Abstract
The in vitro activity of isavuconazole against Mucorales isolates measured by EUCAST E.Def 9.2 and CLSI M38-A2 methodologies was investigated in comparison with those of amphotericin B, posaconazole, and voriconazole. Seventy-two isolates were included: 12 of Lichtheimia corymbifera, 5 of Lichtheimia ramosa, 5 of group I and 9 of group II of Mucor circinelloides, 9 of Rhizomucor pusillus, 26 of Rhizopus microsporus, and 6 of Rhizopus oryzae. Species identification was confirmed by internal transcribed spacer (ITS) sequencing. EUCAST MICs were read on day 1 (EUCAST-d1) and day 2 (EUCAST-d2), and CLSI MICs were read on day 2 (CLSI-d2). Isavuconazole MIC50s (range) (mg/liter) by EUCAST-d1, CLSI-d2, and EUCAST-d2 were 1 (0.125 to 16), 1 (0.125 to 2), and 4 (0.5 to >16), respectively, across all isolates. The similar values for comparator drugs were as follows: posaconazole, 0.25 (≤0.03 to >16), 0.25 (0.06 to >16), and 1 (0.06 to >16); amphotericin, 0.06 (≤0.03 to 0.5), 0.06 (≤0.03 to 0.25), and 0.125 (≤0.03 to 1); voriconazole, 16 (2 to >16), 8 (1 to >16), and >16 (8 to >16), respectively. Isavuconazole activity varied by species: Lichtheimia corymbifera, 1 (0.5 to 2), 1 (1 to 2), and 2 (1 to 4); Lichtheimia ramosa, 0.25 (0.125 to 0.5), 1 (0.5 to 2), and 2 (0.5 to 4); Rhizomucor pusillus, 0.5 (0.5 to 1), 1 (0.125 to 1), and 2 (1 to 2); Rhizopus microsporus, 1 (0.5 to 4), 0.5 (0.125 to 1), and 4 (1 to 8); and Rhizopus oryzae, 1 (0.5 to 4), 1 (0.125 to 2), and 4 (0.5 to 8), respectively, were more susceptible than Mucor circinelloides: group I, 8 (4 to 8), 4 (2 to 4), and 16 (2 to 16), respectively, and group II, 8 (1 to 16), 8 (1 to 8), and 16 (4 to >16), respectively. This was also observed for posaconazole. The essential agreement was best between EUCAST-d1 and CLSI-d2 (75% to 83%). Isavuconazole displayed in vitro activity against Mucorales isolates with the exception of Mucor circinelloides. The MICs were in general 1 to 3 steps higher than those for posaconazole. However, in the clinical setting this may be compensated for by the higher exposure at standard dosing.
INTRODUCTION
Isavuconazole is a new broad-spectrum azole with activity against various yeasts and molds (1). It is administered as a water-soluble prodrug, isavuconazonium sulfate, which is available as cyclodextrin-free intravenous (i.v.) and oral (p.o.) formulations. Following administration, the prodrug is immediately and completely converted by plasma esterases to isavuconazole, which inhibits biosynthesis of ergosterol, an essential component of fungal membranes. Currently, amphotericin B and posaconazole are the only two compounds recommended for treatment of Mucorales infections in Europe, only amphotericin B is recommended for primary treatment, and only amphotericin B is licensed for treatment of these infections (2, 3). The clinical efficacy of isavuconazole against infections due to Mucorales species has been evaluated in a phase III study leading to its approval by the FDA for the primary treatment of mucormycosis (March 2015). An overall success rate of 31.4% (14.3% and 17.1%, complete and partial response, respectively) was reported at the end of treatment among 37 patients with Mucorales monoinfection (4). Twenty-one of these patients were matched and compared with patients from the FungiScope registry treated with an amphotericin B formulation (a third of the patients received conventional amphotericin B) (5). The median treatment duration was 108 days for isavuconazole and 18 days for amphotericin B, with approximately one-third of the patients receiving additional posaconazole therapy in the amphotericin B patient group. Overall survival rates on days 42 and 84 were similar (5).
The in vitro activity of isavuconazole has been studied using the EUCAST and CLSI methodologies against Candida and Aspergillus; however, data on in vitro activity against isolates of the Mucorales order are sparse and particularly so for EUCAST testing (6–9). The purpose of this study was to investigate and compare the in vitro activities against clinical isolates of the Mucorales order by the EUCAST and CLSI reference methodologies and to compare the activities with those of amphotericin B, posaconazole, and voriconazole. Such data are crucial for clarifying the correlation between in vitro and in vivo responses and for future development of epidemiological cutoff values (ECOFFs/ECVs) and clinical breakpoints.
MATERIALS AND METHODS
Mucorales isolates and species identification.
A total of 72 clinical Mucorales isolates were included. The isolates were obtained in 1998 to 2014 from samples or pure cultures referred to the mycology reference laboratory at the Statens Serum Institut, Denmark. Seventy isolates were confirmed to originate from nonsuperficial specimens, whereas no information regarding sample site was available for two samples. All isolates underwent confirmatory molecular species identification by internal transcribed spacer (ITS) DNA sequencing using the universal fungal primers (ITS1, TCGTAGGTGAACCTGCGG, and ITS4, TCCTCCGCTTATTGATATGC [10]) and the online pairwise sequence alignment tool available through the webpage for Centraalbureau voor Schimmelcultures (http://www.cbs.knaw.nl/collections/BioloMICSSequences.aspx). The molecularly confirmed species distribution was as follows: 12 of Lichtheimia corymbifera, 5 of Lichtheimia ramosa, 5 of Mucor circinelloides group I (>99.8% match to CBS strains 106.10 and 195.68), 9 of Mucor circinelloides group II (>99.8% match to CBS strains 542.80 and 416.77), 9 of Rhizomucor pusillus, 26 of Rhizopus microsporus, and 6 of Rhizopus oryzae (see Fig. S1 in the supplemental material).
Susceptibility testing.
