has been increasingly reassessed, since it is more difficult to control than Plasmodium falciparum malaria, and severe clinical syndromes as well as new foci of chloroquine resistance are increasingly reported (3–5). Chloroquine (CQ) is the first-line treatment for P. vivax in most countries where it is endemic. P. vivax resistance to CQ was first reported in 1989 from Papua New Guinea (PNG) (6), rapidly followed by reports from Indonesia in 1991 (7, 8), Myanmar in 1993 and 1995 (9, 10), India in 1995 (11, 12), Malaysian Borneo in 1996 (13), and several South American countries (Guyana, Brazil, and Columbia) from 1996 onwards (14–16). In Vietnam, little evidence of P. vivax susceptibility to CQ has been published so far; one study in Binh Thuan province (southeastern coast region) in the early 2000s reported P. vivax resistance to chloroquine (17), while this was absent in the neighboring Khanh Hoa province (18). The National Malaria Control Program (NMCP) has been closely monitoring antimalarial drug resistance, mainly focused on P. falciparum resistance (19–21), since 1995 in several sentinel sites across the country. Since 2003, P. vivax susceptibility to CQ has been assessed in six sentinel sites, and a rate of between 0 and 5.7% of late parasitological failures has been reported (22).
Vietnam is currently engaged in malaria elimination (23, 24), and the issue of drug resistance is a priority, as P. falciparum resistance to artemisinins has been already reported in five (Tier I) provinces of central Vietnam (25, 26). Moreover, the control of P. vivax is another challenge, as this species is becoming increasingly prevalent (27–30). The main difficulty in controlling vivax malaria lies in the need to radically treat not only blood forms but also the hepatic dormant forms (hypnozoites) that cause relapses for months to years after the initial infection. The World Health Organization (WHO) currently recommends for radical cure a 3-day course of CQ (total of 25 mg/kg) together with a 14-day course of primaquine (PQ) (0.25 mg/kg/day), the recommended treatment in Vietnam since 2009. Nevertheless, between 2007 and 2009, instead of the 14-day course, PQ was given for 10 days at a higher dose (0.5 mg/kg/day) (31). The efficacy of such treatment on liver stages was assessed by following up a cohort of treated P. vivax patients in central Vietnam for 2 years. We report here the results of the first 28-day follow-up done according to the WHO guidelines (32).
MATERIALS AND METHODS
Study site and participants.
The study was carried out between April 2009 and December 2011 at the Tra Leng Commune Health Center (CHC), located in a remote forested area in the southwestern part of Quang Nam province, central Vietnam. A detailed description of the study area and population has been reported elsewhere (33). The study was designed as a 28-day follow-up after treatment of Plasmodium vivax cases with CQ and PQ (32). Male and female patients, aged between 3 and 60 years, presenting at the CHC (or identified through active case detection by the study team) with suspected malaria were screened for eligibility. Inclusion criteria were as follows: axillary temperature of ≥37.5°C and/or history of fever in the previous 48 h, P. vivax monoinfection with asexual parasites confirmed by light microscopy (LM), residency in the study area, and written informed consent from all participants aged 18 years or older (or from parents/guardians for minors). Patients were excluded if they presented general danger signs with severe or complicated malaria, had any acute or chronic concomitant illness, or had already been treated with PQ within the past 30 days. Pregnant or lactating women, patients with known glucose-6-phosphate dehydrogenase (G6PD) deficiency (or history of “black urine” following PQ treatment), or patients with any history of intolerance to the study drugs were excluded. According to the national guidelines, patients were not tested for G6PD deficiency prior to PQ treatment. The prevalence of G6PD genetic polymorphism (Viangchan mutation) was estimated to be below 1.5% in both males and females (33), with no difference between ethnic groups.
Procedures.
Study drugs were provided by the national malaria control program and consisted of CQ tablets of 300 mg chloroquine base (lot no. 08001CN; registration no. VNB-4144-05) and PQ tablets containing 15 mg primaquine base (lot no. 010109; registration no. VD-0877-06).
A general physical examination was performed at inclusion (day 0) and daily during treatment (days 1 to 9); subsequently, patients were examined weekly at days 14, 21, and 28 and during any unscheduled visit. Patients were asked to return daily to the CHC for direct observed therapy with CQ (25 mg base/kg) and PQ (0.5 mg/kg/day) during the first 3 days (days 0 to 2) and then with PQ alone for the remaining 7 days (days 3 to 9). More specifically, signs and symptoms of acute hemolysis (jaundice, black urine, fatigue, tachycardia, shock, etc.) were systematically checked at each visit by the study clinician; adverse drug reactions and concomitant medications were recorded. Patients not attending scheduled visits were visited at home. Any recurrent P. vivax or P. falciparum infection detected by LM during the 28-day follow-up was treated with dihydroartemisinin-piperaquine (DHA-PPQ) for 3 days following national guidelines.
Blood samples (finger prick) were collected at days 0, 1, 2, 3, 7, 14, 21, and 28 for LM (blood films) and later molecular analysis (2 blood spots dried on filter paper). Additional blood samples were taken at days 0, 14, and 28 for hemoglobin (Hb) concentration; at day 7 and any day of recurrent P. vivax infection, 100 μl of blood was taken on a separate filter paper for later measurement of CQ blood level.
Thick and thin films were stained with 3% Giemsa solution for 45 min; parasite density was estimated by counting the number of parasites per 200 white blood cells (WBCs) and assuming 8,000 WBCs/μl. A slide was declared negative if no parasite was found after counting 1,000 WBCs. All slides were read independently by two expert technicians who in case of discrepancy reread the slide until reaching agreement. A later and systematic quality control examination of all blood slides was done by a senior technician at the central level (NIMPE, Hanoi); in case of disagreement, a second senior technician would reread the slide until agreement was reached. The hemoglobin concentration was measured with the HemoCue Hb 301 device (HemoCue AB, Angelholm, Sweden) following the manufacturer's instructions (34). Filter paper blood samples (FPBS) were dried outside direct sunlight, kept in individual sealed plastic bags, and stored at −20°C (NIMPE, Hanoi) until they were processed.
