Abstract
Left untreated, inhalation anthrax is usually fatal. Vegetative forms of Bacillus anthracis survive in blood and tissues during infection due to elaboration of a protective poly-γ-d-glutamic acid (PDGA) capsule that permits uncontrolled bacterial growth in vivo, eventually leading to overwhelming bacillosis and death. As a measure to counter threats from multidrug-resistant strains, we are evaluating the prophylactic and therapeutic potential of the PDGA depolymerase EnvD, a stable and potent enzyme which rapidly and selectively removes the capsule from the surface of vegetative cells. Repeated intravenous administration of 10 mg/kg recombinant EnvD (rEnvD) to mice infected with lethal doses of B. anthracis Ames spores by inhalation prevented the emergence of symptoms of anthrax and death; all animals survived the 5-day treatment period, and 70% survived to the end of the 14-day observation period. In contrast to results in sham-treated animals, the lungs and spleen of rEnvD-dosed animals were free of gross pathological changes. We conclude that rEnvD has potential as an agent to prevent the emergence of inhalation anthrax in infected animals and is likely to be effective against drug-resistant forms of the pathogen.
INTRODUCTION
Bacillus anthracis featured in offensive weapons programs in the United States and former Soviet Union during the last century (1) and has been identified by the World Health Organization, the United Nations, and the Working Group on Civilian Defense (WGCB) as a pathogen of great concern. The WGCB has highlighted a limited number of microorganisms that could cause infections in sufficient numbers to cripple a city or region, and B. anthracis is one of the most serious of such threat agents (2). The bacteria's spores are able to survive in hostile environments for many decades and, in aerosolized form, can travel significant distances on prevailing winds, disseminating over a wide area. Accidental release of anthrax spores as an aerosol from a military facility in Sverdlovsk in 1979 resulted in at least 79 cases of anthrax and 68 deaths, demonstrating the bacteria's lethal potential (3). These traits define B. anthracis as a potential threat agent, attractive to both rogue states and terrorist groups, and a cause of human and animal disease globally. The vegetative bacilli release toxin complexes that cause hemorrhage, edema, and necrosis and are protected from host innate defenses by a capsule comprised of poly-γ-d-glutamic acid (PDGA) (4). In inhalation anthrax, endospores gain access to the alveolar spaces and are ingested by macrophages; they are then transported to regional lymph nodes where spore germination occurs after a variable period of dormancy (4, 5). Toxin-mediated clinical symptoms typically arise soon after the onset of rapid bacillary growth (2).
Effective treatment requires prompt and aggressive antibiotic therapy; a fluoroquinolone and an agent that inhibits protein synthesis such as linezolid are currently recommended by the Centers for Disease Control and Prevention (6). The consensus approach to prophylaxis and treatment of inhalation anthrax could be compromised by the release of B. anthracis carrying engineered antibiotic resistance genes, and occasional reports have emerged of naturally occurring strains resistant to currently useful antibiotics (7, 8). Clearly, new agents or novel therapeutic and prophylactic modalities should be developed as a part of a comprehensive preparedness strategy. We previously demonstrated that parenteral administration of a capsule depolymerase with the capacity to rapidly and selectively remove the protective capsule from the bacterial surface can resolve potentially lethal Escherichia coli infection in the neonatal rat (9, 10). Systemic anthrax is an attractive candidate for this approach as infections are attributable to a single, phylogenetically homogeneous bacterial species, all strains elaborate the unique PDGA capsule essential for pathogenesis (11), and hydrolysis of the outermost layer of the bacilli would confound attempts to circumvent antibiotic chemotherapy by the introduction of antibiotic resistance genes into B. anthracis. Here, we report that early intravenous administration of recombinant EnvD (rEnvD), a recombinant PDGA hydrolase elaborated by a consortium culture of soil bacteria, is able to prevent anthrax in mice infected by the inhalation route.
MATERIALS AND METHODS
Bacteria.
B. anthracis Ames (NR-2324/pXO1+/pXO2+) was obtained from the Biodefense and Emerging Infections Research Resources Repository (Manassas, VA). Spores were prepared by fed batch culture in a 2-liter bioreactor for 26 h at 37°C with stirring at 400 rpm, collected by centrifugation, and washed in sterile distilled water. For spore challenge tests, suspensions (8 × 109 CFU/ml) were prepared in sterile water. Bacillus licheniformis ATCC 9945a was purchased from the American Type Culture Collection and grown in medium E containing 615 μM MnSO4 in an orbital incubator (200 orbits/min) at 37°C (12).