Susceptibility testing was performed using the EUCAST E.Def 9.2 and the CLSI M38-A2 methodologies (11, 12). All isolates were cultured twice on Sabouraud dextrose agar (SSI Diagnostika, Hillerød, Denmark) before susceptibility testing to ensure viability. Stock solutions (5,000 mg/liter) in dimethyl sulfoxide (DMSO) and manufacturers were as indicated: DMSO, Sigma-Aldrich, Vallensbæk Strand, Denmark (catalog no. D8779); isavuconazole, Astellas Pharma Inc., Tokyo, Japan; amphotericin B, Sigma-Aldrich; posaconazole, Merck, Ballerup, Denmark; voriconazole, Pfizer A/S, Ballerup, Denmark. The drug concentration range studied was 0.03 to 16 mg/liter for all compounds. For both methods, plates were made in one batch, immediately frozen (−80°C), and used as soon as thawed. Inoculated plates were incubated at 35°C and read visually (blinded to the species identity) at days 1 (EUCAST-d1) and 2 (EUCAST-d2) for the EUCAST methodology and only at day 2 (CLSI-d2) for the CLSI plates as growth was insufficient at day 1. The MIC was the lowest drug concentration that prevented any discernible growth (100%) as defined in the reference methodologies. The ATCC 6258 strain of Candida krusei was included as a control strain. Amphotericin B, isavuconazole, posaconazole, and voriconazole MIC ranges were as follows with the reference quality control (QC) ranges in parentheses (all values in milligrams per liter): EUCAST-d1, 0.5 (0.125 to 1), ≤0.03 (not established but 0.015 to 0.125 in the work of Howard et al. [13]), ≤0.03 to 0.06 (0.015 to 0.06), and 0.125 to 0.25 (0.03 to 0.25) (14); EUCAST-d2 (no reference ranges established for the day 2 reading), 0.5, ≤0.03 to 0.06, ≤0.03, and 0.25; and CLSI-d2, 0.25 to 0.5 (1 to 4), ≤0.03 to 0.06 (not established), 0.125 (0.125 to 1), and 0.25 to 0.5 (0.125 to 1) (12).
Clinical breakpoints have not been defined for Mucorales isolates. However, as amphotericin B, isavuconazole, posaconazole, and voriconazole have documented clinical efficacy against wild-type Aspergillus fumigatus, we hypothesized that these four agents may also have clinical efficacy against Mucorales isolates for which MICs were within the MIC range for wild-type A. fumigatus. Hence, isolates were classified as potentially susceptible (pot-S) when the MIC was below the defined epidemiological cutoff values for A. fumigatus: amphotericin B, 1 mg/liter for the EUCAST and 2 mg/liter for the CLSI method; posaconazole, 0.25 mg/liter for the EUCAST and 0.5 mg/liter for the CLSI method; isavuconazole, 2 mg/liter for the EUCAST and 1 mg/liter for the CLSI method; and voriconazole, 1 mg/liter for both methods (13, 15–19).
Comparison between EUCAST and CLSI.
The percent essential agreement (±1 2-fold dilution) between the EUCAST and the CLSI methods was calculated for each species. The median and range of 2-fold dilution differences between the two methods were also calculated. In order to calculate the exact differences between the methods, off-scale MICs (≤0.03 and >16 mg/liter) were excluded from this analysis.
The categorical agreement between the two methods was calculated as percentage of isolates classified as pot-S or non-pot-S by both methods. Finally, categorical agreement was also calculated for posaconazole using 1 mg/liter as the MIC cutoff value, recognizing the notable difference in posaconazole susceptibility between Mucor circinelloides and the other species and the fact that a cutoff value at 0.25 mg/liter bisected the combined posaconazole MIC distribution of non-Mucor circinelloides species.
RESULTS
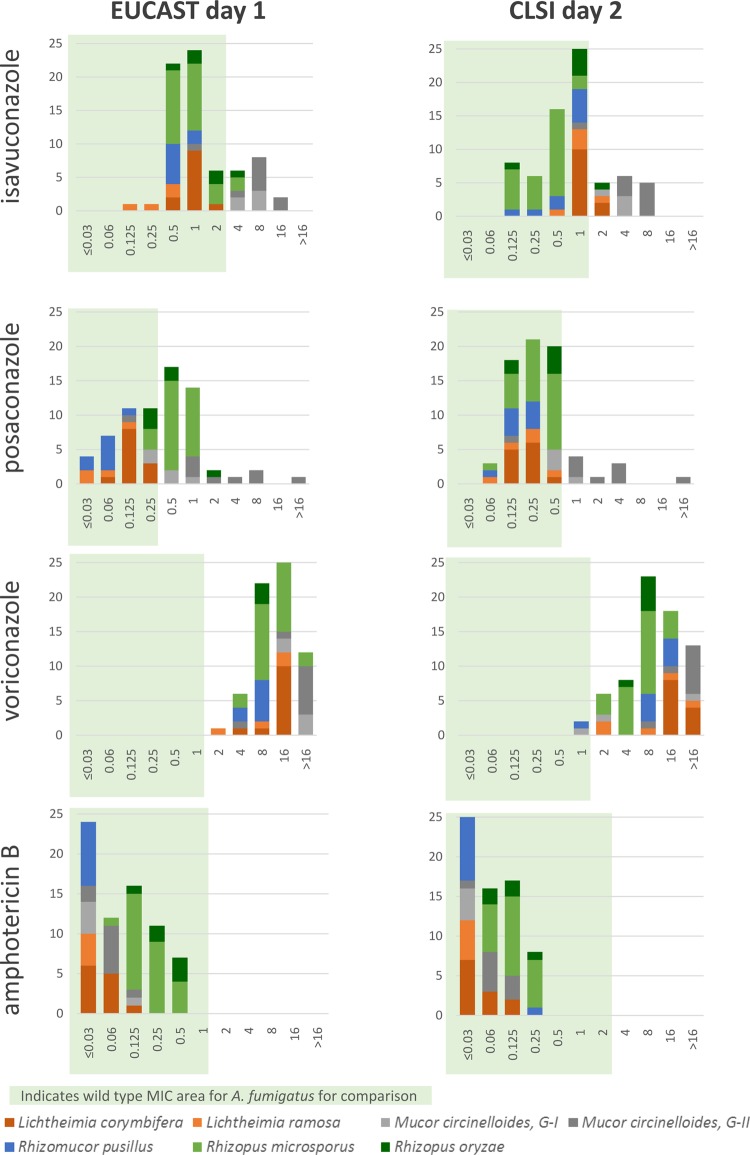
The in vitro activity of isavuconazole was species dependent. The EUCAST-d1 isavuconazole MIC ranges were 0.125 to 4 mg/liter across Lichtheimia corymbifera, Lichtheimia ramosa, Rhizomucor pusillus, Rhizopus microsporus, and Rhizopus oryzae but somewhat higher against Mucor circinelloides groups I and II (1 to 16 mg/liter) (Table 1; Fig. 1). Similarly, the CLSI-d2 isavuconazole MIC ranges were 1 to 8 mg/liter for Mucor circinelloides groups I and II in comparison with 0.125 to 2 mg/liter for the other species. Overall, the isavuconazole MIC50 across all species was 1 mg/liter for both EUCAST-d1 and CLSI-d2 and with almost identical MIC ranges (0.125 to 16 mg/liter for EUCAST-d1 and 0.125 to 8 mg/liter for CLSI-d2). Reading the EUCAST plates after 2 days of incubation elevated the MICs 1 to 2 steps but did not change the overall species-dependent susceptibility pattern (Table 1 and Fig. 2).