The concentrations of CQ and desethylchloroquine (DEC) in dried blood filter paper samples were determined using a validated high-pressure liquid chromatography (HPLC) method with a fluorescence detector at excitation and emission wavelengths of 250 and 400 nm, respectively, a modification of the previous published method (35). Following mincing of the filter paper (Whatman grade 3), extraction was performed using 3 ml of 25% ammonia and 3 ml of ethyl acetate-hexane (1:9). The solution was vortexed for 30 s and centrifuged to separate the organic phase, which was then transferred to another tube and evaporated to dryness. The sample was reconstituted with HPLC mobile phase, and 20 μl was injected into the HPLC system (Waters, USA). We used an X-Bridge Phenyl 5-μm (4.6- by 150-mm) column as the stationary phase. The mobile phase used was diethylamine (0.05%)-acetonitrile (55:45), pumped isocratically at flow rate of 1.0 ml/min and temperature of 30°C. Pyrimethamine was used as an internal standard.
Outcomes.
Efficacy outcomes were classified into early treatment failure (ETF), late clinical failure (LCF), late parasitological failure (LPF), or adequate clinical and parasitological response (ACPR), following the WHO criteria (32). For all efficacy outcomes, no distinction was made between relapse, recrudescence, and reinfection, and any new microscopically detected P. vivax infection after initial parasite clearance was defined as “P. vivax recurrence.” The primary endpoints were the proportion of patients with ACPR by day 28 and the parasite clearance time (PCT). Secondary endpoints included fever and gametocyte clearance times, the proportion of confirmed CQ-resistant P. vivax recurrences (CQ plus DEC concentration of >100 ng/ml), and hematological changes between days 0 and 28.
Data analysis.
The sample size was calculated on the basis of retrospective data (2003 to 2007) reporting LPF ranging from 0% to 5.7% among P. vivax patients treated with CQ (22). Assuming a minimum treatment failure rate of 5% and a loss to follow-up of 10%, a sample size of 204 P. vivax patients would be needed for estimation with a 3% precision and at 5% significance level (“CSample” command/Epi Info 6). The sample size was further increased to comply with the requirements of the cohort evaluation, details of which will be published separately. Data were double entered and cleaned using Epidata version 3.1. The data set was analyzed using STATA version 11 (Stata Corp., College Station, TX). The survey design (survey data set) was taken into account using the svy command in STATA, with villages as strata and household as sampling unit. Descriptive statistics were used to compute baseline sociodemographic characteristics. Ownership of livestock (pigs, buffaloes, and cows) was used as a proxy for the economic status of the household, using a principal-component analysis (33). The PCT was estimated using the daily proportion of patients still parasitemic from day 0 until the day of complete parasite clearance. The proportion of recurrence-free patients by day 28 was assessed by Kaplan-Meier survival analysis. Patients were censored on the day they had last been seen in follow-up. Fever clearance time was estimated by determining the proportion of febrile patients during follow-up among febrile patients at day 0. Similarly, gametocyte clearance was expressed as the proportion of patients with gametocytes during follow-up among gametocyte-positive patients at day 0. Hematological recovery was estimated by computing the median Hb concentration at days 0, 14, and 28 as well as the median of individual Hb differences between day 0 and day 28. Anemia was defined as an Hb concentration of <11 g/dl, for both sexes and all ages (36). The Wilcoxon rank sum test and sign rank test were applied as required to compare Hb medians. A survey logistic regression (“svy” command in STATA) was used to carry out a multivariate adjusted analysis for the risk of anemia before and after treatment (adjusting for all potential confounders, such as sex, age, baseline parasitemia, splenomegaly, and ethnicity). Similarly, survey logistic regression was also used to assess if baseline parasite density (day 0) or age was independently associated with parasite clearance at day 2. A multivariate linear regression model was used to determine the independent effect of the baseline Hb values (day 0 = Hb0) on the relative Hb changes at day 14. Potential risk factors (age, ethnicity, etc.) with a P value of <0.05 in the univariate analysis were included in the multivariate model and retained if the P value was <0.05. Interactions were systematically checked for up to order two. The 5% cutoff was defined as a significant P value for all statistical tests.
Ethical clearance.
Ethical clearance was obtained from both the Ethical Committee of the NIMPE in Hanoi and that of the University of Antwerp. The fundamental principles of ethics in research on human participants were upheld throughout the project. The study objectives and methods were explained to the people's committee, the village's leader, and the local people. All study participants had given their informed consent after the study objectives and procedures, as well as their right to withdraw without prejudice for themselves or their families, were explained. Written informed consent was obtained from parents or guardians of children below 18 years; children between the ages of 12 and 18 years were asked to provide a written assent.
RESULTS
Trial profile and baseline characteristics.
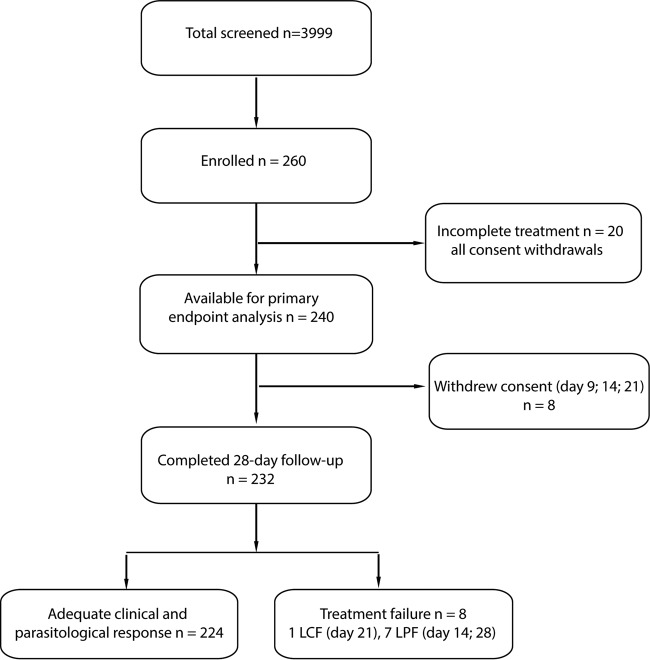
Between April 2009 and December 2010, 260 P. vivax-infected patients were enrolled and given the 10-day radical treatment of PQ (0.50 mg/kg/day) associated with CQ for the first 3 days (total, 25 mg/kg); 240 patients (92.3%; 240/260) completed the treatment and were included in the analysis, and 232 patients completed the 28-day follow-up. All incomplete follow-ups were due to consent withdrawal (Fig. 1) following prolonged absence, mainly because of work requirement in forest fields. Patients were recruited in all four study villages, and the vast majority (78.5%; 204/260) belonged to the M'nong group (Table 1). Males (61.1%; 159/260) slightly outnumbered females, and almost half of the participants (43.1%; 112/260) were children aged 3 to 9 years. The majority of participants had no bed net at home (70.8%; 184/260) and very low socioeconomic status, and all adults were farmers.
