Abstract
Vancomycin-resistant urinary tract infections are often challenging to treat. This retrospective cohort study compared outcomes between patients treated for vancomycin-resistant enterococcal urinary tract infection with an aminopenicillin and those treated with a non-β-lactam antibiotic. Inpatients treated with an enterococcus-active agent for their first symptomatic vancomycin-resistant enterococcal urinary tract infection between 1 January 2012 and 31 December 2013 were considered for inclusion. Patients with colonization, on hospice, or receiving comfort care only were excluded. The primary endpoint of clinical cure was defined as resolution of clinical symptoms, or symptom improvement to the extent that no additional antibacterial drug therapy was necessary, and lack of microbiologic persistence. Secondary endpoints of 30-day readmission or retreatment and 30-day all-cause mortality were also compared. A total of 316 urinary isolates were screened, and 61 patients with symptomatic urinary tract infection were included. Twenty (35%) of the 57 isolates tested were ampicillin susceptible. Thirty-one patients received an aminopenicillin, and 30 received a non-β-lactam. Rates of clinical cure for aminopenicillin versus non-β-lactam treatment were 26/31 (83.9%) and 22/30 (73.3%) (P = 0.315), respectively. Rates of 30-day readmission (6/31, or 19.4%, versus 9/30, or 30%, respectively; P = 0.334), 30-day retreatment (4/31, or 12.9%, versus 4/30, 13.3%, respectively; P = 0.960), and 30-day all-cause mortality (2/31, or 6.5%, versus 1/30, or 3.3%, respectively; P = 0.573) were also not significantly different between groups. Aminopenicillins may be a viable option for treating vancomycin-resistant urinary tract infection regardless of the organism's ampicillin susceptibility. Prospective validation with larger cohorts of patients should be considered.
INTRODUCTION
Enterococci, a group of Gram-positive cocci typically considered normal flora in the human gastrointestinal tract, have increasingly become a major cause of health care-associated infections over the past decade (1–3). In particular, the incidence of vancomycin-resistant enterococci (VRE) has nearly doubled in recent years, with 30% of clinical enterococcal isolates being reported as resistant to vancomycin (4, 5). Of these, nearly all (91%) belong to the Enterococcus faecium species (5). Often resistant to multiple antimicrobials and much more difficult to treat, VRE infections have been associated with higher health care costs as well as with significant morbidity and mortality (6, 7).
Aminopenicillins (APs) are preferred agents for treatment of susceptible VRE urinary tract infections (UTI) for a number of reasons, including cost, safety, and efficacy. They are bacteriostatic against enterococci and have reported mean MICs required to inhibit 90% of organisms (MIC90) against vancomycin-resistant Enterococcus faecalis and Enterococcus faecium of 2 mg/liter (range, 1 to 128 mg/liter) and 128 mg/liter (range, 1 to 256 mg/liter), respectively (6, 8). Isolates are considered susceptible to ampicillin at MICs of ≤8 mg/liter and resistant at MICs of ≥16 mg/liter (6, 8, 9). However, a single dose of amoxicillin results in peak urinary concentrations of 306 to 856 μg/ml (10). Therefore, urinary concentrations after oral administration of high doses of amoxicillin are expected to achieve concentrations well above the MICs of ampicillin-resistant VRE (10, 11).
Despite the plausibility of attaining urine concentrations sufficient to eradicate the bacteria, even those deemed ampicillin resistant, this pharmacokinetic and pharmacodynamic concept has not been validated in a clinical setting. As part of an institutional antimicrobial stewardship initiative to prevent treatment of asymptomatic bacteriuria, our laboratory discontinued routine susceptibility reporting for all enterococci isolated in urine in August 2012. Following the change in laboratory reporting, isolates were identified only as Enterococcus species, vancomycin resistant or susceptible, and a comment to prescribers was added to all reports: “ampicillin IV or amoxicillin oral are predictably reliable for treatment of uncomplicated enterococcal UTI” (where IV is intravenous). Additional susceptibility testing was available from the clinical laboratory upon request. Thus, the purpose of this study was to evaluate the outcome of this stewardship initiative by comparing treatment outcomes between patients treated for a VRE UTI with an aminopenicillin (AP) and those treated with a non-β-lactam (NBL) antibiotic. We were particularly interested in the outcomes of AP therapy among the subgroup of patients with ampicillin-resistant VRE UTI.
MATERIALS AND METHODS
Study setting and design.