TABLE 1.
Overview of MIC ranges, MIC50 values, and proportions of Mucorales species isolates for which MICs fall within the wild-type MIC range for A. fumigatus when susceptibility is tested by EUCAST (E.Def 9.2) and CLSI (M38-A2) methodologiese
| Antifungal compound and species (no. of isolates) | Visual reading result |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| EUCAST, day 1 |
EUCAST, day 2 |
CLSI, day 2 |
|||||||
| Range (mg/liter) | MIC50 (mg/liter) | % of MICs below A. fumigatus ECOFFc | Range (mg/liter) | MIC50 (mg/liter) | % of MICs below A. fumigatus ECOFFc | Range (mg/liter) | MIC50 (mg/liter) | % of MICs below A. fumigatus ECVd | |
| Amphotericin B | |||||||||
| Lichtheimia corymbifera (12) | ≤0.03 to 0.125 | ≤0.03 | 100 | ≤0.03 to 0.25 | 0.125 | 100 | ≤0.03 to 0.125 | ≤0.03 | 100 |
| Lichtheimia ramosa (4a/5) | ≤0.03 | ≤0.03 | 100 | ≤0.03 to 0.06 | 0.06 | 100 | ≤0.03 | ≤0.03 | 100 |
| Mucor circinelloides | |||||||||
| Group I (4/5b) | ≤0.03 to 0.125 | ≤0.03 | 100 | ≤0.03 to 0.125 | 0.06 | 100 | ≤0.03 | ≤0.03 | 100 |
| Group II (9) | ≤0.03 to 0.125 | 0.06 | 100 | 0.06 to 0.25 | 0.125 | 100 | ≤0.03 to 0.125 | 0.06 | 100 |
| Rhizomucor pusillus (8a/9) | ≤0.03 | ≤0.03 | 100 | ≤0.03 to 0.25 | 0.06 | 100 | ≤0.03 to 0.25 | ≤0.03 | 100 |
| Rhizopus microsporus (26) | 0.06 to 0.5 | 0.125 | 100 | 0.25 to 1 | 0.5 | 100 | ≤0.03 to 0.25 | 0.125 | 100 |
| Rhizopus oryzae (6) | 0.125 to 0.5 | 0.25 | 100 | 0.5 to 1 | 0.5 | 100 | ≤0.03 to 0.25 | 0.06 | 100 |
| Total (70/72a,b) | ≤0.03 to 0.5 | 0.06 | 100 | ≤0.03 to 1 | 0.125 | 100 | ≤0.03 to 0.25 | 0.06 | 100 |
| Isavuconazole | |||||||||
| Lichtheimia corymbifera (12) | 0.5 to 2 | 1 | 100 | 1 to 4 | 2 | 67 | 1 to 2 | 1 | 83 |
| Lichtheimia ramosa (4a/5) | 0.125 to 0.5 | 0.25 | 100 | 0.5 to 4 | 2 | 60 | 0.5 to 2 | 1 | 80 |
| Mucor circinelloides | |||||||||
| Group I (4/5b) | 4 to 8 | 8 | 0 | 2 to 16 | 16 | 20 | 2 to 4 | 4 | 0 |
| Group II (9) | 1 to 16 | 8 | 11 | 4 to >16 | 16 | 0 | 1 to 8 | 8 | 11 |
| Rhizomucor pusillus (8a/9) | 0.5 to 1 | 0.5 | 100 | 1 to 2 | 2 | 100 | 0.125 to 1 | 1 | 100 |
| Rhizopus microsporus (26) | 0.5 to 4 | 1 | 92 | 1 to 8 | 4 | 35 | 0.125 to 1 | 0.5 | 100 |
| Rhizopus oryzae (6) | 0.5 to 4 | 1 | 83 | 0.5 to 8 | 4 | 33 | 0.125 to 2 | 1 | 83 |
| Total (70/72a,b) | 0.125 to 16 | 1 | 77 | 0.5 to >16 | 4 | 44 | 0.125 to 2 | 1 | 77 |
| Posaconazole | |||||||||
| Lichtheimia corymbifera (12) | 0.06 to 0.25 | 0.125 | 100 | 0.125 to 0.5 | 0.25 | 75 | 0.125 to 0.5 | 0.25 | 100 |
| Lichtheimia ramosa (4a/5) | ≤0.03 to 0.125 | ≤0.03 | 100 | 0.06 to 0.5 | 0.5 | 40 | 0.06 to 0.5 | 0.25 | 100 |
| Mucor circinelloides | |||||||||
| Group I (4/5b) | 0.25 to 1 | 0.5 | 40 | 0.5 to 8 | 1 | 0 | 0.5 to 1 | 0.5 | 75 |
| Group II (9) | 0.125 to >16 | 2 | 11 | 1 to >16 | >16 | 0 | 0.125 to >16 | 2 | 11 |
| Rhizomucor pusillus (8a/9) | ≤0.03 to 0.125 | 0.06 | 100 | 0.125 to 0.5 | 0.25 | 78 | 0.06 to 0.25 | 0.125 | 100 |
| Rhizopus microsporus (26) | 0.25 to 1 | 0.5 | 12 | 0.5 to >16 | 2 | 0 | 0.06 to 0.5 | 0.25 | 100 |
| Rhizopus oryzae (6) | 0.25 to 2 | 0.5 | 50 | 0.25 to >16 | 0.5 | 17 | 0.125 to 0.5 | 0.5 | 100 |
| Total (70/72a,b) | ≤0.03 to >16 | 0.25 | 47 | 0.06 to >16 | 1 | 26 | 0.06 to >16 | 0.25 | 87 |
| Voriconazole | |||||||||
| Lichtheimia corymbifera (12) | 4 to 16 | 16 | 0 | >16 | >16 | 0 | 16 to >16 | 16 | 0 |
| Lichtheimia ramosa (4a/5) | 2 to 16 | 8 | 0 | 16 to >16 | >16 | 0 | 2 to >16 | 8 | 0 |
| Mucor circinelloides | |||||||||
| Group I (4/5b) | 16 to >16 | >16 | 0 | 8 to >16 | >16 | 0 | 1 to >16 | 2 | 33 |
| Group II (9) | 4 to >16 | >16 | 0 | >16 | >16 | 0 | 8 to >16 | >16 | 0 |
| Rhizomucor pusillus (8a/9) | 4 to 8 | 8 | 0 | 16 to >16 | 16 | 0 | 1 to 16 | 8 | 11 |
| Rhizopus microsporus (26) | 4 to >16 | 8 | 0 | 16 to >16 | >16 | 0 | 2 to 16 | 8 | 0 |
| Rhizopus oryzae (6) | 8 to 16 | 8 | 0 | 16 to >16 | 16 | 0 | 4 to 8 | 8 | 0 |
| Total (70/72a,b) | 2 to >16 | 16 | 0 | 8 to >16 | >16 | 0 | 1 to >16 | 8 | 3 |
EUCAST MICs for one Lichtheimia ramosa isolate and one Rhizomucor pusillus isolate could not be evaluated on day 1 due to insufficient growth.