FIG 1.
Study profile.
TABLE 1.
Baseline demographic, clinical, and parasitological characteristics at enrollment (n = 260)
| Parameter | n | % | 95% CI |
|---|---|---|---|
| Village | |||
| 1 | 101 | 38.85 | 34.83–43.02 |
| 2 | 64 | 24.62 | 21.25–28.33 |
| 3 | 39 | 15.0 | 12.84–17.45 |
| 4 | 56 | 21.54 | 17.63–26.04 |
| Gender | |||
| Male | 159 | 61.15 | 55.76–66.82 |
| Female | 101 | 38.85 | 33.72–44.24 |
| Ethnic group | |||
| M'nong | 204 | 78.46 | 73.96–82.37 |
| Cadong | 56 | 21.54 | 17.63–26.04 |
| Age (yr) | |||
| 3–9 | 112 | 43.08 | 37.45–48.89 |
| 10–19 | 71 | 27.31 | 21.84–33.55 |
| 20–29 | 44 | 16.92 | 12.75–22.11 |
| 30–60 | 33 | 12.69 | 9.36–17.0 |
| Occupation | |||
| None (children <6 yr) | 70 | 26.92 | 22.02–32.47 |
| Farmer | 85 | 32.69 | 27.51–38.34 |
| Pupil | 105 | 40.38 | 34.34–46.74 |
| Bed net in house | |||
| None | 184 | 70.77 | 61.81–78.37 |
| At least one | 76 | 29.23 | 21.63–38.19 |
| Economic statusa | |||
| Lowest | 147 | 56.54 | 47.20–65.43 |
| Low | 26 | 10.0 | 5.70–16.96 |
| Higher | 87 | 33.46 | 25.18–42.9 |
| Clinical symptoms (most frequently reported) | |||
| Fever (axillary temp ≥37.5°C) | 154 | 59.23 | 53.06–65.12 |
| Headache | 94 | 36,15 | 30.42–42.32 |
| Fatigue | 86 | 33.08 | 27.23–39.5 |
| Dizziness | 28 | 10.77 | 7.51–15.22 |
| Nausea | 32 | 12.31 | 8.83–16.90 |
| Enlarged spleen | 16 | 6,15 | 3.58–10.39 |
| Laboratory data | |||
| Asexual parasites/μl, GM (95% CI) | 2754.07 (2271.87–3338.61) | ||
| Gametocytes/μl, GM (95% CI) | 387.72 (324.84–462.80) | ||
| Patients with gametocytes | 224 | 86.15 | 81.37–89.86 |
| Hemoglobin (g/dl), median (IQR) | 11.7 (10.4–13.1) | ||
| Patients with anemia (Hb < 11 g/dl) | 93 | 35.77 | 29.8–42.21 |
Score in tertiles defined as “high,” “medium,” and “low” economic status, following principal-component analysis (33).
More than half of the study patients (59.2%; 154/260) had measurable fever at enrollment; headache (36.1%; 94/260) and fatigue (33.1%; 86/260) were the most common symptoms, and about 6% (16/260) had an enlarged spleen. The mean parasite density at enrollment was 2,754.1/μl (geometric mean [GM]), and gametocytes were found in most of the patients (86.1%; 224/260), though at much lower densities (GM = 387.7/μl). The median hemoglobin concentration at enrollment was 11.7 g/dl, and more than one-third of the patients (35.8%; 93/260) were anemic (Hb, <11g/dl). The treatment was well tolerated, no clinical sign or symptoms of acute hemolysis were observed (despite the occurrence of transient acute hemolysis [see below]), and only few patients (12.3%; 32/260) complained of nausea following PQ administration, though none of them vomited their dose of CQ or PQ.
Primary endpoints.
No ETF was observed; there were eight late treatment failures, i.e., 2 LPFs at day 14, 1 LCF at day 21, and 5 LPFs at day 28 (Table 2). The rate of ACPR at day 28 was 96.6% (95% confidence interval [CI], 93.7 to 98.2). P. vivax recurrence was not associated with delayed parasite clearance, as five of the eight patients with recurrence had cleared parasitemia before 24 h. The mean parasite density at day of recurrence was very low (GM = 41.1/μl; interquartile range [IQR], 23.3 to 855.8).
TABLE 2.
Primary and secondary endpoints
| Endpoint | n (%) | 95% CI |
|---|---|---|
| Primary (n = 240) | ||
| Adequate clinical and parasitological response (KMa) | 224 (96.55) | 93.67–98.15 |
| Cumulative incidence of treatment failures (KM) | 8 (3.45) | 1.85–6.33 |
| Late clinical failure (day 21) | 1 | |
| Late parasitological failure (n = 7) at day: | ||
| 14 | 2 | |
| 28 | 5 | |
| Patients with asexual parasitemia at day: | ||
| 1 | 139 (57.92) | 51.44–64.13 |
| 2 | 17 (7.08) | 4.39–11.23 |
| 3 | 0 | |
| Secondary | ||
| Fever clearance (n = 139 = 100% at day 0) at day: | ||
| 1 | 34 (24.46) | 18.7–31.32 |
| 2 | 5 (3.59) | 1.49–8.41 |
| 3 | 0 | |
| Gametocyte clearance (n = 207 = 100% at day 0) at day: | ||
| 1 | 83 (40.1) | 33.96–46.56 |
| 2 | 11 (5.31) | 3.0–9.23 |
| 3 | 0 | |
| CQ blood concn at day of failure > 100 ng/ml at day: | 3/3 | |
| 14 (LPF) | 114.66 | |
| 21 (LCF) | 133.09 | |
| 28 (LPF) | 125.87 | |
| Hemoglobin recovery, median individual Hb change from day 0–28, g/dl (IQR) for patients: | ||
| All (n = 224) | +0.7 (−0.2–+1.6) | |
| Anemic at day 0 (n = 78) | +1.7 (+0.7–+3.2) | |
| Nonanemic at day 0 (n = 146) | +0.25 (−0.4–+1.0) | |
KM, Kaplan-Meier estimate.