This retrospective cohort study was conducted at Henry Ford Hospital in Detroit, MI, an 802-bed teaching institution with a single, centralized clinical microbiology laboratory. The study was approved by the investigational review board with a waiver of consent. Prior to study initiation, urinary VRE clinical isolates had been identified by the clinical microbiology laboratory and stored in the infectious diseases research laboratory. All urinary isolates positive for VRE between 1 January 2012 and 31 December 2013 were identified with the hospital clinical decision support system (TheraDoc; Premier, Inc., Charlotte, NC) and cross-referenced with the infectious diseases research laboratory database. The clinically evaluable study population was limited to adult inpatients and included only the first isolate of VRE per patient in the study period. Patients who met the definition of a symptomatic UTI and received treatment with an antimicrobial presumed to be active against VRE, defined below, were included in the study population. Patients with colonization, asymptomatic bacteriuria, on hospice service, or receiving comfort care only at the time of VRE identification were excluded.
Definitions.
Symptomatic UTI was defined as a urine culture with any amount of VRE from a patient with pyuria and at least one documented sign or symptom of a UTI (frequency, urgency, new/worse incontinence, dysuria, suprapubic tenderness/abdominal pain, costovertebral angle pain/flank tenderness, or fever). Pyuria was defined as the presence of >10 white blood cells in noncatheterized patients and as >5 white blood cells in catheterized patients on urinalysis. Immunosuppressed patients were defined as those with an absolute neutrophil count of <1,000 cells/mm3, prednisone dose of more than 10 mg daily for longer than 7 days, immunosuppressive therapy following solid-organ or hematologic transplant, and/or HIV/AIDS. Time to active therapy was defined as the difference (in hours) from the time that the urine culture was obtained and the order for the first antibiotic documented or presumed to be active against VRE. Definitive therapy was defined as the antimicrobial agent presumed to be active against VRE that was used for >50% of the entire treatment course (both inpatient and outpatient). AP therapy was considered active in all cases, regardless of the ampicillin susceptibility. Patients were grouped according to definitive therapy with an AP (ampicillin, amoxicillin, ampicillin-sulbactam, or amoxicillin-clavulanate) or with an NBL (ciprofloxacin, linezolid, daptomycin, nitrofurantoin, or fosfomycin). Antimicrobials were dosed according to institutional antimicrobial stewardship guidelines for all patients. For patients with normal renal function, 500 mg of amoxicillin orally (per os, p.o.) every 8 h or 875 mg amoxicillin p.o. every 12 h and 1 to 2 g of ampicillin intravenously every 4 to 6 h are recommended by institutional guidelines. The primary endpoint for analysis was clinical cure, defined as resolution of clinical symptoms of UTI, or symptom improvement to the extent that no additional antibacterial drug therapy for UTI was necessary, and lack of microbiologic persistence. Microbiologic persistence was defined as growth of the bacterial pathogen found at baseline after completion of therapy; eradication was presumed in patients with clinical improvement and no microbiological follow-up. Secondary outcomes were 30-day retreatment, all-cause readmission, and 30-day all-cause mortality.
Microbiology methods.
Prior to the change in laboratory reporting, identification and susceptibility testing were performed by the clinical microbiology laboratory via standard methods utilizing Vitek-2 (bioMérieux, Inc., Durham, NC) and reported in the electronic medical record. The report display included genus and species name, MICs, and susceptibility interpretations (i.e., susceptible [S], intermediate [I], or resistant [R]) for ampicillin, nitrofurantoin, ciprofloxacin, and vancomycin according to Clinical and Laboratory Standards Institute (CLSI) guidelines (9). After the laboratory reporting change, the clinical microbiology laboratory identified the isolate only as Enterococcus sp. via basic biochemical tests, and the organism was plated on a brain heart infusion (BHI) agar at a vancomycin concentration of 6 μg/ml (Remel/Thermo Fisher Scientific, Lenexa, KS) in order to screen for vancomycin resistance. Any colony growth on the BHI plate was considered positive and reported as vancomycin resistant on the final microbiology report.
The infectious diseases research lab at our health system maintains a −70°C research laboratory freezer repository which contains all clinical isolates of VRE. Isolates screened for inclusion in this evaluation were subcultured from the research repository for further identification and ampicillin susceptibility. Carbohydrate fermentation broths were used to identify the enterococcal isolates to the species level. Manual broth microdilution to determine ampicillin MICs was performed using Mueller-Hinton II broth and 96-well microtiter plates using standard laboratory procedures according to the CLSI, with ampicillin resistance defined as an MIC of ≥16 μg/ml (9).