CLSI MICs for one Mucor circinelloides group I isolate could not be evaluated due to no growth on day 2.
Amphotericin B, 1 mg/liter; posaconazole, 0.25 mg/liter; isavuconazole, 2 mg/liter; voriconazole, 1 mg/liter (13, 15–17).
Amphotericin B, 2 mg/liter; posaconazole, 0.5 mg/liter; isavuconazole, 1 mg/liter; voriconazole, 1 mg/liter (18, 19).
MIC ranges and MIC50 values for Mucorales species isolates were determined by EUCAST (E.Def 9.2) and CLSI (M38-A2) methodologies.
FIG 1.
Isavuconazole, posaconazole, voriconazole, and amphotericin B MICs against various Mucorales species determined by EUCAST (endpoint reading after 1 day of incubation) and CLSI (endpoint reading after 2 days of incubation) methodologies. MIC ranges for wild-type A. fumigatus are shown as shaded areas for comparison. x axes, MIC (milligrams per liter); y axes, number of isolates.
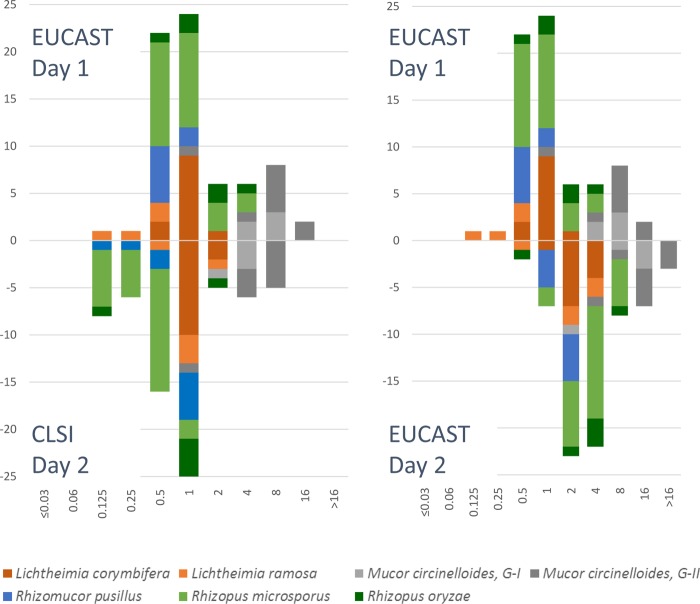
FIG 2.
Comparison between isavuconazole MICs obtained by the EUCAST E.Def 9.2 (above the x axis) and CLSI M38 (below the x axis) methods (left diagram) and between EUCAST day 1 (above the x axis) and day 2 (below the x axis) readings (right diagram).
The overall MIC50s for amphotericin B and posaconazole were 0.06 and 0.25 mg/liter, respectively, when determined by either the EUCAST-d1 or the CLSI-d2 method and again with almost identical MIC ranges (Table 1). The in vitro activity of posaconazole varied by species, with Mucor circinelloides group II being the least susceptible species by both methods (MIC50, 2 mg/liter; range, 0.125 to >16 mg/liter). In comparison, the amphotericin B in vitro activity was more uniform with species-specific MIC50s between ≤0.03 and 0.25 mg/liter when determined by EUCAST-d1, ≤0.03 to 0.125 mg/liter by CLSI-d2, and 0.06 to 0.5 mg/liter by EUCAST-d2. Finally, the MIC ranges for voriconazole were 2 to >16, 1 to >16, and 8 to >16 mg/liter obtained by EUCAST-d1, CLSI-d2, and EUCAST-d2 reading, respectively, with MIC50s of 8 to >16 mg/liter.
Based on the hypothesis that Mucorales isolates could be regarded as potentially susceptible (pot-S) when the MIC was within the wild-type MIC range for A. fumigatus, the proportion of such (pot-S) isolates was calculated (Table 1). All isolates were pot-S to amphotericin B independently of which susceptibility test was used, but only 0 to 3% were classified as pot-S to voriconazole. For isavuconazole, 77% of the isolates were pot-S by EUCAST-d1 and CLSI-d2 testing with significant variation between the species, e.g., 0 and 11% were pot-S for Mucor circinelloides groups I and II, respectively, but 80 to 100% were pot-S for the other species. For posaconazole, 47% and 87% were pot-S by EUCAST-d1 and CLSI-d2 testing, respectively, including all Lichtheimia and Rhizomucor pusillus isolates but only 11% of Mucor circinelloides group II isolates. For the other species (Mucor circinelloides group I, Rhizopus microsporus, and Rhizopus oryzae), more were classified as pot-S by the CLSI-d2 (75 to 100%) than by the EUCAST-d1 (12 to 50%) methodology.
The best essential agreement was found between the CLSI-d2 method and the EUCAST-d1 method, with overall essential agreement ranging from 75% for isavuconazole to 83% for amphotericin B. The median (range) 2-fold dilution differences were 0 (−3 to 4) (Table 2). The essential agreement was highest for Lichtheimia corymbifera (100% across amphotericin B, posaconazole, and isavuconazole and 7/8, 88%, for voriconazole) and lowest for amphotericin B against Rhizopus oryzae (1/6, 17%), isavuconazole against Rhizopus microsporus (14/26, 54%), and posaconazole against Mucor circinelloides group II (3/7, 43%) and Rhizomucor pusillus (5/8, 63%). The essential agreement between the CLSI-d2 method and the EUCAST-d2 method was 38% to 61% and lowest for isavuconazole.
TABLE 2.