Recombinant EnvD.
The enzyme was expressed, refolded, and purified as described previously (12). Endotoxin was removed using Proteus Endotoxin Removal columns (Abd Serotec, Oxford, United Kingdom), and removal was confirmed with a Pierce LAL (Limulus amebocyte lysate) Chromogenic Endotoxin Quantitation kit (Thermo Fisher, Rockford, USA). Purified rEnvD was stored in 20 mM Tris (pH 8.5) at −20°C until required.
Impact of rEnvD on bacterial viability.
A culture (50 ml) from a single, heavily mucoid colony of B. licheniformis 9945a was grown to an optical density at 600 nm (OD600) of 0.6 and examined by light microscopy to ensure that only vegetative bacilli were present. Two aliquots of 1 ml were removed, and rEnvD was added to one aliquot to give a final protein concentration of 1 μg/ml. An equal volume of phosphate-buffered saline (PBS) was added to the second aliquot. Both samples were incubated at 37°C for 15 min, serially diluted in PBS, and plated onto Luria-Bertani agar. Plates were incubated at 37°C for 16 h, and bacteria were enumerated.
Stability of rEnvD in serum.
Aliquots of rEnvD (final concentration of 100 nM in 1.6-ml Eppendorf tubes) were incubated at 37°C in serum from BALB/c mice (total volume, 200 μl; Sigma) for up to 24 h, and EnvD activity was determined at regular intervals by Förster resonance energy transfer (FRET) utilizing the fluorescently labeled synthetic peptide substrate 5-FAM-(d-Glu-γ-)5-K(QXL520)-NH2 (where FAM is 6-carboxyfluorescein) as previously described (12). Two tubes were used for each time point to provide duplicate readings. In some experiments heat-inactivated (56°C for 30 min) serum was used, and some assays were conducted in the presence of Roche complete protease inhibitor cocktail (Roche, Basel, Switzerland) at concentrations specified by manufacturer's guidelines.
Serum half-life (t1/2).
Pairs of female adult BALB/c mice were dosed with 10 mg/kg rEnvD in 180 μl of 20 mM Tris, pH 8.5, by tail vein injection. Paired animals were sacrificed over a 24-h period, blood was withdrawn by cardiac puncture, and serum was obtained. Serum was diluted 2-fold with 0.1 M Tricine and 0.1% CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate), pH 8.5, to a final volume of 200 μl, and 100 μl was transferred to each well of a black 96-well microtiter plate. All assays were run in duplicate. rEnvD activity was measured using the FRET assay described above with the exception that fluorescence was measured over a 4-min period. The concentration of enzyme in each sample was determined using a standard curve prepared in mouse serum. The area under the curve (AUC) was calculated by GraphPad Prism (GraphPad Software Inc., La Jolla, CA) using the trapezoid rule.
Infection of mice with B. anthracis.
All animal studies were carried out in accordance with the United Kingdom's Animals (Scientific Procedures) Act 1986 and the Codes of Practice for the Housing and Care of Animals used in Scientific Procedures 1989, following approval by the local ethical committee and the United Kingdom's Home Office. Female BALB/c mice (minimum age, 10 weeks; approximate body weight, 20 g; food and water available ad libitum) were obtained from Charles River (Canterbury, United Kingdom) and infected by aerosol (13). Groups of 10 mice were challenged with 10 to 50 minimum lethal doses (50% lethal dose [LD50], 6 × 104 CFU; presented dose, ∼1.45 × 106 CFU) of B. anthracis spores with an AeroMP-Henderson apparatus. The challenge aerosol was generated using a six-jet Collison Nebulizer (BGI, Inc., Waltham, MA); the aerosol was mixed with conditioned air in the spray tube and delivered to the nose of each animal through an exposure tube in which nonanesthetized mice were held in restraint tubes. Samples of the aerosol were obtained with an AGI30 glass impinger (Ace Glass, Inc., Vineland, NJ), and the mean particle size was determined with an aerodynamic particle sizer (TSI Instruments, Ltd., High Wycombe, United Kingdom). These processes were controlled and monitored from an AeroMP management platform (Biaera Technologies, Hagerstown, MD). All-glass impinger samples were titrated by serial dilution and plated on Trypticase soy agar prior to incubation at 37°C for 16 to 24 h.