Statistical analysis.
We estimated that a total sample size of 124 patients was needed to detect a 20% difference in the proportion of clinical success between groups, assuming 91% clinical success (12) among controls, with 80% power and a two-sided 5% level of significance.
All statistical analyses were performed using SPSS Software, version 21.0 (SPSS, Inc., Chicago, IL). Categorical variables were compared via a chi-square or Fisher's exact test, and continuous variables were compared with the Student t test or Mann-Whitney U test, as appropriate. A P value of <0.05 was considered statistically significant for all comparisons.
RESULTS
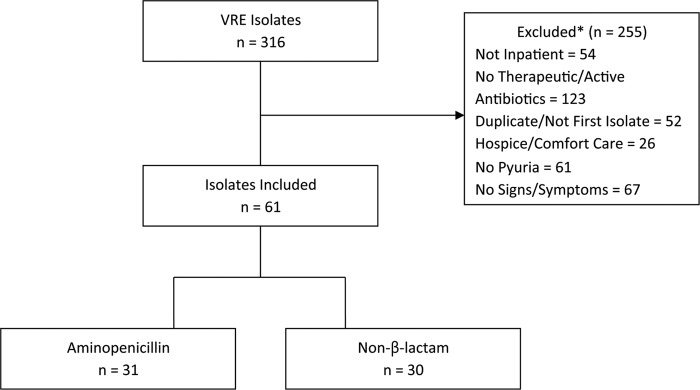
A total of 316 VRE urinary isolates were identified from the study period (Fig. 1). The identification of species and ampicillin MIC were available on 299/316 (94%) and 291/316 (92%) of all isolates screened, respectively. The most common organisms included E. faecium (208/316, 65%) and E. faecalis (91/316, 29%). For the 291 isolates, 91 (31%) of the isolates were ampicillin susceptible, and the median ampicillin MIC was 256 mcg/ml (interquartile range [IQR], 2 to 256 mcg/ml).
FIG 1.
Patient inclusion and exclusion criteria. Categories of excluded patients (*) are not mutually exclusive.
A final cohort of 61 patients met clinically evaluable inclusion criteria for symptomatic UTI, with 31 in the AP group and 30 in the NBL group. Of the isolates tested, 33% (20/57) were ampicillin susceptible. Baseline characteristics, including comorbidities, urologic abnormalities, and setting of acquisition, were similar between groups (Table 1). Patients in the AP group were slightly older (68 versus 58.5 years for the NBL treatment group; P = 0.047) and had a shorter length of stay (6 versus 19.5 days for the NBL treatment group; P = 0.006). Additionally, patients receiving an NBL for definitive therapy were more likely to be in the intensive care unit (ICU) (9, or 29%, for the AP group versus 17, or 56.7% for the NBL group; P = 0.029), have a urinary catheter present (11, or 35.5%, for the AP group versus 20, or 66.7%, for the NBL group; P = 0.015), or have a documented antibiotic allergy (2, or 6.5%, for the AP group, versus 13, or 43.3%, for the NBL group; P = 0.001). Among the AP group, 13/31 (41.9%) patients were infected with E. faecium, and 18/31 (58.1%) were infected with E. faecalis, whereas 23/26 (88.5%) patients were infected with E. faecium and 3/26 (11.5%) were infected with E. faecalis in the NBL group (P < 0.001). Four patients in the NBL group were infected with other Enterococcus species.
TABLE 1.