At day 1, more than half of the patients (57.9%) were still parasitemic, at day 2 only 7.1% were, and at day 3 none of them had detectable parasitemia. Parasite clearance at day 2 was significantly associated with a higher asexual parasite density at day 0 (odds ratio [OR] = 1.79; 95% CI, 1.14 to 2.82; P = 0.012) but not with age.
Secondary endpoints.
All patients were afebrile and without gametocytemia by day 3 (Table 2). Dried blood samples for measuring CQ blood concentrations were available (at day 7 and the day of recurrence) for 5 of the 8 patients with vivax malaria recurrence, and among these, three had interpretable results. The CQ blood concentrations at day 7 ranged from 365.1 to 1,347.1 ng/ml, confirming adequate drug absorption. The three CQ blood concentrations at time of recurrence were 114.7 ng/ml (day 14), 133.1 ng/ml (day 21), and 125.9 ng/ml (day 28); all of them were above the 100-ng/ml threshold, confirming CQ resistance.
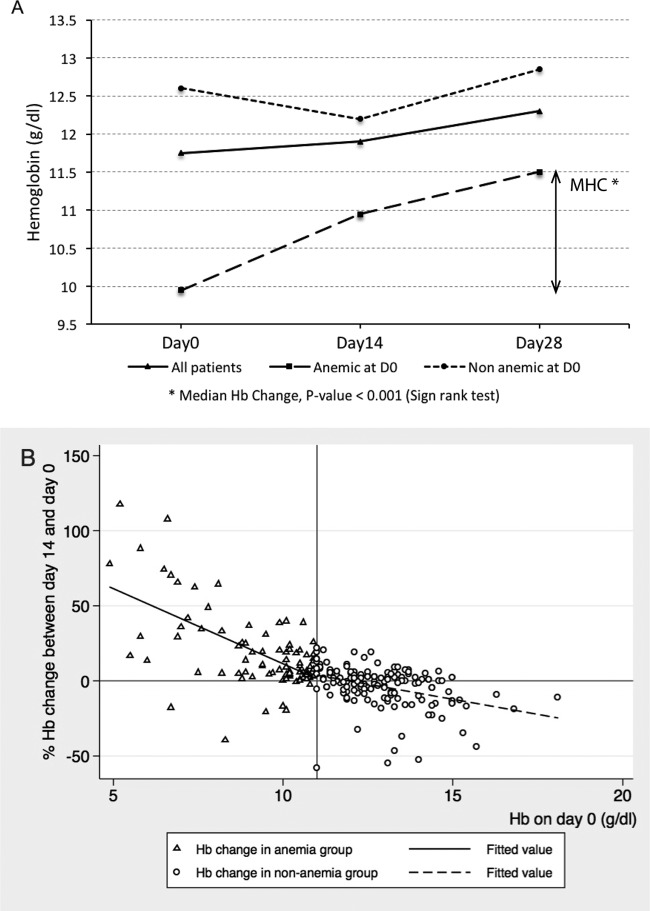
The median Hb value at day 0 among patients with ACPR (n = 224) was 11.7 g/dl (IQR = 10.5 to 13.1), and children were significantly more at risk of anemia (50.0%; 49/98) than older patients (23.0%; 29/126) even after adjusting for baseline parasitemia (adjusted OR [AOR] = 3.60; 95% CI, 1.88 to 6.88; P < 0.001). By day 28, the median Hb increased to 12.3 g/dl (IQR = 11.3 to 13.4), and the median value of individual Hb changes between day 0 and day 28 was +0.7 g/dl (IQR = −0.2 to +1.6). Among anemic patients at day 0 (n = 78), the median Hb was 9.9 g/dl (IQR = 8.3 to 10.5), and it significantly increased to 11.5 g/dl (IQR = 10.8 to 12.1) by day 28 (sign rank test, P < 0.001), with a median change of +1.7 g/dl (IQR = +0.7 to +3.2) (Fig. 2A). This change was slightly lower in children (median = 1.5 g/dl; IQR, 0.7 to 2.5) than in adults (median = 1.9; IQR, 1.1 to 4.2) (Wilcoxon rank sum test, P = 0.08). After treatment, 24.5% (24/98) of children and 8.7% (11/126) of adults were still anemic (AOR = 3.5; 95% CI, 1.7 to 7.0; P = 0.001). Patients who were still anemic by day 28 were treated with hematinic drugs (ferrous sulfate and folic acid).
FIG 2.
(A) Median hemoglobin (Hb) concentration at days 0, 14, and 28 (n = 224 patients with ACPR); (B) relative Hb change (between day 0 and day 14) according to baseline Hb values (cutoff for anemia, Hb concentration of <11.0 g/dl) (n = 240). Relative Hb change on day 14 (%) by linear regression: (i) anemia group, coefficient β = −10.00; 95% CI, −12.81 to −7.19; P < 0.001; (ii) nonanemia group, β = −6.46; 95% CI, −7.41 to −5.51; P < 0.001. A significant interaction was found between Hb change at day 14 and anemia status at day 0 (interaction term β = −5.99; 95% CI, −8.87 to −3.11; P < 0.001).
In order to better understand the relationship between Hb changes, age and baseline Hb values (Hb0), we plotted the individual changes at day 14 relative to day 0 (%) (Fig. 2B) as a function of Hb0 and carried out a multivariate linear regression analysis adjusting for the potential confounding effect of age. The final model showed that relative Hb changes at day 14 were independently (and negatively) associated with Hb0 (P < 0.001) and that age was not a confounder, since it was associated only with the exposure and not with the outcome variable. Moreover, the linear regression model showed that the effect of Hb0 on relative Hb changes at day 14 was significantly different between anemic and nonanemic patients at day 0 (interaction term coefficient β = −5.99; P < 0.001). Indeed, while in nonanemic patients the Hb decreased by 6.5% for every increase in Hb0 unit (β = −6.45; P < 0.001), in the anemic group, the Hb increased by 10% for every decrease in Hb0 unit (β = −10.00; P < 0.001). Interestingly, 9 patients experienced more than a 25% reduction in Hb by day 14, ranging from −58.2% to −32.8%, without any sign or symptom of hemolysis detected during the 28-day follow-up. All but one of these patients had normal Hb values at day 0, and the majority (6/9) of them had recovered a normal Hb value by day 28.