Intravenous (i.v.) administration of rEnvD was initiated 12 h after spore challenge. The dosing regimen was guided by the stability of the enzyme in commercial mouse serum and by the rate of clearance of rEnvD from the circulation of adult female BALB/c mice. Each mouse received rEnvD (0.5 to 10 mg/kg) by injection at regular intervals up to 120 h after spore challenge; groups were comprised of 10 individual animals. Control mice received i.v. injections of PBS (180 μl) at these time points. Additional groups of 10 mice received oral doses of ciprofloxacin (118 mg/kg every 12 h for 14 days). Animals were monitored and assigned a clinical score at least twice daily up to 14 days after spore challenge and at least four times daily during critical periods (13). Clinical scores were based on severity of symptoms (ruffled fur, closed eyes, arched back, immobility, and weight loss). Animals surpassing a threshold score were euthanized humanely by pentobarbital overdose. Surviving mice from each group were euthanized at day 14 after challenge. Postmortem, blood, lung, and spleen samples were taken for enumeration of bacterial load, as follows: tissues were weighed and homogenized in sterile water using a Precellys 24 tissue homogenizer (Bertin Technologies, Villeurbanne, France), the homogenates were serially diluted in sterile water and plated onto Trypticase soy agar, and the plates were incubated at 37°C for 16 to 24 h before enumeration. Further lung and spleen samples were placed in 10% neutral buffered formalin for pathological evaluation. An additional group of 10 mice was employed to evaluate pathological changes 6 days after spore challenge; animals were culled 24 h after receiving their final dose of rEnvD on day 5, and blood and tissue samples were removed. A Kaplan-Meier log rank test was used to determine the significance of differences in survival between groups of animals, and GraphPad Prism software (GraphPad, La Jolla, CA, USA) was employed. For histological evaluation, formalin-fixed tissue samples were processed to paraffin wax, and 3- to 5-μm sections were cut and stained with hematoxylin and eosin. Sections were examined by light microscopy and evaluated subjectively. Slides were randomized by a third party before microscopic examination to avoid prior knowledge of group or treatment.
RESULTS
rEnvD is a promising candidate for in vivo attenuation of B. anthracis capsule expression.
Unusually, envD resides on the genome of a strain of Pusillimonas noertemannii, but the enzyme is produced only when the bacteria are cocultured with a strain of Pseudomonas fluorescens (12, 14). rEnvD shows strong sequence homology to bacterial dienelactone hydrolases, and its enzymatic activity is restricted to the hydrolysis of γ-linkages in d- and l-glutamic acid-containing polymers (kcat, 72.6 h−1; Km, 0.65 μM; kcat/Km, 3.08 × 104 M−1 s−1 at 37°C). The enzyme retained enzymatic activity after accelerated storage at 37°C for 30 days and completely removed the capsule from B. anthracis Pasteur strain within 5 min at 37°C (12). Exposure of Bacillus licheniformis ATCC 9945a (induced to elaborate a PDGA polymer) to rEnvD resulted in rapid stripping of the capsule (12), but the viability of this surrogate strain was not significantly altered (2.5 × 108 to 2.9 × 108 CFU/ml over 15 min; n = 6; Student's t, P > 0.05).