Baseline patient characteristics
| Characteristic | Value for the treatment groupa |
P value | |
|---|---|---|---|
| AP (n = 31) | NBL (n = 30) | ||
| Baseline demographics | |||
| Age (yr) | 68 (56–83)b | 58.5 (47–70)b | 0.047 |
| Female | 19 (61.3) | 20 (66.7) | 0.662 |
| Antibiotic allergy | 2 (6.5) | 13 (43.3) | 0.001 |
| ICU | 9 (29.0) | 17 (56.7) | 0.029 |
| Comorbidities | |||
| Diabetes mellitus | 15 (48.4) | 14 (46.7) | 0.893 |
| Chronic kidney disease | 19 (61.3) | 16 (53.3) | 0.530 |
| Immunosuppressed | 5 (16.1) | 11 (36.7) | 0.068 |
| Benign prostatic hyperplasia | 2 (6.5) | 4 (13.3) | 0.367 |
| Urologic abnormalities | |||
| Urinary catheter present at time VRE cultured from urine | 11 (35.5) | 20 (66.7) | 0.015 |
| Chronic indwelling urinary catheter prior to admission | 7 (22.6) | 6 (20.0) | 0.806 |
| Urinary hardware | 2 (6.5) | 1 (3.3) | 0.573 |
| Structural/functional abnormality | 6 (19.4) | 5 (16.7) | 0.785 |
| Recent urinary instrumentation | 7 (22.6) | 5 (16.7) | 0.561 |
| Renal calculi | 4 (12.9) | 1 (3.3) | 0.173 |
| History of recurrent UTI | 5 (16.1) | 2 (6.7) | 0.246 |
| Setting of acquisition | |||
| Community-acquired | 2 (6.5) | 1 (3.3) | 0.573 |
| Healthcare-associated | 16 (51.6) | 10 (33.3) | 0.149 |
| Hospital-acquired | 13 (41.9) | 19 (63.3) | 0.094 |
Values are displayed as number of patients (percent of population) except as otherwise noted.
Values are mean (IQR).
Within the AP group, the most common agent selected for definitive therapy was amoxicillin (21 patients, or 67.7%), followed by intravenous ampicillin (7 patients, or 22.6%), ampicillin-sulbactam (2 patients, or 6.5%), and amoxicillin-clavulanate (1 patient, or 3.2%). In the NBL group, the most common agent selected for definitive therapy was linezolid (22/30, or 73.3%), followed by daptomycin (7 patients, or 23.3%) and fosfomycin (1 patient, or 3.3%); no patients received nitrofurantoin or ciprofloxacin. The median time to active therapy was not different between groups (58.95 versus 52.24 h; P = 0.806).
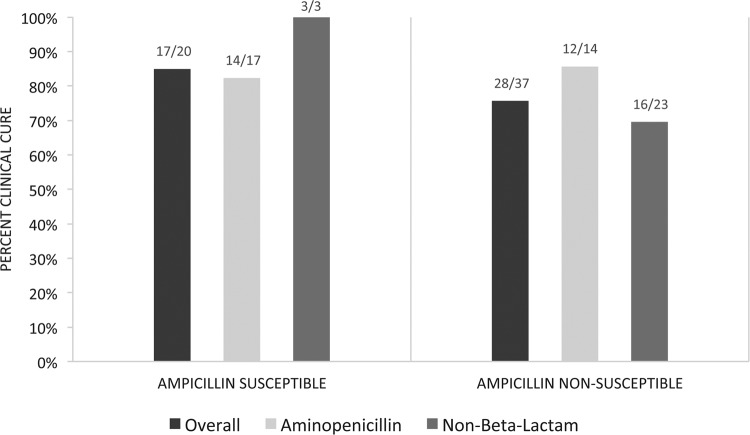
There was no significant difference detected in the primary endpoints, with rates of clinical cure of 26/31 (83.9%) patients in the AP group and 22/30 (73.3%) in the NBL group (P = 0.363). Microbiologic persistence occurred in 1/31 (3.2%) of AP-treated patients and in 7/30 (23.3%) of NBL-treated patients (P = 0.026). Rates of 30-day readmission (6/31, or 19.4%, for the AP group versus 9/30, or 30%, for the NBL group; P = 0.334), 30-day retreatment (4/31, or 12.9%, for the AP group versus 4/30, or 13.3%, for the NBL group; P = 0.960), and 30-day all-cause mortality (2/31, or 6.5%, for the AP group versus 1/30, or 3.3%, for the NBL group; P = 0.573) were also not significantly different between groups. Clinical cure rates for patients treated with an AP versus an NBL according to ampicillin susceptibility are described in Fig. 2. MICs for the 14 nonsusceptible isolates treated with aminopenicillin therapy included one isolate with an MIC of 128 μg/ml, six isolates with an MIC of 256 μg/ml, and seven isolates with an MIC of 512 μg/ml. Clinical cure was not achieved in two patients. Both were infected with Enterococcus faecium, and the ampicillin MICs were 256 and 512 μg/ml.
FIG 2.
Clinical cure by treatment group and ampicillin susceptibility.
Subgroup analysis.