Similar results were found for the association between Hb0 and relative Hb changes by day 28, with a significant interaction (interaction term β = P < 0.001) and a slightly stronger effect of Hb0 among anemic patients (β = −14.00; P < 0.001) and a smaller effect (β = −3.38%; P < 0.001) in the nonanemic group (data not shown).
DISCUSSION
This study confirms for the first time P. vivax CQ resistance in Vietnam, as three patients with recurrent P. vivax infections were found to have CQ blood concentrations above the MIC (100 ng/ml of whole blood). Suspected P. vivax resistance to chloroquine was observed in Binh Thuan province in southern Vietnam in the late 1990s, with 16% treatment failure after a 3-day course of CQ (25 mg/kg) (17), but could not be confirmed because CQ blood concentrations were not available. Indeed, the latter is necessary (37), as recurrent infections could be the consequence of inadequate drug concentration due to suboptimal drug quality and dosage or low intestinal absorption rather than CQ resistance. For this study, these factors can be excluded, as the day 7 CQ concentrations were within the optimal range, at least for the five patients with available results at day 7.
Since the first reports from Papua New Guinea (PNG) in 1989 (6, 8, 38, 39), P. vivax resistance to chloroquine has rapidly reached unacceptably high levels in Indonesia and PNG, prompting the WHO to recommend artemisinin-based combination therapies for P. vivax (40). Moreover, a recent systematic review showed that P. vivax resistance to chloroquine can be found in most countries where vivax malaria is endemic, across continents (41). The apparently low cumulative risk of recurrence by day 28 estimated in our study together with the absence of ETF suggest a low grade of resistance compared to that in other Southeast Asian countries, particularly Indonesia, where ETFs ranged from 6% to 24% and 28-day recurrence rates from 18% to 100% (41). Similarly, the recurrence rate may be considered negligible compared to that (16%) observed in Binh Thuan province about 15 years ago (17). Nevertheless, when considering that CQ was coadministered with high-dose (0.5-mg/kg/day) PQ, which has also an effect on P. vivax asexual blood stages (42, 43), the estimation of P. vivax resistance to chloroquine provided here is probably much lower than its true prevalence. Indeed, in Indonesia adding PQ to CQ decreased the day 28 treatment failure from 78% to 15% (39). Therefore, our seemingly low-grade resistance is the CQ failure when combined with high-dose PQ, while the true failure related to CQ resistance is probably higher, possibly up to 5-fold higher (39). Therefore, P. vivax resistance to chloroquine in Quang Nam province is probably similar to that reported 15 years ago from Binh Thuan province (17). As CQ (monotherapy) efficacy measured in 6 sentinel sites in central and southern Vietnam between 2006 and 2011 has been consistently at 100% (24), it is possible that P. vivax resistance to chloroquine in Vietnam has not reached the high levels observed in PNG and Indonesia. Indeed, despite the lack of power, with sample sizes between 25 and 65 patients (24), which are far below the minimum of 75 recommended by the WHO (32), it is unlikely that high-grade resistance would have been missed. Therefore, P. vivax resistance to chloroquine was present in central and southern Vietnam since at least the late 1990s, and unlike in PNG and Indonesia, it did not evolve to high-grade levels. The most likely explanation for such a difference could be the much lower CQ pressure, as artemisinin derivatives have been used since the early 1990s for the treatment of multidrug-resistant P. falciparum.
When considering the timing of the observed recurrent infections, the two LPFs at day 14 are probably recrudescences, as P. vivax infections recurring before day 16 are almost certainly due to a recrudescence from the primary infection (37). Infections recurring later may be either recrudescences or relapses, with CQ-resistant parasites if the CQ blood level is above the MICs (37). As this is an area of extremely low transmission, more than one infectious bite within 1 month is unlikely, though it cannot be excluded as farmers often stay overnight in their forest fields, where they are at higher risk of exposure to the main vector, Anopheles dirus (44, 45). Genotyping alone is usually of limited help to distinguish between recrudescence and relapse/new infection, since relapses can occur with either the same or different clones (46).
Vivax malaria-associated anemia was common, and hematological recovery at day 28 depended on baseline Hb. Indeed, the more pronounced hematological recovery was observed among patients who were anemic before treatment. This observation is similar to a recent report from PNG (47). In addition, young children were at a much higher risk of anemia than older patients, and this risk remained high after treatment, illustrating the importance of an efficacious radical treatment for P. vivax in children (48). The linear regression model showed that age was indirectly associated with Hb changes only through its significant association with Hb0. Moreover, in anemic patients, the lowest Hb0 values corresponded to the more marked Hb increase during follow-up; for the other patients, the higher the Hb0, the more marked was the Hb decrease during follow-up. This inverse relation could be partly explained by the increased hemolytic risk in older red blood cells (49) and by the suppressive activity of hemozoin (digested Hb) on erythropoiesis (50). It is possible that anemic patients were infected for longer periods and at day 0 had already reached their lowest Hb value. This would have resulted in a more robust bone marrow response than in nonanemic, recently infected patients (47). Transient asymptomatic Hb reductions (≥50%) after PQ treatment, as either radical cure or single gametocytocidal dose, have been observed among G6PD-deficient and nondeficient African children (51–53).
A quick post hoc genotyping (54) was carried out to screen for the four most commonly reported G6PD mutations in Vietnam (Vieng Chang, Canton, Union, and Kaiping) among the 9 patients who experienced a >25% reduction in Hb by day 14 (together with 9 randomly selected control patients [having no change in Hb]). Only one patient was found positive for the Vieng Chan mutation, i.e., a 26-year-old male of Cadong ethnicity with a transient Hb decrease of 52.9% by day 14 (Hb0 = 14.0 g/dl) followed by a full recovery at day 28 (Hb28 = 13.6 g/dl). It is not possible to exclude, among these 9 patients, the presence of other G6PD variants (i.e., Vietnam I, Vietnam II, Gaohe Gaozhou, Coimbra, etc.) also reported in different ethnic groups from central Vietnam (55, 56). This will be further investigated by carrying out an in-depth analysis of the G6PD genetic polymorphism in all 240 study patients in relation to their Hb changes. Moreover, the observed transient but potentially life-threatening hemolysis (>50% Hb change at day 14) questions the national policy, which currently does not recommend G6PD testing prior to radical PQ treatment. To better estimate the risk of hemolysis linked to PQ use, there is the urgent need of determining the prevalence of the G6PD-deficient phenotype together with the G6PD genetic polymorphisms among different ethnic minorities living in areas of residual malaria endemicity.