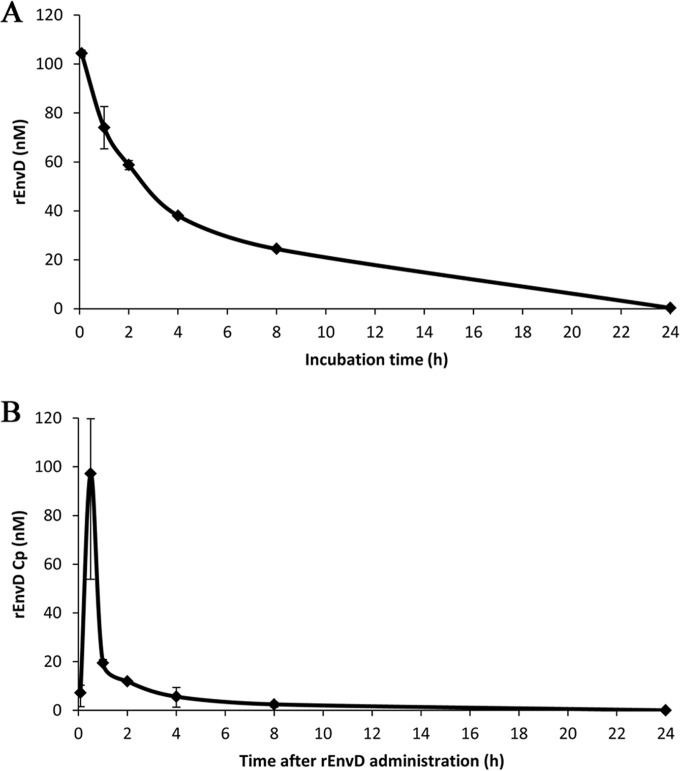
There is limited capacity for repeated parenteral injections in small animals. To guide the design of dosing regimens for the administration of rEnvD to infected BALB/c mice, we determined the retention of depolymerase activity in mouse serum and the serum half-life (t1/2) following intravenous (i.v.) administration. The reduction in rEnvD activity following incubation at 37°C in murine serum as determined by FRET assay (12) followed first-order kinetics, with a t1/2 of 177 min (Fig. 1A); approximately 20% of activity remained after 8 h of incubation. Neither heat inactivation of serum nor the presence of protease inhibitors had any impact on the rate of reduction of activity. Elimination of rEnvD from the blood circulation of BALB/c mice was biphasic, with an initial rapid decrease in serum concentration (0.5 h to 1 h) followed by a slower elimination phase (2 h to 24 h) characteristic of first-order kinetics (Fig. 1B). This elimination profile is typical of agents administered by the intravenous route (15), with a rapid decrease in serum concentration due to distribution from the central circulation into the peripheral body tissues (alpha phase) followed by a gradual decrease in plasma concentration attributable to metabolism and excretion of the drug (beta phase). The AUC was determined as 118 nM · h/liter. Based on these data, we established a dosing regimen in which mice received rEnvD (0.5 to 10 mg/kg body weight) by i.v. injection 12 h, 24 h, 48 h, 72 h, 96 h, and 120 h after spore challenge.
FIG 1.
In vitro stability in serum and elimination of rEnvD from the circulation of BALB/c mice. (A) Stability of rEnvD in BALB/c mouse serum. Enzyme (100 nM) was incubated at 37°C in serum, and activity was determined by FRET assay (12). Enzyme activity in relative fluorescence units was converted to concentration of active enzyme by comparison to a standard curve. Error bars represent the range of three separate determinations; the t1/2 in serum of rEnvD was 2.95 h (177 min). (B) rEnvD in serum obtained from mice intravenously dosed with 10 mg/kg rEnvD. Serum was obtained by terminal bleed, and enzyme activity was determined by FRET assay. Serum concentration (Cp) of rEnvD was obtained by comparison to a standard curve. Error bars represent the range of three separate determinations performed in duplicate. The half-life, t1/2, was calculated by determination of the elimination rate constant (Ke) and transformation of data to the natural log (ln) to produce a line of best fit for each phase, with the slope equal to Ke: t1/2 = ln (2)/Ke. The t1/2 was 0.22 h (13 min) for the initial alpha phase between 0.5 h and 1 h, and it was 2.71 h (163 min) for the beta phase between 2 h and 24 h.
rEnvD administration prevents inhalation anthrax in aerosol-challenged mice.