Comparison between EUCAST and CLSI methods for antifungal susceptibility testing of Mucoralesd
| Comparison and species | No. of strains | % essential agreement, median (range) 2-fold difference |
% categorical agreement |
||||||
|---|---|---|---|---|---|---|---|---|---|
| AMB | POSA | ISA | VORI | AMB | POSAa | ISA | VORI | ||
| EUCAST day 1 vs CLSI | |||||||||
| Lichtheimia corymbifera | 12 | 100, 0 (−1 to 1) | 100, −0.5 (−1 to 0) | 100, 0 (−1 to 1) | 88, 0 (−2 to 0) | 100 | 100/100 | 83 | 100 |
| Lichtheimia ramosa | 4 | 100, 0 (0–0) | 75, −1 (−3 to 1) | 75, −1 (−3 to 1) | 75, 0.5 (0–2) | 100 | 100/100 | 100 | 100 |
| Mucor circinelloides | |||||||||
| Group I | 4 | 100, 0 (0–0) | 100, 0 (−1 to 0) | 75, 1 (0–2) | 0, 3b | 100 | 50/75 | 100 | 67 |
| Group II | 9 | 100, 0 (−1 to 1) | 43, 0 (−2 to 3) | 100, 0 (0–1) | 100, −1b | 100 | 100/67 | 100 | 100 |
| Rhizomucor pusillus | 8 | 100, 0 (0–0) | 63, −1 (−2 to 1) | 88, 0 (−1 to 2) | 88, −0.5 (−1 to 2) | 100 | 100/100 | 100 | 88 |
| Rhizopus microsporus | 26 | 73, 1 (−1 to 4) | 73, 1 (0–2) | 54, 1 (0–3) | 75, 1 (−1 to 3) | 100 | 12/100 | 92 | 100 |
| Rhizopus oryzae | 6 | 17, 2 (1–2) | 83, 0.5 (−1 to 2) | 67, 0.5 (0–2) | 100, 1 (0–1) | 100 | 50/83 | 67 | 100 |
| All | 70 | 83, 0 (−1 to 4) | 76, 0 (−3 to 3) | 75, 1 (−3 to 3) | 81, 0 (−2 to 3) | 100 | 59/93 | 91 | 97 |
| EUCAST day 2 vs CLSI | |||||||||
| Lichtheimia corymbifera | 12 | 58, 1 (0–2) | 100, 1 (−1 to 1) | 75, 1 (0–2) | NDc | 100 | 75/100 | 67 | 100 |
| Lichtheimia ramosa | 4 | 100, 1 (0–1) | 80, 0 (0–2) | 80, 1 (0–2) | 0, 3b | 100 | 40/100 | 80 | 100 |
| Mucor circinelloides | |||||||||
| Group I | 4 | 75, 1 (0–2) | 50, 2 (0–3) | 25, 2 (1–3) | ND | 100 | 25/75 | 100 | 67 |
| Group II | 9 | 89, 1 (0–2) | 50 (0 and 3b) | 67, 1 (1–2) | ND | 100 | 89/89 | 89 | 100 |
| Rhizomucor pusillus | 8 | 100, 1 (0–1) | 89, 1 (0–2) | 67, 1 (0–3) | 86, 1 (0–4) | 100 | 78/100 | 100 | 89 |
| Rhizopus microsporus | 26 | 35, 2 (0–5) | 16, 3 (1–5) | 4, 3 (1–6) | 33, 2 (0–3) | 100 | 0/35 | 35 | 100 |
| Rhizopus oryzae | 6 | 0, 3 (2–4) | 80, 1 (0–2) | 17, 2 (1–2) | 80, 1 (1–2) | 100 | 17/83 | 50 | 100 |
| All | 70 | 58, 1 (0–5) | 61, 1 (−1 to 5) | 38, 2 (0–6) | 59, 1 (0–4) | 100 | 39/72 | 63 | 97 |
Categorical agreement using the cutoffs of 0.25 mg/liter and 1 mg/liter.
Fewer than 3 isolates with on-scale MICs. The 2-fold dilution differences are presented for each isolate.
ND, not determined because of off-scale MICs.
Abbreviations: AMB, amphotericin B; POSA, posaconazole; ISA, isavuconazole; VORI, voriconazole.
The overall categorical agreement between the CLSI-d2 method and the EUCAST-d1 method ranged from 91% for isavuconazole, 93% for posaconazole (with the 1 mg/liter cutoff), to 100% for amphotericin B. For isavuconazole, the lowest categorical agreement was found for Rhizopus oryzae (4/6, 67%). For posaconazole, the categorical agreement between EUCAST-d1 and CLSI-d2 was calculated using the A. fumigatus ECOFF of 0.25 mg/liter as well as 1 mg/liter to avoid bisecting the non-Mucor circinelloides MIC distributions. The agreement was highest using the 0.25-mg/liter cutoff for Mucor circinelloides overall (85% versus 69%) and Mucor circinelloides group II in particular (100% versus 67%) but using 1 mg/liter for Rhizopus microsporus (12% versus 100%), Rhizopus oryzae (50% versus 83%), and Mucor circinelloides group I (50% versus 75%).
Finally, the pharmacokinetic characteristics of isavuconazole in comparison with those for the other mold-active azoles were compared (Table 3) (20–27). The isavuconazole minimum concentration of drug in serum (Cmin) (3.91 mg/liter) and area under the concentration-time curve (AUC) (97.9 mg · h/liter) were 6- to 3-fold higher than the similar parameters for posaconazole oral solution (0.64 mg/liter and 17.2 mg · h/liter, respectively) and i.v. formulation (1.07 mg/liter and 34.3 mg · h/liter, respectively) (Table 3).
TABLE 3.