In exploratory subgroup analysis, we evaluated clinical cure rates among patients admitted to the ICU and with chronic kidney disease (CKD), diabetes mellitus (DM), and indwelling urinary catheters. Clinical cure was observed in 8/9 (89%) ICU patients who received an AP versus 13/17 (76%) patients who received an NBL (odds ratio [OR], 0.406; 95% confidence interval [CI], 0.038 to 4.310). Among patients with CKD, clinical cure was 18/19 (95%) with an AP and 12/16 (75%) with an NBL (OR, 0.167; 95% CI, 0.017 to 1.679). Among the population with DM, clinical cure was observed in 14/15 (93%) with an AP versus 9/14 (64%) with an NBL (OR, 0.129; 95% CI, 0.013 to 1.288). Finally, clinical cure was observed in 11/11 (100%) patients with an indwelling urinary catheter who received an AP versus 15/20 (75%) who received an NBL (OR, 1.73; 95% CI, 1.247 to 2.409).
DISCUSSION
We observed that in a population of strictly defined VRE UTI, aminopenicillins appear to be effective, regardless of susceptibility. Clinical cure with aminopenicillin therapy was observed in 84% of all cases and in 86% of patients with ampicillin-resistant isolates, with no statistical difference detected between results for those treated with non-β-lactams. AP therapy was associated with improved microbiologic eradication. While severity of illness was not evenly distributed between treatment groups, among the subset of ICU patients (n = 26), clinical cure with aminopenicillins was 89% (8/9), similar to that observed for treatment with non-β-lactams (76%, or 13/17).
In the absence of urine-specific breakpoints, our work provides additional in vitro and clinical evidence for ampicillin as a potential first-line agent for VRE UTI, regardless of susceptibility interpretation (12). A recent study by Khair and colleagues demonstrated that vancomycin resistance did not impact clinical outcomes in a cohort of patients with enterococcal bacteriuria (13). In 1974, Stamey and colleagues (14) demonstrated that urine antibiotic concentrations were a better predictor than serum concentrations for clinical cure. Specific to aminopenicillins, the mechanism of ampicillin resistance in E. faecium has been reported as the low-affinity penicillin binding protein 5 (PBP5) (15). Despite resistance, an investigation of 310 enterococci isolated from urine suggested that ampicillin MICs for enterococci, including ampicillin-resistant strains, were all within one dilution of 256 mcg/ml (10). After a single 250-mg dose of amoxicillin, peak urine concentrations in volunteers with normal renal function ranged from 306 to 856 μg/ml (11). Multiple doses of amoxicillin at a dose of 500 mg are expected to achieve urine concentrations well over the MICs of enterococci labeled resistant by CLSI guidelines, potentially overcoming resistance conferred by the low-affinity PBP5.
As with any study, this investigation is not without limitations. This is a single-center and retrospective study. Type 2 error is likely in pilot studies of this small, underpowered sample size; however, these data are a necessary first step to ensure safety and effectiveness before the practice can be recommended more widely. As such, we employed strict criteria for inclusion and outcome ascertainment to increase internal validity. Only patients with clearly documented urinary symptoms were included; however, it is still possible that colonized patients may have been misclassified. Likewise, a composite endpoint of clinical and microbiological response was used to minimize misclassification of outcomes. We used ampicillin as a surrogate for susceptibility instead of the administered agent in some cases and did not perform molecular testing on the isolates to evaluate for clonality. Although our institutional laboratory comment is phrased to promote use of aminopenicillins for “uncomplicated enterococcal UTI,” our analysis did not exclude complicated UTI. A larger data set would be necessary to further distinguish the role of aminopenicillin therapy based upon UTI type. A final limitation of this study is lack of randomization and the potential for introduction of confounders. More patients treated with non-β-lactams had a history of antibiotic allergy, were admitted to the ICU, or had a urinary catheter in place at the time of VRE isolation. While we did not calculate severity-of-illness scores, such as a Charlson comorbidity index, or assess other coinfections, we attempted to evaluate for variables known or suspected to affect our outcome of clinical cure with exploratory analysis in the subpopulations of patients in the ICU or with CKD, diabetes, and urinary catheters.
Despite these limitations, the importance of this study is that it provides some evidence that patient outcomes have not been negatively impacted by our local laboratory reporting change and antimicrobial stewardship initiative to encourage use of ampicillin for VRE urinary tract infections. The streamlined laboratory reporting procedure allows for a modest reduction in laboratory technician time and cost utilization dedicated to enterococcal urinary isolates. And while this may not be generally applicable to all practice settings, it provides adequate evidence to support larger, adequately powered studies of similar stewardship approaches. In addition, clinical microbiology labs may be able to use this work to justify repurposing their limited resources.