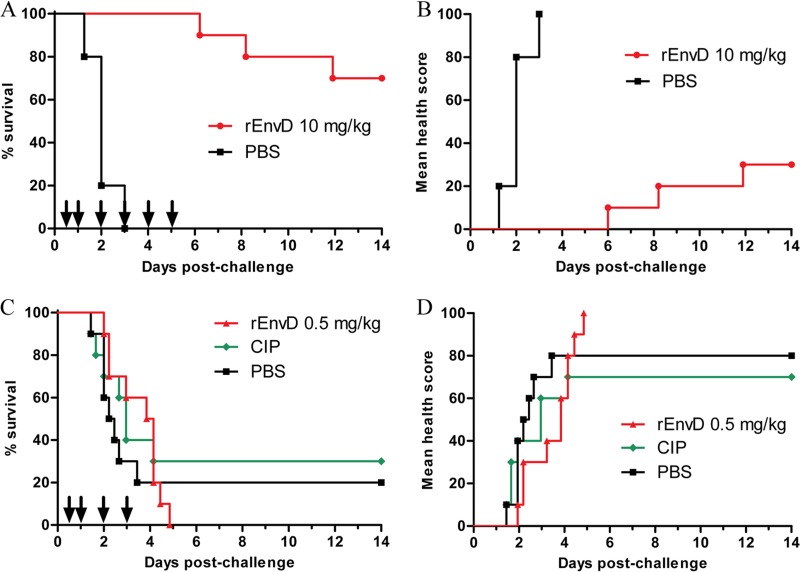
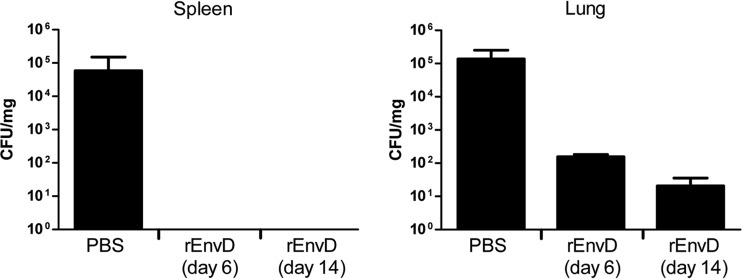
Typically, all animals in the sham-treated (PBS) control groups met humane endpoints within 72 h (median time to death, 48 h), whereas all mice treated with 10 mg/kg of rEnvD survived the treatment period (P < 0.0001; log rank test) (Fig. 2A). Nine days after termination of treatment, 70% of rEnvD-treated mice had survived (P < 0.0001, compared to survival of control animals). The protective effect of rEnvD was reflected in the comparative health status of the mice: abnormal clinical signs were absent from rEnvD-treated animals during the 5-day period of enzyme administration (Fig. 2B). At 6 and 14 days postchallenge and in contrast to results in controls, bacteria were not cultured from the blood or spleen of surviving rEnvD-treated mice from the group assigned for harvesting of tissues; sham-treated mice were found to carry a high B. anthracis bioburden in the blood (mean, 2.4 × 104 CFU/ml) and spleen (mean, 5.84 × 104 CFU/mg) at the time of postmortem examination (based on severity threshold score). High numbers of viable bacteria were also present in the lung of sham-treated animals at the same time point (mean, 1.37 × 105 CFU/mg). In comparison to levels in controls, a significant (P = 0.006311) reduction in the lung bioburden was noted in EnvD-treated animals 6 days after spore challenge (mean, 1.55 × 102 CFU/mg), and the bioburden was lower (mean, 2.07 × 101 CFU/mg) in the lung of surviving animals 14 days after challenge (Fig. 3).
FIG 2.
Impact of intravenous administration of rEnvD on inhalation anthrax in mice. Combined Kaplan-Meier survival plots (A and C) and cumulative mean clinical observation scores (B and D) for rEnvD-dosed, infected BALB/c mice. Mice were infected with B. anthracis Ames on day 0 by aerosol, followed by tail vein administration of either 10 mg/kg rEnvD or PBS vehicle (A and B) and of either 0.5 mg/kg rEnvD or PBS (C and D) at the times indicated by arrows. Ciprofloxacin (CIP; 118 mg/kg) was also administered orally for 14 days (C and D). Clinical observations were scored as described previously (13) and were based on severity of symptoms (ruffled fur, closed eyes, arched back, immobility, and weight loss).
FIG 3.
B. anthracis (CFU/mg tissue) in the spleen and lung of mice following rEnvD or PBS administration by the intravenous route (n = 7 to 10; values are means ± 1 standard deviation). PBS controls were culled when the clinical score reached threshold levels as the animals were then close to death (13). Tissues were weighed and homogenized in sterile water, the homogenates were serially diluted in sterile water and plated onto Trypticase soy agar, and the plates were incubated at 37°C for 16 to 24 h.
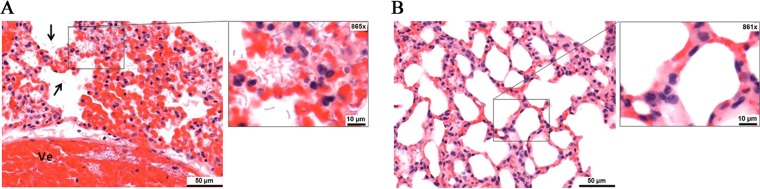
Microscopic changes attributable to infection with B. anthracis were observed in the lung and spleen of all control animals. In the lung, prominent pulmonary congestion and patchy hemorrhage, expanding septal cavities, and numerous bacilli located in alveolar spaces, walls, and within vessel lumina (bacteremia) (Fig. 4A) were observed. In the spleen, numerous bacilli within the red pulp sinusoids and vascular lumina were present in the control animals (data not shown). Further, splenic white pulp contained prominent degeneration and loss of lymphocytes, characterized by nuclear fragmentation and cellular paucity. In contrast, animals receiving rEnvD and surviving until study endpoints were found to be clear of gross pathological changes, and bacilli were not visible within lung (Fig. 4B) or spleen tissue.