The main limitation of our study is the concomitant use of PQ and CQ, which most likely resulted in a substantial underestimation of the true CQ-related cumulative risk of recurrence by day 28. As per WHO recommendations (32), P. vivax resistance to chloroquine can be accurately estimated only by standard 28-day in vivo studies with CQ monotherapy, with PQ being withheld until day 28. Strictly speaking, resistance could not be confirmed in all eight vivax malaria recurrences, as CQ blood levels results were available for only three patients. However, given the pharmacokinetics of CQ (37), it is likely that the other patients also had adequate CQ blood levels. In addition, the concomitant administration of PQ and its synergistic effect on asexual blood stages could also explain why no association was found between the PCT and the risk of recurrence, unlike that reported in a recent meta-analysis by Price and colleagues (41). For all these reasons, a new study has been initiated using CQ monotherapy to accurately determine its in vivo and in vitro efficacy for treating P. vivax infections.
Another limitation of our study is the 24-h sampling schedule, which was not optimal for an accurate determination of the PCT. For future studies, 8- to 12-h sampling and a baseline parasite density of at least 250/μl are needed to accurately determine parasite clearance (32, 57).
Conclusion.
In conclusion, this is the first confirmed evidence of P. vivax resistance to chloroquine in central Vietnam, an area where we recently reported P. falciparum resistance to artemisinins (25). P. vivax resistance to chloroquine should continue to be monitored in different sentinel sites of central Vietnam, using standardized and sufficiently powered in vivo protocols with CQ monotherapy and with PQ therapy delayed to day 28. Vietnam has committed to malaria elimination by 2030, and within this context, antimalarial drug resistance, not only P. falciparum resistance to arteminins but also P. vivax resistance to CQ, is as a major threat. New treatment guidelines based on short and highly effective drug regimens as well as regional and Plasmodium genus-wide integrated strategies for the containment of antimalarial drug resistance in the Greater Mekong Subregion need to be urgently developed.
ACKNOWLEDGMENTS
We thank Germana Bancone and her colleagues at the Shoklo Malaria Research Unit (Thailand) for the post hoc G6PD genotyping. We also thank the M'nong and Cadong communities in the Tra Leng and Tra Don communes for their contribution to the present study, as well as the local authorities for their strong and continuous support.
This study was funded by the UBS Optimus Foundation and the Framework Agreement project (FA3) of the Belgium Cooperation (DGCD).
REFERENCES
- 1.Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. 2010. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Diseases 4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts L, Enserink M. 2007. Malaria. Did they really say ... eradication? Science 318:1544–1545. [DOI] [PubMed] [Google Scholar]
- 3.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. 2007. Vivax malaria: neglected and not benign. Am J Trop Med Hyg 77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 4.Baird JK. 2004. Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother 48:4075–4083. doi: 10.1128/AAC.48.11.4075-4083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird JK. 2009. Resistance to therapies for infection by Plasmodium vivax. Clin Microbiol Rev 22:508–534. doi: 10.1128/CMR.00008-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rieckmann KH, Davis DR, Hutton DC. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183–1184. [DOI] [PubMed] [Google Scholar]
- 7.Baird JK, Basri H, Purnomo Bangs MJ, Subianto B, Patchen LC, Hoffman SL. 1991. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am J Trop Med Hyg 44:547–552. [DOI] [PubMed] [Google Scholar]
- 8.Murphy GS, Basri H, Purnomo, Andersen EM, Bangs MJ, Mount DL, Gorden J, Lal AA, Purwokusumo AR, Harjosuwarno S, Hoffman SL. 1993. Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet 341:96–100. doi: 10.1016/0140-6736(93)92568-E. [DOI] [PubMed] [Google Scholar]
- 9.Myat Phone K, Myint O, Myint L, Thaw Z, Kyin Hla A, Nwe Nwe Y. 1993. Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma). Trans R Soc Trop Med Hyg 87:687. doi: 10.1016/0035-9203(93)90294-Z. [DOI] [PubMed] [Google Scholar]
- 10.Marlar T, Myat Phone K, Aye Yu S, Khaing Khaing G, Ma S, Myint O. 1995. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans R Soc Trop Med Hyg 89:307–308. doi: 10.1016/0035-9203(95)90556-1. [DOI] [PubMed] [Google Scholar]
- 11.Garg M, Gopinathan N, Bodhe P, Kshirsagar NA. 1995. Vivax malaria resistant to chloroquine: case reports from Bombay. Trans R Soc Trop Med Hyg 89:656–657. doi: 10.1016/0035-9203(95)90432-8. [DOI] [PubMed] [Google Scholar]
- 12.Singh RK. 2000. Emergence of chloroquine-resistant vivax malaria in south Bihar (India). Trans R Soc Trop Med Hyg 94:327. doi: 10.1016/S0035-9203(00)90344-4. [DOI] [PubMed] [Google Scholar]
- 13.Ahlm C, Wistrom J, Carlsson H. 1996. Chloroquine-resistant Plasmodium vivax malaria in Borneo. J Travel Med 3:124. doi: 10.1111/j.1708-8305.1996.tb00719.x. [DOI] [PubMed] [Google Scholar]
- 14.Phillips EJ, Keystone JS, Kain KC. 1996. Failure of combined chloroquine and high-dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana, South America. Clin Infect Dis 23:1171–1173. doi: 10.1093/clinids/23.5.1171. [DOI] [PubMed] [Google Scholar]