Human pharmacokinetic data for the mold-active azole compoundsc
| Drug | Route or form of administration | Patient group (reference) | Dosage | Day(s) when steady state reached | Bioavailabilityd | Mean Cmax, mg/literd | Mean Cmin, mg/literd | Mean Cav, mg/literd | Mean total body CL/F (liters/h)d | Mean t1/2 (h)d | Mean AUC24 (mg · h/liter)d | Fraction unbound (%) | Mean V/F (liters/kg)d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isavuconazole | Oral or i.v. | Patients with IA (n = 222) (20) | 200 mg TID on days 1–2, 200 mg QD | 14 | 0.98–1 | 3.91 (49) | 2.4 (44) | 100 (50–150) | 97.9 (58) | 1 | >5 (44) | ||
| Posaconazole | Oral suspension | Febrile neutropenic patients or patients with refractory invasive fungal diseases (n = 23) (21) | 400 mg BID | 7–10 | 0.54–0.75 | 0.85 (82) | 0.64 (98) | 0.72 (86) [6.70–2,256] | 76.1 (78) [14.9–256] | 31.7 (42) [12.4–67.3] | 17.2 (86) [3.1–53.6] | 2 | 44 (84) [5.8–187] |
| Gastroresistant tablet (day 8) | Neutropenic patients receiving cytotoxic chemotherapy for AML or MDS (n = 32) (22) | 300 mg BID on day 1, 300 mg QD | 7–10 | 0.54–0.75 | 1.96 (33) | [0.343–2.55] | 1.46 (38) | 35 (41) [11.8–62.3] | |||||
| i.v. (day 14) | Neutropenic patients receiving cytotoxic chemotherapy for AML or MDS (n = 19) (23) | 300 mg BID on day 1, 300 mg QD | 1 | 2.61 (39) | 1.07 (50) | 1.43 (42) | 34.3 (42) | ||||||
| Voriconazole | Oral | Adult patients with IA (n = 43) (24) | 400 mg BID on day 1, 200 mg BID | 5–7 | 0.82 (15) | 3.57 (48.5)a | 0.83 (197) | 11.52 (73)a | 11.31 (87.3)a | 36 (119) | 2.6 (96) | ||
| Adult patients with proven or probable IA on combination therapy with anidulafungin (n = 454) (25) | 6 mg/kg of body wt BID on day 1, 4 mg/kg BID for 7 days, 300 mg BID | 5–7 | 0.64 (24) | 2.04 (54) | 5.30 (11) | 66 (45) | 45–55b | 2.38 (15–26) | |||||
| i.v. | Adult patients with IA (n = 43) (24) | 6 mg/kg BID on day 1, 4 mg/kg BID | 1 | 2.54 (231) | 90.4 (168) | 45–55b | 2.6 (96) | ||||||
| Adult patients with proven or probable IA on combination therapy with anidulafungin (n = 454) (25) | 6 mg/kg BID on day 1, 4 mg/kg BID | 1 | 3.10 (52) | 5.30 (11) | 102 (43) | 2.38 (15–26) |
Data obtained from reference 26.
Data obtained from reference 27.
Abbreviations: IA, invasive aspergillosis; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; TID, three times daily; QD, once daily; BID, twice daily; Cmax, maximum concentration of drug in serum; Cmin, minimum concentration of drug in serum; Cav, average concentration of drug in serum; CL, clearance; F, bioavailability; t1/2, half-life; AUC24, area under the concentration-time curve at 24 h; V, volume of distribution.
Pharmacokinetic data in parentheses are percent coefficients of variation; data in brackets are ranges.
DISCUSSION
Overall, the MICs correlated with the well-accepted clinical antifungal spectrum associated with efficacy and failure. Thus, the MICs for voriconazole, which has no clinical efficacy against Mucorales infections, were high and above the MIC range correlated with clinical efficacy for A. fumigatus (15, 17, 19). In contrast, amphotericin B MICs fell in the MIC range that for other mold and yeast species normally would predict susceptibility (15, 16, 18, 28). This observation is somewhat reassuring as susceptibility testing of molds is challenging and the correlation with clinical outcome is often debated.
By EUCAST and CLSI susceptibility testing, isavuconazole MICs for the Mucorales isolates were similar to those found for Aspergillus species, with the exception of Mucor circinelloides, which was notably less susceptible than the other species across all methods and endpoints (13, 29). Accordingly, 83 to 100% of the isolates were classified as pot-S using EUCAST-d1 and CLSI-d2 across all isolates except Mucor circinelloides. This observation suggests species-specific differential clinical efficacy against the clinically relevant Mucorales species. A similar pattern was found for posaconazole, which was also found to be less active against Mucor circinelloides and against Mucor circinelloides group II in particular, and even for voriconazole, the MICs against Mucor circinelloides were the highest ones. However, in addition to this species-specific differential activity, some additional and method-dependent differential activity was noted. For example, Rhizopus microsporus was clearly less susceptible than Lichtheimia species and Rhizomucor pusillus to posaconazole when susceptibility was tested by the EUCAST method but not when tested by the CLSI method. Similarly, Rhizopus spp. were more susceptible to isavuconazole than Lichtheimia spp. when tested by the CLSI method but not when tested by the EUCAST method. The clinical impact of these observations, if any, remains to be understood, but they clearly demonstrate that clinical breakpoints have to be species as well as method specific in order to provide the same categorization of isolates as susceptible or resistant. Whereas no species-specific in vivo outcome data have been published for infections due to Mucor species isolates, in vivo data suggest isavuconazole efficacy against isolates of Rhizopus. Thus, a successful clinical outcome of rhinocerebral mucormycosis by a Rhizopus oryzae isolate with a MIC of 1 mg/liter has been reported after isavuconazole salvage therapy with trough plasma levels maintained at 1.3 to 3.24 mg/liter (30). Moreover, preclinical studies showed that high doses of isavuconazole were as effective as high-dose liposomal amphotericin B against experimental mucormycosis by a Rhizopus delemar isolate with a MIC of 0.125 mg/liter (31).
The isavuconazole MIC50s across the isolates were 2 dilution steps higher than those for posaconazole and 4 steps higher than those for amphotericin B. Direct comparisons of MICs across compounds are, however, not meaningful because bioavailability and pharmacokinetic and pharmacodynamics parameters associated with clinical efficacy are different among compounds and drug classes (Table 3) (20–27). For the azole drugs, outcome is best predicted by the AUC/MIC ratio. Noticeably, the AUC for isavuconazole is 4 to 6 times higher than that for posaconazole, which may compensate for the 2-dilution-step-lower MIC and explain the clinical efficacy observed in the clinical trial despite higher MICs (Table 3). Some support for this hypothesis was further derived from the observations made when adopting the ECOFF/ECVs for these four agents for A. fumigatus as potential breakpoints for susceptibility. Thus, all isolates were rightfully classified as susceptible to amphotericin B and virtually none were classified as susceptible to voriconazole, and interestingly, more isolates were classified as potentially susceptible to isavuconazole than to posaconazole independently of which susceptibility testing method was used. Therefore, this study provides some in vitro support for the assumption that isavuconazole may be an appropriate choice for most Mucorales species with the exception of Mucor circinelloides.
The EUCAST susceptibility plates were read on day 1, whereas the CLSI plates were read on day 2 due to a lack of visible growth after the first day of incubation. This difference is most likely explained by the 10-fold-lower inoculum used for the CLSI method and the 10-fold-lower glucose concentration, test conditions which are associated with lower growth rates for Candida species. When the reading of the EUCAST plates was repeated on day 2, the MICs rose approximately 2 dilutions for the three azoles and 1 dilution for amphotericin B, leading to a marked decrease in the categorical agreement with CLSI-d2 results for isavuconazole and posaconazole. Similarly, MICs reported in the literature for day 2 readings are in general higher than the EUCAST-d1 MICs presented here (6, 7). It is a well-known phenomenon that MICs rise with extended time of incubation and also that MIC endpoints may vary considerably across methods and endpoint criteria in general and also specifically for isavuconazole and Mucorales (32). Hence, standardization is key and future clinical breakpoints should be specific for the method, species, and incubation time used.