FIG 4.
Pathology of lung tissue 6 days after inhalation of spores. Samples were fixed in 10% neutral buffered formalin, processed to paraffin wax, sectioned to 3 to 5 μm, stained with hematoxylin and eosin, and examined by light microscopy. Slides were randomized by a third party before microscopic examination to avoid prior knowledge of group or treatment. (A) Images from animals receiving only PBS vehicle. The region shows iatrogenic thickening of the alveolar walls due to the collapsed nature of the tissue. Arrows indicate bacilli located in alveolar spaces. Ve, vessel lumina. (B) Images from a region of inflated lung from animals receiving 10 mg/kg rEnvD over 5 days.
Experiments with 5 mg/kg rEnvD dosed over 3 days also demonstrated a high degree of protection from anthrax infection (100% survival at 3 days; 60% survival at 14 days), but 0.5 mg/kg rEnvD did not prevent the emergence of clinical symptoms and death. rEnvD administered for 5 days provided better protection than orally dosed ciprofloxacin administered by the oral route throughout the 14-day period (Fig. 2C and D).
DISCUSSION
This study provides clear evidence that prompt serial i.v. administration of small quantities of EnvD prevents the onset and progression of inhalation anthrax in a robust murine model of invasive disease. Even though the strain employed in this study is highly toxigenic, removal of the capsule during the early stages of infection appears sufficient to confound the pathogenic potential of the invading bacteria and further supports the key role of the protective PDGA capsule in anthrax pathogenesis (4, 11), highlighting the requisite nature of the capsule for in vivo dissemination of vegetative bacilli. The study also adds to growing evidence that prophylaxis and treatment of severe systemic infections can be realized by agents that do not kill the target bacterial population per se but modify the phenotype of the pathogen in a way that is beneficial to the host (16). Further, this approach has the potential to deliver exquisitely selective therapeutics that are unaffected by the presence of antibiotic resistance mechanisms.
Treatment of bacterial infections with capsule depolymerases was first explored over 80 years ago by Dubos, Avery, and colleagues at the Rockefeller Institute for Medical Research. They used an enzyme preparation from cultures of a peat soil bacterium to selectively remove the polysaccharide capsule, the pathogen's principle means of defense against immune attack, from the surface of type III pneumococci (17). Intraperitoneal administration of enzyme extracts to mice prior to challenge with type III pneumococci gave rise to type III-specific protection (18), and i.v. administration to rabbits with type III dermal infections resulted in early termination of the normally fatal infection (19). The enzyme also prevented dissemination, sterilized the blood, and promoted early recovery in nonhuman primates infected by the intratracheal and intrabronchial routes (20). In addition to our previous work on systemic neonatal E. coli infections (9, 10), capsule depolymerases have been shown to resolve potentially lethal experimental Klebsiella pneumoniae K1 infections in mice (21).
Recently, other attempts have been made to exploit PDGA depolymerases as antianthrax therapeutics. CapD is a γ-glutamyltranspeptidase elaborated by B. anthracis and catalyzes the attachment of PDGA to peptidoglycan, but it also functions as a depolymerase, effecting the release of diffusible PDGA fragments from the surface of producer strains (22). CapD mediates removal of the capsule and induces macrophage uptake and neutrophil killing in vitro (23). Intraperitoneal coinjection of CapD and vegetative B. anthracis Ames bacteria afforded some protection against infection in mice, but no significant protection could be demonstrated when the enzyme was administered after challenge with Ames spores (24), almost certainly due to the labile nature of CapD (12, 20). rEnvD is a far more robust enzyme (12) and a better candidate for therapeutic development.