- 15.Alecrim MdG, Alecrim W, Macedo V. 1999. Plasmodium vivax resistance to chloroquine (R2) and mefloquine (R3) in Brazilian Amazon region. Rev Soc Brasil Med Trop 32:67–68. [DOI] [PubMed] [Google Scholar]
- 16.Soto J, Toledo J, Gutierrez P, Luzz M, Llinas N, Cedeno N, Dunne M, Berman J. 2001. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am J Trop Med Hyg 65:90–93. [DOI] [PubMed] [Google Scholar]
- 17.Phan GT, de Vries PJ, Tran BQ, Le HQ, Nguyen NV, Nguyen TV, Heisterkamp SH, Kager PA. 2002. Artemisinin or chloroquine for blood stage Plasmodium vivax malaria in Vietnam. Trop Med Int Health 7:858–864. doi: 10.1046/j.1365-3156.2002.00948.x. [DOI] [PubMed] [Google Scholar]
- 18.Taylor WR, Doan HN, Nguyen DT, Tran TU, Fryauff DJ, Gomez-Saladin E, Kain KC, Le DC, Baird JK. 2000. Assessing drug sensitivity of Plasmodium vivax to halofantrine or choroquine in southern, central Vietnam using an extended 28-day in vivo test and polymerase chain reaction genotyping. Am J Trop Med Hyg 62:693–697. [DOI] [PubMed] [Google Scholar]
- 19.Ngo T, Duraisingh M, Reed M, Hipgrave D, Biggs B, Cowman AF. 2003. Analysis of pfcrt, pfmdr1, dhfr, and dhps mutations and drug sensitivities in Plasmodium falciparum isolates from patients in Vietnam before and after treatment with artemisinin. Am J Trop Med Hyg 68:350–356. [PubMed] [Google Scholar]
- 20.Thanh NV, Cowman AF, Hipgrave D, Kim TB, Phuc BQ, Cong LD, Biggs BA. 2001. Assessment of susceptibility of Plasmodium falciparum to chloroquine, quinine, mefloquine, sulfadoxine-pyrimethamine and artemisinin in southern Viet Nam. Trans R Soc Trop Med Hyg 95:513–517. doi: 10.1016/S0035-9203(01)90023-9. [DOI] [PubMed] [Google Scholar]
- 21.Phuc BQ, Caruana SR, Cowman AF, Biggs BA, Thanh NV, Tien NT, Thuan le K. 2008. Prevalence of polymorphisms in DHFR, DHPS, PFMDR1 and PFCRT genes of Plasmodium falciparum isolates in Quang Tri Province, Vietnam. Southeast Asian J Trop Med Public Health 39:959–962. [PubMed] [Google Scholar]
- 22.Tien NT. 2007. Reviewing the monitoring of antimalarial drug efficacy and resistance in sentinel sites in Vietnam. Abstr Int Colloq ITM/NIMPE, p 71 http://www.itg.be/internet/colloq2007/abstracts.pdf.
- 23.Ministry of Health, National Malaria Control and Elimination Programme. 2011, posting date. National strategy for Malaria Control and Elimination for the period of 2012–2015. http://static1.1.sqspcdn.com/static/f/471029/25390280/1409624888740/National+Strategy+for+Malaria+Control+and+Elimination+2012-2015.pdf?token=3BLd15GIHwidLPtf6Nl9xL1wf7o%3D.
- 24.National Institute of Malariology, Parasitology and Entomology. Review of antimalaria drug efficacy over the last 10 years in Vietnam. NIMPE Report to WHO in 2012. Internal report NIMPE, Hanoi, Vietnam. [Google Scholar]
- 25.Thriemer K, Hong NV, Rosanas-Urgell A, Phuc BQ, Ha do, Pockele ME, Guetens P, Van NV, Duong TT, Amambua-Ngwa A, D'Alessandro U, Erhart A. 2014. Delayed parasite clearance after treatment with dihydroartemisinin-piperaquine in Plasmodium falciparum malaria patients in central Vietnam. Antimicrob Agents Chemother 58:7049–7055. doi: 10.1128/AAC.02746-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO. January 2014, posting date. Status report on artemisinin resistance. http://www.who.int/malaria/publications/atoz/update-artemisinin-resistance-jan2014/en/ Accessed March 2015. [Google Scholar]
- 27.WHO. 2014. World malaria report, p 162 http://www.who.int/malaria/publications/country-profiles/profile_vnm_en.pdf.
- 28.Erhart A, Ngo D, Phan V, Ta T, Van Overmeir C, Speybroeck N, Obsomer V, Le X, Le K, Coosemans M, D'Alessandro U. 2005. Epidemiology of forest malaria in central Vietnam: a large scale cross-sectional survey. Malaria J 8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erhart A, Thang ND, Bien TH, Tung NM, Hung NQ, Hung LX, Tuy TQ, Speybroeck N, Cong LD, Coosemans M, D'Alessandro U. 2004. Malaria epidemiology in a rural area of the Mekong Delta: a prospective community-based study. Trop Med Int Health 9:1081–1090. doi: 10.1111/j.1365-3156.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen HV, van den Eede P, van Overmeir C, Thang ND, Hung L, D'Alessandro XU, Erhart A. 2012. Marked age-dependent prevalence of symptomatic and patent infections and complexity of distribution of human Plasmodium species in central Vietnam. Am J Trop Med Hyg 87:989–995. doi: 10.4269/ajtmh.2012.12-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vietnam NMCP. Malaria treatment guideline (Vietnamese version). http://www.impe-qn.org.vn/impe-qn/vn/portal/InfoDetail.jsp?area=58&cat=1066&ID=1322. [Google Scholar]
- 32.WHO. 2009. Methods for surveillance of antimalarial drug efficacy. http://whqlibdoc.who.int/publications/2009/9789241597531_eng.pdf.
- 33.Thanh PV, Van Hong N, Van Van N, Van Malderen C, Obsomer V, Rosanas-Urgell A, Grietens KP, Xa NX, Bancone G, Chowwiwat N, Duong TT, D'Alessandro U, Speybroeck N, Erhart A. 2015. Epidemiology of forest malaria in Central Vietnam: the hidden parasite reservoir. Malaria J 14:86. doi: 10.1186/s12936-015-0601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.HemoCue. 2014. HemoCue Hb 301 operating manual. http://www.hemocue.com/en/hemocue-point-of-care-products/anemia-screening-and-hemoglobin/hb-301-system.
- 35.Cheomung A, Na-Bangchang K. 2011. HPLC with ultraviolet detection for the determination of chloroquine and desethylchloroquine in whole blood and finger-prick capillary blood dried on filter paper. J Pharm Biomed Anal 55:1031–1040. doi: 10.1016/j.jpba.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 36.WHO. 2001. Iron deficiency anaemia assessment, prevention, and control. A guide for programme managers. http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf.