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by a grant from Basilea.
We thank Birgit Brandt for excellent technical assistance.
M.C.A. has received research grants and travel grants from and has been paid for talks on behalf of Astellas, Basilea, Gilead, Merck Sharp & Dohme, and Pfizer. She has been on the advisory board for Merck and Gilead. R.H.J. has received grant support from Gilead Sciences and travel grants from Gilead, Merck Sharp & Dohme, Pfizer, and Astellas. J.M. has received research grants, travel grants, and honoraria from Astellas, Gilead, Merck Sharp & Dohme, and Pfizer.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01919-15.
REFERENCES
- 1.Miceli MH, Kauffman CA. 15 July 2015. Isavuconazole: a new broad-spectrum triazole antifungal agent. Clin Infect Dis doi: 10.1093/cid/civ571. [DOI] [PubMed] [Google Scholar]
- 2.Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Freiberger T, Guinea J, Guarro J, de Hoog S, Hope W, Johnson E, Kathuria S, Lackner M, Lass-Flörl C, Lortholary O, Meis JF, Meletiadis J, Muñoz P, Richardson M, Roilides E, Tortorano AM, Ullmann AJ, van Diepeningen A, Verweij P, Petrikkos G. 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect 20(Suppl 3):S5–S26. doi: 10.1111/1469-0691.12371. [DOI] [PubMed] [Google Scholar]
- 3.Skiada A, Lanternier F, Groll AH, Pagano L, Zimmerli S, Herbrecht R, Lortholary O, Petrikkos GL. 2013. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica 98:492–504. doi: 10.3324/haematol.2012.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marty FM, Perfect JR, Cornely OA, Mullane KM, Rahav G, Lee M, Ito M, Maher R, Zeiher B, Ostrosky-Zeichner L. 2014. An open-label phase 3 study of isavuconazole (VITAL): focus on mucormycosis, poster 824 IDWeek 2014 Abstr. [Google Scholar]
- 5.Vehreschild MJGT, Vehreschild JJ, Marty FM, Perfect J, Ostrosky-Zeichner L, Rahav G, Zeiher B, Lee M, Maher R, Lovell C, Engelhardt M, Cornely OA. 2014. Primary treatment of invasive mucormycosis (IM) with isavuconazole (VITAL Study) or amphotericin formulations (FungiScope): case-matched analysis, poster 1152 56th ASH Annu Meet. [Google Scholar]
- 6.Perkhofer S, Lechner V, Lass-Flörl C. 2009. In vitro activity of isavuconazole against Aspergillus species and zygomycetes according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob Agents Chemother 53:1645–1647. doi: 10.1128/AAC.01530-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson GR, Wiederhold NP. 2010. Isavuconazole: a comprehensive review of spectrum of activity of a new triazole. Mycopathologia 170:291–313. doi: 10.1007/s11046-010-9324-3. [DOI] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Guinea J, Hagen F, Meis JF, Thompson GR, Turnidge J. 2015. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for the Cryptococcus neoformans-Cryptococcus gattii species complex using the CLSI M27-A3 broth microdilution method. Antimicrob Agents Chemother 59:666–668. doi: 10.1128/AAC.04055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guinea J, Peláez T, Recio S, Torres-Narbona M, Bouza E. 2008. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob Agents Chemother 52:1396–1400. doi: 10.1128/AAC.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Bolchacova E, Voigt K, Crous PW, Miller AN, Wingfield MJ, Aime MC, An K-D, Bai F-Y, Barreto RW, Begerow D, Bergeron M-J, Blackwell M, Boekhout T, Bogale M, Boonyuen N, Burgaz AR, Buyck B, Cai L, Cai Q, Cardinali G, Chaverri P, Coppins BJ, Crespo A, Cubas P, Cummings C, Damm U, de Beer ZW, de Hoog GS, Del-Prado R, Dentinger B, Dieguez-Uribeondo J, Divakar PK, Douglas B, Duenas M, Duong TA, Eberhardt U, Edwards JE, Elshahed MS, Fliegerova K, Furtado M, Garcia MA, Ge Z-W, Griffith GW, Griffiths K, Groenewald JZ, Groenewald M, Grube M, Gryzenhout M, Guo L-D, Hagen F, Hambleton S, Hamelin RC, Hansen K, Harrold P, Heller G, Herrera C, Hirayama K, Hirooka Y, Ho H-M, Hoffmann K, Hofstetter V, Hognabba F, Hollingsworth PM, Hong S-B, Hosaka K, Houbraken J, Hughes K, Huhtinen S, Hyde KD, James T, Johnson EM, Johnson JE, Johnston PR, Jones EBG, Kelly LJ, Kirk PM, Knapp DG, Koljalg U, Kovacs GM, Kurtzman CP, Landvik S, Leavitt SD, Liggenstoffer AS, Liimatainen K, Lombard L, Luangsa-ard JJ, Lumbsch HT, Maganti H, Maharachchikumbura SSN, Martin MP, May TW, McTaggart AR, Methven AS, Meyer W, Moncalvo J-M, Mongkolsamrit S, Nagy LG, Nilsson RH, Niskanen T, Nyilasi I, Okada G, Okane I, Olariaga I, Otte J, Papp T, Park D, Petkovits T, Pino-Bodas R, Quaedvlieg W, Raja HA, Redecker D, Rintoul TL, Ruibal C, Sarmiento-Ramirez JM, Schmitt I, Schussler A, Shearer C, Sotome K, Stefani FOP, Stenroos S, Stielow B, Stockinger H, Suetrong S, Suh S-O, Sung G-H, Suzuki M, Tanaka K, Tedersoo L, Telleria MT, Tretter E, Untereiner WA, Urbina H, Vagvolgyi C, Vialle A, Vu TD, Walther G, Wang Q-M, Wang Y, Weir BS, Weiss M, White MM, Xu J, Yahr R, Yang ZL, Yurkov A, Zamora J-C, Zhang N, Zhuang W-Y, Schindel D. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci U S A 109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arendrup MC, Hope W, Howard SJ. 2014. EUCAST definitive document E.Def 9.2 method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. EUCAST, Växjö, Sweden. [Google Scholar]
- 12.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI document M38-A2 CLSI, Wayne, PA. [Google Scholar]
- 13.Howard SJ, Lass-Flörl C, Cuenca-Estrella M, Gomez-Lopez A, Arendrup MC. 2013. Determination of isavuconazole susceptibility of Aspergillus and Candida species by the EUCAST method. Antimicrob Agents Chemother 57:5426–5431. doi: 10.1128/AAC.01111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope W. 2012. EUCAST technical note on the EUCAST definitive document E.Def 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts E.Def 7.2 (EUCAST-AFST). Clin Microbiol Infect 18:E246–E247. doi: 10.1111/j.1469-0691.2012.03880.x. [DOI] [PubMed] [Google Scholar]