Current evidence suggests that although the toxin complex undoubtedly plays a vital role in anthrax pathogenesis, probably by suppression of the immune response in early stages of the disease, death occurs from overwhelming bacteremia and sepsis due to uncontrolled bacterial proliferation and release of proinflammatory mediators (25). Thus, a therapeutic window may be available if treatment is initiated before extensive bacterial division occurs in the blood. Our results support this hypothesis: depolymerase administration initiated 12 h after aerosol challenge provided significant protection against systemic anthrax and prevented bacteremia and dissemination of bacilli to the spleen. This finding concurs with that of a previous report that the capsule is essential for hematogenous bacillary spread as capsule-negative mutants did not migrate to the spleen in experimental infections (11). In the current study, deaths generally occurred following cessation of treatment. Viable bacteria were present in the lung of rEnvD-treated mice after the treatment period, and animals that subsequently succumbed to infection almost certainly died due to delayed germination of latent spores and after enzyme had been cleared from the blood circulation. B. anthracis spores are known to persist in the lung for extended periods. For example, latent spores have been isolated from the lung tissue of nonhuman primates months after initial exposure (26). The size of the mouse restricts the number of i.v. injections that can be given over a relatively short period of time, and this issue will be addressed using larger species, such as the rabbit. In addition, the mouse is particularly susceptible to death from systemic anthrax due to uncontrolled in vivo bacterial growth and a high quantitative level of bacteremia (27), factors which do not favor an anticapsule therapeutic strategy. The relative susceptibility of humans to toxemia and infection in anthrax is poorly documented; but the rabbit is used as an equivalent to human infection (27), and examination of rEnvD in this species will be an important next step.
ACKNOWLEDGMENTS
Funding was provided by a project grant GA2014-001R from the British Society for Antimicrobial Chemotherapy, Centre for Defence Enterprise contract DSTLX1000088481 from the Defence Science and Technology Laboratory, and project grant HF5E PoC-13-020 from UCL Business.
This work was supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
REFERENCES
- 1.Leitenberg M, Zilinskas RA. 2012. The Soviet biological weapons program: a history. Harvard University Press, Cambridge, MA. [Google Scholar]
- 2.Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, McDade J, Osterholm MT, Parker G, Perl TM, Russell PK, Tonat K. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236–2252. doi: 10.1001/jama.287.17.2236. [DOI] [PubMed] [Google Scholar]
- 3.Meselson M, Guillemin J, Hugh-Jones M, Langmuir A, Popova I, Shelokov A, Yampolskaya O. 1994. The Sverdlovsk anthrax outbreak of 1979. Science 266:1202–1208. doi: 10.1126/science.7973702. [DOI] [PubMed] [Google Scholar]
- 4.Mock M, Fouet A. 2001. Anthrax. Annu Rev Microbiol 55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 5.Lincoln R, Hodges DR, Klein F, Mahlandt BG, Jones WI Jr, Haines BW, Rhian MA, Walker JS. 1965. Role of the lymphatics in the pathogenesis of anthrax. J Infect Dis 115:481–494. doi: 10.1093/infdis/115.5.481. [DOI] [PubMed] [Google Scholar]
- 6.Adalja AA, Toner E, Inglesby TV. 2015. Clinical management of potential bioterrorism-related conditions. N Engl J Med 372:954–962. doi: 10.1056/NEJMra1409755. [DOI] [PubMed] [Google Scholar]
- 7.Turnbull PCB, Sirianni NM, LeBron CI, Samaan MN, Sutton FN, Reyes AE, Peruski LF. 2004. MICs of selected antibiotics for Bacillus anthracis, Bacillus cereus, Bacillus thuringiensis, and Bacillus mycoides from a range of clinical and environmental sources as determined by the Etest. J Clin Microbiol 42:3626–3634. doi: 10.1128/JCM.42.8.3626-3634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ågren J, Finn M, Bengtsson B, Segerman B. 2014. Microevolution during an anthrax outbreak leading to clonal heterogeneity and penicillin resistance. PLoS One 9:e89112. doi: 10.1371/journal.pone.0089112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mushtaq N, Redpath MB, Luzio JP, Taylor PW. 2004. Prevention and cure of systemic Escherichia coli K1 infection by modification of the bacterial phenotype. Antimicrob Agents Chemother 48:1503–1508. doi: 10.1128/AAC.48.5.1503-1508.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mushtaq N, Redpath MB, Luzio JP, Taylor PW. 2005. Treatment of experimental Escherichia coli infection with recombinant bacteriophage-derived capsule depolymerase. J Antimicrob Chemother 56:160–165. doi: 10.1093/jac/dki177. [DOI] [PubMed] [Google Scholar]