- 37.Baird JK, Leksana B, Masbar S, Fryauff DJ, Sutanihardja MA, Suradi Wignall FS, Hoffman SL. 1997. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine levels. Am J Trop Med Hyg 56:621–626. [DOI] [PubMed] [Google Scholar]
- 38.Schuurkamp GJ, Spicer PE, Kereu RK, Bulungol PK. 1989. A mixed infection of vivax and falciparum malaria apparently resistant to 4-aminoquinoline: a case report. Trans R Soc Trop Med Hyg 83:607–608. doi: 10.1016/0035-9203(89)90370-2. [DOI] [PubMed] [Google Scholar]
- 39.Schuurkamp GJ, Spicer PE, Kereu RK, Bulungol PK, Rieckmann KH. 1992. Chloroquine-resistant Plasmodium vivax in Papua New Guinea. Trans R Soc Trop Med Hyg 86:121–122. doi: 10.1016/0035-9203(92)90531-G. [DOI] [PubMed] [Google Scholar]
- 40.WHO. 2015. Guidelines for the treatment of malaria, 3rd ed http://www.who.int/malaria/publications/atoz/9789241549127/en/.
- 41.Price RN, von Seidlein L, Valecha N, Nosten F, Baird JK, White NJ. 2014. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis 14:982–991. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pukrittayakamee S, Vanijanonta S, Chantra A, Clemens R, White NJ. 1994. Blood stage antimalarial efficacy of primaquine in Plasmodium vivax malaria. J Infect Dis 169:932–935. doi: 10.1093/infdis/169.4.932. [DOI] [PubMed] [Google Scholar]
- 43.Baird JK, Basri H, Subianto B, Fryauff DJ, McElroy PD, Leksana B, Richie TL, Masbar S, Wignall FS, Hoffman SL. 1995. Treatment of chloroquine-resistant Plasmodium vivax with chloroquine and primaquine or halofantrine. J Infect Dis 171:1678–1682. doi: 10.1093/infdis/171.6.1678. [DOI] [PubMed] [Google Scholar]
- 44.Van Bortel W, Trung HD, Hoi Le, Van Ham XN, Van Chut N, Luu ND, Roelants P, Denis L, Speybroeck N, D'Alessandro U, Coosemans M. 2010. Malaria transmission and vector behaviour in a forested malaria focus in central Vietnam and the implications for vector control. Malaria J 9:373. doi: 10.1186/1475-2875-9-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peeters Grietens K, Xuan X, Van Bortel W, Duc T, Ribera J, Ba Nhat T, Van K, Le Xuan H, D'Alessandro U, Erhart A. 2010. Low perception of malaria risk among the Ra-glai ethnic minority in south-central Vietnam: implications for forest malaria control. Malaria J 9:23. doi: 10.1186/1475-2875-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imwong M, Snounou G, Pukrittayakamee S, Tanomsing N, Kim JR, Nandy A, Guthmann JP, Nosten F, Carlton J, Looareesuwan S, Nair S, Sudimack D, Day NP, Anderson TJ, White NJ. 2007. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis 195:927–933. doi: 10.1086/512241. [DOI] [PubMed] [Google Scholar]
- 47.Taylor WR, Widjaja H, Basri H, Tjitra E, Ohrt C, Taufik T, Baso S, Hoffman SL, Richie TL. 2013. Haemoglobin dynamics in Papuan and non-Papuan adults in northeast Papua, Indonesia, with acute, uncomplicated vivax or falciparum malaria. Malaria J 12:209. doi: 10.1186/1475-2875-12-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Douglas NM, Lampah DA, Kenangalem E, Simpson JA, Poespoprodjo JR, Sugiarto P, Anstey NM, Price RN. 2013. Major burden of severe anemia from non-falciparum malaria species in Southern Papua: a hospital-based surveillance study. PLoS Med 10:e1001575. doi: 10.1371/journal.pmed.1001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beutler E. 1994. G6PD deficiency. Blood 84:3613–3636. [PubMed] [Google Scholar]
- 50.Casals-Pascual C, Kai O, Cheung JO, Williams S, Lowe B, Nyanoti M, Williams TN, Maitland K, Molyneux M, Newton CR, Peshu N, Watt SM, Roberts DJ. 2006. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood 108:2569–2577. doi: 10.1182/blood-2006-05-018697. [DOI] [PubMed] [Google Scholar]
- 51.Shekalaghe SA, Bousema JT, Kunei KK, Lushino P, Masokoto A, Wolters LR, Mwakalinga S, Mosha FW, Sauerwein RW, Drakeley CJ. 2007. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Trop Med Int Health 12:547–553. doi: 10.1111/j.1365-3156.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 52.Shekalaghe SA, ter Braak R, Daou M, Kavishe R, van den Bijllaardt W, van den Bosch S, Koenderink JB, Luty AJ, Whitty CJ, Drakeley C, Sauerwein RW, Bousema T. 2010. In Tanzania, hemolysis after a single dose of primaquine coadministered with an artemisinin is not restricted to glucose-6-phosphate dehydrogenase-deficient (G6PD A-) individuals. Antimicrob Agents Chemother 54:1762–1768. doi: 10.1128/AAC.01135-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor WR, White NJ. 2004. Antimalarial drug toxicity: a review. Drug Safety 27:25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- 54.Nuchprayoon I, Sanpavat S, Nuchprayoon S. 2002. Glucose-6-phosphate dehydrogenase (G6PD) mutations in Thailand: G6PD Viangchan (871G→A) is the most common deficiency variant in the Thai population. Hum Mutat 19:185. doi: 10.1002/humu.9010. [DOI] [PubMed] [Google Scholar]
- 55.Hue NT, Charlieu JP, Chau TT, Day N, Farrar JJ, Hien TT, Dunstan SJ. 2009. Glucose-6-phosphate dehydrogenase (G6PD) mutations and haemoglobinuria syndrome in the Vietnamese population. Malaria J 8:152. doi: 10.1186/1475-2875-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuoka H, Thuan DT, van Thien H, Kanbe T, Jalloh A, Hirai M, Arai M, Dung NT, Kawamoto F. 2007. Seven different glucose-6-phosphate dehydrogenase variants including a new variant distributed in Lam Dong Province in southern Vietnam. Acta Med Okayama 61:213–219. [DOI] [PubMed] [Google Scholar]
- 57.Flegg JA, Guerin PJ, White NJ, Stepniewska K. 2011. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malaria J 10:339. doi: 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]