- 15.Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope WW. 2013. Breakpoints for antifungal agents: an update from EUCAST focussing on echinocandins against Candida spp. and triazoles against Aspergillus spp. Drug Resist Updat 16:81–95. doi: 10.1016/j.drup.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope WW, Flörl C, Arikan S, Barchiesi F, Bille J, Chryssanthou E, Groll A, Gaustad P, Järv H, Klimko N, Lortholary O, Matos T, Moore C, Schmalreck A, Velegraki A, Verweij P. 2012. EUCAST technical note on Aspergillus and amphotericin B, itraconazole, and posaconazole. Clin Microbiol Infect 18:E248–E250. doi: 10.1111/j.1469-0691.2012.03890.x. [DOI] [PubMed] [Google Scholar]
- 17.Hope WW, Cuenca-Estrella M, Lass-Flörl C, Arendrup MC. 2013. EUCAST technical note on voriconazole and Aspergillus spp. Clin Microbiol Infect 19:E278–E280. doi: 10.1111/1469-0691.12148. [DOI] [PubMed] [Google Scholar]
- 18.Espinel-Ingroff A, Cuenca-Estrella M, Fothergill A, Fuller J, Ghannoum M, Johnson E, Pelaez T, Pfaller MA, Turnidge J. 2011. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B and Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). Antimicrob Agents Chemother 55:5150–5154. doi: 10.1128/AAC.00686-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espinel-Ingroff A, Diekema DJ, Fothergill A, Johnson E, Pelaez T, Pfaller MA, Rinaldi MG, Canton E, Turnidge J. 2010. Wild-type MIC distributions and epidemiological cutoff values for the triazoles and six Aspergillus spp. for the CLSI broth microdilution method (M38-A2 document). J Clin Microbiol 48:3251–3257. doi: 10.1128/JCM.00536-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai A, Kovanda L, Kowalski D, Lu Q, Townsend R. 2014. Isavuconazole (ISA) population pharmacokinetic modeling from phase 1 and phase 3 clinical trials and target attainment analysis, abstr A-697 Abstr 54th Intersci Conf Antimicrob Agents Chemother. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ullmann AJ, Cornely OA, Burchardt A, Hachem R, Kontoyiannis DP, Töpelt K, Courtney R, Wexler D, Krishna G, Martinho M, Corcoran G, Raad I. 2006. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob Agents Chemother 50:658–666. doi: 10.1128/AAC.50.2.658-666.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duarte RF, López-Jiménez J, Cornely OA, Laverdiere M, Helfgott D, Haider S, Chandrasekar P, Langston A, Perfect J, Ma L, van Iersel MLPS, Connelly N, Kartsonis N, Waskin H. 2014. Phase 1b study of new posaconazole tablet for prevention of invasive fungal infections in high-risk patients with neutropenia. Antimicrob Agents Chemother 58:5758–5765. doi: 10.1128/AAC.03050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maertens J, Cornely OA, Ullmann AJ, Heinz WJ, Krishna G, Patino H, Caceres M, Kartsonis N, Waskin H, Robertson MN. 2014. Phase 1B study of the pharmacokinetics and safety of posaconazole intravenous solution in patients at risk for invasive fungal disease. Antimicrob Agents Chemother 58:3610–3617. doi: 10.1128/AAC.02686-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hope WW. 2012. Population pharmacokinetics of voriconazole in adults. Antimicrob Agents Chemother 56:526–531. doi: 10.1128/AAC.00702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P, Mould DR. 2014. Population pharmacokinetic analysis of voriconazole and anidulafungin in adult patients with invasive aspergillosis. Antimicrob Agents Chemother 58:4718–4726. doi: 10.1128/AAC.02808-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geist MJP, Egerer G, Burhenne J, Riedel K-DD, Weiss J, Mikus G. 2013. Steady-state pharmacokinetics and metabolism of voriconazole in patients. J Antimicrob Chemother 68:2592–2599. doi: 10.1093/jac/dkt229. [DOI] [PubMed] [Google Scholar]
- 27.Vanstraelen K, Pauwels S, Oyaert M, Maertens J, Spriet I. 2015. Key elements in determining voriconazole protein binding characteristics: comment on “Determination of plasma unbound fraction of voriconazole in patients treated with a prophylactic or a curative treatment.” Ther Drug Monit 37:551–553. doi: 10.1097/FTD.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 28.Lass-Flörl C, Arendrup MC, Rodriguez-Tudela JL, Cuenca-Estrella M, Donnelly P, Hope W. 2011. EUCAST technical note on amphotericin B. Clin Microbiol Infect 17:E27–E29. doi: 10.1111/j.1469-0691.2011.03644.x. [DOI] [PubMed] [Google Scholar]
- 29.Espinel-Ingroff A, Chowdhary A, Gonzalez GM, Lass-Flörl C, Martin-Mazuelos E, Meis J, Peláez T, Pfaller MA, Turnidge J. 2013. Multicenter study of isavuconazole MIC distributions and epidemiological cutoff values for Aspergillus spp. for the CLSI M38-A2 broth microdilution method. Antimicrob Agents Chemother 57:3823–3828. doi: 10.1128/AAC.00636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ervens J, Ghannoum M, Graf B, Schwartz S. 2014. Successful isavuconazole salvage therapy in a patient with invasive mucormycosis. Infection 42:429–432. doi: 10.1007/s15010-013-0552-6. [DOI] [PubMed] [Google Scholar]
- 31.Luo G, Gebremariam T, Lee H, Edwards JE, Kovanda L, Ibrahim AS. 2014. Isavuconazole therapy protects immunosuppressed mice from mucormycosis. Antimicrob Agents Chemother 58:2450–2453. doi: 10.1128/AAC.02301-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verweij PE, González GM, Wiedrhold NP, Lass-Flörl C, Warn P, Heep M, Ghannoum MA, Guinea J. 2009. In vitro antifungal activity of isavuconazole against 345 Mucorales isolates collected at study centers in eight countries. J Chemother 21:272–281. doi: 10.1179/joc.2009.21.3.272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.