- 11.Drysdale M, Heninger S, Hutt J, Chen Y, Lyons CR, Koehler TM. 2005. Capsule synthesis by Bacillus anthracis is required for dissemination in murine inhalation anthrax. EMBO J 24:221–227. doi: 10.1038/sj.emboj.7600495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negus D, Taylor PW. 2014. A poly-γ-d-glutamic acid depolymerase that degrades the protective capsule of Bacillus anthracis. Mol Microbiol 91:1136–1147. doi: 10.1111/mmi.12523. [DOI] [PubMed] [Google Scholar]
- 13.Hatch GJ, Bate SR, Crook A, Jones N, Funnell SG, Vipond J. 2014. Efficacy testing of orally administered antibiotics against an inhalational Bacillus anthracis infection in BALB/c mice. J Infect Dis Ther 2:175. [Google Scholar]
- 14.Stabler RA, Negus D, Pain A, Taylor PW. 2013. Draft genome sequences of Pseudomonas fluorescens BS2 and Pusillimonas noertemannii BS8, soil bacteria that cooperate to degrade the poly-γ-d-glutamic acid anthrax capsule. Genome Announc 1:e00057-12. doi: 10.1128/genomeA.00057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shargel L, Yu ABC. 1993. Applied biopharmaceutics and pharmacokinetics, 3rd ed Appleton and Lange, Norwalk, CT. [Google Scholar]
- 16.Taylor PW, Bernal P, Zelmer A. 2009. Modification of the bacterial phenotype as an approach to counter the emergence of multidrug-resistant pathogens, p 43–78. In Bonilla AR, Muniz KP (ed), Antibiotic resistance: causes and risk factors, mechanisms and alternatives. Nova Science Publishers, Hauppauge, NY. [Google Scholar]
- 17.Dubos R, Avery OT. 1931. Decomposition of the capsular polysaccharide of pneumococci type III by a bacterial enzyme. J Exp Med 54:51–71. doi: 10.1084/jem.54.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avery OT, Dubos R. 1931. The protective action of a specific enzyme against type III pneumococcus infection in mice. J Exp Med 54:73–89. doi: 10.1084/jem.54.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodner K, Dubos R, Avery OT. 1932. The action of a specific enzyme upon the dermal infection of rabbits with type III pneumococcus. J Exp Med 55:393–404. doi: 10.1084/jem.55.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francis T, Terrell EE, Dubos R, Avery OT. 1934. Experimental type III pneumococcus pneumonia in monkeys. II. Treatment with an enzyme which decomposes the specific capsular polysaccharide of pneumococcus type III. J Exp Med 59:641–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin TL, Hsieh PF, Huang YT, Lee WC, Tsai YT, Su PA, Pan YJ, Hsu CR, Wu MC, Wang JT. 2014. Isolation of a bacteriophage and its depolymerase specific for K1 capsule of Klebsiella pneumoniae: implication in typing and treatment. J Infect Dis 210:1734–1744. doi: 10.1093/infdis/jiu332. [DOI] [PubMed] [Google Scholar]
- 22.Candela T, Fouet A. 2005. Bacillus anthracis CapD, belonging to the γ-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol Microbiol 57:717–726. doi: 10.1111/j.1365-2958.2005.04718.x. [DOI] [PubMed] [Google Scholar]
- 23.Scorpio A, Chabot DJ, Day WA, O'Brien DK, Vietri NJ, Itoh Y, Mohamadzadeh M, Friedlander AM. 2007. Poly-γ-glutamate capsule-degrading enzyme treatment enhances phagocytosis and killing of encapsulated Bacillus anthracis. Antimicrob Agents Chemother 51:215–222. doi: 10.1128/AAC.00706-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scorpio A, Tobery SA, Ribot WJ, Friedlander AM. 2008. Treatment of experimental anthrax with recombinant capsule depolymerase. Antimicrob Agents Chemother 52:1014–1020. doi: 10.1128/AAC.00741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coggeshall KM, Lupu F, Ballard J, Metcalf JP, James JA, Farris D, Kurosawa S. 2013. The sepsis model: an emerging hypothesis for the lethality of inhalation anthrax. J Cell Mol Med 17:914–920. doi: 10.1111/jcmm.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson DW, Peacock S, Belton FC. 1956. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J Hyg (Lond) 54:28–36. doi: 10.1017/S0022172400044272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goossens PL. 2009. Protective antigen as a correlative marker for anthrax in animal models. Mol Aspects Med 30:467–480. doi: 10.1016/j.mam.2009.07.005. [DOI] [PubMed] [Google Scholar]