Conclusion.
The results of this study support the pharmacokinetic plausibility that aminopenicillins achieve sufficient urinary concentrations to eradicate VRE and therefore may be a viable option for treating VRE UTI, regardless of the organism's ampicillin susceptibility. This validates our current laboratory procedures and encourages antimicrobial stewardship practices while minimizing harm to our patients. Prospective validation with larger cohorts of patients should be considered.
REFERENCES
- 1.Reik R, Tenover FC, Klein E, McDonald LC. 2008. The burden of vancomycin-resistant enterococcal infections in US hospitals, 2003 to 2004. Diagn Microbiol Infect Dis 62:81–85. doi: 10.1016/j.diagmicrobio.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Zirakzadeh A, Patel R. 2006. Vancomycin-resistant enterococci: colonization, detection, and treatment. Mayo Clin Proc 81:529–536. doi: 10.4065/81.4.529. [DOI] [PubMed] [Google Scholar]
- 3.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK, the National Healthcare Safety Network Team, Participating National Healthcare Safety Network Facilities. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey AM, Zilberberg MD. 2009. Secular trends of hospitalization with vancomycin-resistant enterococcus infection in the United States, 2000–2006. Infect Control Hosp Epidemiol 30:184–186. doi: 10.1086/593956. [DOI] [PubMed] [Google Scholar]
- 5.Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. 2007. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn Microbiol Infect Dis 58:163–170. doi: 10.1016/j.diagmicrobio.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Heintz BH, Halilovic J, Christensen CL. 2010. Vancomycin-resistant enterococcal urinary tract infections. Pharmacotherapy 30:1136–1149. doi: 10.1592/phco.30.11.1136. [DOI] [PubMed] [Google Scholar]
- 7.Rice LB. 2001. Emergence of vancomycin-resistant enterococci. Emerg Infect Dis 7:183–187. doi: 10.3201/eid0702.010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhanel GG, Laing NM, Nichol KA, Palatnick LP, Noreddin A, Hisanaga T, Johnson JL, Hoban DJ, NAVRESS Group. 2003. Antibiotic activity against urinary tract infection (UTI) isolates of vancomycin-resistant enterococci (VRE): results from the 2002 North American Vancomycin Resistant Enterococci Susceptibility Study (NAVRESS). J Antimicrob Chemother 52:382–388. doi: 10.1093/jac/dkg352. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed CLSI document M7-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 10.Cole M, Ridley B. 1978. Absence of bioactive metabolites of ampicillin and amoxicillin in man. J Antimicrob Chemother 4:580–582. doi: 10.1093/jac/4.6.580. [DOI] [PubMed] [Google Scholar]
- 11.Williamson JC, Craft DW, Butts JD, Raasch RH. 2002. In vitro assessment of urinary isolates of ampicillin-resistant enterococci. Ann Pharmacother 36:246–250. doi: 10.1345/aph.1A085. [DOI] [PubMed] [Google Scholar]
- 12.Shultz J, Klinker K, Borgert S, Ramphal R. 2012. An analysis of the use of ampicillin for urinary tract infections caused by vancomycin-resistant enterococcus, abstr K-274 Abstr 52nd Intersci Conf Antimicrob Agents Chemother. [DOI] [PubMed] [Google Scholar]
- 13.Khair HN, VanTassell P, Henderson JP, Warren DK, Marschall J, CDC Epicenters Program. 2013. Vancomycin resistance has no influence on outcomes of enterococcal bacteriuria. J Hosp Infect 85:183–188. doi: 10.1016/j.jhin.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamey TA, Fair WR, Timothy MM, Millar MA, Mihara G, Lowery YC. 1974. Serum versus urinary antimicrobial concentrations in cure of urinary-tract infections. N Engl J Med 291:1159–1163. doi: 10.1056/NEJM197411282912204. [DOI] [PubMed] [Google Scholar]
- 15.Fontana R, Aldegheri M, Ligozzi M, Lopez H, Sucari A, Satta G. 1994. Overproduction of a low-affinity penicillin-binding protein and high-level ampicillin resistance in Enterococcus faecium. Antimicrob Agents Chemother 38:1980–1983. doi: 10.1128/AAC.38.9.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]